Heat Enhancement of Ethylene Glycol/Water Mixture in the Presence of Gyroid TPMS Structure: Experimental and Numerical Comparison
Abstract
1. Introduction
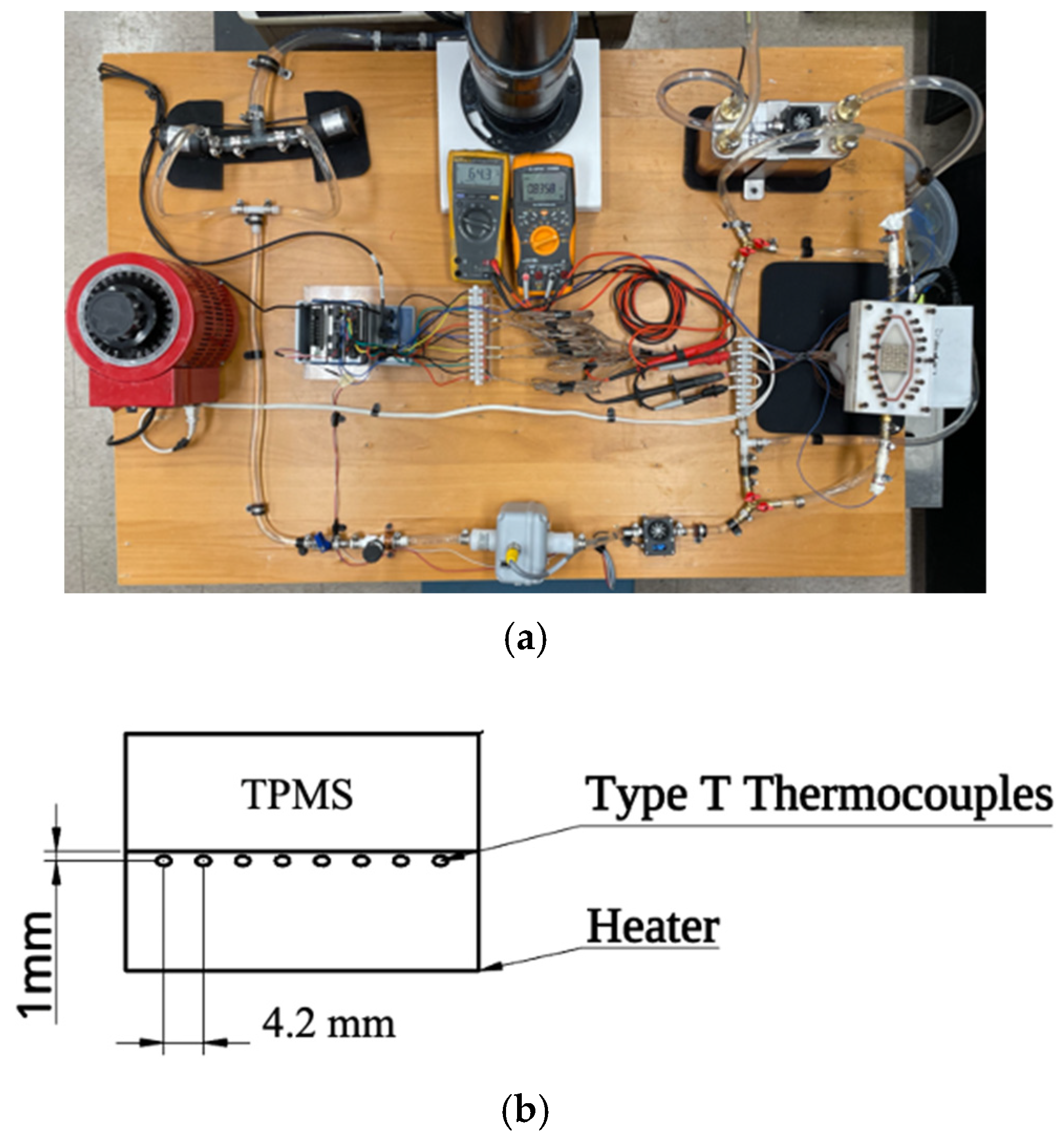
2. Experimental Study
2.1. Experiment Description
2.2. Sensitivity Analysis
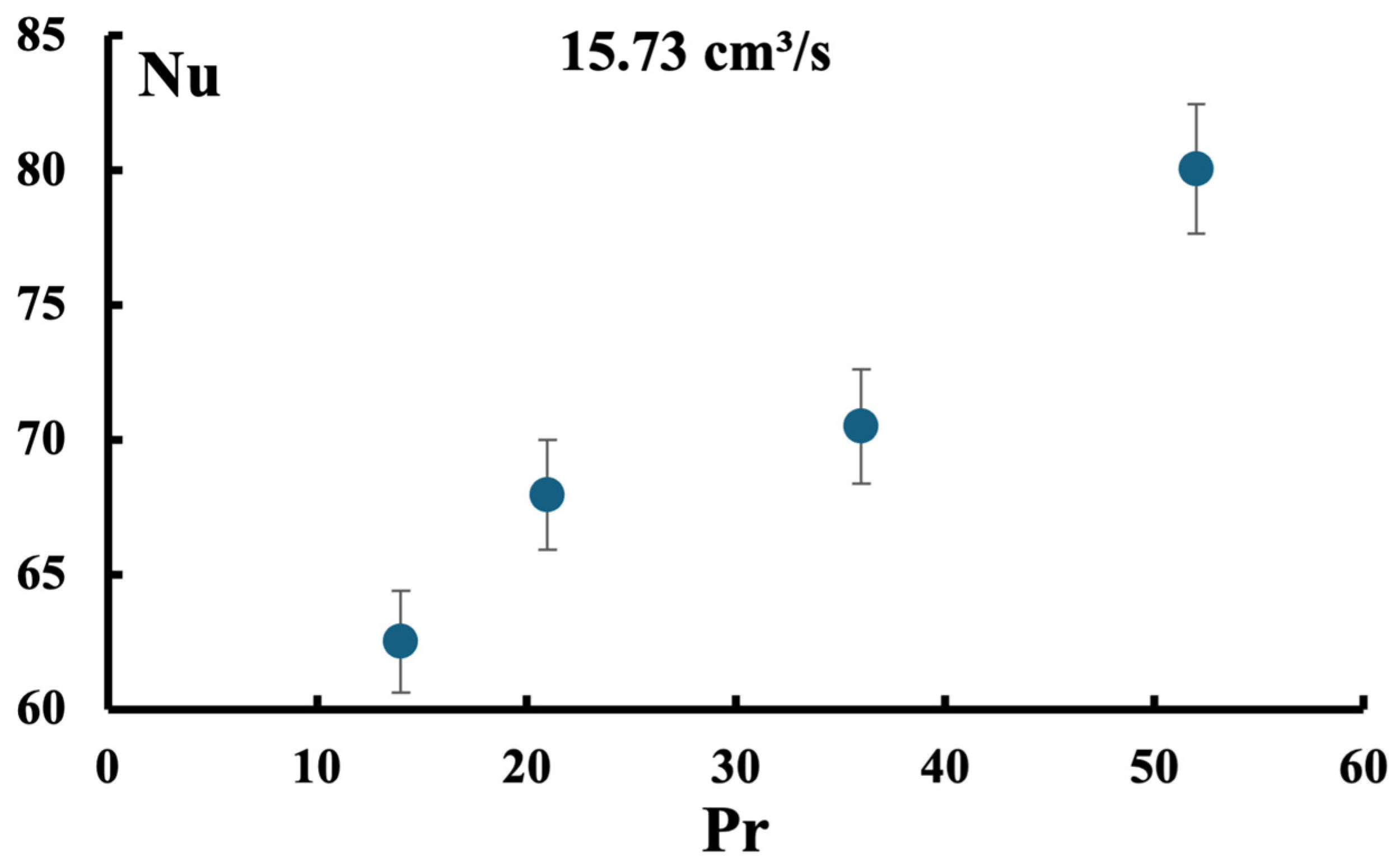
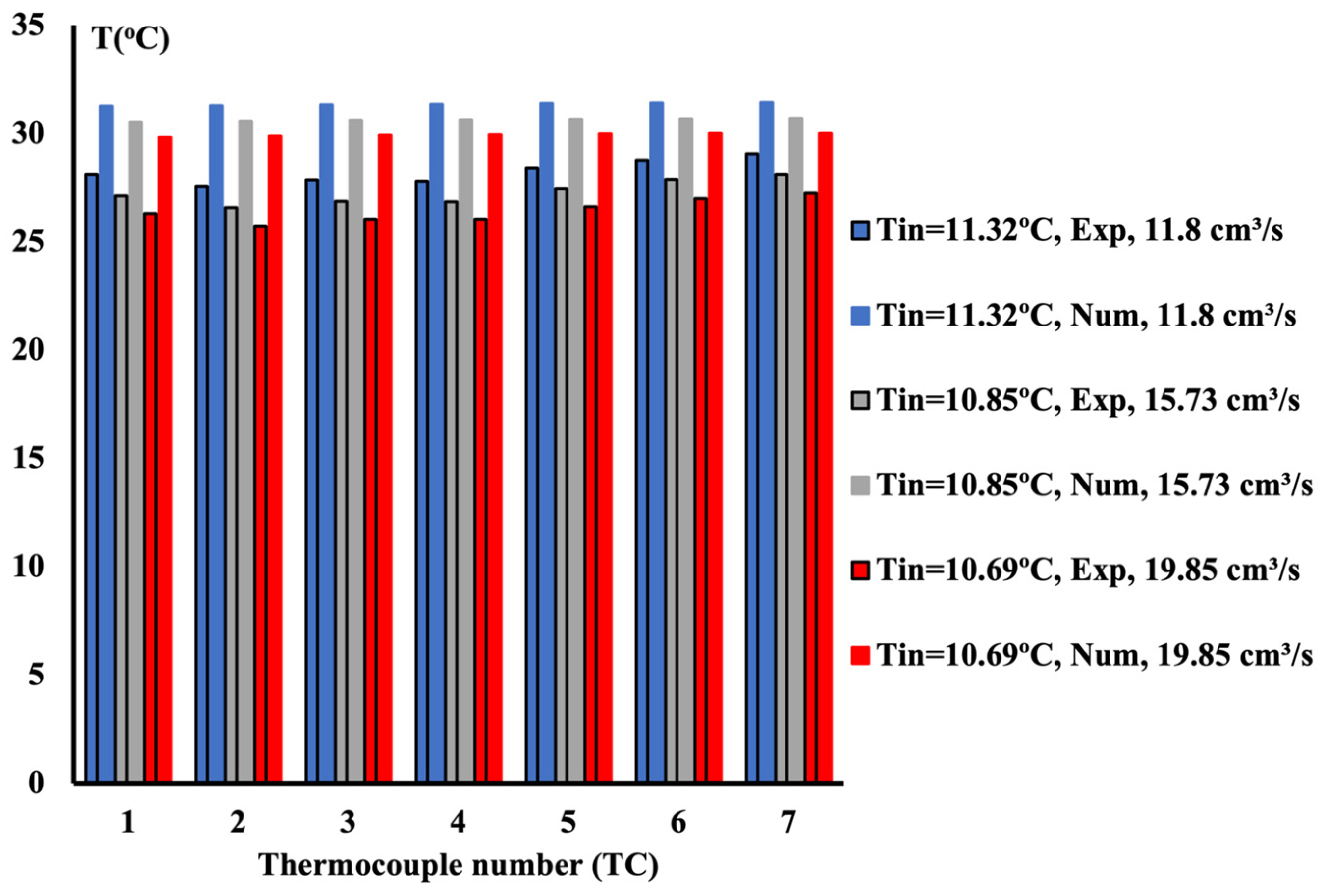
2.3. Experimental Results for EG/Water Using Aluminum TPMS
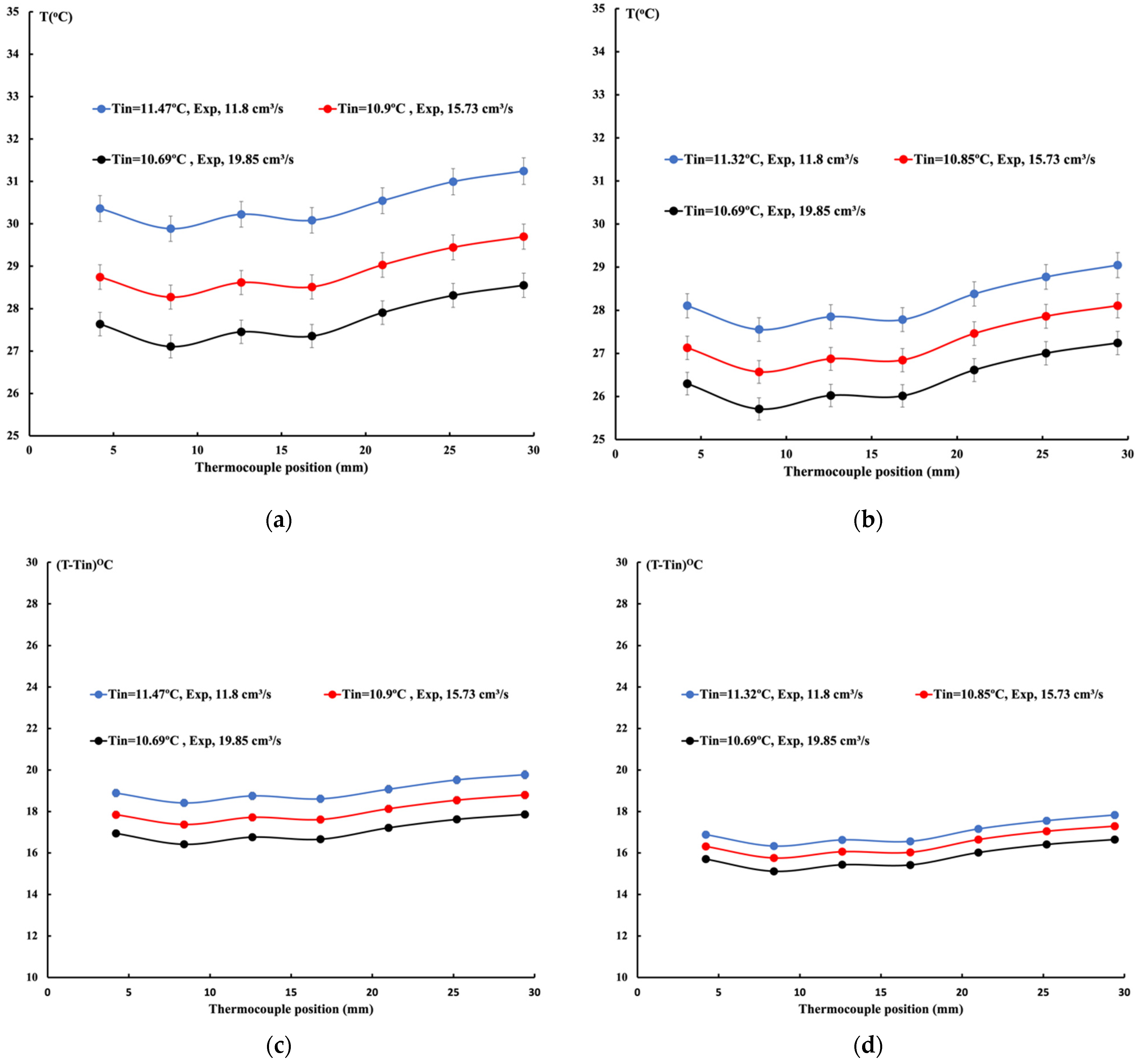
2.3.1. 10%vol Ethylene Glycol/Water Mixture
2.3.2. 20%vol Ethylene Glycol/Water Mixture
2.3.3. 30%vol Ethylene Glycol/Water Mixture
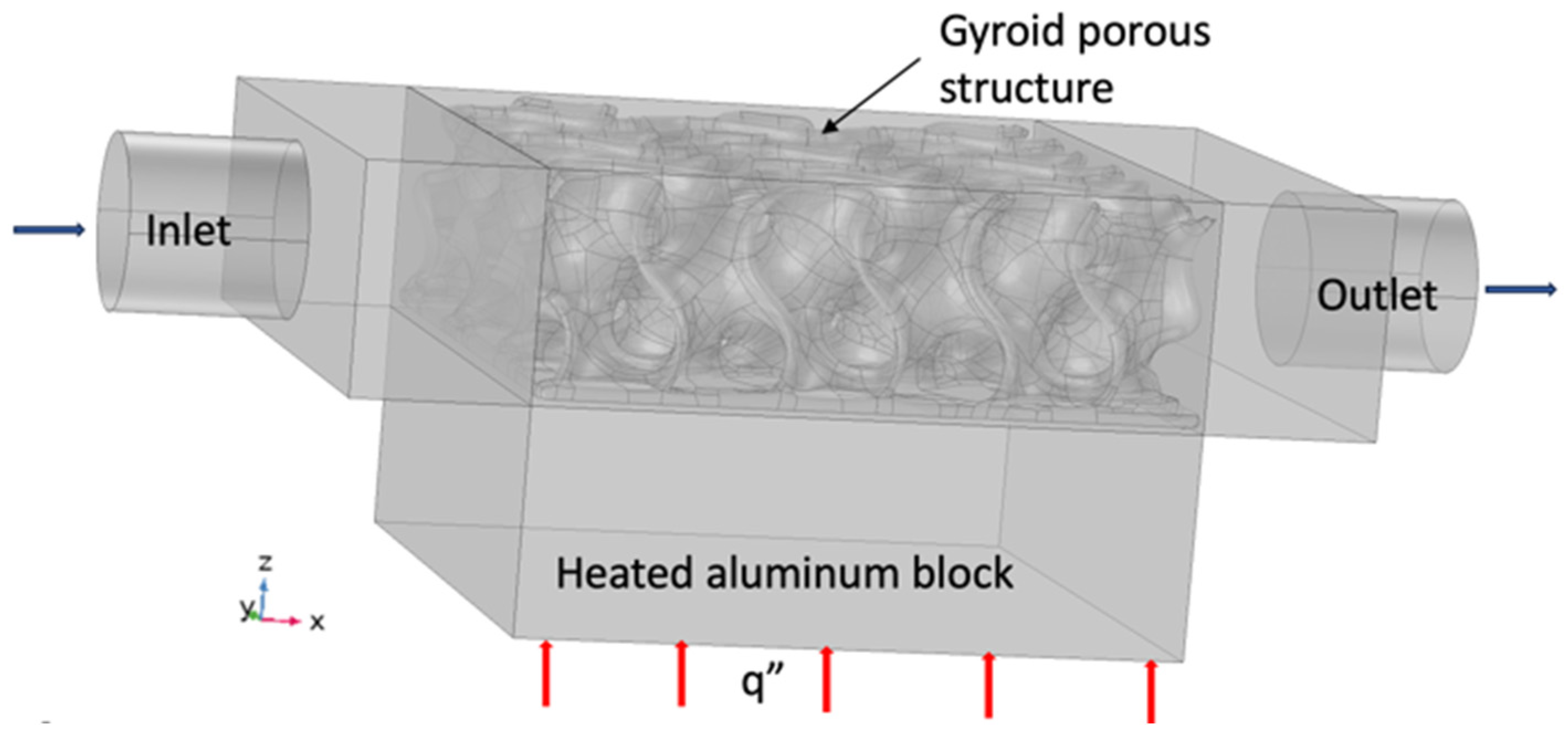
3. Finite Element Study
Boundary Conditions of the System
- (i)
- The velocity u = uin in the x direction is applied at the inlet.
- (ii)
- At the inlet, the temperature of the fluid enters the test section at T = Tin.
- (iii)
- At the outlet, an open boundary is applied where the stresses are equal to zero.
- (iv)
- The bottom surface of the aluminum block is heated with a heat flux q″, as shown in red.
- (v)
- All external surfaces are assumed adiabatic, , and for the flow, no-slip boundary conditions are applied.
4. Comparison Between Experimental Data and Numerical Model
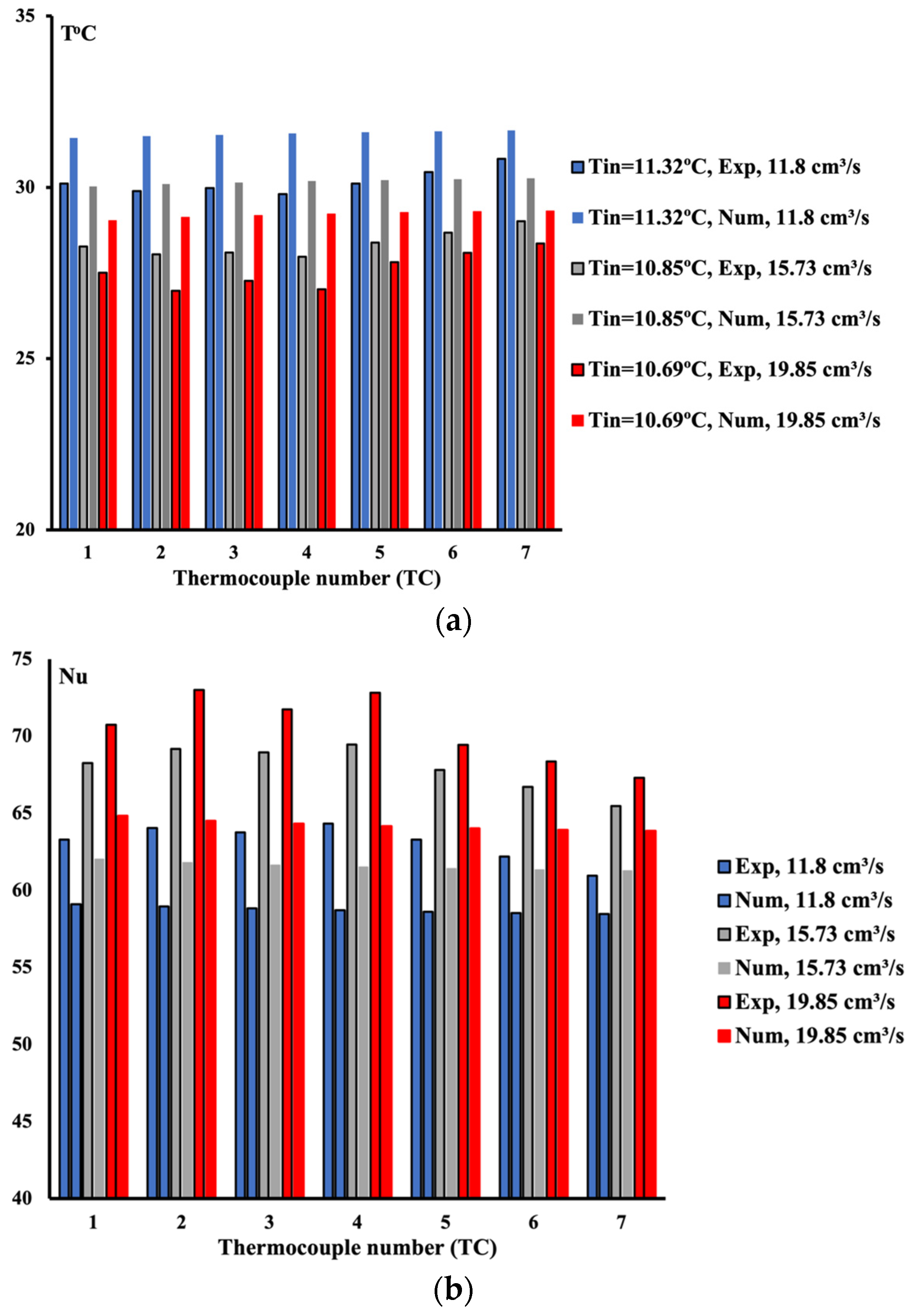
4.1. 10%vol Ethylene Glycol/Water Mixture
4.2. 20%vol Ethylene Glycol/Water Mixture
5. Conclusions
- The presence of triply periodic minimal surfaces maintains a uniform cooling mechanism regardless of the fluid mixture used;
- The heat enhancement presented by the Nusselt number is nearly identical regardless of the mixture tested experimental and verified numerically;
- Due to the viscous effect, the higher the ethylene glycol concentration can secure a more significant pressure drop, thus a higher friction coefficient;
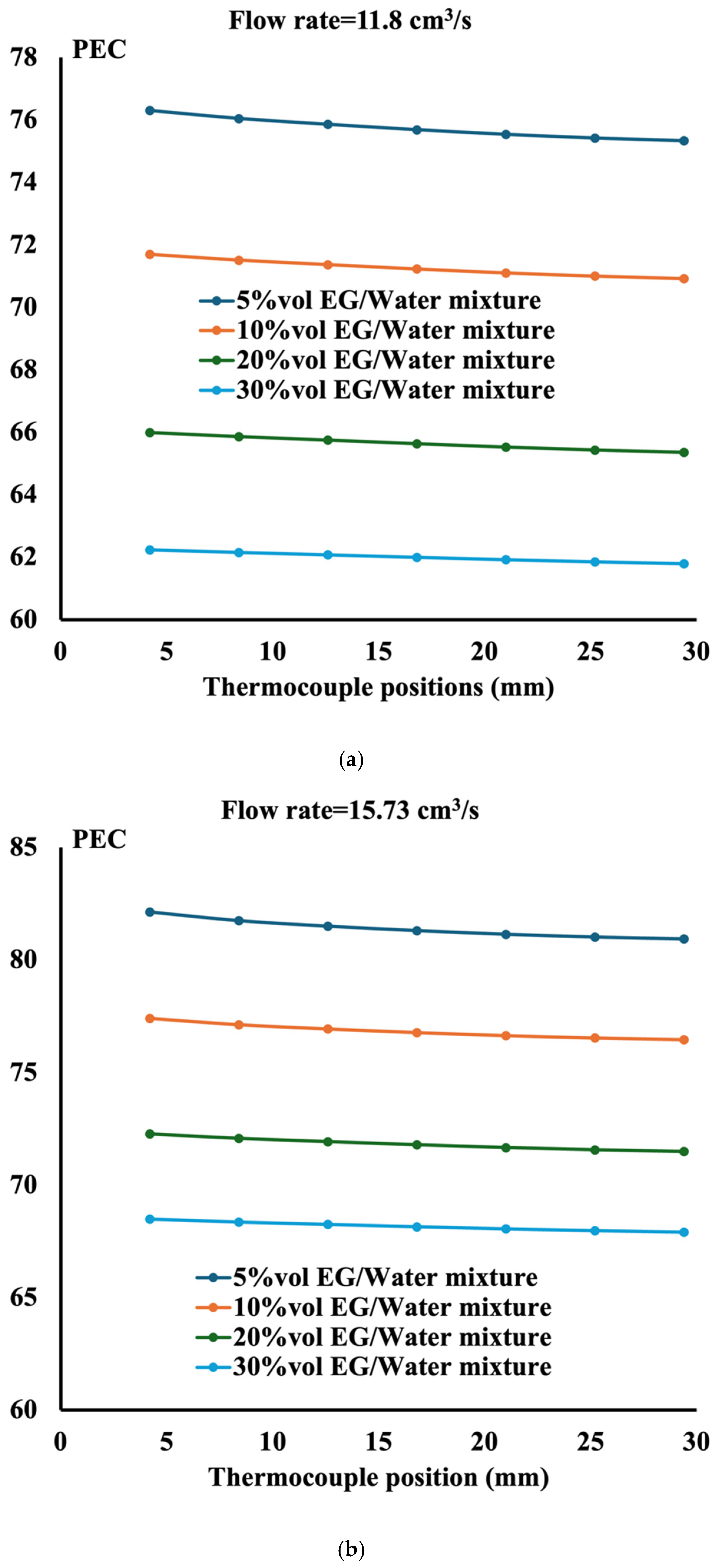
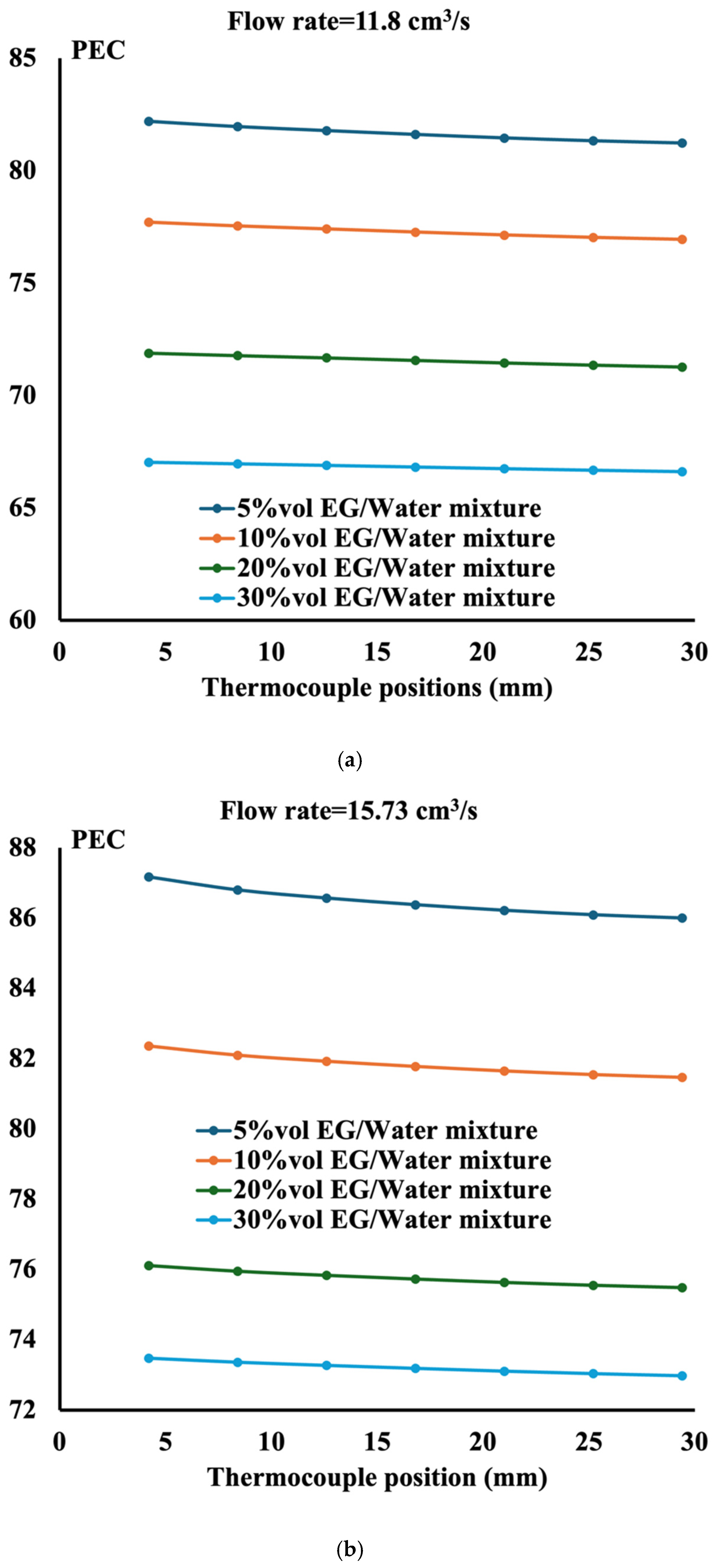
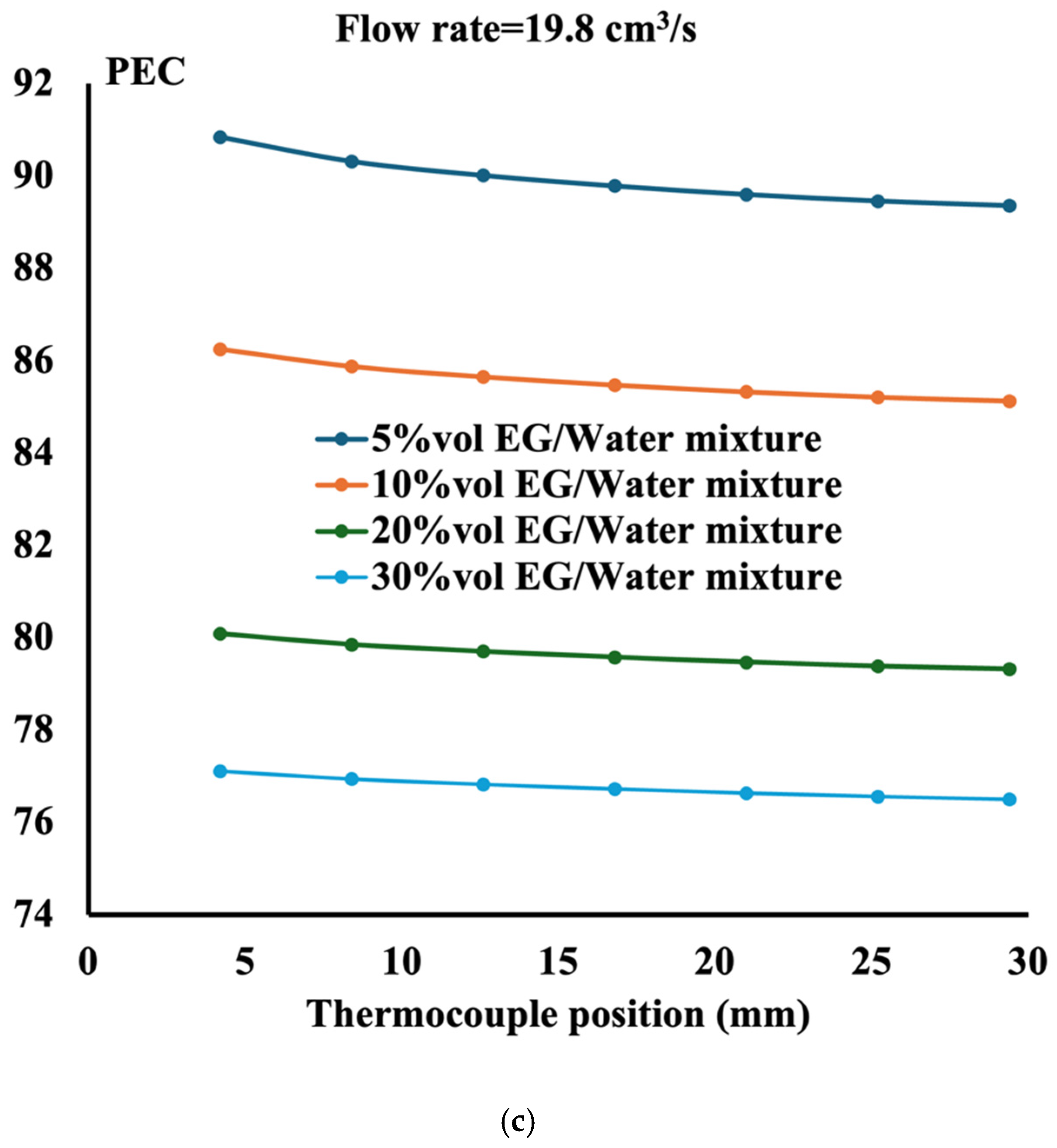
- The performance evaluation criteria show that the highest magnitude is obtained at the highest flow rate and the lowest ethylene glycol concentration;
- The performance of the ethylene glycol water mixture is less than that of distilled water. However, if the environment requires liquid to sustain low temperature, then a 5%vol ethylene glycol water mixture is recommended;
- As the concentration of ethylene glycol increases, for a constant flow rate, the drop in pressure increases, respectively;
- The performance evaluation criteria reach the maximum value of 90 when the mixture is composed of 5%vol ethylene glycol in water with aluminum TPMS;
- In the presence of a silver TPMS, the maximum performance evaluation criterion is around 95 with a 5% ethylene glycol/water mixture.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| The pressure difference (N/m2) | Fluid density | ||
| T | Temperature in °K | Fluid viscosity | |
| Tin | Inlet temperature (°K) | Cp | Fluid specific heat |
| uin | Inlet velocity (m/s) | kf | Fluid conductivity |
| Nu | Local Nusselt number | h | Local heat transfer coefficient (W/m2·K) |
| D | Inlet cylinder diameter (m) | q″ | Heat flux applied to the bottom of the aluminum block (W/m2·K) |
| p | Pressure (N/m2) | u, v, w | Velocity in x, y and z direction (m/s) |
| Tout | Outlet Temperature (°K) | PEC | Performance Evaluation Criterion |
| L | TPMS length (m) | Re = | Reynolds number |
| TC | Thermocouple |
References
- Welsford, C.A.; Delisle, C.S.; Plant, R.D.; Saghir, M.Z. Effects of nanofluid concentration and channeling on the thermal effectiveness of highly porous open-cell foam metals: A numerical and experimental study. J. Therm. Anal. Calorim. 2020, 140, 1507–1517. [Google Scholar] [CrossRef]
- Hajialibabaei, M.; Saghir, M.Z. A critical review of the straight and wavy microchannel heat sink and the application in lithium-ion battery thermal management. Int. J. Thermofluids 2022, 14, 100153. [Google Scholar] [CrossRef]
- Kerme, E.D.; Hajialibabei, M.; Saghir, M.Z.; El-Ketan, O. Experimental investigation of porous gyroid structure: Effect of cell size and porosity on performance. Therm. Sci. Eng. Prog. 2024, 53, 102728. [Google Scholar] [CrossRef]
- Sanitjai, S.; Goldstein, R.J. Heat transfer from a circular cylinder to mixtures of water and ethylene glycol. Int. J. Heat Mass Transf. 2004, 47, 4785–4794. [Google Scholar] [CrossRef]
- Gowen, R.A.; Smith, J.W. The effect of the Prandtl number on temperature profiles for heat transfer in turbulent pipe flow. Chem. Eng. Sci. 1967, 22, 1701–1711. [Google Scholar] [CrossRef]
- Obot, N.T.; Das, L.; Rabas, T.J. Smooth-and Enhanced-Tube Heat Transfer and Pressure Drop: Part I. Effect of Prandtl Number with Air, Water, and Glycol/Water Mixtures(No. ANL/ET/CP-102885); Argonne National Lab.: Lemont, IL, USA, 2000. [Google Scholar]
- Obot, N.T.; Das, L.; Vakili, D.E.; Green, R.A. Effect of Prandtl number on smooth-tube heat transfer and pressure drop. Int. Commun. Heat Mass Transf. 1997, 24, 889–896. [Google Scholar]
- Keklikcioglu, O.; Dagdevir, T.; Ozceyhan, V. Second law analysis of a mixture of ethylene glycol/water flow in modified heat exchanger tube by passive heat transfer enhancement technique. J. Therm. Anal. Calorim. 2020, 140, 1307–1320. [Google Scholar] [CrossRef]
- Jafari, M.; Farajollahi, A.; Gazori, H. The experimental investigation concerning the heat transfer enhancement via a four-point star swirl generator in the presence of water–ethylene glycol mixtures. J. Therm. Anal. Calorim. 2021, 144, 167–178. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, A.; Zhang, J.; Wang, Y. Experimental investigation on the friction characteristics of water–ethylene glycol mixture flow in internal helical finned horizontal tubes. Exp. Therm. Fluid Sci. 2017, 89, 1–8. [Google Scholar] [CrossRef]
- Dehghandokht, M.; Khan, M.G.; Fartaj, A.; Sanaye, S. Flow and heat transfer characteristics of water and ethylene glycol–water in a multi-port serpentine meso-channel heat exchanger. Int. J. Therm. Sci. 2011, 50, 1615–1627. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, Y.; Liu, C. Investigation on liquid thermal conductivity of ethylene glycol (EG)/water mixtures: A comparative experimental and molecular dynamics simulation study. J. Mol. Liq. 2023, 384, 122268. [Google Scholar] [CrossRef]
- Joy, B.; Philip, J.; Zachariah, R. Investigations on serpentine tube type solar photovoltaic/thermal collector with different heat transfer fluids: Experiment and numerical analysis. Sol. Energy 2016, 140, 12–20. [Google Scholar] [CrossRef]
- Go, E.M.; Shin, E.; Cha, J.; Kwak, S.K. Estimation of heat transfer coefficient of water and ethylene glycol mixture in nanopipe via non-equilibrium coarse-grained molecular dynamics. J. Ind. Eng. Chem. 2019, 77, 128–134. [Google Scholar] [CrossRef]
- Ahdavi, M.; Sharifpur, M.; Ghodsinezhad, H.; Meyer, J.P. Experimental and numerical study of the thermal and hydrodynamic characteristics of laminar natural convective flow inside a rectangular cavity with water, ethylene glycol–water and air. Exp. Therm. Fluid Sci. 2016, 78, 50–64. [Google Scholar] [CrossRef]
- Yang, J.; Jacobi, A.; Liu, W. Heat transfer correlations for single-phase flow in plate heat exchangers based on experimental data. Appl. Therm. Eng. 2017, 113, 1547–1557. [Google Scholar] [CrossRef]
- Zhou, N.; Feng, H.; Guo, Y.; Chen, H.; Liu, W.; Peng, H.; Lei, Y.; Deng, S.; Xu, Y. Experimental study on the spray cooling heat transfer performance and dimensionless correlations for ethylene glycol water solution. Appl. Therm. Eng. 2022, 214, 118824. [Google Scholar] [CrossRef]
- Gemicioğlu, B.; Demircan, T. Experimental analysis of the effects of using different water-ethylene glycol mixture rates on heat transfer performance in a heat. Eskişehir Osman. Üniversitesi Mühendislik Mimar. Fakültesi Derg. 2021, 29, 145–157. [Google Scholar] [CrossRef]
- Thiyana, T.; Junaedi, A.; Rahman, M.A.; Sukarman, S.; Khoirudin, K.; Azizah, R.L. The Heat Transfer Coefficient in a Copper Pipe Flow System Using a 40/60 Volume Ratio Ethylene Glycol/Water (EG/H2O) Blended Fluid. J. Tek. Mesin Mech. Xplore 2023, 4, 37–46. [Google Scholar] [CrossRef]
- Mohan, S.V.; Kuriakose, J.; Nidhin, A.R.; Varghese, B.; Fidus, F. Experimental Comparison of Heat Transfer in a Down Flow Louvered Fin Auto Radiator using Water, Water and Ethylene Glycol Mixture & Water, Ethylene Glycol and Sugarcane Juice Mixture as Coolants. Glob. Res. Dev. J. Eng. 2017, 2, 215–223. [Google Scholar]
- Peyghambarzadeh, S.M.; Hashemabadi, S.H.; Hoseini, S.M.; Jamnani, M.S. Experimental study of heat transfer enhancement using water/ethylene glycol based nanofluids as a new coolant for car radiators. Int. Commun. Heat Mass Transf. 2011, 38, 1283–1290. [Google Scholar] [CrossRef]
- Kanthimathi, T.; Bhramara, P.; Abhiram, G. Experimental investigations on thermal performance of double pipe heat exchanger using EG-water-based sic nanofluid. J. Enhanc. Heat Transf. 2020, 27, 249–266. [Google Scholar] [CrossRef]
- Pérez-Tavernier, J.; Vallejo, J.P.; Cabaleiro, D.; Fernández-Seara, J.; Lugo, L. Heat transfer performance of a nano-enhanced propylene glycol: Water mixture. Int. J. Therm. Sci. 2019, 139, 413–423. [Google Scholar] [CrossRef]
- Saghir, M.Z.; Kilic, G.A. Experimental Forced Convection Study Using a Triply Periodic Minimal Surface Porous Structure with a Nanofluid: Comparison with Numerical Modeling. Appl. Sci. 2024, 14, 7594. [Google Scholar] [CrossRef]
- Hajialibabei, M. Experimental and Numerical Study on Thermal Performance and Optimization of Novel Wavy and Straight Channel Heat Sinks for Better Performance. Ph.D. Thesis, Toronto Metropolitan University, Toronto, ON, Canada, 2024. [Google Scholar]







| Parameters | Components | Uncertainty |
|---|---|---|
| Temperature | T-type thermocouple | ±0.75% |
| Flow rate | Digital flow meters | ±0.44% |
| Nu number | - | ±2.44% |
| Liquid Mixture | Density (kg/m3) | Dynamic Viscosity Kg/m·s | Heat Capacity J/kg·C | Thermal Conductivity W/m·K | Prandtl Number |
|---|---|---|---|---|---|
| 5%vol EG | 1004.12 | 0.002 | 4092 | 0.595 | 14 |
| 10%vol EG | 1010.05 | 0.003 | 4002 | 0.577 | 21 |
| 20%vol EG | 1021.89 | 0.0051 | 3822 | 0.540 | 36 |
| 30%vol EG | 1033.735 | 0.00713 | 3642 | 0.504 | 52 |
| Liquid Mixture | Flow Rate (cm3/s) | Inlet Velocity (m/s) | Reynolds Number |
|---|---|---|---|
| 5%vol EG | 11.8 | 0.15 | 744 |
| 15.7 | 0.2 | 993 | |
| 19.6 | 0.25 | 1240 | |
| 10%vol EG | 11.8 | 0.15 | 498 |
| 15.7 | 0.2 | 664 | |
| 19.6 | 0.25 | 829 | |
| 20%vol EG | 11.8 | 0.15 | 301 |
| 15.7 | 0.2 | 402 | |
| 19.6 | 0.25 | 502 | |
| 30%vol EG | 1.96 | 0.025 | 36 |
| 15.7 | 0.2 | 290 |
| Flow Rate = 15.73 cm3/s, Aluminum Gyroid | |||||||
|---|---|---|---|---|---|---|---|
| TC | Position (mm) | 10%vol EG/Water | 10%vol EG/Water | 20%vol EG/Water | 20%vol EG/Water | 30%vol EG/Water | 30%vol EG/Water |
| T (°C) | (T-Tin) °C | T (°C) | (T-Tin) °C | T (°C) | (T-Tin) °C | ||
| 1 | 4.2 | 28.27 | 17.42 | 28.74 | 17.84 | 28.05 | 16.88 |
| 2 | 8.4 | 28.04 | 17.19 | 28.27 | 17.37 | 27.37 | 16.20 |
| 3 | 12.6 | 28.09 | 17.24 | 28.61 | 17.71 | 27.88 | 16.71 |
| 4 | 16.8 | 27.97 | 17.12 | 28.51 | 17.61 | 27.81 | 16.64 |
| 5 | 21 | 28.39 | 17.54 | 29.02 | 18.12 | 28.38 | 17.21 |
| 6 | 25.2 | 28.67 | 17.82 | 29.44 | 18.54 | 28.79 | 17.62 |
| 7 | 29.4 | 29.01 | 18.16 | 29.69 | 18.79 | 29.03 | 17.86 |
| Temperature T(°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | Flow Rate = 11.8 cm3/s | Flow Rate = 15.73 cm3/s | Flow Rate = 19.85 cm3/s | ||||||
| Exp. | Num. | %Diff | Exp. | Num. | %Diff | Exp. | Num. | %Diff | |
| 1 | 30.11 | 31.44 | 4.41 | 28.27 | 30.02 | 6.20 | 27.50 | 29.03 | 5.55 |
| 2 | 29.89 | 31.49 | 5.35 | 28.04 | 30.09 | 7.31 | 26.98 | 29.12 | 7.94 |
| 3 | 29.97 | 31.53 | 5.20 | 28.09 | 30.14 | 7.27 | 27.26 | 29.18 | 7.01 |
| 4 | 29.80 | 31.57 | 5.92 | 27.97 | 30.18 | 7.90 | 27.02 | 29.22 | 8.15 |
| 5 | 30.11 | 31.61 | 4.98 | 28.39 | 30.21 | 6.429 | 27.82 | 29.26 | 5.19 |
| 6 | 30.44 | 31.64 | 3.93 | 28.67 | 30.24 | 5.457 | 28.08 | 29.29 | 4.29 |
| 7 | 30.83 | 31.66 | 2.69 | 29.01 | 30.26 | 4.29 | 28.36 | 29.31 | 3.34 |
| Temperature T(°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | Flow Rate = 11.8 cm3/s | Flow Rate = 15.73 cm3/s | Flow Rate = 19.85 cm3/s | ||||||
| Exp. | Num. | %Diff | Exp. | Num. | %Diff | Exp. | Num. | %Diff | |
| 1 | 27.37 | 29.70 | 8.509 | 26.30 | 28.96 | 10.10 | 25.40 | 28.32 | 11.48 |
| 2 | 26.97 | 29.74 | 10.28 | 25.84 | 29.02 | 12.27 | 24.92 | 28.39 | 13.92 |
| 3 | 27.23 | 29.78 | 9.33 | 26.08 | 29.05 | 11.38 | 25.16 | 28.44 | 13.02 |
| 4 | 27.09 | 29.81 | 10.03 | 25.93 | 29.09 | 12.19 | 25.00 | 28.48 | 13.89 |
| 5 | 27.84 | 29.84 | 7.19 | 26.76 | 29.12 | 8.79 | 25.87 | 28.51 | 10.17 |
| 6 | 28.09 | 29.87 | 6.32 | 27.01 | 29.14 | 7.87 | 26.09 | 28.53 | 9.36 |
| 7 | 28.39 | 29.89 | 5.28 | 27.29 | 29.16 | 6.84 | 26.37 | 28.55 | 8.27 |
| Temperature T (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | Flow Rate = 11.8 cm3/s | Flow Rate = 15.73 cm3/s | Flow Rate = 19.85 cm3/s | ||||||
| Exp. | Num. | %Diff | Exp. | Num. | %Diff | Exp. | Num. | %Diff | |
| 1 | 30.35 | 33.29 | 9.67 | 28.74 | 31.65 | 10.11 | 27.63 | 30.67 | 10.98 |
| 2 | 29.88 | 33.34 | 11.56 | 28.27 | 31.70 | 12.15 | 27.10 | 30.73 | 13.40 |
| 3 | 30.22 | 33.37 | 10.43 | 28.61 | 31.75 | 10.95 | 27.45 | 30.78 | 12.14 |
| 4 | 30.08 | 33.41 | 11.09 | 28.51 | 31.79 | 11.50 | 27.35 | 30.82 | 12.70 |
| 5 | 30.54 | 33.45 | 9.52 | 29.02 | 31.82 | 9.63 | 27.90 | 30.86 | 10.59 |
| 6 | 30.99 | 33.48 | 8.03 | 29.44 | 31.85 | 8.19 | 28.31 | 30.88 | 9.10 |
| 7 | 31.24 | 33.51 | 7.25 | 29.69 | 31.87 | 7.34 | 28.55 | 30.90 | 8.25 |
| Temperature T (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | Flow Rate = 11.8 cm3/s | Flow Rate = 15.73 cm3/s | Flow Rate = 19.85 cm3/s | ||||||
| Exp. | Num. | %Diff | Exp. | Num. | %Diff | Exp. | Num. | %Diff | |
| 1 | 28.10 | 31.25 | 11.22 | 27.12 | 30.51 | 12.49 | 26.29 | 29.83 | 13.45 |
| 2 | 27.55 | 31.29 | 13.55 | 26.56 | 30.55 | 15.01 | 25.70 | 29.89 | 16.26 |
| 3 | 27.85 | 31.31 | 12.45 | 26.87 | 30.58 | 13.83 | 26.02 | 29.92 | 15.00 |
| 4 | 27.78 | 31.35 | 12.84 | 26.84 | 30.61 | 14.05 | 26.01 | 29.95 | 15.16 |
| 5 | 28.37 | 31.38 | 10.58 | 27.45 | 30.64 | 11.59 | 26.61 | 29.98 | 12.66 |
| 6 | 28.77 | 31.41 | 9.16 | 27.85 | 30.66 | 10.06 | 27.00 | 30.00 | 11.12 |
| 7 | 29.04 | 31.43 | 8.21 | 28.10 | 30.68 | 9.16 | 27.23 | 30.02 | 10.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saghir, M.Z.; Yahya, M.; Ortiz, P.D.; Impellizzeri, S.; Al-Ketan, O. Heat Enhancement of Ethylene Glycol/Water Mixture in the Presence of Gyroid TPMS Structure: Experimental and Numerical Comparison. Processes 2025, 13, 228. https://doi.org/10.3390/pr13010228
Saghir MZ, Yahya M, Ortiz PD, Impellizzeri S, Al-Ketan O. Heat Enhancement of Ethylene Glycol/Water Mixture in the Presence of Gyroid TPMS Structure: Experimental and Numerical Comparison. Processes. 2025; 13(1):228. https://doi.org/10.3390/pr13010228
Chicago/Turabian StyleSaghir, Mohamad Ziad, Mohamad Yahya, Pedro D. Ortiz, Stefania Impellizzeri, and Oraib Al-Ketan. 2025. "Heat Enhancement of Ethylene Glycol/Water Mixture in the Presence of Gyroid TPMS Structure: Experimental and Numerical Comparison" Processes 13, no. 1: 228. https://doi.org/10.3390/pr13010228
APA StyleSaghir, M. Z., Yahya, M., Ortiz, P. D., Impellizzeri, S., & Al-Ketan, O. (2025). Heat Enhancement of Ethylene Glycol/Water Mixture in the Presence of Gyroid TPMS Structure: Experimental and Numerical Comparison. Processes, 13(1), 228. https://doi.org/10.3390/pr13010228









