The Effect of Oxidation on Coal’s Molecular Structure and the Structure Model Construction of Oxidized Coal Molecular
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal Samples
2.2. Main Research Methods
2.2.1. Vitreous Reflectance of Coal Samples
2.2.2. X-Ray Photoelectron Spectroscopy (XPS) Tests
2.2.3. Fourier Transform Infrared (FT-IR) Spectroscopy Tests
2.2.4. Solid-State 13C-NMR Tests
3. Results and Discussion
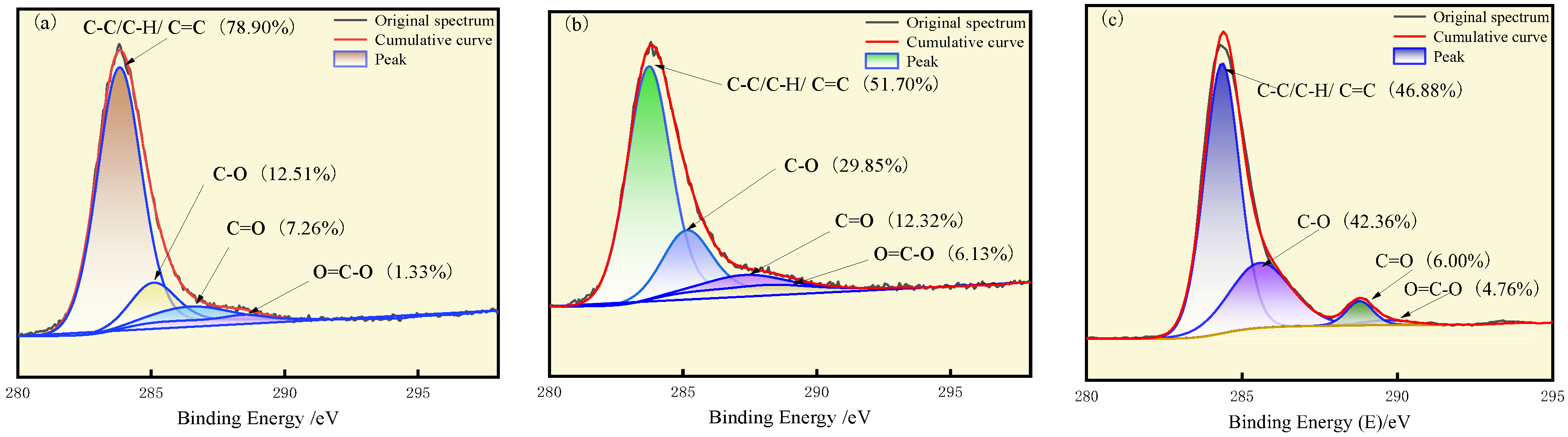
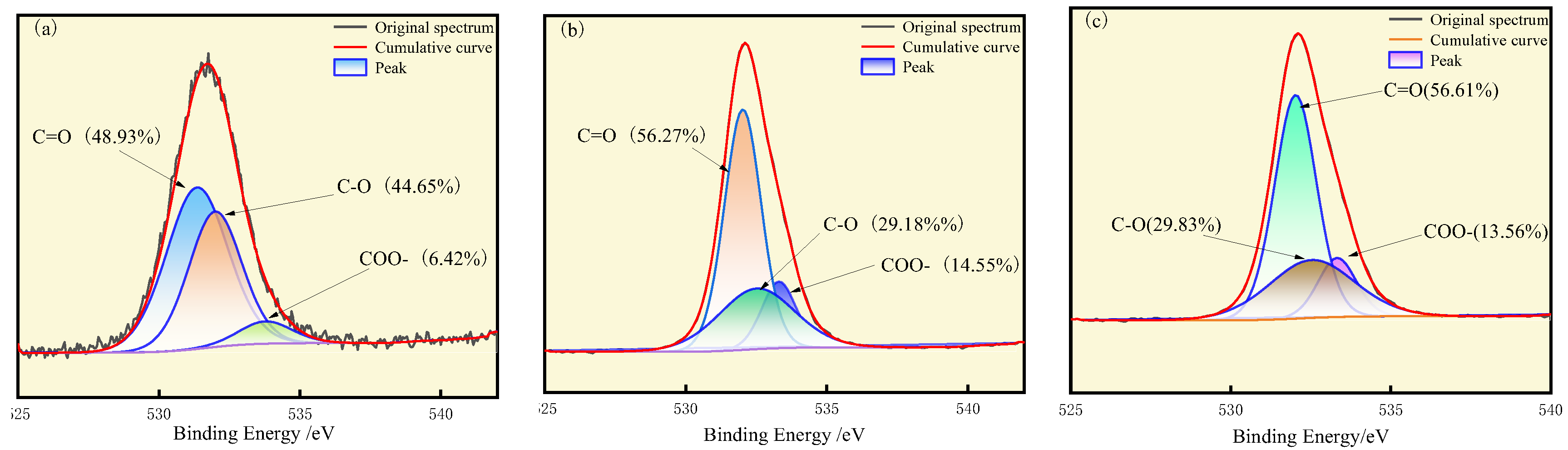
3.1. XPS Analysis
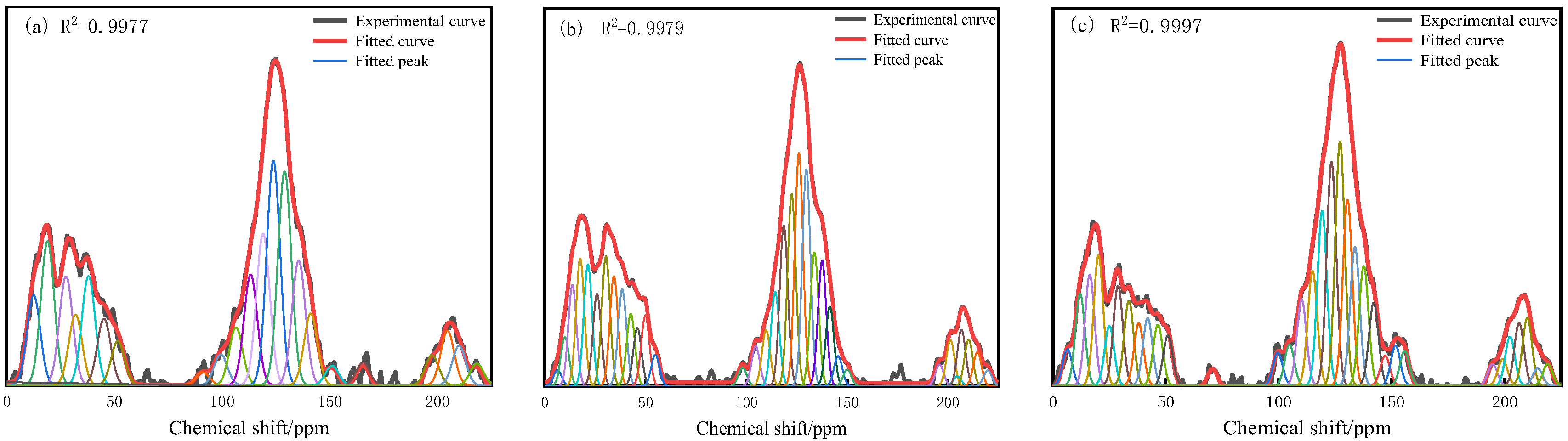
3.2. 13C-NMR Analysis
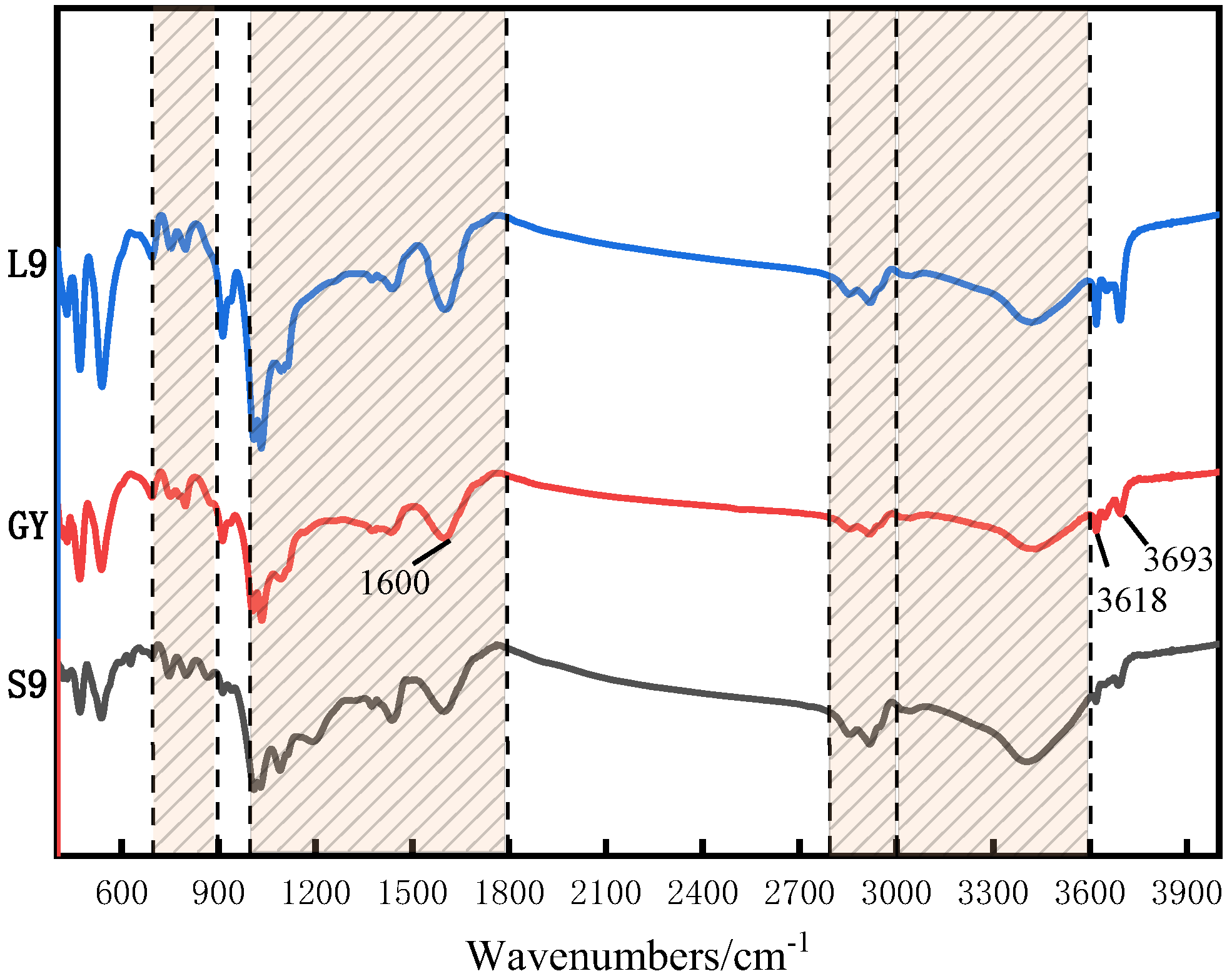
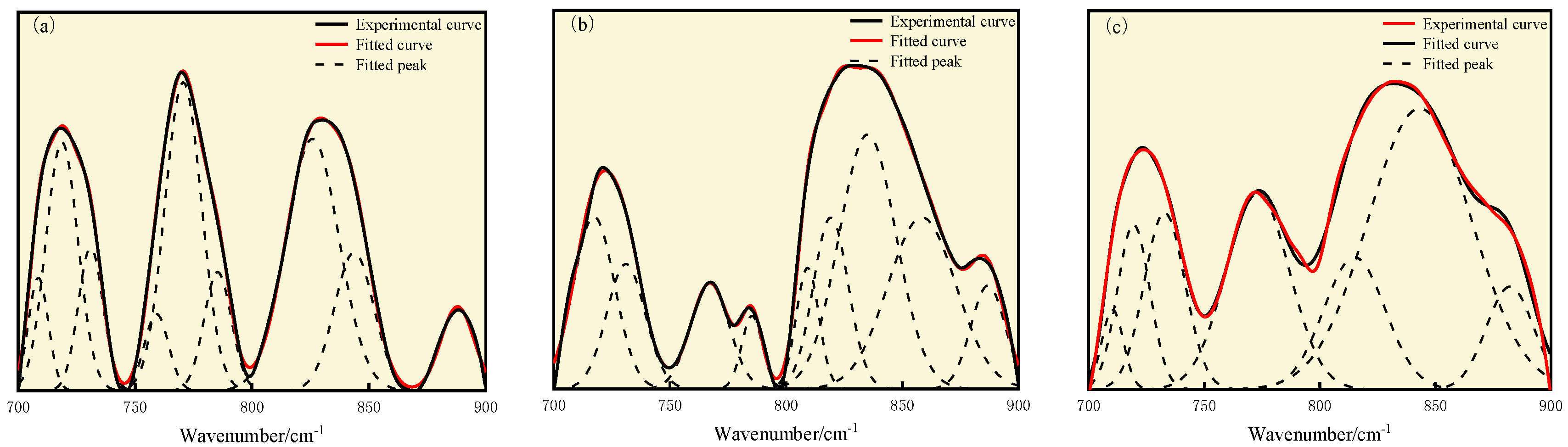
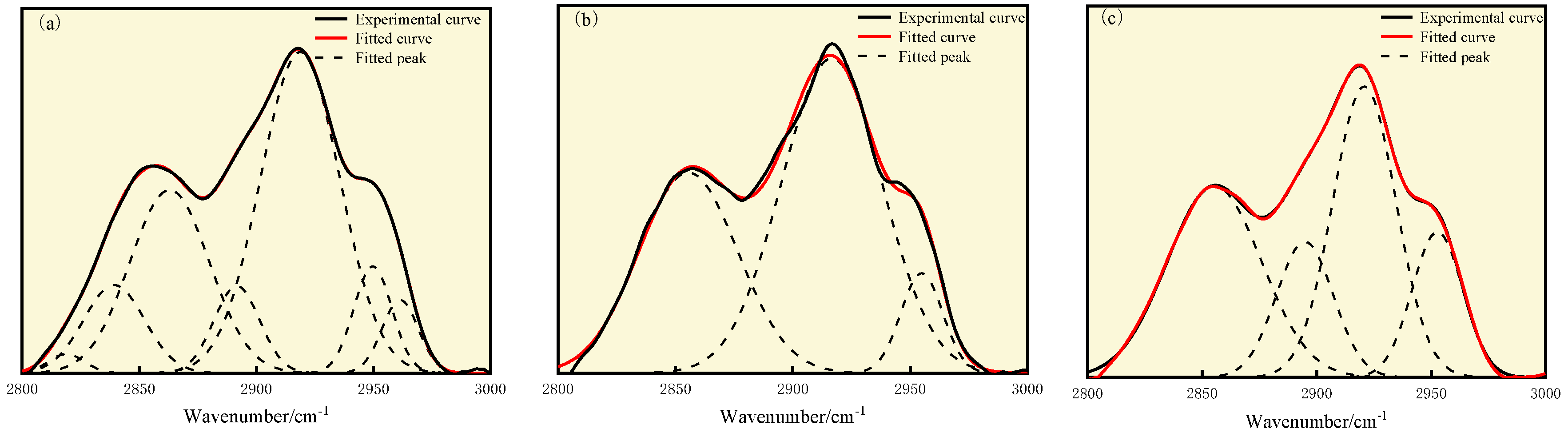
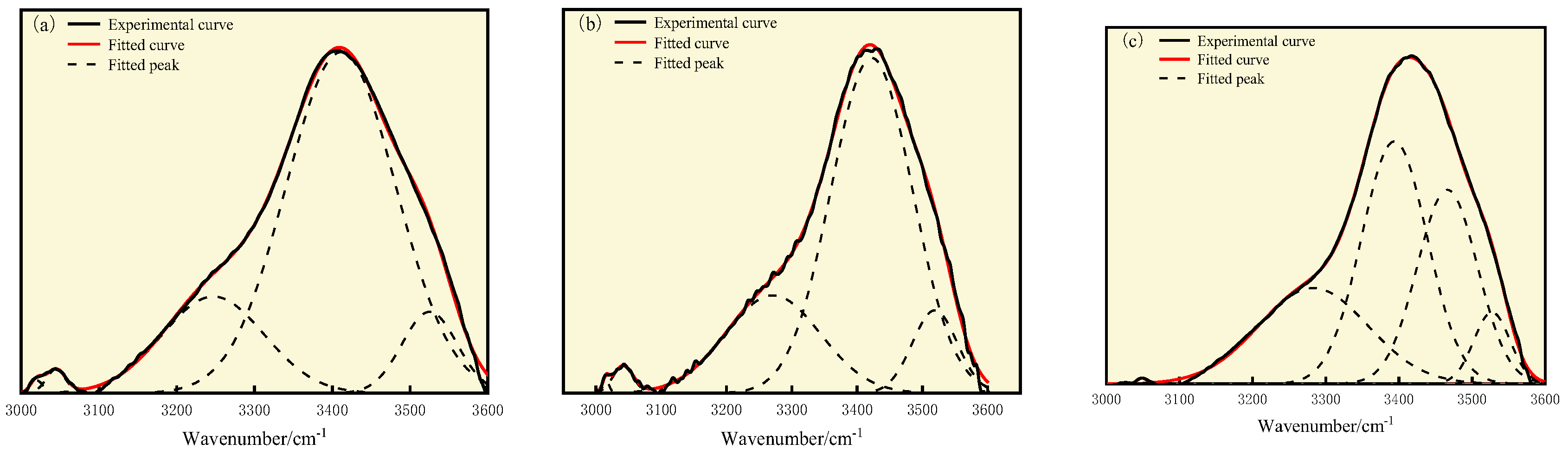
3.3. FT-IR Analysis
4. Construction and Optimization of Coal Molecular Structure Models
4.1. Aromatic Structure
4.2. Fat Structure
4.3. Heteroatom Structure
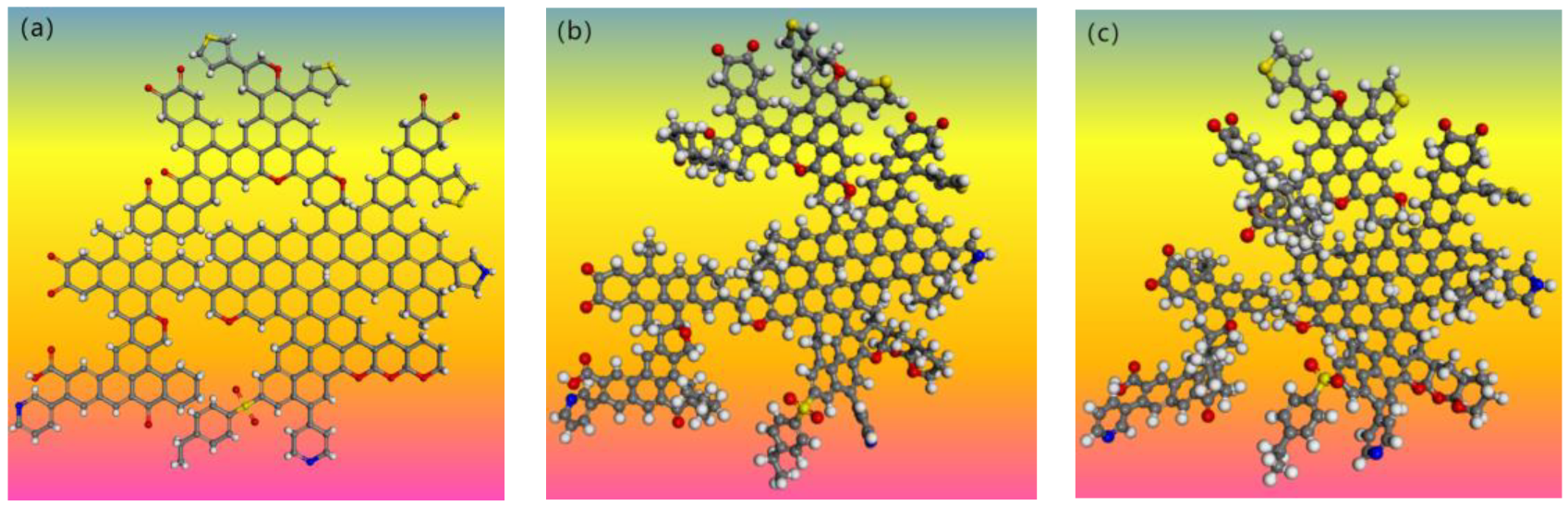
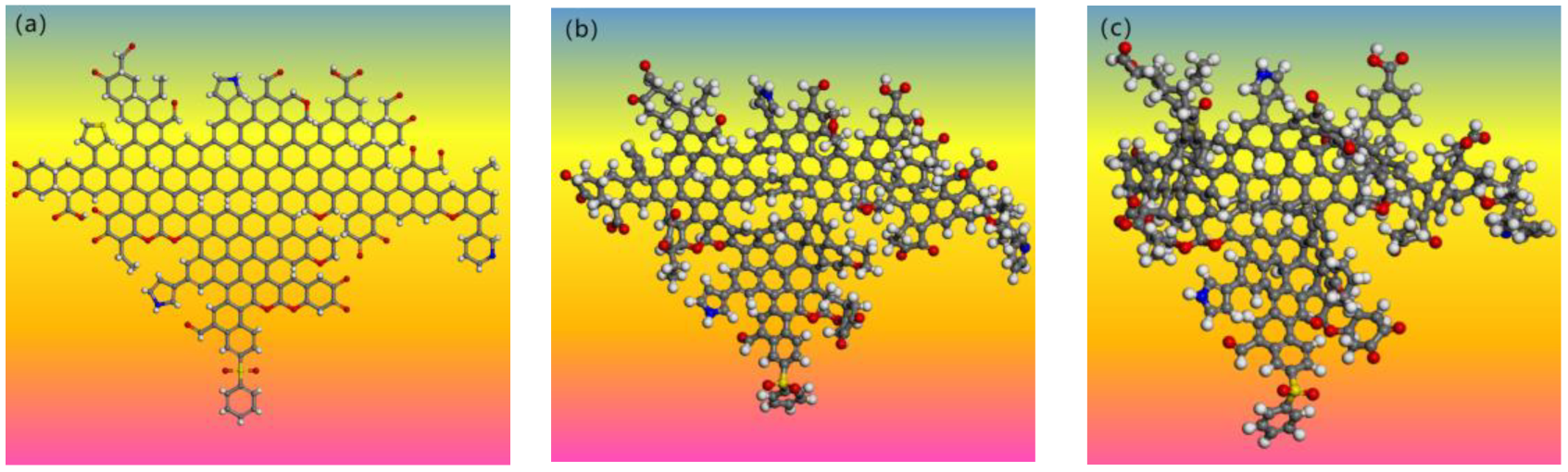
4.4. Modeling of Coal Molecular Structure
4.5. Optimization of Macromolecular Model for Oxidized Coal
4.6. The Influence of Oxidation on the Molecular Structure of Coal
5. Conclusions
- (1)
- S9, GY, and L9 are all 1/3 coking coal. The coal was comprehensively characterized by elemental analysis, FT-IR, XPS, and 13C-NMR, with oxidation degrees of 21.10%, 48.30%, and 53.12%, respectively. The molecular formula of S9 coal is C228H165N3O21S4, with a molecular weight of 3411. The molecular formula of GY coal is C244H171N3O31S2, with a molecular weight of 3705. The molecular formula of L9 coal is C225H177N3O33S2, with a molecular weight of 3515. Finally, energy optimization was performed on the three coal samples, and the rationality of their structural models was verified through nuclear magnetic resonance prediction and elemental analysis.
- (2)
- The XBP of S9, GY, and L9 coal samples are 0.3786, 0.3351, and 0.2228, respectively. The average methylene chain lengths are 4.9569, 2.6843, and 1.9055, respectively. As the degree of oxidation increases, the XBP gradually decreases and the Cn gradually decreases. This indicates that the condensation degree of aromatic compounds is gradually decreasing, indicating that the oxidation process will destroy the bridging carbon structure, reduce the number of bridging carbons, and make the spatial arrangement of molecules more dispersed.
- (3)
- Oxidation leads to the formation of nitrogen oxides from pyrrole and pyridine nitrogen. The proportion of nitrogen oxides increases with oxidation, accounting for 10.08%, 17.18%, and 19.88%, respectively, in the three coal types. Thiophene sulfur undergoes oxidation to sulfoxide, which is subsequently oxidized to sulfone. As oxidation progresses, the proportion of thiophene compounds gradually decreases.
- (4)
- Although existing technologies still have certain limitations in constructing coal molecular structure models, it has certain guiding significance for further analyzing the impact of oxidation on coal molecular structure patterns and exploring the influence of the oxidation degree on coal samples.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Li, Q.; Han, Y. A numerical investigation on kick control with the displacement kill method during a well test in a deep-water gas reservoir: A case study. Processes 2024, 12, 2090. [Google Scholar] [CrossRef]
- Belousov, A.; Lushpeev, V.; Sokolov, A. Hartmann–Sprenger Energy Separation Effect for the Quasi-Isothermal Pressure Reduction of Natural Gas: Feasibility Analysis and Numerical Simulation. Energies 2024, 17, 2010. [Google Scholar] [CrossRef]
- Bondarenko, A.V.; Islamov, S.R.; Ignatyev, K.V.; Mardashov, D.V. Laboratory studies of polymer compositions for well-kill under increased fracturing. Perm J. Pet. Min. Eng. 2020, 20, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, S.; Demir, U.; Sahbazet, O. The effects of dodecylamine, kerosene and pH on batch flotation of Turkey’s Tuncbilek coa. Int. J. Miner. Process. 2008, 88, 65–71. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Coal oxidation at low temperatures: Oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. [Google Scholar] [CrossRef]
- Shobhana, D. Enhancement in hydrophobicity of low rank coal by surfactants—A critical overvie. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar]
- Xia, W.; Xie, G.; Liang, C. Flotation behavior of different size fractions of fresh and oxidized coals. Powder Technol. 2014, 267, 80–85. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Analysis of the mechanism of the low-temperature oxidation of coal. Combust. Flame 2003, 134, 107–117. [Google Scholar] [CrossRef]
- Eterigho-Ikelegbe, O.; Yoro, K.O.; Bada, S. Coal as a filler in polymer composites: A review. Resour. Conserv. Recycl. 2021, 174, 105756. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Hao, Z. Dissolution behavior and chemical characteristics of low molecular weight compounds from tectonically deformed coal under tetrahydrofuran extraction. Fuel 2019, 257, 116030. [Google Scholar] [CrossRef]
- Kawashima, H.; Takanohashi, T. Modification of model structures of upper freeport coal extracts using 13C-NMR chemical shift calculation. Energy Fuels 2001, 15, 591–598. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Li, P. Investigation on solubility of multicomponents from semi-anthracite coal and its effect on coal structure by Fourier transform infrared spectroscopy and X-ray diffraction. Fuel Process. Technol. 2018, 174, 123–131. [Google Scholar] [CrossRef]
- Feng, W.; Li, Z.; Gao, H.; Wang, Q.; Bai, H.; Li, P. Understanding the Molecular Structure of HSW Coal at Atomic Level: A Comprehensive Characterization from Combined Experimental and Computational Study. Green Energy Environ. 2021, 6, 150–159. [Google Scholar] [CrossRef]
- Lian, L.; Qin, Z.; Li, C.; Zhou, J.; Chen, Q.; Yang, X.; Lin, Z. Molecular Model Construction of the Dense Medium Component Scaffold in Coal for Molecular Aggregate Simulation. ACS Omega 2020, 5, 13375–13383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, Q.; Wei, S.; Yun, T.; Yan, G.; Yan, K. Large Scale Molecular Model Construction of Xishan Bituminous Coal. Energy Fuels 2017, 31, 1310–1317. [Google Scholar] [CrossRef]
- Zhang, J.; Weng, X.; Han, Y.; Li, W.; Cheng, J.; Gan, Z.; Gu, J. The Effect of Supercritical Water on Coal Pyrolysis and Hydrogen Production: A Combined ReaxFF and DFT Study. Fuel 2013, 108, 682–690. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Liu, J.; Wang, Z.; Gong, X.; Guo, L.; Song, W. Pyrolysis of Liulin coal simulated by GPU-based ReaxFF MD with cheminformatics analysis. Energy Fuels 2014, 28, 522–534. [Google Scholar] [CrossRef]
- Hatcher, P.G. Chemical structural models for coalified wood (vitrinite) in low rank coal. Org. Geochem. 1990, 16, 959–968. [Google Scholar] [CrossRef]
- Meng, J.Q.; Zhong, R.Q.; Li, S.C.; Yin, F.F.; Nie, B.S. Molecular model construction and study of gas adsorption of Zhaozhuang coal. Energy Fuel 2018, 32, 9727–9737. [Google Scholar] [CrossRef]
- Guo, S.; Geng, W.; Yuan, S.; Yi, C.; Dong, Z.; Xu, J. Understanding the Molecular Structure of Datong Coal by Combining Experimental and Computational Study. J. Mol. Struct. 2023, 1279, 135035. [Google Scholar] [CrossRef]
- Li, N.; Zhu, M.; Zhang, J.; He, B.; Li, Z.; Wu, Y.; Wu, J.; Zhang, H.; Bai, H. Features and insights for molecular structure of Chinese Taixi anthracite at atomic scale. J. Mol. Struct. 2024, 1308, 138071. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Yao, W.; Jiang, X.; Jiang, X. Molecular Characterization of Henan Anthracite Coal. Energy Fuels 2019, 33, 6215–6225. [Google Scholar] [CrossRef]
- Qin, X.; Yang, T.; Liu, Z. Molecular structure, bond cleavage and their relation of four low rank coals. Fuel Process. Technol. 2022, 236, 107391. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Xue, Y.; Yan, C.; Han, S. Chemical Structural Characteristics of High Inertinite Coal. Fuel 2021, 286, 119283. [Google Scholar] [CrossRef]
- GB/T212 2008; Proximate Analysis of Coal. Standards Press of China: Beijing, China, 2008.
- GB/T476 2008; Determination of Carbon and Hydrogen in Coal. Standards Press of China: Beijing, China, 2008.
- GB/T6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. Standards Press of China: Beijing, China, 2008.
- Xiang, J.; Zeng, F.; Bin, L.I. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation. J. Fuel Chem. Technol. 2013, 41, 391–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Zhong, Q. A large-scale molecular model of Fenghuangshan anthracite coal. Fuel 2021, 295, 120616. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, X.; Peng, Y. Understanding and improving the flotation of coals with different degrees of surface oxidation. Powder Technol. 2017, 321, 190–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Jing, K. Study on the pore structure and oxygen-containing functional groups devoting to the hydrophilic force of dewatered lignite. Appl. Surf. Sci. 2015, 324, 90–98. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Tan, H. Co-pyrolysis of pyridine and pyrrole as nitrogenous compounds model of coal. Asian J. Chem. 2010, 22, 6998. [Google Scholar]
- Meng, L.; Zhang, X.; Li, N. Study on sulfur transformation during the drying of Lignite and sulfur distribution in pyrolysis. J. Anal. Appl. Pyrolysis 2024, 180, 106535. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, M. Model construction and optimization of coal molecular structure. J. Mol. Struct. 2023, 1290, 135960. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Yan, J.; Lei, Z.; Li, Z. Molecular structure characterization of low-medium rank coals via XRD, solid state 13C-NMR and FTIR spectroscopy. Fuel 2020, 268, 117038. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.; Chen, S. Structural characterization and molecular model construction of lignite: A case of xianfeng coal. Energies 2024, 17, 1049. [Google Scholar] [CrossRef]
- Fang, X.U.; Hui LI, U.; Qing, W. Comparison of Huolinhe lignite structural features by using 13C-NMR & FTIR techniques. CIESC J. 2017, 68, 4272. [Google Scholar]
- Qian, L.; Tao, C.; Ma, C. Construction of a macromolecular structure model for Zhundong subbituminous coal. J. Mol. Struct. 2022, 1248, 131496. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, G.; Zhao, Y. Construction of the molecular structure model of the Shengli lignite using TG-GC/MS and FT-IR spectrometry data. Fuel 2017, 203, 924–931. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Dai, C. Molecular structure modeling and comparative analysis of macerals inertinite concentrates from Lingwu and Qinghua bituminous coals. J. China Coal Soc. 2022, 47 (Suppl. S1), 171–183. [Google Scholar]
- Fu, Y. Study on structural evolution characteristics of structural coal macromolecular structure based on infrared spectroscopy and nuclear magnetic resonance. Fresenius Environ. Bull. 2020, 29, 9043–9054. [Google Scholar]
- Jia, J.; Wang, D.; Li, B. Study on molecular structure characteristic and optimization of molecular model construction of coal with different metamorphic grade. J. Mol. Struct. 2024, 1295, 136655. [Google Scholar] [CrossRef]





| Coal Sample | Proximate Analysis (%) | Ultimate Analysis (%) | Atomic Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cdaf | Hdaf | Odaf | Ndaf | Sdaf | H/C | O/C | N/C | S/C | |
| S9 | 1.30 | 12.38 | 27.75 | 58.57 | 79.14 | 4.79 | 10.76 | 1.36 | 3.95 | 0.73 | 0.10 | 0.01 | 0.02 |
| GY | 2.41 | 26.12 | 22.49 | 48.98 | 78.92 | 4.63 | 13.81 | 1.49 | 1.16 | 0.70 | 0.13 | 0.02 | 0.01 |
| L9 | 5.94 | 29.44 | 23.14 | 41.48 | 76.26 | 5.03 | 15.47 | 1.38 | 1.87 | 0.79 | 0.15 | 0.02 | 0.01 |
| Coal Sample | Romax/% | Standard Deviation/% |
|---|---|---|
| S9 | 0.93 | 0.056 |
| GY | 0.96 | 0.079 |
| L9 | 0.90 | 0.050 |
| Coal Sample | O/% | C/% | Si/% | Al/% | N/% |
|---|---|---|---|---|---|
| S9 | 14.78 | 73.62 | 2.47 | 7.54 | 1.59 |
| GY | 24.71 | 62.10 | 6.79 | 4.59 | 1.80 |
| L9 | 26.86 | 59.95 | 5.86 | 5.76 | 1.58 |
| Peak Number | Chemical Shift | Peak Area/% | Peak Number | Chemical Shift | Peak Area/% |
|---|---|---|---|---|---|
| 1 | 12.46 | 4.95 | 12 | 119.01 | 8.32 |
| 2 | 18.92 | 8.90 | 13 | 123.80 | 12.32 |
| 3 | 27.50 | 5.98 | 14 | 129.00 | 11.72 |
| 4 | 31.89 | 3.89 | 15 | 135.54 | 6.85 |
| 5 | 37.94 | 5.99 | 16 | 141.12 | 3.94 |
| 6 | 45.16 | 3.66 | 17 | 151.04 | 1.08 |
| 7 | 51.58 | 2.43 | 18 | 165.35 | 1.26 |
| 8 | 90.94 | 0.72 | 19 | 197.44 | 1.65 |
| 9 | 99.32 | 1.69 | 20 | 204.61 | 2.00 |
| 10 | 106.43 | 3.17 | 21 | 209.99 | 2.17 |
| 11 | 113.23 | 6.08 | 22 | 218.13 | 1.20 |
| Peak Number | Chemical Shift | Peak Area/% | Peak Number | Chemical Shift | Peak Area/% |
|---|---|---|---|---|---|
| 1 | 6.38 | 0.59 | 18 | 118.59 | 5.93 |
| 2 | 10.01 | 1.81 | 19 | 122.50 | 7.10 |
| 3 | 13.74 | 3.74 | 20 | 126.10 | 8.63 |
| 4 | 17.61 | 4.72 | 21 | 129.78 | 8.02 |
| 5 | 21.42 | 4.50 | 22 | 133.79 | 4.94 |
| 6 | 25.97 | 3.41 | 23 | 137.61 | 4.64 |
| 7 | 30.33 | 4.80 | 24 | 141.43 | 2.93 |
| 8 | 34.32 | 4.06 | 25 | 145.54 | 1.11 |
| 9 | 38.48 | 3.58 | 26 | 150.05 | 0.61 |
| 10 | 42.68 | 2.68 | 27 | 195.87 | 0.79 |
| 11 | 45.98 | 2.15 | 28 | 201.25 | 1.70 |
| 12 | 49.91 | 2.62 | 29 | 204.57 | 0.37 |
| 13 | 54.87 | 1.16 | 30 | 206.71 | 2.09 |
| 14 | 98.15 | 0.69 | 31 | 210.25 | 1.73 |
| 15 | 104.63 | 1.48 | 32 | 214.60 | 1.26 |
| 16 | 109.79 | 2.07 | 33 | 219.70 | 0.59 |
| 17 | 114.30 | 3.49 |
| Peak Number | Chemical Shift | Peak Area/% | Peak Number | Chemical Shift | Peak Area/% |
|---|---|---|---|---|---|
| 1 | 4.04 | 0.42 | 18 | 119.43 | 6.69 |
| 2 | 7.62 | 1.21 | 19 | 123.67 | 8.76 |
| 3 | 12.69 | 3.77 | 20 | 127.56 | 9.40 |
| 4 | 17.12 | 4.33 | 21 | 130.68 | 5.37 |
| 5 | 20.73 | 4.41 | 22 | 133.32 | 3.13 |
| 6 | 26.36 | 2.81 | 23 | 133.33 | 1.97 |
| 7 | 29.87 | 3.23 | 24 | 137.38 | 4.80 |
| 8 | 34.74 | 3.37 | 25 | 142.01 | 3.24 |
| 9 | 40.26 | 3.19 | 26 | 147.29 | 1.07 |
| 10 | 45.45 | 2.58 | 27 | 151.85 | 1.46 |
| 11 | 50.52 | 2.17 | 28 | 155.76 | 1.26 |
| 12 | 70.69 | 0.63 | 29 | 195.25 | 0.97 |
| 13 | 99.70 | 1.22 | 30 | 200.97 | 2.03 |
| 14 | 104.77 | 1.47 | 31 | 205.48 | 2.34 |
| 15 | 110.23 | 3.27 | 32 | 209.54 | 2.95 |
| 16 | 115.13 | 2.91 | 33 | 214.29 | 1.26 |
| 17 | 115.16 | 1.43 | 34 | 218.90 | 0.86 |
| Coal Sample | fa | fac | fa′ | faH | faN | faP | faS | faB | fal | fal* | falH | falO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S9 | 64.18 | 8.28 | 55.90 | 44.03 | 24.8 | 1.08 | 3.94 | 18.57 | 34.82 | 12.85 | 19.53 | 2.43 |
| GY | 60.17 | 8.53 | 51.64 | 29.39 | 22.25 | 0.61 | 8.68 | 12.96 | 39.83 | 15.37 | 23.30 | 1.16 |
| L9 | 67.87 | 10.41 | 57.46 | 35.16 | 22.30 | 2.73 | 9.11 | 10.47 | 32.13 | 14.14 | 17.36 | 0.63 |
| Coal Sample | Wavenumber/cm−1 | Proportion/% | Assignment |
|---|---|---|---|
| S9 | 708.64, 718.72, 731.01 | 26.53 | Disubstituted benzene ring |
| 759.16, 770.61, 785.34 | 31.80 | Trisubstituted benzene ring | |
| 825.87, 843.69 | 36.51 | Tetrasubstituted benzene ring | |
| 887.91 | 5.17 | Pentasubstituted benzene ring | |
| GY | 717.35, 731.10 | 21.08 | Disubstituted benzene ring |
| 767.21, 785.34, 809.23 | 14.91 | Trisubstituted benzene ring | |
| 819.24, 835.09, 858.45 | 57.83 | Tetrasubstituted benzene ring | |
| 886.89 | 6.18 | Pentasubstituted benzene ring | |
| L9 | 710.34, 719.42, 732.83 | 20.89 | Disubstituted benzene ring |
| 773.15 | 18.16 | Trisubstituted benzene ring | |
| 814.99, 843.23 | 54.14 | Tetrasubstituted benzene ring | |
| 882.28 | 6.81 | Pentasubstituted benzene ring |
| Coal Sample | Wavenumber/cm−1 | Proportion/% | Assignment |
|---|---|---|---|
| S9 | 1018.95 | 0.40 | ash content |
| 1059.89 | 7.00 | C-O-C vibration | |
| 1105.94, 1127.39, 1145.06, 1170.25, 1237.38, 1292.67 | 40.50 | C-O vibration of phenols, alcohols, ethers, and esters | |
| 1354.25, 1390.60 | 14.04 | CH3-Ar | |
| 1410.46, 1472.14 | 7.06 | CH3-, CH2- | |
| 1494.07, 1526.55, 1562.75, 1637.31 | 19.00 | C=C vibration of aromatic hydrocarbons | |
| 1677.83 | 6.64 | Conjugate C=O vibration | |
| 1722.47 | 4.30 | C=C vibration of aromatic esters | |
| 1753.48 | 0.98 | C=O stretching vibration of carboxylic acid | |
| GY | 1018.95 | 0.93 | ash content |
| 1066.05 | 4.71 | C-O-C vibration | |
| 1139.85, 1204.06 | 33.43 | C-O vibration of phenols, alcohols, ethers, and esters | |
| 1334.11 | 38.58 | CH3-Ar | |
| 1478.99 | 4.13 | CH3-, CH2- | |
| 1525.75 | 7.50 | Aromatic hydrocarbon C=C vibration | |
| 1682.46 | 6.68 | Conjugate C=O vibration | |
| 1748.88 | 4.04 | Aromatic ester C=C vibration | |
| L9 | 1019.65 | 0.42 | ash content |
| 1068.38 | 5.95 | C-O-C vibration | |
| 1138.71, 1221.54, 1311.75 | 49.54 | C-O vibration of phenols, alcohols, ethers, and esters | |
| 1393.15 | 20.63 | CH3-Ar | |
| 1476.43 | 2.99 | CH3-, CH2- | |
| 1523.80 | 11.52 | Aromatic hydrocarbon C = C vibration | |
| 1673.46 | 4.82 | Conjugate C=O vibration | |
| 1733.21 | 4.13 | Aromatic ester C=C vibration |
| Coal Sample | Wavenumber/cm−1 | Proportion/% | Assignment |
|---|---|---|---|
| S9 | 2818.89, 2839.28 | 10.63 | Symmetric stretching vibration of CH2 |
| 2862.99 | 25.62 | Symmetric stretching vibration of CH3 | |
| 2891.69 | 7.03 | CH stretching vibration | |
| 2918.74 | 44.88 | Asymmetric stretching vibration of CH2 | |
| 2949.79, 2961.46 | 11.84 | Asymmetric stretching vibration of CH3 | |
| GY | 2855.48 | 35.61 | Symmetric stretching vibration of CH3 |
| 2916.56 | 56.86 | Asymmetric stretching vibration of CH2 | |
| 2954.97 | 7.52 | Asymmetric stretching vibration of CH3 | |
| L9 | 2855.34 | 34.95 | Symmetric stretching vibration of CH3 |
| 2894.85 | 15.00 | CH stretching vibration | |
| 2920.86 | 35.78 | Asymmetric stretching vibration of CH2 | |
| 2952.73 | 14.27 | Asymmetric stretching vibration of CH3 |
| Coal Sample | Wavenumber/cm−1 | Proportion/% | Assignment |
|---|---|---|---|
| S9 | 3019.97, 3044.50 | 1.18 | Hydrogen bonds formed by hydroxyl and nitrogen atoms |
| 3247.00 | 18.89 | Cyclic hydroxyl group | |
| 3411.10 | 71.47 | Self-associating hydroxyl group | |
| 3525.06 | 8.46 | OH-π | |
| GY | 3015.60, 3041.28 | 1.52 | Hydrogen bonds formed by hydroxyl and nitrogen atoms |
| 3271.02 | 22.73 | Cyclic hydroxyl group | |
| 3422.31 | 66.72 | Self-associating hydroxyl group | |
| 3519.07 | 9.02 | OH-π | |
| L9 | 3282.86 | 25.75 | Cyclic hydroxyl group |
| 3393.79 | 39.07 | Self-associating hydroxyl group | |
| 3466.18 | 28.71 | Hydroxyl–hydroxyl hydrogen bond | |
| 3527.12 | 6.35 | OH-π |
| Aromatic Unit Structure | Number | ||
|---|---|---|---|
| S9 | GY | L9 | |
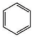 | 0 | 1 | 4 |
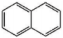 | 2 | 2 | 5 |
 | 3 | 3 | 2 |
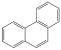 | 4 | 4 | 2 |
 | 2 | 1 | 1 |
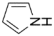 | 1 | 2 | 2 |
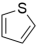 | 3 | 1 | 1 |
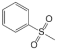 | 1 | 1 | 1 |
| Coal Sample | Type | C/% | H/% | O/% | N/% | S/% |
|---|---|---|---|---|---|---|
| S9 | Actual | 79.14 | 4.79 | 10.76 | 1.36 | 3.95 |
| Model | 80.28 | 4.88 | 9.85 | 1.23 | 3.76 | |
| GY | Actual | 78.92 | 4.63 | 13.81 | 1.49 | 1.16 |
| Model | 79.10 | 4.65 | 13.39 | 1.13 | 1.73 | |
| L9 | Actual | 76.26 | 5.03 | 15.47 | 1.38 | 1.87 |
| Model | 76.88 | 5.08 | 15.02 | 1.20 | 1.82 |
| Coal Sample | State | Total Energy | Valence Energy | Bond | Angle | Torsion | Non-Bond Energy | Van der Waals | Electrostatic |
|---|---|---|---|---|---|---|---|---|---|
| S9 | Initial | 6938.79 | 4757.49 | 1839.89 | 166.02 | 2747.98 | 935.07 | 858.01 | 77.06 |
| Final | 2951.41 | 3065.77 | 74.81 | 167.94 | 2809.13 | 74.29 | 144.02 | −69.73 | |
| GY | Initial | 7862.33 | 5352.45 | 2203.36 | 300.49 | 2842.26 | 1220.76 | 1180.80 | 39.96 |
| Final | 3057.82 | 3206.08 | 66.91 | 235.91 | 2879.13 | 13.66 | 146.31 | −132.64 | |
| L9 | Initial | 6732.53 | 1264.68 | 2215.81 | 153.95 | 2528.37 | 564.34 | 693.06 | −128.71 |
| Final | 2470.55 | 2911.33 | 59.87 | 163.14 | 2664.91 | −310.14 | 141.76 | −451.90 |
| Coal Sample | S9 | GY | L9 |
|---|---|---|---|
| Romax/% | 0.93 | 0.96 | 0.90 |
| /% | 21.10% | 48.30% | 53.12% |
| Molecular formula | C228H165N3O21S4 | C244H171N3O31S2 | C225H177N3O33S2 |
| Relative molecular mass | 3411 | 3705 | 3515 |
| XBP | 0.3786 | 0.3351 | 0.2228 |
| Cn | 4.9569 | 2.6843 | 1.9055 |
| Aromatic carbon ratio/% | 55.90 | 51.64 | 57.46 |
| Aromatic structure | The aromatic structure is relatively complete and stable, and the condensation degree of aromatic compounds is high. | The aromatic structure in coal is disrupted, and the condensation degree of aromatic compounds is between S9 and L9. | The aromatic structure in oxidized coal is more significantly damaged by oxidation, and the condensation degree is lower. |
| Fat structure | Mainly composed of methylene groups | Mainly composed of methylene groups | Mainly composed of methyl and methylene groups, with a significant increase in oxygen-containing functional groups |
| Heteroatom structure | Mainly pyridine nitrogen | Mainly pyrrole nitrogen | Mainly pyrrole nitrogen, with an increase in nitrogen oxides and an increase in sulfate content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, F.; Cao, Z.; Li, R.; Hu, Y. The Effect of Oxidation on Coal’s Molecular Structure and the Structure Model Construction of Oxidized Coal Molecular. Processes 2025, 13, 187. https://doi.org/10.3390/pr13010187
Li D, Yang F, Cao Z, Li R, Hu Y. The Effect of Oxidation on Coal’s Molecular Structure and the Structure Model Construction of Oxidized Coal Molecular. Processes. 2025; 13(1):187. https://doi.org/10.3390/pr13010187
Chicago/Turabian StyleLi, Dahu, Fangjia Yang, Zhao Cao, Ruoqi Li, and Yiwen Hu. 2025. "The Effect of Oxidation on Coal’s Molecular Structure and the Structure Model Construction of Oxidized Coal Molecular" Processes 13, no. 1: 187. https://doi.org/10.3390/pr13010187
APA StyleLi, D., Yang, F., Cao, Z., Li, R., & Hu, Y. (2025). The Effect of Oxidation on Coal’s Molecular Structure and the Structure Model Construction of Oxidized Coal Molecular. Processes, 13(1), 187. https://doi.org/10.3390/pr13010187







