1. Introduction
Oil refining, transportation, and storage are primary sources of pollution, and oil sludge is formed during these processes. Oil sludge processing creates additional oil resources and is a necessary way to protect the environment. Under the conditions of continuous exploitation of oil resources and the accompanying environmental pollution, the necessary task of environmental protection is becoming increasingly urgent [
1,
2,
3,
4]. Thus, the treatment and reuse of oil sludge in the oil industry has become relevant in recent years. This article examines the characteristics and environmental impacts of oil sludge, as well as the traditional and newly developed oil sludge treatment technologies.
Oil sludge is a dispersion system consisting of water, hydrocarbons, mechanical impurities, and metals [
5]. Oil sludge contains oil, asphaltenes, mechanical impurities, and salts of heavy metals. The mass ratio of the components in oil sludge is as follows: hydrocarbons, 35–58% (as a percentage by weight); water, 25–31%; mechanical impurities, 25–25%; metals, 3–15% [
6]. The composition of oil sludge is very complex. The hydrocarbon part of oil sludge contains alkanes, aromatic compounds, asphaltenes, anthracenes, pyrene, and other toxic and harmful substances [
7].
Oil sludge is toxic waste. The volatilization of oil components in oil sludge increases the concentration of hydrocarbons in the air of adjacent areas and pollutes surface and ground water. Oil sludge contains a large number of toxic and harmful organic compounds such as hydrocarbons, phenols, anthracene, and benzene compounds. Some substances have carcinogenic, teratogenic, and mutagenic effects on the environment [
5]. The traditional technologies for cleaning oil sludge include solvent extraction technology, chemical cleaning technology, and pyrolysis technology.
Classic methods for processing heavy oil waste are thermal cracking, catalytic cracking [
8], and vibration cracking, which create new marketable products, namely motor fuels. A cheap economical method for utilizing oil waste is proposed, which involves initiating thermal processes. By combining these processes, the yield of light fractions is increased.
The thermal process of oil sludge conversion is more convenient than thermal catalytic conversion. Due to the low quality of the raw materials, large capital investments and operating costs are required. A more rational approach to thermal processes makes it possible to use them effectively, allowing for the introduction of new trends in oil sludge processing.
Through reaction analysis, it was shown that a more effective destructive effect on high-molecular hydrocarbons is achieved via an increase in temperature, i.e., a thermodestructive method. With the addition of external energy, hydrocarbon molecules become mobile and reactive. Developing and disposing of oil waste [
9] at high temperatures is one of the ways to obtain light fractions. The future integration and combination of various methods can fully ensure the disposal of oil sludge with a yield of 100%. The use of various disposal methods helps improve the environment with less harm to the ecology of a given region.
To remove some hydrocarbons, the Fenton method is used, which reduces the time required for state regulation and energy costs. However, the oil sludge from the formation contains a large amount of polycyclic aromatic hydrocarbons. In this regard, for the destruction of these hydrocarbons, large amounts of hydrogen peroxide and Fe
3+ ions are used in the method. Research shows that the resulting hydroxyl radicals are capable of decomposing organic pollutants such as petroleum hydrocarbons. When processing toxic methanol-containing oil sludge, mixing with demulsifiers in a separate container is required. High interphase activity associated with solid particles, asphaltenes, and naphthenic acids contributes to the formation of a thick, rigid, and stable emulsion layer on the surface of the oil sludge. This process provides physical separation of oil sludge into liquid and solid phases, with subsequent separation of the liquid into oil and water fractions [
10,
11,
12,
13].
Various researchers have shown that ultrasonic treatment during the distillation of oil sludge increases the yield of light products due to the breakdown of heavy polycyclic aromatic hydrocarbons [
14].
Many researchers in this field consider thermal cracking to be a free radical process, where the production and conversion of oil sludge components are sequential and sequential-parallel reactions.
The extraction of oil sludge is based on the principle of “miscibility”. Organic solvents with properties similar to crude oil are selected to extract petroleum substances from oil sludge. Oil sludge extraction is a typical liquid–solid extraction, which is the process of selecting a suitable extraction solution, mixing it with waste, and then distilling the solvent–oil mixture and separating the oil from the solvent [
15]. The solvent is reused after condensation. Common extractants include trichloromethane, propane-triethylamine, and reforming oil. However, the use of chlorine-containing extractants pollutes the resulting secondary hydrocarbons.
Solvent extraction is an effective recyclable treatment method that effectively separates oily sludge into recyclable hydrocarbons, fine solids, and semi-solids [
16] and is particularly good for treating large quantities of oil sludge.
Presently, one of the main obstacles to the large-scale application of oil sludge treatment methods is the high consumption of organic extractants; the large amount of organic solvents used can easily cause secondary pollution. The supercritical liquid extraction method makes it possible to effectively [
17] reduce the dosage of extractants and shorten the extraction time. However, it is not suitable for large-scale application due to the harsh operating conditions. Thus, searching for highly effective, accessible, and inexpensive extractants or developing new alternative methods for processing oil sludge is relevant.
The purpose of this work is to study the physical and chemical characteristics of tank oil sludge and determine the optimal method for processing it.
The novelty of this work lies in the increased yield of light products of oil sludge processing by 18% using ultrasonic treatment and extraction methods, which ensure the maximum splitting of heavy hydrocarbons into light motor fuels.
This study presents the results of oil extraction from oil sludge in a benzene–hexane mixture at a ratio of 1:1 and a study of the effect of ultrasonic treatment with a frequency of 100 kHz. The ultrasound process aimed to achieve the highest possible yield of light fractions and a decrease in the volume of solid residues. Ultrasonic treatment of oil sludge prevents the aggregation of polarized particles into large structures and disperses them into small molecules. Benzene also effectively dissolves complex cyclic aromatic hydrocarbons and leads to the decomposition of the original components into light ones. After ultrasonic treatment, the resulting mixture is distilled.
3. Results and Discussions
The composition of oil sludge from the Uzen field was studied, and it is presented in
Table 1. Mechanical impurities in the oil were determined according to GB/T 511-2010 [
27]. Cleaning of mechanical impurities was carried out by preliminary dissolution in benzene and then washing the sediment on the filter with a solvent, followed by drying and weighing. The water content in the oil sludge was determined according to ASTM D6304-16 [
28]. Removal of asphaltenes was carried out by dissolving the oil sludge in light gasoline and hexane, and separation of neutral resins was carried out by adsorption on silica gel.
The organic part of the oil sludge reached 86%, whereases the content of mechanical impurities was 6.4% and that of water was 6.2%.
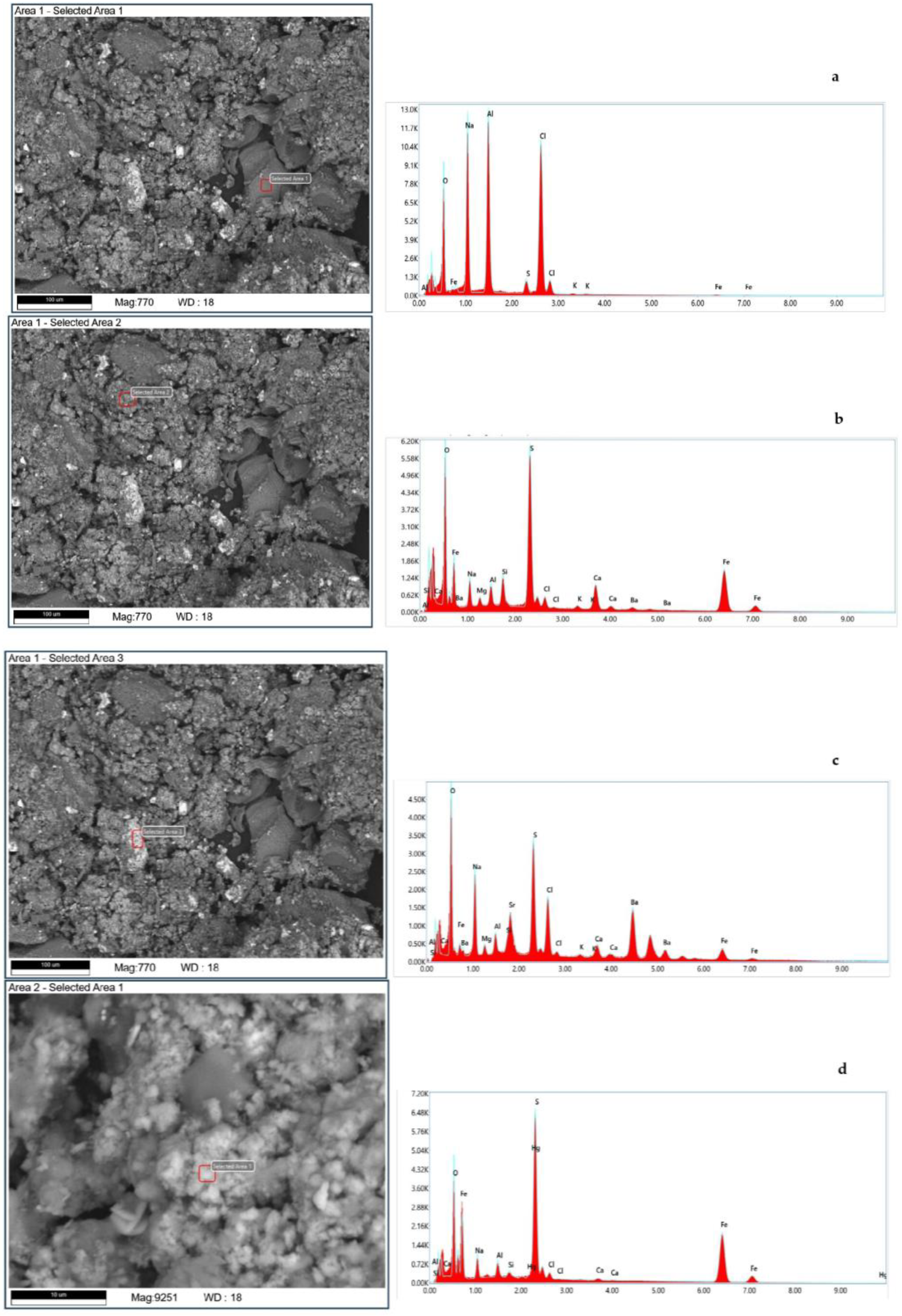
Figure 1,
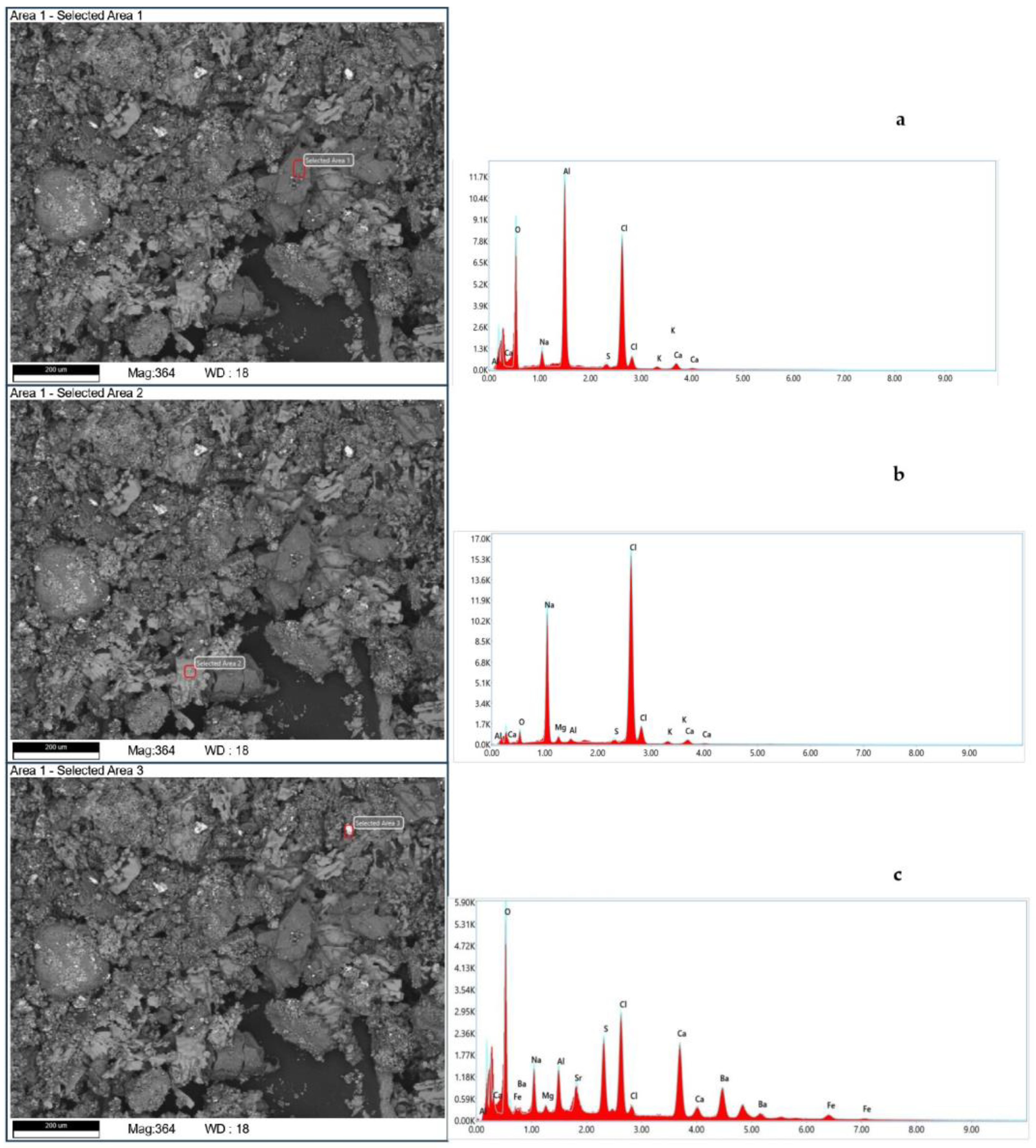
Figure 2 and
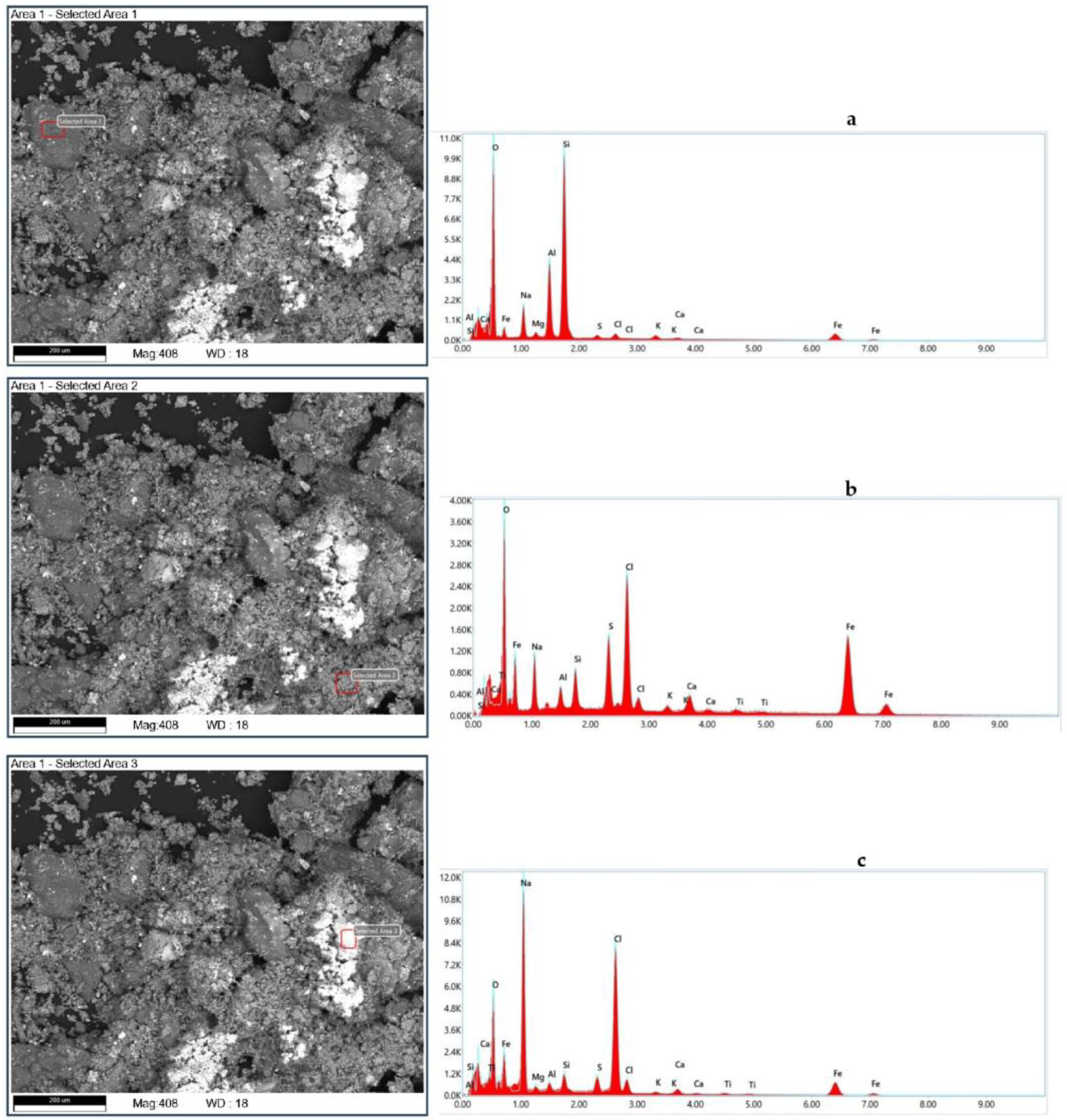
Figure 3 show the scanning electron microscopy (SEM) and elemental analysis results of the tank oil sludge samples.
Prior to analysis, the untreated (original oil sludge) and refined reservoir oil sludge (liquid) and residual solids (solid oil sludge) were dried on a thermal balance, resulting in weight losses of 26.9% (for untreated oil), 62.87% (for refined oil), and 14.65% (for residue), mainly due to volatile hydrocarbons impregnating the sludges.
Figure 1a,b show micrographs of the untreated reservoir oil sludge. The untreated samples of reservoir oil sludge contained solid particles. The elemental composition of individual sections of the reservoir oil sludge contained inorganic elements (
Figure 1c). Both organic and inorganic elements were present on the surface of the solid particles of oil sludge
Figure 1d.
Table 2 shows the qualitative elemental composition of the untreated oil sludge, obtained using SEM analysis. All experiments were carried out three times, and the average results are shown. As can be seen from the results, the main component is oxides. Specifically, BaO and SO
3 are the most common compounds and K
2O and MgO are the least common. Among these oxides, we can see chlorine in the amount of 5.9%.
Figure 2 shows scanning electron micrographs of the purified tank oil sludge and the elemental analysis.
Figure 2a,b show the surfaces of the purified tank oil sludge. The surface of purified tank oil sludge shown in
Figure 2c is without features.
However, we cannot see a significant difference between them in terms of their morphological structure. The qualitative elemental composition results for this oil sludge are recorded in
Table 3.
Table 3 shows the mean results of the elemental composition of the purified sample, as obtained by SEM analysis. As can be seen from the qualitative elemental analysis, after purification, most inorganic salt impurities were eliminated. The main salts remaining included Al, Cl, and oxides.
The process of cleaning the tank oil sludge from container impurities was carried out by vacuum filtration.
Figure 3 shows images of the mechanical particles obtained using a microscope scan and elementary analysis.
Figure 3a shows the surface of the mechanical particles.
Figure 3b,c show the morphological structure of harder particles.
Table 4 shows the mean results of the elemental composition of the mechanical impurities of the sample after the purification process, as obtained by SEM analysis. All three samples were composed of a clay matrix of silicoaluminates with particles of 100–200 μm in diameter.
The samples’ composition was characterized mainly by inorganic salts such as sulphates, pyrite, oxides (aluminum, sodium, iron, barium, calcium, strontium), and chlorides (mainly aluminum and sodium), whose origin can be attributed to soil alteration near lakes of a brackish character.
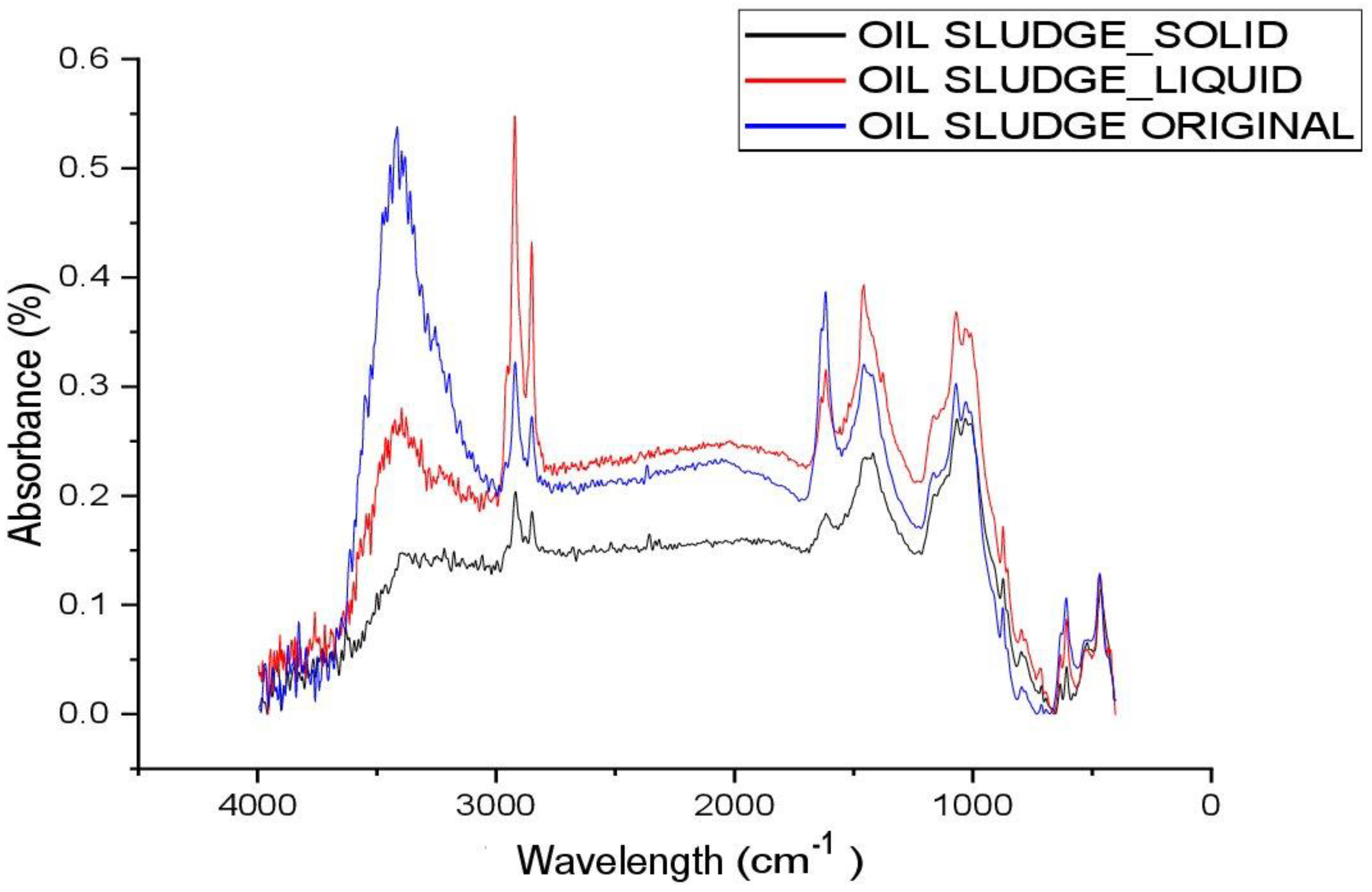
Figure 4 shows the FT-IR results of the untreated (original oil sludge) and purified tank oil sludge (liquid) and the mechanical impurities (solid oil sludge). In the IR spectra of the samples in
Figure 4, an absorption band is observed at 3416 cm
−1, characteristic of free acidic hydroxyl groups. The absorption peaks at 2922 and 2846 cm
−1 are due to the bound hydroxyl groups of carboxylic acids. Low-intensity absorption bands in the region of 1618 cm
−1 are attributed to vibrations of condensed aromatic hydrocarbons. It should be noted that the absorption bands at 608 cm
−1 are quite high in intensity, which appear due to stretching vibrations of methyl groups. The low intensity of absorption bands in the region of 1704 and 1606 cm
−1 is due to condensed aromatic hydrocarbons; such absorption bands belong to arylalkyl ketones (Ar-CO-R) and represent stretching vibrations of C-O-C and C-OH in carboxyl groups. Absorption bands with average intensity compared to natural bitumen deposit in the region of 1455 and 1376 cm
−1 belong to vibrations of methyl groups and characterize the degree of branching of paraffins. The wide absorption band at 1000 cm
−1 of high intensity is due to the presence of Si-O-Si bonds of the mineral part of the rock or S-O sulfoxide groups. Absorption bands in the range of 775–645 cm
−1 clearly indicate monosubstituted aromatic structures in the composition of asphaltenes, resins, and asphaltogenic acids [
25].
The tables below show the correspondence between functional groups and I, their corresponding peaks found in the IR spectra. As can be seen from
Table 5, the spectra of the three analyzed samples have the same functional groups, with the exception of the peak related to the stretching of the OH group in the solid oil sludge sample.
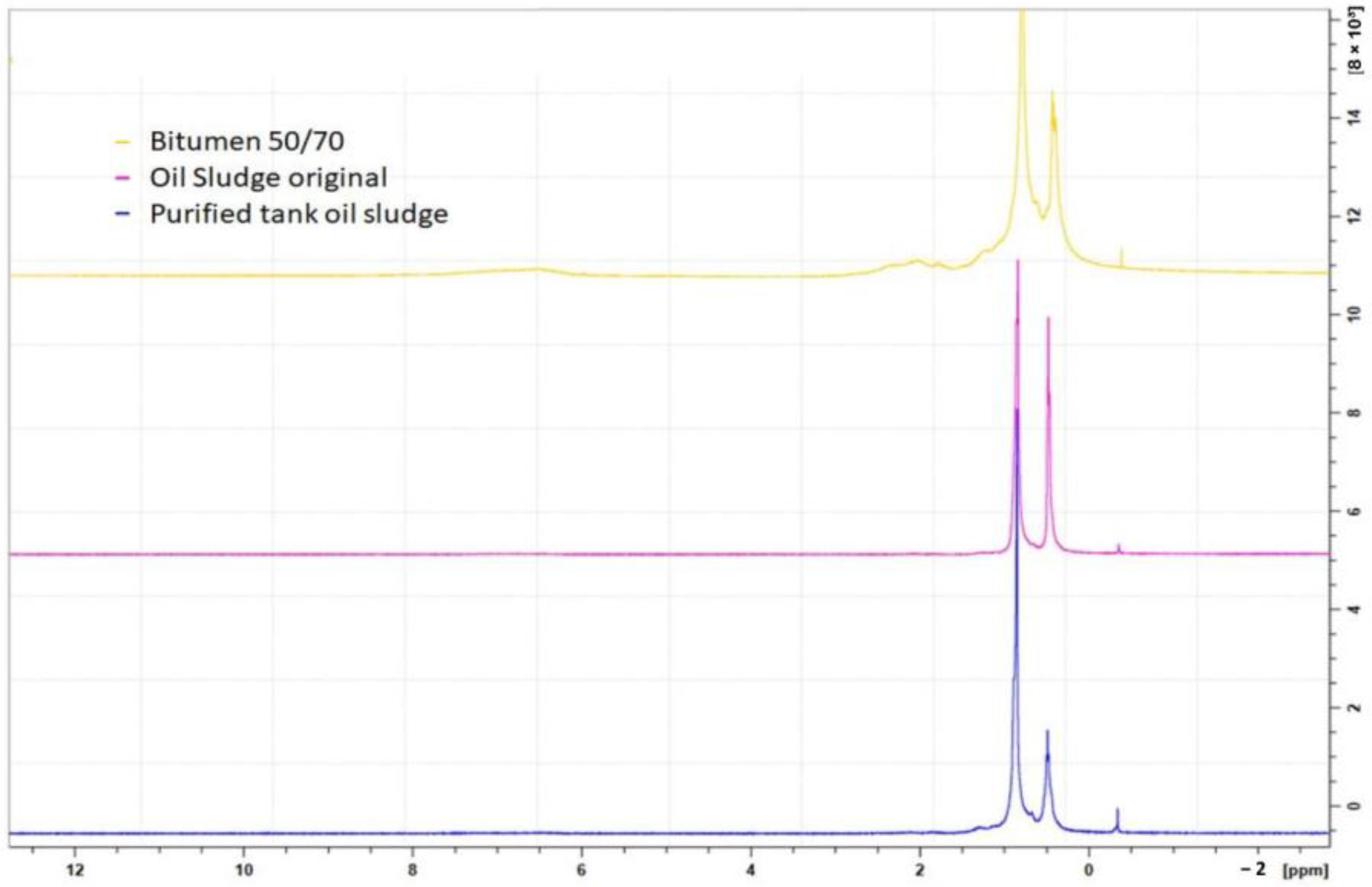
The spectra in
Figure 5 are
1H NMR spectra of crude and treated tank oil sludge samples, in which two signals are visible in the aliphatic region, indicating that the oil sludge samples contain mainly this type of hydrocarbon while in the aliphatic zone.
The qualitative and quantitative compositions of the distillation products before and after ultrasonic treatment were determined by chromatographic analysis. The chromatographic analysis results of the samples are presented in
Table 6,
Table 7 and
Table 8.
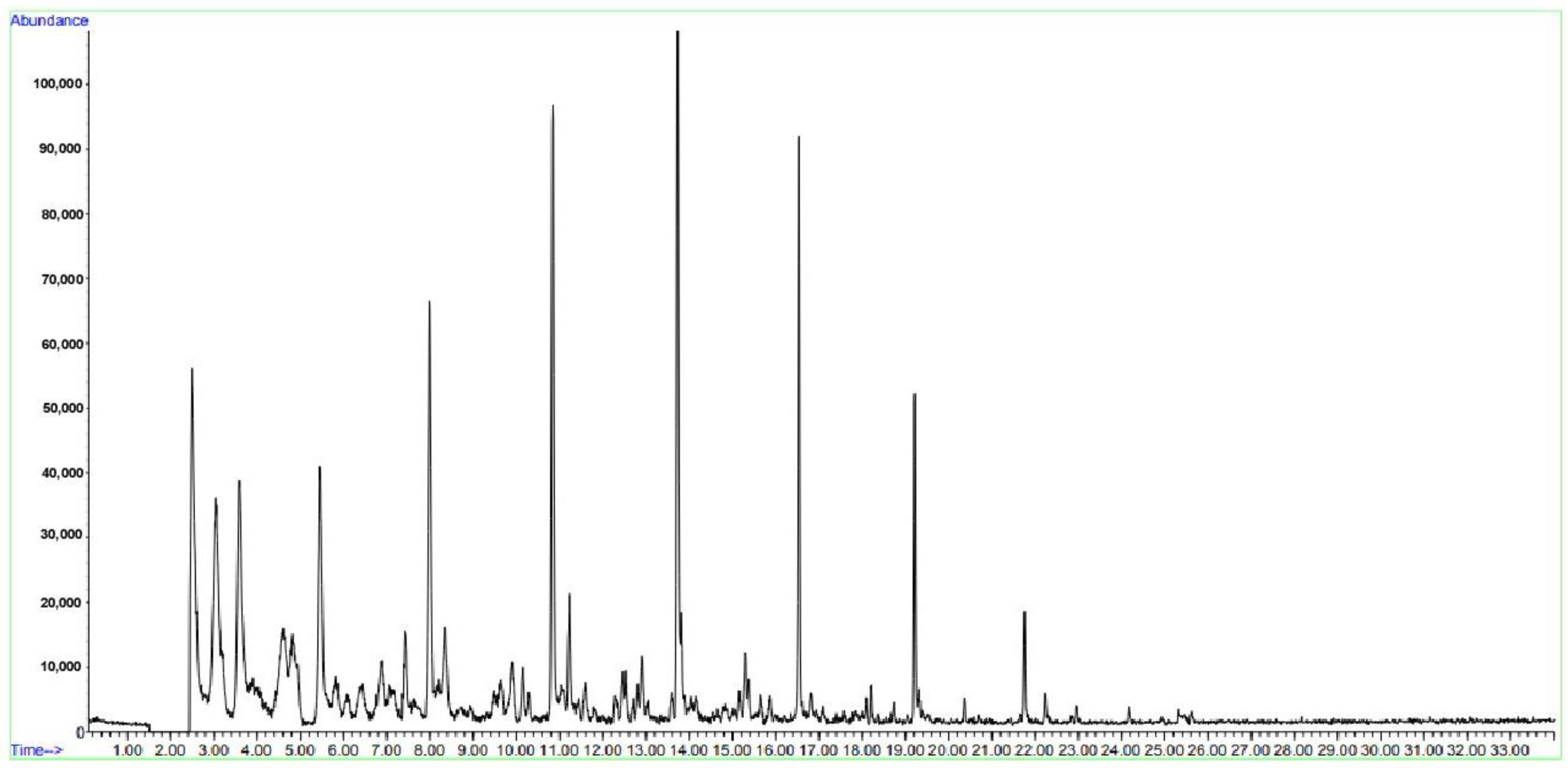
Table 6 presents the results of distillation of oil sludge from tank No. 1. Sample No. 2 of tank oil sludge was treated with ultrasound after mixing with a solvent. It has been established that oil sludge is a multicomponent mixture consisting of paraffin-naphthenic series substances and aromatic hydrocarbons, the intense peaks of which appear with a release time from 3 to 16 min (
Figure 6).
The study of the influence of ultrasonic treatment and the choice of mode on the composition of oil sludge from distillation products was carried out by studying the yield of individual low-molecular hydrocarbons. In order to clarify the effect of ultrasonic treatment, hydrocarbons such as cyclohexane, octane, 2,2-dimethylpentane, and naphthalene were studied in different modes and temperature conditions. We also studied the total yield of light fractions.
In order to determine the potential for the yield of light oil products, atmospheric distillation of the bottom sediments of oil reservoirs was carried out.
From the data in
Table 7, it is clear that during storage, the fractional composition of the oil sludge changed; the content of light fractions decreased and the content of high-boiling fractions increased due to the formation of complex molecules with a high content of heavy polycyclic aromatic hydrocarbons and resinous asphaltene substances. There was a small amount of normal paraffin hydrocarbons, which is also evidenced by the gelatinous-mastic structure of the oil sludge from sludge reservoirs. The group and fractional composition indicates the presence of resins, paraffin-naphthenic hydrocarbons, and heavy aromatic polycyclic hydrocarbons.
In the sonicated sample, the main identifiable components were alkanes of normal and isomeric [
29] structure, as well as some representatives of polycyclic aromatic compounds. At the first stage of analysis, the concentrations of non-alkane compounds in the final extract were significant.
Table 8 presents the products of the third fraction, obtained at temperatures of 180–220 °C. Yields of hexylcyclohexane and pentylcyclohexane in the products increased during ultrasonic treatment. Light hydrocarbons and their isomers also predominated in the distillation products. When the temperature increased to 225 °C and above, a light beige liquid consisting of 25 wt.% heavy paraffin was distilled.
Figure 6 shows a chromatogram of the distillation products of oil sludge treated with ultrasound, obtained by GC–MS. As shown in
Figure 6, all components were released in up to 18 min, and naphthalene and its isomers were not present in the distillation products.
According to the obtained analyses, it is clear that exposure to ultrasound has a beneficial effect on the composition of oil sludge. Naphthalene acts as a carcinogenic agent in the environment and in humans, which affects the liver and the nervous system, and can cause cataracts and retinal hemorrhage. Naphthalene and its isomers as volatile substances increase the risk of development of laryngeal and colorectal cancers.
It has been experimentally established that ultrasonic treatment of tank oil sludge, when heated to 60 °C, enhances the effect of destruction of high-molecular-weight hydrocarbons and their interaction. According to the GC and mass spectrometric analysis, during the distillation of the ultrasonic-treated oil sludge extract, an increase in the yield of cyclohexane, octane, and 2,2-dimethylpentane and a decrease in the yield of naphthalene were observed.
At a frequency of 100 kHz for 30 min, ultrasonic treatment helps convert the composition of hydrocarbons into light hydrocarbons. Without ultrasonic treatment, the destruction of the structure of naphthalene and its derivatives begins at a distillation temperature of 450 °C. In addition, in samples treated with ultrasound at temperatures from 350 to 380 °C, the yield of naphthalene decreases to 3% of the total mass of hydrocarbons.
From these studies, it follows that tank oil sludge contains a large amount of heavy polycyclic aromatic hydrocarbons. The optimal conditions for ultrasonic treatment [
30] of barn oil sludge have been determined as a duration of 30 min at 100 kHz, which increases the yield of light hydrocarbons during the process [
18] of atmospheric distillation. It has been established that ultrasonic treatment reduces the yield of heavy polycyclic aromatic hydrocarbons. Polycyclic aromatic hydrocarbons decompose into smaller aromatic hydrocarbons. With an increase in the power of ultrasonic treatment, the yield of hydrocarbon isocompounds decreases.
4. Conclusions
The method of processing and disposing of oil sludge requires enhanced control since the problem of its use has not been solved in production conditions [
31]. The lack of separate collection and storage of oil sludge is a big problem. By separately storing oil sludge depending on the composition [
32] at the stage of formation, groups of waste suitable for recycling can be distinguished. Further, the processing of mixed and old oil sludge requires additional purification.
In this work, the purification of tank oil sludge from mechanical impurities was carried out. The composition of mechanical impurities in the oil sludge was studied.
During extraction with hexane, medium and lighter hydrocarbons passed into the solution. During extraction in a mixture of hexane and benzene, depending on the ratio, heavy aromatic hydrocarbons and their derivatives were extracted. Ultrasonic treatment increased the selectivity of the formation of hydrocarbon isocompounds and promoted the maximum yield of light hydrocarbon fractions as motor fuels.
The optimal conditions for ultrasonic treatment of tank oil sludge were determined as a duration of 30 min at 100 kHz, which increased the yield of light hydrocarbons during atmospheric distillation. It has been established that ultrasonic treatment reduces the yield of heavy polycyclic aromatic hydrocarbons, which decompose into smaller aromatic hydrocarbons.
The residual high-carbon mass is less carcinogenic since many heavy hydrocarbons are removed during extraction.
A study of the composition of the oil sludge was carried out in laboratory conditions using the methods of chromatography and mass spectrometry, and the physicochemical properties of the oil sludge were studied in order to select the most effective method of processing in order to obtain secondary products.
Fractions obtained at temperatures of 65 and 85 °C were used, with 200 g employed to dissolve a new portion of tank oil sludge for processing. Fractions obtained at temperatures of 120 and 180 °C can be used as light motor fuel. In distillation products at a temperature of 180 °C, the yield of isocompounds was 20.5%, and at a temperature of 220 °C—17.2%. In the following fractions synthesized at temperatures of 230–330 °C, naphthenes were found in large quantities. In fractions obtained at temperatures of 350 °C and above, polycyclic aromatic hydrocarbons predominated.