Assessment of Yield, Flavonoid and Phytosterol Contents, and Fatty Acid Composition of Baru Almond Oil (Dipteryx alata Vogel) by Supercritical CO2 Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Proximate Analysis and Micronutrient
2.3. Baru Oil Extraction Process
2.3.1. Soxhlet Extraction of Baru Oil
2.3.2. Supercritical Extraction of Baru Oil
2.4. Flavonoids Content
2.5. Fatty Acid Profile and Phytosterol Content
2.6. Statistical Analysis
3. Results
3.1. Proximate Composition of Baru Almond
3.2. Nutrients
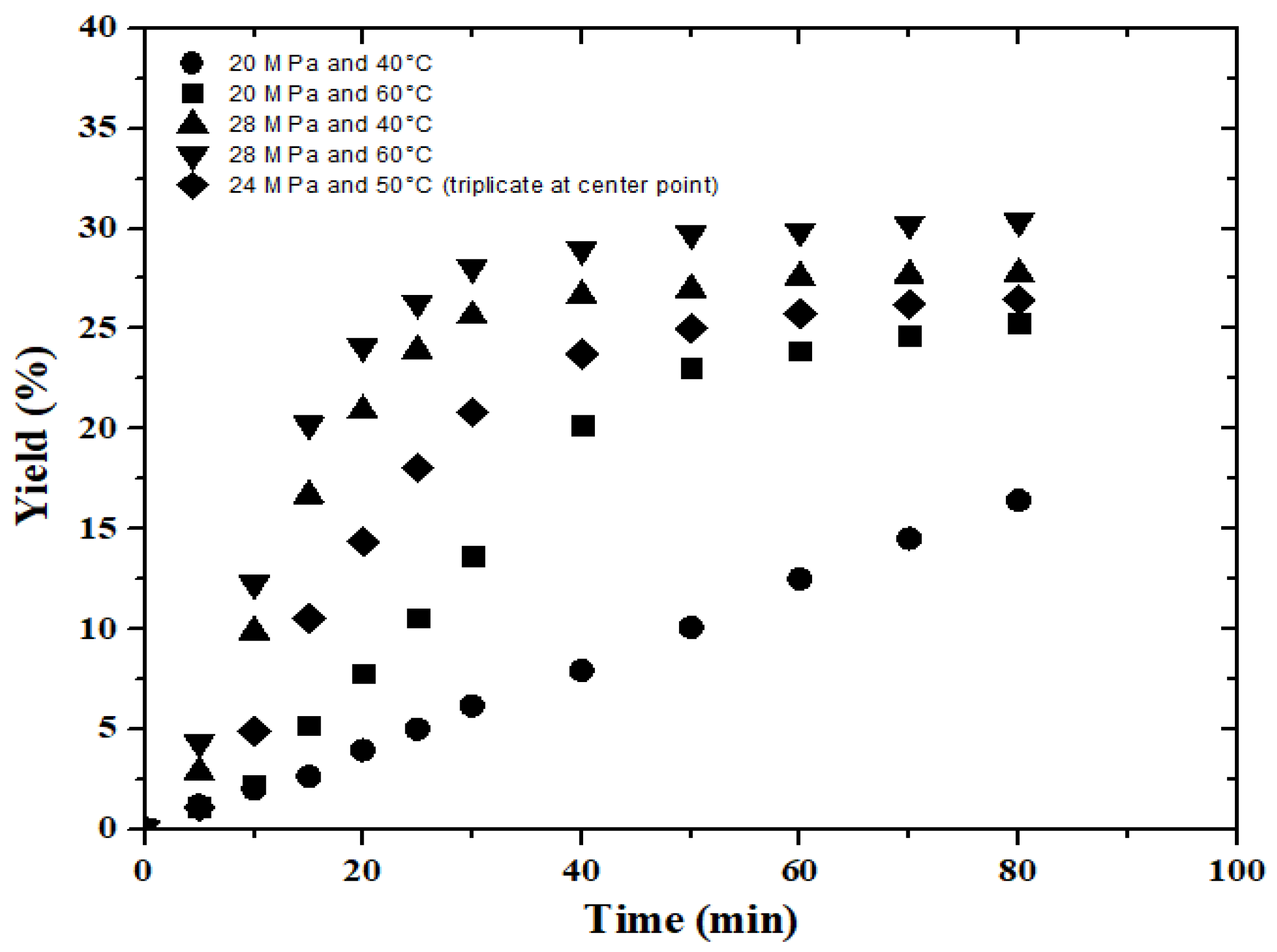
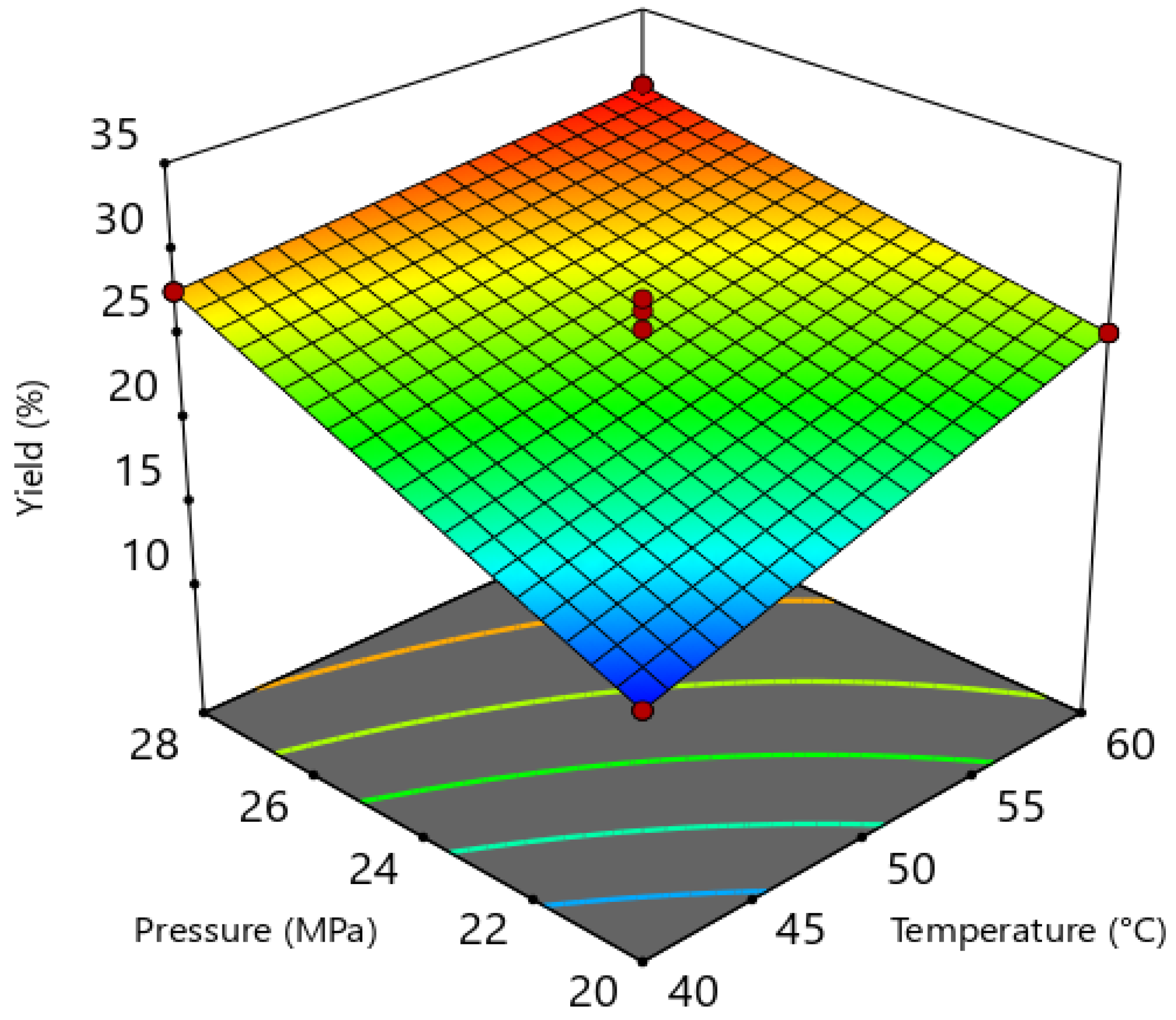
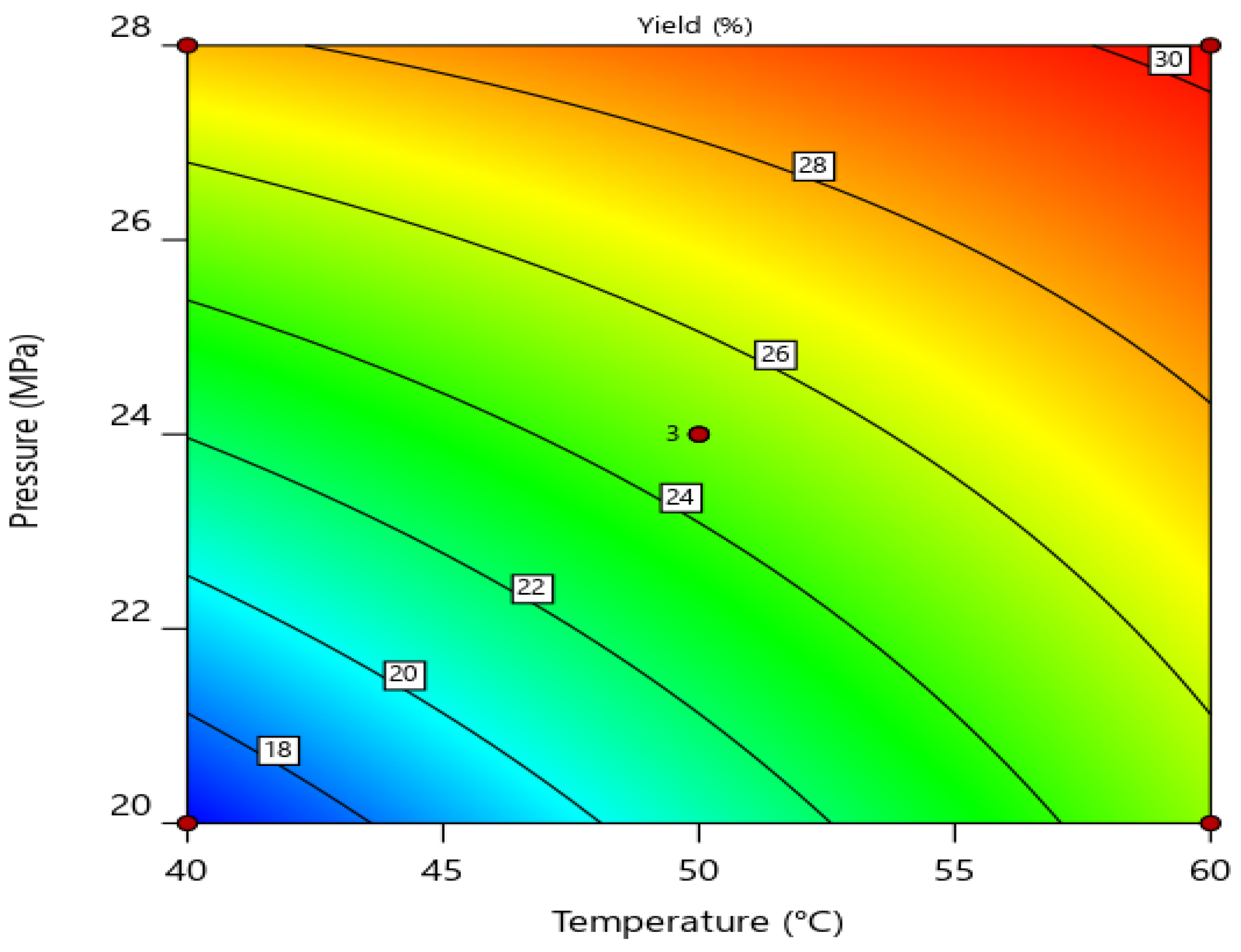
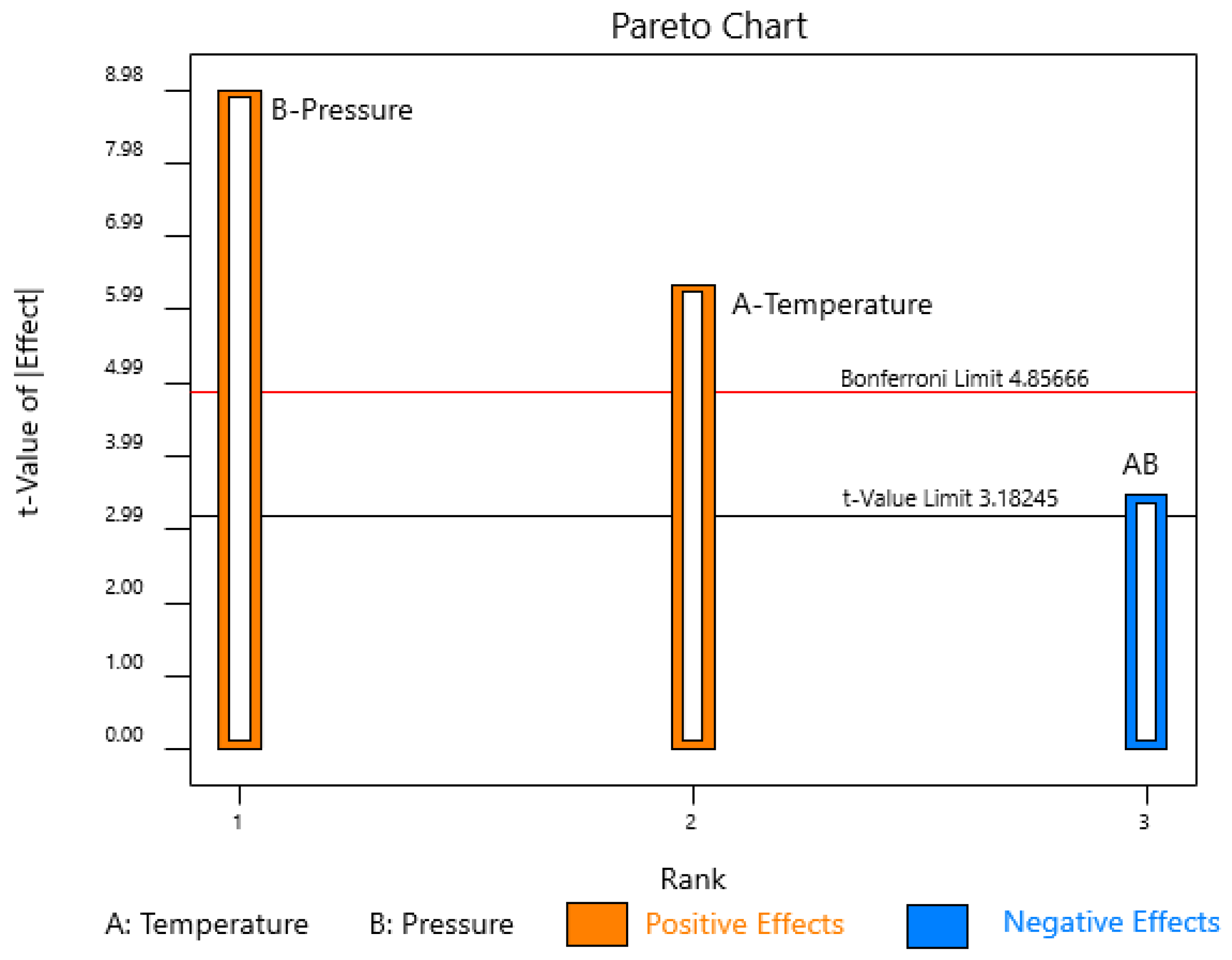
3.3. Oil Extraction Yield
3.4. Phytosterol Compounds
3.5. Flavonoids
3.6. Fatty Acid Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves-Santos, A.M.; Fernandes, D.C.; Naves, M.M.V. Baru (Dipteryx alata Vog.) Fruit as an Option of Nut and Pulp with Advantageous Nutritional and Functional Properties: A Comprehensive Review. NFS J. 2021, 24, 26–36. [Google Scholar] [CrossRef]
- Vera, R.; Soares Junior, M.S.; Naves, R.V.; de Souza, E.R.B.; Fernandes, E.P.; Caliari, M.; Leandro, W.M. Características Químicas de Amêndoas de Barueiros (Dipteryx alata Vog.) de Ocorrência Natural No Cerrado Do Estado de Goiás, Brasil. Rev. Bras. Frutic. 2009, 31, 112–118. [Google Scholar] [CrossRef]
- Takemoto, E.; Okada, I.A.; Garbelotti, M.L.; Tavares, M.; Aued-Pimentel, S. Composição Química Da Semente e Do Óleo de Baru (Dipteryx alata Vog.) Nativo Do Município de Pirenópolis, Estado de Goiás1. Rev. Inst. Adolfo Lutz 2001, 60, 113–117. [Google Scholar] [CrossRef]
- Paulo, L.; Fernandes, R.; Gandra, K.; Minim, V.; Minim, L.; Grimaldi, R.; Vidigal, M. Baru Seed Extracted Oil (Dipteryx alata Vog.): Chemical Composition and Thermal and Oxidative Stability. J. Braz. Chem. Soc. 2023, 34, 664–672. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.U. Simultaneous Analysis of Tocopherols, Cholesterol, and Phytosterols Using Gas Chromatography. J. Food Sci. 2002, 67, 1696–1700. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Oliveira de Souza, L.I.; Bezzera-Silva, P.C.; do Amaral Ferraz Navarro, D.M.; da Silva, A.G.; dos Santos Correia, M.T.; da Silva, M.V.; de Figueiredo, R.C.B.Q. The Chemical Composition and Trypanocidal Activity of Volatile Oils from Brazilian Caatinga Plants. Biomed. Pharmacother. 2017, 96, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Rakel, D. Benign Prostatic Hyperplasia. In Integrative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 601–607.e1. [Google Scholar]
- Sekar, G. Effect of β-Sitosterol on Insulin Resistance & Protein Expression of Insulin Signalling Molecules in Quadriceps Muscle of High Fat Diet-Induced Type-2 Diabetic Rats. Bioinformation 2022, 18, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.H.; Scheidt, G.N.; Portella, A.C.; Arruda, E.J.; Costa, R.B. O Baru (Dipteryx alata Vog.) Como Alternativa de Sustentabilidade Em Área de Fragmento Florestal Do Cerrado, No Mato Grosso Do Sul. Interações (Campo Gd.) 2009, 10, 31–39. [Google Scholar] [CrossRef]
- Fetzer, D.L.; Cruz, P.N.; Hamerski, F.; Corazza, M.L. Extraction of Baru (Dipteryx alata Vogel) Seed Oil Using Compressed Solvents Technology. J. Supercrit. Fluids 2018, 137, 23–33. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; Osorio-Tobón, J.F.; Johner, J.C.F.; Meireles, M.A.A. A Comparative and Economic Study of the Extraction of Oil from Baru (Dipteryx alata) Seeds by Supercritical CO2 with and without Mechanical Pressing. Heliyon 2021, 7, e05971. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.; De Aguiar, A.C.; Viganó, J.; Boeing, J.S.; Visentainer, J.V.; Martínez, J. Supercritical CO2 Extraction of Cumbaru Oil (Dipteryx alata Vogel) Assisted by Ultrasound: Global Yield, Kinetics and Fatty Acid Composition. J. Supercrit. Fluids 2016, 107, 75–83. [Google Scholar] [CrossRef]
- AOCS (American Oil Chemists’ Society). Official Methods and Recommended Practices of the AOCS, 5th ed.; Firestone, D., Ed.; American Oil Chemists’ Society: Urbana, IL, USA, 1998; ISBN 0935315977. [Google Scholar]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos (Métodos Químicos e Biológicos), 3rd ed.; Imprensa Universitária da UFV: Viçosa, MG, Brazil, 2002. [Google Scholar]
- Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, SP, Brasil, 2008; Volume 1. [Google Scholar]
- EMBRAPA. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; da Silva, F.C., Ed.; Centro Nacional de Pesquisa de Solos: Brasilia, DF, Brasil, 2009; Volume 1. [Google Scholar]
- Silva, M.O.; Camacho, F.P.; Ferreira-Pinto, L.; Giufrida, W.M.; Vieira, A.M.S.; Visentaine, J.V.; Vedoy, D.R.L.; Cardozo-Filho, L. Extraction and Phase Behaviour of Moringa oleifera Seed Oil Using Compressed Propane. Can. J. Chem. Eng. 2016, 94, 2195–2201. [Google Scholar] [CrossRef]
- Barbosa, I.M.V.; Morais, J.R.W.G.; Cardoso, V.L. OTIMIZAÇÃO DO TEMPO NA PRODUÇÃO DE LIPASE POR Candida Rugosa EM MELAÇO DE SOJA. In Proceedings of the Anais do X Congresso Brasileiro de Engenharia Química, Vassouras, RJ, Brazil, 1 December 2014; Editora Edgard Blücher: São Paulo, SP, Brasil, 2014; pp. 645–650. [Google Scholar]
- Iwassa, I.J.; Saldaña, M.D.A.; Cardozo-Filho, L.; Silva, C. Yield and Quality Parameters of Pretreated Crambe Seed Oil Extracted Using C3H8, CO2 and C3H8 + CO2 Mixtures under Pressurized Conditions. J. Supercrit. Fluids 2021, 175, 105277. [Google Scholar] [CrossRef]
- Mello, A.F.A.; Hoscheid, J.; Raspe, D.T.; Stevanato, N.; Silva, C. Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization. AppliedChem 2024, 4, 56–69. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. Design of Experiments. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2010; pp. 1–22. [Google Scholar]
- Vallilo, M.I.; Tavares, M.; Aued, S. Composição Química Da Polpa e Da Semente Do Fruto Do Cumbaru (Dipteryx alata Vog.)—Caracterização Do Óleo Da Semente. Rev. Do Inst. Florest. 1990, 2, 115–125. [Google Scholar] [CrossRef]
- Campidelli, M.L.L.; Carneiro, J.D.S.; Souza, E.C.; Magalhães, M.L.; Nunes, E.E.C.; Faria, P.B.; Franco, M.; Vilas Boas, E.V.B. Effects of the Drying Process on the Fatty Acid Content, Phenolic Profile, Tocopherols and Antioxidant Activity of Baru Almonds (Dipteryx alata Vog.). Grasas Y Aceites 2020, 71, 343. [Google Scholar] [CrossRef]
- Marques, F.G.; de Oliveira Neto, J.R.; da Cunha, L.C.; de Paula, J.R.; Bara, M.T.F. Identification of Terpenes and Phytosterols in Dipteryx alata (Baru) Oil Seeds Obtained through Pressing. Rev. Bras. Farmacogn. 2015, 25, 522–525. [Google Scholar] [CrossRef][Green Version]
- Polmann, G.; Badia, V.; Danielski, R.; Ferreira, S.R.S.; Block, J.M. Non-Conventional Nuts: An Overview of Reported Composition and Bioactivity and New Approaches for Its Consumption and Valorization of Co-Products. Future Foods 2021, 4, 100099. [Google Scholar] [CrossRef]
- Santos, K.A.; Klein, E.J.; Fiorese, M.L.; Palú, F.; Silva, C.; Silva, E.A. Extraction of Morus Alba Leaves Using Supercritical CO2 and Ultrasound-Assisted Solvent: Evaluation of β-Sitosterol Content. J. Supercrit. Fluids 2020, 159, 104752. [Google Scholar] [CrossRef]
- Vasquez, W.V.; Hernández, D.M.; del Hierro, J.N.; Martin, D.; Cano, M.P.; Fornari, T. Supercritical Carbon Dioxide Extraction of Oil and Minor Lipid Compounds of Cake Byproduct from Brazil Nut (Bertholletia excelsa) Beverage Production. J. Supercrit. Fluids 2021, 171, 105188. [Google Scholar] [CrossRef]
- Hussain, A.; Kaler, J.; Ray, S.D. The Benefits Outweigh the Risks of Treating Hypercholesterolemia: The Statin Dilemma. Cureus 2023, 15, e33648. [Google Scholar] [CrossRef]
- Viana, H.N.A.C.; Sganzerla, W.G.; Castro, L.E.N.; Veeck, A.P.d.L. Characterization of Baru (Dipteryx alata Vog.) and Application of Its Agro-Industrial by-Product in the Formulation of Cookies. J. Agric. Food Res. 2023, 12, 100577. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Destandau, E.; Lesellier, E. Optimization of Supercritical Fluid Extraction of Polar Flavonoids from Robinia pseudoacacia L. Heartwood. J. CO2 Util. 2023, 70, 102440. [Google Scholar] [CrossRef]
| Component | This Work | Literature | |||
|---|---|---|---|---|---|
| Chani-Paucar et al. [12] | Vallilo et al. [23] | Campidelli et al. [24] | Takemoto et al. [3] | ||
| Moisture | 5.05 ± 0.32% | 3.5% | 5.8% | 6.6% | 6.1% |
| Ash | 3.10 ± 0.01% | 3.2% | 2.8% | 1.55% | 2.7% |
| Lipids | 37.1 ± 0.45% | 41.9% | 41.6% | 31.7% | 38.2% |
| Proteins | 22.01 ± 0.34% | 29.9% | 23.4% | 22.9% | 23.9% |
| Carbohydrates | 32.60 ± 0.91% | 12.25% | 23.0% | 37.13% | 15.8% |
| This Work | Yield (%) (Literature) | ||||||
|---|---|---|---|---|---|---|---|
| Santos et al. [13] | Chani-Pauçar et al. [12] | Fetzer et al. [11] | Fetzer et al. [11] | ||||
| Test | Pressure (MPa) | Temperature (°C) | Yield (%) | CO2 35 MPa/50 °C | CO2 35 MPa/45 °C | CO2 + Ethanol 25 MPa/80 °C | Propane 10 MPa/60 °C |
| 1 | 20 | 40 | 16.4 | 22.8% | 29% | 32.6 | 36.8 |
| 2 | 20 | 60 | 25.3 | ||||
| 3 | 28 | 40 | 27.7 | ||||
| 4 | 28 | 60 | 30.3 | ||||
| 5 | 24 | 50 | 26.6 | ||||
| 6 | 24 | 50 | 25.5 | ||||
| 7 | 24 | 50 | 27.3. | ||||
| Soxhlet | Atmospheric | 40 | 38.3 ± 0.4 a (hexane) | 24.1 | 39.70 ± 0.32 | 39.70 ± 0.32 | |
| Terms | Sum of Squares | Degrees of Freedom | Mean Squares | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Model | 109.41 | 3 | 36.47 | 44.29 | 0.0222 | 0.9852 |
| T | 33.06 | 1 | 33.06 | 40.16 | 0.0240 | |
| P | 66.42 | 1 | 66.42 | 80.68 | 0.0122 | |
| T.P | 9.92 | 1 | 9.92 | 12.05 | 0.0739 | |
| Curvature | 4.07 | 1 | 4.07 | 4.95 | 0.1561 | |
| Pure Error | 1.65 | 2 | 0.8233 | |||
| Cor Total | 115.13 | 6 |
| Phytosterols (mg/100 g) | |||||||
|---|---|---|---|---|---|---|---|
| Test 1 | Campesterol | Stigmasterol | β-Sitosterol | Total (This Work) | |||
| This Work | Literature | This Work | Literature | This Work | Literature | ||
| 1 | 11.3 ± 1.5 b,c | 5.5 [25] | 31.5 ± 0.7 b | 14.21 [25] 12.3 [26] | 83.8 ± 2.0 b | 63.9 [25] 145 [26] | 126.6 ± 2.7 b |
| 2 | 16.3 ± 0.7 a | 46.9 ± 0.6 a | 106.2 ± 2.9 a | 169.5 ± 4.2 a | |||
| 3 | 7.7 ± 0.3 d | 25.9 ± 1.2 c | 61.4 ± 0.0 d | 95.0 ± 1.5 c | |||
| 4 | 13.5 ± 0.6 a,b | 31.1 ± 1.3 b | 72.0 ± 3.6 c | 116.6 ± 5.5 b,c | |||
| 5–7 | 9.8 ± 0.3 c,d | 30.3 ± 1.2 b | 67.6 ± 0.5 c,d | 107.7 ± 0.3 c,d | |||
| Soxhlet | 7.8 ± 0.2 d | 24.2 ± 0.5 c | 65.4 ± 0.6 c,d | 97.4 ± 0.8 d,e | |||
| Flavonoids (mg/100 g) | ||
|---|---|---|
| Test 1 | This Work | Literature |
| 1 | 254.94 ± 1.05 b | 29 ± 1.7 [30] |
| 2 | 276.46 ± 2.63 a | |
| 3 | 264.66 ± 2.29 b | |
| 4 | 277.12 ± 2.07 a | |
| 5–7 | 274.83 ± 6.95 a | |
| Fatty Acid | This Work | Literature | ||||
|---|---|---|---|---|---|---|
| 28 MPa/60 °C | Soxhlet | Santos et al. [13] | Chani-Paucar et al. [12] | Fetzer et al. [11] | ||
| Palmitic acid | C16:0 | 7.1 ± 0.11 b | 7.9 ± 0.01 a | 7.8 ± 0.08 | 7.6 ± 0.1 | 5.56 |
| Stearic acid | C18:0 | 6.9 ± 0.06 b | 8.1 ± 0.19 a | 4.8 ± 0.06 | 5.7 ± 0.1 | 5.16 |
| Oleic acid | C18:1 | 51.1 ± 0.46 b | 54.3 ± 0.33 a | 48.8 ± 0.04 | 50 ± 1 | 52.4 |
| Linoleic acid | C18:2 | 28.5 ± 0.2 a | 21.7 ± 0.03 b | 26.0 ± 0.17 | 27 ± 1 | 23.9 |
| Arachidic acid | C20:0 | 1.4 ± 0.05 b | 1.7 ± 0.03 a | 1.2 ± 0.02 | 1.4 ± 0.1 | 1.3 |
| Gadoleic acid | C20:1 | 1.9 ± 0.01 b | 2.4 ± 0.04 a | 2.3 ± 0.03 | 2.9 ± 0.1 | |
| Behenic acid | C22:00 | 3.0 ± 0.02 b | 3.9 ± 0.1 a | 4.1 ± 0.06 | 3.0± 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.C.; Lopes, G.; Filho, A.C.; Postaue, N.; Belisário, C.; Paim, L.L.; Cardozo-Filho, L.; da Silva, C.; Ferreira-Pinto, L.; Favareto, R. Assessment of Yield, Flavonoid and Phytosterol Contents, and Fatty Acid Composition of Baru Almond Oil (Dipteryx alata Vogel) by Supercritical CO2 Extraction. Processes 2024, 12, 1729. https://doi.org/10.3390/pr12081729
Ferreira AC, Lopes G, Filho AC, Postaue N, Belisário C, Paim LL, Cardozo-Filho L, da Silva C, Ferreira-Pinto L, Favareto R. Assessment of Yield, Flavonoid and Phytosterol Contents, and Fatty Acid Composition of Baru Almond Oil (Dipteryx alata Vogel) by Supercritical CO2 Extraction. Processes. 2024; 12(8):1729. https://doi.org/10.3390/pr12081729
Chicago/Turabian StyleFerreira, Ana Carolina, Guilherme Lopes, Antonio Carlos Filho, Najla Postaue, Celso Belisário, Leonardo Lataro Paim, Lúcio Cardozo-Filho, Camila da Silva, Leandro Ferreira-Pinto, and Rogério Favareto. 2024. "Assessment of Yield, Flavonoid and Phytosterol Contents, and Fatty Acid Composition of Baru Almond Oil (Dipteryx alata Vogel) by Supercritical CO2 Extraction" Processes 12, no. 8: 1729. https://doi.org/10.3390/pr12081729
APA StyleFerreira, A. C., Lopes, G., Filho, A. C., Postaue, N., Belisário, C., Paim, L. L., Cardozo-Filho, L., da Silva, C., Ferreira-Pinto, L., & Favareto, R. (2024). Assessment of Yield, Flavonoid and Phytosterol Contents, and Fatty Acid Composition of Baru Almond Oil (Dipteryx alata Vogel) by Supercritical CO2 Extraction. Processes, 12(8), 1729. https://doi.org/10.3390/pr12081729







