Abstract
Magnetic ion-imprinted polymers (MIIPs) are an innovative material that combines the selectivity of ion imprinting with the ease of separation provided by magnetic properties. Recent advancements in MIIPs have shown that they have higher selectivity coefficients compared to non-imprinted materials. The synthesis of MIIPs involves creating specific recognition sites for target ions in magnetic nanomaterials. Various nanomaterials, such as graphene oxide, carbon nanotubes, and silica, have been incorporated into the IIPs to improve their analytical performance for different environmental applications, including metal extraction, monitoring, detection, and quantification. This review stresses the need to develop new monomers with a high affinity for the target analyte and to find supporting materials with groups that facilitate the effective removal of the target analyte. It also explores the influence of experimental parameters on metal determination.
1. Introduction
Environmental pollution caused by heavy metals is an appealing case study for many researchers. Over the years, there has been a significant increase in scientific research on removing these toxins [1]. Heavy metals such as Arsenic (As), copper (Cu), cadmium (Cd), and lead (Pb) are hazardous and pose a threat to both people and other living organisms. These metals are non-biodegradable, persistent, and poisonous [2,3]. Industries such as mining, tannery, and fertiliser production are the primary sources of environmental heavy metal pollution [4]. The amount and duration of exposure determine the level of hazard caused by these metals [4]. Heavy metals can travel through the food chain and accumulate in the human body, causing damage to organs and the central nervous system [5,6]. This has prompted various organisations to regulate the levels of these metals in different environmental compartments to ensure people’s safety. The World Health Organization (WHO) and Environmentals Protection Agency (EPA) have set the maximum permissible discharge of heavy metal at less than 2.0 mg L−1 [7]. However, despite this limit, large quantities of heavy metals beyond the permitted threshold are still being released into the environment every year. This can lead to severe health and environmental problems as these metals move through the food chain [7]. Therefore, studying heavy metals and their environmental impact is of utmost importance.
Nanoparticles have recently gained recognition for their effectiveness in removing heavy metals quickly and efficiently [8]. Magnetic nano-adsorbents are becoming increasingly popular and are well documented in the literature [9,10]. Fe3O4 is commonly used as a heavy metal adsorbent due to its many advantages over other nanomaterials, including resistance to agglomeration, eco-friendliness [11], diversity in surface functionalisation [8] large surface area [12], low cost, and simple separation using an external magnet [13]. The adsorption properties of magnetic nanoparticles can be improved by adding functional groups through surface coating and ion imprinting [12]. For instance, ref. [14] combined metal-organic frameworks with magnetite, while ref. [15] employed hydrogel nanocomposite for the removal of Cu(II), Pb(II), MV, and CR, achieving high removal percentages. Moreover, magnetic graphene oxide combined with multi-walled carbon nanotubes was utilised for removing three heavy metals [16]. Magnetic iron magnesium oxide nanocomposites were synthesised and demonstrated outstanding adsorption capabilities, with maximum adsorption capacities of 1476.4 mg·g−1 for Pb(II) [17].
Using ion-imprinted polymers (IIPs) as nanocomposites is fascinating because it offers improved selectivity [12]. IIPs are primarily used in wastewater treatment [18], sensors [19], and solid-phase extraction for heavy metals [20]. IIPs have been supported by various materials over the years, such as Fe3O4, silica [21], and graphene oxide [22]. The latest trend in IIPs involves using magnetic nanoparticles as supporting materials. This method requires surface imprinting, where a layer of polymer of a certain thickness is applied to the surface of the supporting material. A suitable matrix is essential for high adsorption capacity [23]. The recognition site on the surface of the polymer aids in the elution and increases the mass transfer of the polymer [24]. Researchers have demonstrated that IIPs are a promising solution to the problem of wastewater treatment, and their application in sensor technology has shown great potential. Furthermore, IIPs are highly effective in solid-phase extraction for heavy metals. It is imperative to obtain the relevant data for synthesising and applying magnetic ion-imprinted polymers. The recommended search engines for this purpose are Google Scholar, Scopus, Research Gate, and Science Direct. The use of appropriate keywords such as surface-imprinted polymers, magnetic nanocomposites, selective ion adsorption, ion-imprinted polymers, polymerisation techniques, water treatment, and imprinted nanocomposites is crucial. The search results must include combinations such as surface-imprinted polymers using carbon nanotubes, surface imprinting using graphene as a matrix, synthesis of magnetic ion-imprinted polymers, adsorption of metals using magnetic nanocomposites, and application of magnetic ion-imprinted polymers in environmental samples. This review paper thoroughly discusses the synthesis, adsorption, pre-concentration, and sensing of MIIPs. Furthermore, future opportunities and challenges of magnetic IIPs for removing heavy metals are also discussed.
2. Synthesis of Magnetic Ion-Imprinted Polymers
The concept of imprinted polymers dates to 1949, when Dickey first proposed it, and is now a firmly established approach to generating molecularly polymer materials that have specific recognition [25]. This imprinting technique is based on the template-monomer pre-complex formation, and an initiator is added to kick-start the reaction. At the same time, a crosslinker forms a rigid structure, and upon removal of the template, three-dimensional cavities with specificity to the template are generated [26]. The pre-complex mentioned is created by interactions that can be either covalent, semi-covalent, or non-covalent. Covalent leads to a homogenous distribution of binding sites but makes template removal difficult. Non-covalent comprises electrostatic forces, van der Waals forces, and hydrogen bonding; it is simple and convenient, allows for a broader range of functional monomers, and is, hence, the most employed. While semi-covalent comprises both covalent and non-covalent characteristics, it has the advantage of efficient rebinding properties [27]. Molecularly imprinted polymers have sparked interest in the fields of solid-phase extraction [28], catalysis [29], antibodies [30], and receptors [31]. IIPs are derived from MIPs. The principles of IIPs and MIPs are similar, with the only difference being the type of recognition substance, which can be ions or molecules, and how they interact with monomers or ligands [32]. The first IIP was developed by Nishide by crosslinking poly(4-vinylpyridine) with metal ions (Cu2+, Fe3+, Co2+, Zn2+, Ni2+, and Hg2+) [33]. IIP has been applicable in solid phases [34], catalysis [35], chromatographic separation [36], and other fields since its development. These materials have the advantages of resistance to environmental extremes and specific selectivity [34]. IIPs are synthesised by bulk, precipitation, emulsion, and surface imprinting techniques. The synthesis of ion-imprinted polymers (IIPs) involves using bulk, precipitation, emulsion, and surface imprinting techniques. However, conventional IIPs are associated with issues such as non-uniform shapes and sizes, challenges in template removal, poor affinity of the imprinting sites for the target species [35], and low production yield [36]. A key challenge lies in the inaccessibility of the imprinted sites deeply embedded within the polymer matrix, resulting in reduced mass transfer efficiency for the target ions [37]. The surface ion imprinting technique has demonstrated considerable promise. It employs a suitable carrier that facilitates the formation of a polymer layer on this carrier, enabling easy access to the active site [38]. The mechanism of ion-imprinted polymers involves a complex formation between the ligand and the template, in which the ligand binds to the supporting material and also interacts with the functional groups of monomers; then, a suitable crosslinking agent is used to form a rigid polymer on the surface of the supporting material that, upon washing, leaves cavities that can selectively recapture the target metal in the presence of other competing species. The ion-ligand or template monomer complexation during the pre-polymerisation results in the selectivity of these materials [39]. The coordination number and shape of the ligand towards the template determine the selectivity of IIP cavities towards the template [40]. The ion’s radius, charge, and size determine the cavities’ shape, size, and functionalities [23]. These materials are cost-effective; they can be effectively stored for long time while retaining their affinity for the target ion [41]. Even though new IIPs with new capabilities have been developed throughout the years, there are still limitations and shortfalls. Their improvement lies in finding better ligands with less toxicity, good supporting materials, and monomers that can effectively form bonds with different ion templates. The polymerisation reagents are explored in the following section.
2.1. Templates
IIPs are made by using metal ions as the target analyte or template. To ensure successful polymerisation, the template must meet specific criteria. Firstly, it should be chemically inactive and not act as an inhibitor during the crosslinking or polymerisation processes. Additionally, it should remain stable at the given temperature for polymerisation or crosslinking to occur [42]. Metal ions imprinted in recent studies include, cadmium [43], copper [44], nickel [45], and gallium [46]. This technique is not limited to one template; multiple templates [47] and double templates have been studied [48,49,50]. Dual-template IIPs are more popular than multi-template. Metal ions are grouped according to their properties and their existence in the environment, for example, Cd2+ and Pb2+ [20,51], Fe3+ and Cu2+ [52], silver1+, and lead2+ [53]. Heavy metals can also be imprinted with organic compounds, such as cadmium and salicylic acid [54], λ-cyhalothrin, and copper [55]. This imprinting is good as metals co-exist with organics in actual samples. It reduces the effort of preparing the imprinted polymers for individual analytes. The ionic radius and properties of the metals should be considered when grouping these metals because that can decrease the selectivity of polymers [56]. Multi-template magnetic ion-imprinted polymers and surface imprinting can overcome limitations by providing supporting materials with functional groups that allow selective recognition of multiple target ions without compromising the polymer’s selectivity [57]. However, multiple ions can lead to decreased selectivity due to overlapping imprinted sites, and removing multiple template ions can be more challenging.
2.2. Monomers and Crosslinkers
A monomer is a substance used in polymerisation. It quickly assembles around a template and forms different interactions such as electrostatic, hygroscopic, and hydrogen bond forces. These forces improve the polymer’s ability to adsorb target ions and recognise specific ions [58]. The monomer and template are grafted onto the carrier’s surface during surface imprinting to form a thin organic layer [23]. The choice of monomer in IIPs is determined by the molecular structure of the template or the metal complex [59]. Selecting a complex ligand and monomer in ion imprinting is crucial, as it directly affects selectivity. Therefore, the functional groups on monomers must interact specifically and strongly with the template ion to create effective binding sites [60]. Table 1 shows that monomers can be classified into two types: those with double bonds and those with functional groups, such as amino and hydroxyl groups, that can bind with metal ions.
Most IIPs are synthesised using either a single or two functional monomers, as shown in Table 1. These single monomers can recognise most templates. For instance, methacrylic acid (MAA) has been able to recognise a wide range of elements such as As(III), Ni(II), Pb(II), Ag(I), and more, as shown in Table 1. However, more recently, bifunctional and multidentate monomers, such as acrylic acid, vinyl pyridines, methacrylate, and ox silanes, have been used as the typical monomers, resulting in low selectivity [40]. For example, N, N, N-tri(2-carboxyethyl)-3-(2-aminoethylamino) propyl-trimethoxy silane provides five ligating atoms to complex a metal target [61]. All in all, polyfunctional monomers offer better selectivity [61]. However, it is challenging because environmental/industrial samples contain complex matrices and co-existing metal ions. Hence, multiple functional monomers are needed for multi-point interactions [62]. Also, more than one monomer can be used for a single polymerisation, e.g., Ref. [62] employed three monomers, gelatine, 8-hydroxyquinoline, and chitosan, to remove Cu(II) from an aqueous solution. Chitosan and acrylic acid were used to remove Ni(II) [63]. Alternative monomers, except for commercial ones, can be used. These monomers include ally-rich amines [22], ally chloride [40], chitosan [64], waste beer yeast [65], and papain [48]. Cost, toxicity, availability, solubility, and thermal stability are key factors when choosing monomers. Computational simulations can simplify the selection of the appropriate monomer for a specific template. This is done by calculating the monomer ratio based on the binding energy of the complex. This technique is mainly used in molecularly imprinted polymers (MIPs) and is not commonly used in ion imprinting. The crosslinking agents play a crucial role in determining the properties of IIPs, including their capacity and selectivity. Monomers with inadequate cross-linking capabilities can result in flexible or collapsing binding sites. Therefore, they are essential to consider when designing MIPs [66].

Table 1.
Monomers that are used in ion-imprinted polymers.
Table 1.
Monomers that are used in ion-imprinted polymers.
| Template | Monomers and Ligands | Crosslinker | Supporting Material | Polymerisation Technique | References |
|---|---|---|---|---|---|
| Cd(II) and Pb(II) | Popain | APTES | Fe3O4-SiO2 | Surface imprinting combined with sol-gel | [48] |
| Cd(II) | Waste beer yeast | TEOS | Fe3O4-SiO2 | Surface imprinting combined with sol-gel | [65] |
| Au(III) | TEOS and Y-MAPS | EDGMA | Hybrid monolithinic vinyl functionalised Fe3O4 | One-pot synthesis (sol-gel combined with free radical polymerisation | [67] |
| Ag(I) | Methacrylic acid | EDGMA | Core-shell of Fe3O4, SiO2, and TIO2 | Sol-gel combined with surface imprinting | [68] |
| Li(I) | Sing N-propylacrylamide and benzo-12-crown 4-ether | EDGMA | Magnetic carbon nanosphere | Surface imprinting | [69] |
| Cd(II) | 2-Phosphonobutane-1,2,4-tricarboxylic acid | N,N′-Methylenebisacrylamide | Fe3O4@SiO2 | Surface imprinting and chemical grafting | [37] |
| Cr(VI) | 4-vinyl pyridine | EDGMA | Fe3O4 | Surface imprinting | [32] |
| Ni(II) | Chitosan+ Acrylic acid | N′ N-methylene bis-acrylamide | Fe3O4 Multi-walled carbon nanotubes | Inverse emulsion system | [63] |
| Ni(II) | Citric acid | Polyvinyl alcohol | Bentonite/CoFe2O4/SiO2 @ Polyvinyl alcohol | Surface imprinting | [70] |
| Cd(II) and Pb(II) | Methacrylic acid | EDGMA | Fe3O4@SiO2@NH2 | Ultrasonic-mediated precipitation polymerisation | [51] |
| Ni(II) | Methacrylic acid | EDGMA | Fe3O4@SiO2–NH2 | Surface imprinting | [71] |
| Pb(II) | Itaconic acid | EDGMA | Fe3O4@itonic acid | Surface imprinting | [72] |
2.3. Solid Matrix
Surface imprinting is a technique that uses magnetic nanoparticles as supporting materials to create a polymer on the surface of these materials [67]. Some supporting materials include chitosan, graphene oxide, silicon dioxide, carbon nanotubes, and organic frameworks. These materials are carefully selected based on their specific properties, such as having a large surface area, being stable, insoluble, and containing many active groups that can be complex with heavy metals [56]. The supporting material is critical in enhancing the adsorption efficiency by regenerating cavities on a large surface area. Furthermore, the template used in the process should have a high affinity for the functional groups of the supporting material.
2.3.1. Magnetic Carbon Nanotubes
Carbon nanotubes, discovered by S. Iijima in 1991, have since been widely used due to their exceptional properties, such as high chemical stability, large surface area, and outstanding physisorption efficiency [68,69]. Adding magnetic nanoparticles to carbon nanotubes enhances their surface functional groups, allowing for the formation of additional tubes by opening some of the end caps. Figure 1 illustrates the synthesis of a magnetic nanotube ion-imprinted polymer.
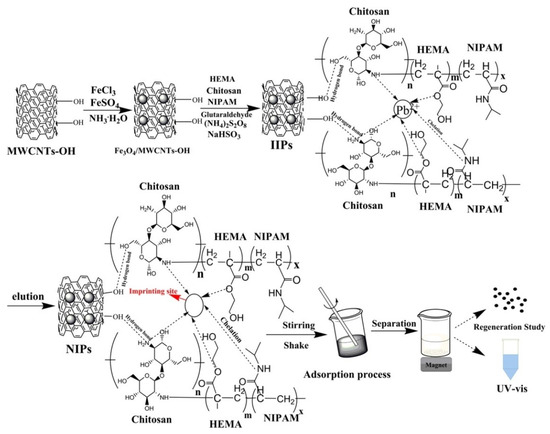
Figure 1.
Ion imprinting with a multi-walled carbon nanotube [24].
Magnetic multi-walled nanotube IIP nanoparticles with a maximum sorption capacity of 48.1 mg g−1 were used to detect lead ions selectively [70]. Another example was a functionalised carbon nanotube-based multi-ion-imprinted polymer with dithizone ligand, which formed chelates with many metals. Acrylamide was added as a co-monomer, was cross-linked with methylene bisacrylamide, and exhibited maximum adsorption capacities of 105, 91.8, 75.0, and 63.5 mg g−1 for Hg(II), Cd(II), Cu(II), and Ni(II), respectively [71]. The oxidising surface of multi-walled carbon nanotubes with carboxylic acid end groups was used to create a 3D Fe3O4@MWCNT-CdIIP terminated with an IIP. The adsorption capacity was 109 mg g−1 (2.5 times that of a non-imprinted polymer) [69]. Magnetic carbon nanotube IIPs have a significantly higher adsorption capacity and efficiency than regular IIPs, with an adsorption capacity typically of 10 mg g−1 [34]. Furthermore, carbon nanotubes were used to construct electrochemical sensors with good stability, conductivity, electron transfer, stability under acidic conditions, and easy separation with an external magnetic field without additional centrifugation or filtration procedures [72]. A multi-ion-imprinted polymer has been created to recognise four different metals selectively: Hg(II), Cd(II), Cu(II), and Ni(II). Amino groups (-NH2) have been added to their surface to improve the reactivity of carbon nanotubes. This functionalisation process can help these nanotubes interact with metals, and the amino groups can even be used further to modify the surface of metal nanoparticles or surfaces. This material has a good adsorption capacity, considering the low adsorption capacities of multi-template polymers. The adsorption capacities of the multi-IIP were 105.34, 91.79, 75.03, and 63.54 mg g−1 of Hg(II), Cd(II), Cu(II), and Ni(II), respectively [71]. Carbon nanotubes can be combined with other nanomaterials, such as graphene, to improve the chemical stability and selectivity of the carbon nanotube IIP.
2.3.2. Magnetic Silica
Silicon dioxide (silica) is a frequent supporting material in heavy metal surface imprinting. Its porous structure has a large surface area, strong stability, easy functionalisation with diverse chemicals and polymers, and low toxicity [73]. Silica can be coupled with various organ silane coupling agents such as 3-(2-amino ethyl amino) propyl trimethoxy silane [74], 2-((2-(3-(trimethoxysilyl)propylamino)ethylimino)methyl)phenol [75], -(2-amino ethyl amino) propyl trimethoxy silane, and together with the initiators, create the initiation system, which promotes the attachment of functional monomers and templates to silica [76]. Moreover, the crosslinking agent tetraethyl orthosilicate is mainly used with organosilanes. The synthesis, as well as the polymerisation reagents, produce materials with varying functionalities and performances. For example, Cd(II) ions were imprinted on the aminoethyl chitosan surface and coated with Fe3O4@SiO2 nanoparticles. The maximum adsorption capacity determined by Langmuir of Cd(II)-IIP was 26.1, and the NIP was 6.7 mg/g, which was low [77]. Fe3O4@SiO2 as a magnetic core, followed by precipitation polymerisation using a 2-hydroxyethyl methacrylate polymer, with a maximum adsorption capacity of 65.75 mg g−1 for Pb(II) [78]. IIPs formed by silicon dioxide have similar kinetics; however, IIPs formed by combining silicon dioxide with materials such as graphene oxide perform better due to additional functional groups. Mesoporous silica was coupled with magnetic graphene oxide for selective capturing of Cu(II), and the combination of the two materials resulted in a greater adsorption capacity of 195.3 mg−1 for Cu(II) [75]. Magnetic Fe3O4 NPs were also synthesised and treated with silica with an amine (−NH2) coating before generating an IIP by precipitation polymerisation in the second stage. The polymer showed an excellent adsorption capacity of 169.49 mg/g [79]. Fe3O4@SiO2 nanocomposite was used to preconcentrate lead in real water and cosmetic samples using styrene as a functional monomer. The sol-gel method gave flexible hollow paces and high surface area-specific binding sites to lead [80]. Silicon dioxide supporting material with magnetite was also successfully used in the multi-template analysis of cadmium and lead and obtained a maximum adsorption capacity of 41.7 for Cd2+ and 76.4 mg/g for Pb2+ [48]. A typical synthesis of magnetic silicone dioxide IIP is shown in Figure 2.
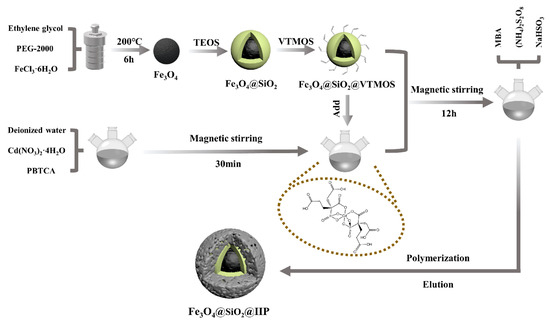
Figure 2.
Synthesis of Fe3O4@SiO2@IIP using the surface imprinting technique and chemical grafting method [37].
2.3.3. Magnetic Graphene Oxide
Graphene oxide (GO) is a two-dimensional graphene derivative. It has been used in electrochemical research, adsorption [81], and sensors [82] due to its low cost and long-term stability [81]. It is used in surface imprinting due to its vast surface area [74], high dispersity [83], extraordinary mechanical strength, hydrophilic nature, and reactive functional groups (hydroxyl, carboxyl, and epoxy). The surface of GO is modified during imprinting to add heteroatoms with a high affinity for heavy metals, such as nitrogen, sulphur, and oxygen. It provides sites for complexing with the target analyte during imprinting. GO forms complexes with metal ions through electrostatic interaction, hydrogen bonding, and coordination [84]. GO-IIPs dispense quickly, and Fe3O4 is introduced to recover the composite magnetically. Great effort has been made to provide multi-imprinting sites; hence, graphene oxide has been combined with other solid matrices. Fe3O4/GO as support was used with MAA monomer and EDGMA as the crosslinker to extract As(III), with a maximum capacity of 49.4 mg/g and good selectivity [85]. The combination of graphene oxide and water will further enlarge its surface area and strengthen its adsorption capacity. The fabrication of a lead ion-imprinted polymer based on magnetic lamellar graphene oxide (MnFe2O4@SiO2/GO-IIP) to selectively remove Pb(II) ions from an aqueous solution was successful, and the adsorption capacity of 58.82 mg g−1 was reached [86]. Figure 3 shows graphene oxide-chitosan IIP for removing copper(II). Magnetic graphene oxide was modified by the silane coupling agent KH570, which was used to remove Li+ from Salt Lake [87]. In addition, surface-imprinted Fe3O4@GO@IIP was preferentially synthesised to adsorb Ni(II) from aqueous solutions [88]. For the electrochemical monitoring of copper(II) ions, a graphite oxide/imprinted polymer composite electrode was designed. The electrode demonstrated a highly selective potentiometric response to Cu2+ compared to closely related metals such as alkaline and heavy metal cations [89]. Lanthanum ion-imprinted polymer adsorption equilibrium was achieved in just 20 min using magnetic graphene oxide as a carrier. The polymerisation process is shown in Figure 3. This method also demonstrated excellent reusability [90] and can be used for various templates, including metals, semi-metals, and lanthanides.
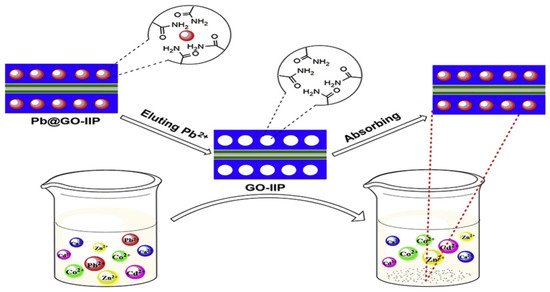
Figure 3.
Surface modification of graphene oxide with IIP for selective removal of Pb(II) [23].
2.3.4. Magnetic Chitosan
Natural polymers such as alginate, starch, and cellulose are commonly used and can be modified using methods such as graft copolymerisation, esterification, and oxidation. However, these polymers may need more structural stability to withstand harsh conditions [91]. Chitin is derived from crabs, shrimp, krill shells, and fungi and is hydrolysed to produce commercial chitosan [92]. It is amongst the most abundant polymers. It comprises monomers with primary amine and hydroxide groups and secondary hydroxyl groups. These groups can be easily modified without affecting the degree of polymerisation. Chitosan is an inexpensive substance used in various fields, such as medicine, biochemistry, and environmental protection, for a long time [93]. Even though it is non-toxic [94], its application potential is limited due to its inability to dissolve in water and most organic solvents [94]. Chitosan has sparked considerable interest in developing IIPs for heavy metal removal and can be used in IIPs to create microspheres, fibres, membranes, and hydrogels [95]. It is utilised in ion imprinting as a monomer, a supporting material, or an additive for nanocomposite [96]. To enhance the stability of chitosan in acidic environments and allow for a wide pH range for nickel adsorption, chitosan has been modified by incorporating it with magnetite, as demonstrated by [64]. Magnetite provides functional groups and is easy to operate, making it a good choice to combine with chitosan [97]. Cu(II) ions imprinted on magnetic chitosan beads were created to remove Cu(II) ions from wastewater. Chitosan was used as the functional monomer, Cu(II) ions as the template, Fe3O4 as the magnetic core, and epichlorohydrin and glutaraldehyde as the crosslinkers. Beads often have better mechanical strength and result in uniform size and shape, ensuring high reproducibility of 10 times without a significant loss [98]. Forming a membrane is difficult with chitosan due to its flexible structure in its polymer chain; it combines with a synthetic polymer (polyacrylonitrile) to form an electro-spun nanofibrous membrane. The membrane selectively removed metals and was also used for antibacterial fouling properties [99]. We also synthesised a chitosan/(vinyl alcohol) IIP for Ni(II) adsorption. This composite had an impressive adsorption capacity of 500 mg/g. Another study by [100] created a selective IIP by coprecipitating GO and magnetic chitosan nanocomposite. To selectively remove Cd(II) and Ni(II) from the aqueous solution, a dual-template imprinted nanoparticle material (MCTS@GO@DIIP) was synthesised using the surface ion-imprinted technique. The material was created on a GO slice structure coated with MnFe2O4 magnetic chitosan film [101]. Double imprinting results in decreased adsorption and the selectivity of the target ions, and the adsorption capacities were 33.91 and 39.35 mg g−1 for Cd(II) and Ni(II), respectively. Chitosan was used as a co-monomer with acrylic acid to introduce additional binding to Ni(II) as the template and crosslinked with N′ N-methylene bis-acrylamide, achieving a maximum capacity of 19.86 mg/g [63]. Some challenges associated with using chitosan in IIP could stem from variations due to differences in its biological source and processing conditions. Chitosan must be functionalised with other monomers and solid matrices to enhance and improve its selectivity.
2.3.5. Other Supporting Materials
Some materials are commonly used as a matrix in surface imprinting, but not all materials are used for this purpose. For instance, metal-organic frameworks are synthesised using a ligand or metal ions, and they have been used as supporting materials in IIPs. However, they are not typically used as a matrix in surface imprinting. Recent studies have shown that metal-organic frameworks can increase adsorption capacity as a supporting material and are used as a matrix to imprint various metal ions [95]. Moreover, it has been used without the magnetic component to help with the centrifugation. It has a high specific surface area and is easily functionalised; it has low selectivity as its adsorption ability relies on the complexation effect of its functional groups with a metal ion [102]. Clay materials such as halloysite nanoclay have proven to be excellent matrices for molecularly imprinted polymers for organics. They have a tubular-like structure and functional groups and can cheaply extract organic and inorganic compounds. They can also be an option for IIPs.
3. Application of Magnetic Ion-Imprinted Polymers
3.1. Electrochemical Detection of Metals
The IIP electrochemical sensor must be able to differentiate the electrochemical activities of the target analyte from those of other metals [103]. The synthesis of IIPs on electrode surfaces, such as glassy carbon electrodes and carbon paste electrodes, focuses on two primary methods: direct and indirect synthesis. The direct method includes in situ polymerisation, where functional monomers, template ions, and initiators are combined under specific conditions and applied to an electrode for polymerisation, and electropolymerisation, which involves polymerising functional monomers in the presence of template ions on an electrode surface under an electric potential or current. The indirect method involves synthesising IIPs through bulk, precipitation, or suspension polymerisation, removing the template ions, and then attaching the IIPs to the electrode [59]. There are challenges in integrating IIPs on electrode surfaces, such as modification strategy, renewal, low adsorption capacities, non-specific binding, heterogeneity of binding sites, and compatibility with the readout system [104]. To address this, surface-imprinted polymers coat IIPs onto certain carrier materials, such as Fe3O4, which is cost-effective and environmentally friendly and can be retrieved using an external magnet [67]. As shown in Figure 4, synthesising electrodes with magnetic ion-imprinted polymers as a modifying agent involves the separation of the magnetic IIPs on the surface of the electrode. Figure 4(I) shows the incorporation of Fe3O4 with silicon dioxide, which is then polymerised with MAA monomer in the presence of PAN. The formed mixture was then crosslinked with EDGMA, forming the AgFe3O4@SiO2-IIP. When the IIP was formed, it was dispersed in a beaker with a sample containing various matrices. The IIP was selective towards the target analyte. The adsorbent was separated from the liquid using an external magnet, as indicated in Figure 4(II) [105]. This procedure does not combine carbon paste with IIP particles, effectively cleaning and reusing the carbon paste electrode.
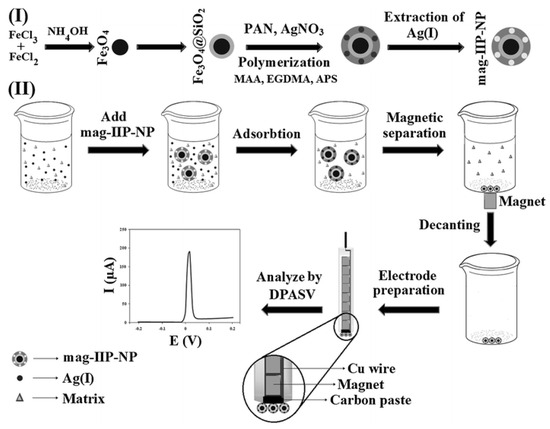
Figure 4.
Synthesis of mag-IIPs electrochemical sensor [105].
IIP can be attached to various nanomaterial substrates with increased surface area and ex-traction solid performance. A glassy carbon electrode enhanced with synthesised ion-imprinted polymer (IIP) nanoparticles forms the Fe3O4@SiO2@IIP sensor designed for detecting lead ions (Pb2+). Employing differential pulse voltammetry, Fe3O4@SiO2@IIP/glassy carbon electrode had a higher current density than Fe3O4@SiO2@NIP/GCE due to IIP’s selective cavities, as indicated in Figure 5. This sensor exhibits high sensitivity and selectivity towards Pb2+, with a notable detection limit of 0.05 ng/mL and a broad detection range of 0.1–80 ng/mL. Its performance superiority is partly due to the enhanced electron transfer capability of the IIP nanoparticles. In contrast to its counterpart using non-imprinted polymers (Fe3O4@SiO2@NIP), the IIP-based sensor shows significantly better peak current density, indicating higher activity and selectivity. The magnetic nanoparticles enhanced the sensor’s performance and stability [103].
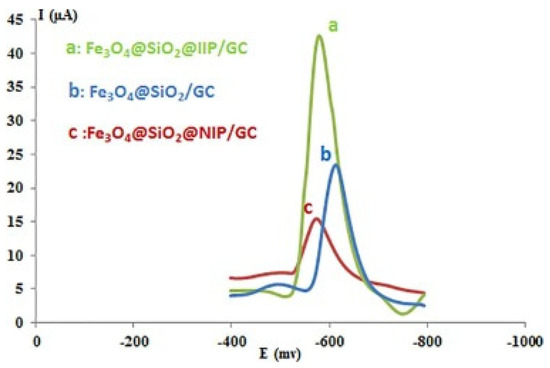
Figure 5.
CV curves for Fe3O4@SiO2@IIP/GCE, Fe3O4@SiO2@NIP/GCE, and Fe3O4@SiO2/GCE [103].
The magnetic ion-imprinted polymer (IIP) sensor was synthesised based on Fe3O4/SiO2/CS and modified on a glassy carbon electrode. The SiO2 coating enhances resistance to harsh environments. Electrochemical impedance spectroscopy showed that the ion-imprinting sites promote electron transfer, improving sensor sensitivity. The peak current for Fe3O4/SiO2/CS/Nafion/GCE IIP is nearly four times higher than for non-imprinted polymers (NIP) due to cavities left by etched Cu(II) acting as re-absorption sites. The sensor has a broad linear detection range (0.01 to 20 μmol L−1) and an ultra-low limit of detection (LOD) of 0.005 μmol L−1. It demonstrates high selectivity for Cu(II) even in the presence of other interfering ions. Recovery experiments confirm its applicability in natural samples [67]. The magnetic ion-imprinted polymer (IIP) sensor is designed for selective detection of Co2+ ions using nanobeads created by polymerising specific compounds around a Co2+ complex on magnetic nanoparticles. This sensor, which utilises electrochemical methods for evaluation, shows improved conductivity and the ability to create channels for ion diffusion after Co2+ removal, enhancing its detection capability. It offers excellent sensitivity, with a detection limit of 0.1 nM and a quantification limit of 0.33 nM, along with good reproducibility and stability after multiple uses. The sensor’s performance is highly effective and comparable to existing Co2+ sensors, proving to be a reliable tool for detecting ultra-trace levels of Co2+ in various samples such as water, blood, and urine [106]. Magnetic silver ion-imprinted polymer nanoparticles were developed for the selective detection of silver(I) ions using electrochemical analysis. Utilising differential pulse voltammetry on a magnetic carbon paste electrode, these nanoparticles captured Ag(I) ions and provided a precise measure of their concentration. The magnetic IIP nanoparticles boast a superior adsorption capacity, yielding a robust electroanalytical signal. This sensor is notable for its excellent selectivity against other metal ions, even in high excess, allowing for efficient detection of Ag(I) in a variety of water sources, including dams, aqueducts, and well water, which proves its versatile applicability in real-world conditions [105]. Table 2 summarises the performance of magnetic electrochemical sensors.

Table 2.
Electrochemical detection of various magnetic ion-imprinted materials.
3.2. Preconcentration of Heavy Metals
The use of IIP in preconcentration is an alternative to conventional methods that generate secondary pollution, low efficiency, and costly materials [108]. Magnetic IIPs serve as an attractive case study due to their excellent selectivity and have been used in several studies for the preconcentration of heavy metals. Solvothermal with one-pot synthesis produced Au(III) poly(1-vinyl imidazole) functionalised gold ion-imprinted polymer-based. The hybrid monolithic interlayer formation contributed to the excellent chemical and mechanical stability of the MIIP, enhancing its durability and effectiveness. The maximum adsorption capacities of the MIP and NIP were 92.8 and 45.9 mg g−1. A selectivity study using ions similar to the target ion showed that Au(III) had recoveries ranging from 93–96%, indicating excellent anti-interference ability in complex matrices. The MIIP can be reused up to 15 times and has a small detection limit (LOD) of 0.002 μg L−1, making the method highly accurate. The measured value of 4.56 μg g−1 was close to the certified value of 4.68 μg g−1, confirming the reliability of the adsorbent. Furthermore, the method was successfully applied to various environmental and mineral samples, demonstrating its versatility and practical utility in real-world applications [109]. The polymer is grafted onto multi-walled carbon nanotubes using carboxylic acid end groups. Functional monomers, such as methacrylic acid and a crosslinker, N, N methylene bisacrylamide, are used in polymerisation. The synthesised polymer shows an adsorption capacity of 109 mg/g, significantly higher (2.5 times) than non-imprinted polymers. The preconcentration factor was 50, enhancing sensitivity and selectivity. The method is applicable for determining and removing Cd(II) from food, tap water, and wastewater, making it versatile for environmental and food safety monitoring. Cd(II)-loaded IIP was further utilised to remove anionic dyes with >95% removal [69]. Despite the improved thermal and mechanical stability provided by the surface coating on MWCNTs, there is still some susceptibility to swelling and shrinking. This can affect the reproducibility and reliability of the adsorption capacity, particularly in varying environments. Also, the detection limit of 1.13 µg/L is high; other methods can offer lower detection limits, making them more suitable for trace-level analysis. MIIPs typically have a magnetic core (often Fe3O4 nanoparticles) coated with a silica shell that provides a surface for further functionalisation. The imprinted sites were highly selective for the target ions, such as Cd(II), Al(III), or Be(II), due to their specific size, shape, and functional group orientation that match the template ions used during synthesis. The method had LODs of 3.2 and 0.9 ng mL−1 [110]. The analytical performance of various magnetic IIPs is summarised in Table 3.

Table 3.
Analytical performance of various MIIPs.
3.3. Adsorption Performance of IIPs
Several key factors can impact the sorption efficiency of IIPs when used for wastewater treatment. These factors include ligand groups, degree of cross-linking, and properties specific to the wastewater, such as its pH level and the presence of additional contaminants [35]. IIPs are widely recognised for their ability to bind specific analytes selectively. To evaluate their adsorption performance, IIPs can be compared to non-imprinted polymers (NIPs), produced using the same technique but without adding the template ion. The equations used to calculate adsorption performance are listed below. The percentage removal is an approximate measure of adsorption performance, as shown in Equation (1):
C and Ce are the initial adsorbate and concentration at equilibrium. Equation (2) is the equilibrium concentration of the adsorbate Qe (mg/g) at the amount of adsorbate adsorbed at the equilibrium.
where m (g) is the dry mass of the used adsorbent; and V (L) is the volume of the adsorbate solution.
To evaluate the ability of the template ion to be recognised by the IIP in the presence of interfering species, such as other cations, competitive experiments are conducted. These experiments can involve multicomponent mixtures. Equation (3) represents the distribution coefficient, while Equations (4) and (5) are utilised to calculate the selectivity coefficient and the relative selectivity coefficient.
Kd is the partition coefficient, k is the selectivity coefficient, and k′ is the selectivity coefficient. Ci and Cf are the initial and final concentrations of the analyte, respectively.
Different parameters are studied in adsorption, the most common being pH, concentration, and mass of the adsorbent.
Influential Parameters in the Adsorption of Metals
- Sample pH
The pH value is an essential factor affecting metal ions’ preconcentration in various matrices. The species and surface charge of the adsorbent change as the pH value changes, which involves the adsorption process. Therefore, the optimal adsorption conditions depend on the adsorbing material’s surface properties and the adsorbate’s characteristics [113]. Most researchers have found that metal ions have an optimum pH range of 6–8 [62,85]. Additionally, heavy metals tend to have lower adsorption rates at higher pH levels due to the hydroxide causing the target ions to precipitate rather than adsorb [12,114]. At low pH levels, the H+ proton and the target analyte compete for the same adsorption sites on the material. However, the H+ proton can bind to the imprinted cavities, creating a repulsive force with the charged ion. As the pH of the solution increases, the electrostatic and repulsive forces decrease, reducing the competition between metal ions and protons for the adsorption sites. This ultimately leads to an increase in the adsorption capacity of the material [86].
- Adsorbent dose
Apart from pH, the amount of adsorbent used and the concentration of the adsorbate are critical factors that determine the effectiveness of adsorption. The amount of adsorbent used is a crucial economic parameter, and using a smaller dose of adsorbent in experiments is desirable from a financial standpoint. Increasing the amount of adsorbent increases the number of available sites or cavities for metal ions [78]. At constant concentration, it was also observed that the magnetic IIP’s high dosage generated more sorption sites, increasing the capacity. Ref. [115] study examined the impact of concentration on the pre-concentration of Co2+. The concentration levels varied from 200 to 1400 mg L−1. Higher concentrations increased cation migration to adsorption sites, with the optimum value achieved at 1000 mg L−1. Conversely, increasing the adsorbent dose increased the number of unsaturated sites available. The IIP performs better due to matrix cavities, producing higher adsorption capacity and selectivity. The distribution coefficient (Kd) and selectivity coefficient (K) of the IIP were 2.9 times that of the NIP [86] and also [101] had a similar observation where the selectivity coefficient of Ni(II) was 2.98 times that of the NIP. The performance between NIP and IIP is summarised in Table 3.
- Contact time
The contact time is an essential factor in determining the efficiency and effectiveness of the adsorption process. This parameter affects the mass transfer and interaction between the adsorbent and the analytes. MIIPs reach equilibrium more quickly, requiring minimal time. For instance, the imprinted polymer reached equilibrium at 90 min, while the non-imprinted polymer took 120 min, which is a significant difference. Additionally, the adsorption process was rapid within the first 20 min, attributed to the many unoccupied reactive sites on the surface. Equilibrium reached 90 min, with a slower increase in adsorption from 20 to 90 min due to decreased availability of reactive sites [12]. Ref. [116] also demonstrated rapid adsorption kinetics in just 35 min due to the dendritic mesoporous structure, which is highly accessible to cadmium ions. However, Ref. [37] showed that the non-imprinted (Fe3O4@SiO2@NIP) reached equilibrium first due to a smaller surface area than Fe3O4@SiO2@IIP, which had more adsorption sites created during the imprinting and had a higher adsorption capacity. As a result, it requires more time to reach equilibrium due to the more available adsorption sites.
- Initial metal ion concentration
The adsorption capacity of the MIIP materials increases with the concentration of Cu2+ ions up to approximately 60 mg L−1, after which it plateaus due to saturation of adsorption sites. NIP showed lower adsorption capacities for copper ions than imprinted [36]. Similarly, for Pb(II) ions, a polyacrylonitrile-chitosan electro-spun nanofibrous membrane demonstrated significantly higher adsorption capabilities than the NIP, especially at higher temperatures (318 K). This enhanced performance is attributed to the IIP’s stronger affinity for Pb(II) ions [99]. Additionally, the adsorption capacity for both copper and lead ions increases with higher initial metal ion concentrations in the solution until a saturation point is reached, emphasising the efficiency of MIIP materials in heavy metal ion adsorption under various conditions [117].
- Temperature
The temperature of the sample enhances the adsorption capacity of the metals. In the adsorption of Ni(II) using a sulfonic acid-based MIIP, the adsorption capacity also increased when the temperature increased. Higher temperatures can alter the pore structure of the adsorbent, potentially increasing pore size and allowing more Ni(II) ions to diffuse into the material. They can also enhance the chemical affinity between Ni(II) ions and the surface of the adsorbent, leading to more effective chemical interactions during the adsorption process [118]. The adsorption of Cd(II) onto MDMS@MAH-Cd-IIP is spontaneous, with higher temperatures enhancing the adsorption capacity. The endothermic process requires heat input, increasing randomness at the solid-liquid interface. These thermodynamic insights indicate that optimising the temperature can significantly improve the efficiency of Cd(II) adsorption onto the material [116]. The thermodynamic parameters indicate that Cd(II) adsorption onto IIPs is a spontaneous and feasible process dominated by physisorption. The endothermic nature of the process and the increase in system entropy further support the idea that higher temperatures improve adsorption efficiency. This suggests the potential for optimising temperature conditions to maximise the adsorption capacity of IIPs for Cd(II) removal [119].
Magnetic Cd(II)-IIP was imprinted on the surface of aminoethyl chitosan, coated with Fe3O4@SiO2 nanoparticles. It fitted well with the Langmuir isotherm model, suggesting that it was a monolayer and homogeneous adsorption process. Magnetic IIPs are synthesised to have a homogeneous distribution of active sites due to imprinting. These active sites are specific to the target ion, resulting in a surface where all sites have similar adsorption energies, fitting the Langmuir model assumption of a homogeneous surface [77]. It can be observed from Table 4 that most of the MIIPs fit well with Langmuir. Even though the author [65] had 30 mg, which is a bigger adsorbent dose than 5 mg [77], the equilibrium of the bigger mass was reached fast because the adsorbent might have more accessible active sites, so it can be concluded that variations in synthesis lead to differences in surface characterisation and overall adsorption efficiency.

Table 4.
Adsorption of magnetic nanoparticles on ion-imprinted polymers.
4. Conclusions and Prospects
Recently, significant progress has been made in the development of MIIPs. Various techniques with different surface modifications have been developed to produce MIIPs with desirable functionalities. Most MIIPs are synthesised with iron oxides (Fe3O4), providing the magnetic properties necessary for easy separation. MIIPs with high adsorption capacity and better selectivity can be created by applying multiple surface modifications. The imprinted cavities in MIIPs reduce interference from other ions, enhancing the accuracy of analytical methods and purification processes and resulting in a low detection limit. MIIPs can be regenerated and reused multiple times, maintaining effectiveness and reducing overall costs. These materials effectively identify toxic metal ions from water, soil, vegetables, mineral samples, and agricultural samples, showcasing their versatility in environmental cleanup and pollution control. The future of MIIPs lies in incorporating nanomaterials, which could enhance their properties and functionalities, leading to new applications in various fields such as biomedicine. Continued research into the molecular and structural properties of MIIPs will improve our understanding of their behaviour and enhance their design. Developing greener synthesis methods for MIIPs will reduce their environmental impact and make them more sustainable. The use of MIIPs does not always solve the problem of low selectivity; hence, new effective strategies should be developed. There is a gap in multi-template ion-imprinted polymers, which can offer different groups from various supporting materials and ligands interacting with the template while simultaneously removing and pre-concentrating the metal ions with better selectivity and higher adsorption capacity. Poor selectivity and difficulties removing the template from the IIP could be reasons for limited studies on multi-template IIPs.
Author Contributions
N.B.M.: Conceptualisation, visualisation, investigation, validation, formal analysis, writing—original draft. P.N.N.: Conceptualisation, supervision, funding acquisition, visualisation, resources, Writing—review and editing. L.N.: writing—review and editing, funding acquisition, supervision, conceptualisation, and resources. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support provided by the Department of Science and Innovation-National Research Foundation-South African Research Chair Initiative (DSI-NRF SARChI) programme (grant number 91230), NRF-Thuthuka (South Africa, grant no: 138375), and NRF-Freestanding, Innovation, and Scarce Skills for Doctoral students (grant no: 130347).
Acknowledgments
The authors thank the Chemical Sciences Department (DFC) at the University of Johannesburg for providing the instruments and laboratory facilities necessary for conducting this project. Additionally, they acknowledge the financial support provided by the Department of Science and Innovation-National Research Foundation-South African Research Chair Initiative (DSI-NRF SARChI) programme (grant number 91230), NRF-Thuthuka (South Africa, grant no: 138375), and NRF-Freestanding, Innovation, and Scarce Skills for Doctoral students (grant no: 130347).
Conflicts of Interest
The authors confirm that they do not have any known conflicting financial interests or personal relationships that could have impacted the outcomes presented in this study.
Abbreviations
| MIIPs | Magnetic ion-imprinted polymers |
| WHO | The World Health Organisation |
| EPA | Environmental Protection Agency |
| Fe3O4 | Iron oxide |
| Fe@MgO | Iron magnesium oxide |
| IIPs | ion-imprinted polymers |
| Fe3O4@SiO2-IIP | Iron oxide-coated silicon dioxide ion-imprinted polymers |
| SMACNT-MIIP | silanised magnetic amino-functionalised carbon nanotube-based multi-ion-imprinted polymer |
| Fe3O4@SiO2@AECS-IIP | Functionalised silica-coated magnetite aminoethyl chitosan imprinted polymers |
| NIP | Non-imprinted polymer |
| Fe3O4@VTES-IIP | Iron(III) oxide functionalised with vinyltriethoxysilane ion-imprinted polymer |
| Fe3O4@SBA-15-NH2-IIP | Magnetic SBA silica NH2 ion-imprinted polymer |
| Fe3O4@MWCNT-IIP | Magnetic multi-walled carbon nanotube ion-imprinted polymer |
| Fe3O4/SiO2/CS-IIP | Magnetic silica chitosan ion-imprinted polymer |
| Fe3O4/MWCNTs-COOH | Iron(III) oxide/multi-walled carbon nanotubes functionalised with carboxyl groups. |
| IIP/MNPs-oxine/GCE | Ion-imprinted polymer/magnetic nanoparticles modified with oxine/glassy carbon electrode |
| APTES | 3-Aminopropyl)triethoxysilane |
| TEOS | Tetraethyl orthosilicate |
| EDGMA | Ethylene glycol dimethacrylate |
| Bentonite/CoFe2O4/SiO2 @ Polyvinyl alcohol | Bentonite/cobalt ferrite/silicon dioxide encapsulated with polyvinyl alcohol |
| Fe3O4@SiO2–NH2 | Iron(III) oxide coated with silica and functionalised with amine groups |
| Fe3O4@ITA | Itaconic acid-coated magnetite nanoparticles |
| TIO2 | Titanium dioxide |
| Fe3O4@MWCNT-IIP | Magnetic multiwalled ion-imprinted polymer |
| MnFe2O4@SiO2/GO-IIP | Manganese ferrite silicon dioxide graphene oxide ion-imprinted polymer |
| MnFe2O4 | Manganese oxide |
| GO | Graphene oxide |
| MDMS@MAH-Cd-IIP | Magnetic dendritic mesoporous silica functionalised with maleic anhydride cadmium ion-imprinted polymer |
References
- Nazaripour, M.; Reshadi, M.A.M.; Mirbagheri, S.A.; Nazaripour, M.; Bazargan, A. Research Trends of Heavy Metal Removal from Aqueous Environments. J. Environ. Manag. 2021, 287, 112322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Liu, L.; Yu, H. Application of Co-Pyrolysis Biochar for the Adsorption and Immobilization of Heavy Metals in Contaminated Environmental Substrates. J. Hazard. Mater. 2021, 420, 126655. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Simultaneous Adsorption of Heavy Metals and Organic Dyes by β-Cyclodextrin-Chitosan Based Cross-Linked Adsorbent. Carbohydr. Polym. 2021, 255, 117486. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Gopinath, K.P.; Kumar, P.S. Adsorptive Separation of Toxic Metals from Aquatic Environment Using Agro Waste Biochar: Application in Electroplating Industrial Wastewater. Chemosphere 2021, 262, 128031. [Google Scholar] [CrossRef] [PubMed]
- Arthi, D.; Jose, J.M.A.; Gladis, E.H.E.; Shinu, P.M.S.; Joseph, J. Removal of Heavy Metal Ions from Water Using Adsorbents from Agro Waste Materials. Mater. Today Proc. 2021, 45, 1794–1798. [Google Scholar] [CrossRef]
- Yuan, Y.; An, Z.; Zhang, R.; Wei, X.; Lai, B. Efficiencies and Mechanisms of Heavy Metals Adsorption on Waste Leather-Derived High-Nitrogen Activated Carbon. J. Clean. Prod. 2021, 293, 126215. [Google Scholar] [CrossRef]
- Xu, C.; Shi, S.; Wang, X.; Zhou, H.; Wang, L.; Zhu, L.; Zhang, G.; Xu, D. Electrospun SiO2-MgO Hybrid Fibers for Heavy Metal Removal: Characterization and Adsorption Study of Pb(II) and Cu(II). J. Hazard. Mater. 2020, 381, 120974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast Adsorption of Heavy Metal Ions onto Functionalized Lignin-Based Hybrid Magnetic Nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Fato, F.P.; Li, D.W.; Zhao, L.J.; Qiu, K.; Long, Y.T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Baloch, H.A.; Griffin, G.J.; Srinivasan, M.P.; Mubarak, N.M.; Abdullah, E.C.; Mazari, S.A.; Tanksale, A. Iron Oxide Nanomaterials for the Removal of Heavy Metals and Dyes From Wastewater. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy Metal Ions Removal from Industrial Wastewater Using Magnetic Nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, S.; Zhou, R.; Wang, C.; Song, Z.; Miller, R.H.B.; Hao, T.; Yang, R. Synthesis of Ion Imprinted Magnetic Nanocomposites and Application for Novel Selective Recycling of Ni(II). J. Clean. Prod. 2021, 314, 127999. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, K.; Chen, Q.; Wang, A.; Chen, W. Application of Magnetic Ferrite Nanoparticles for Removal of Cu(II) from Copper-Ammonia Wastewater. J. Alloys Compd. 2019, 773, 140–149. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs Nanocomposites for Rapid Removal of Heavy Metal Ions and Dyes from Water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Alipour, E.; Sadeghi, M. Superabsorbent Magnetic Fe3O4-Based Starch-Poly (Acrylic Acid) Nanocomposite Hydrogel for Efficient Removal of Dyes and Heavy Metal Ions from Water. J. Polym. Res. 2019, 26, 271. [Google Scholar] [CrossRef]
- Wang, L.; Hu, D.; Kong, X.; Liu, J.; Li, X.; Zhou, K.; Zhao, H.; Zhou, C. Anionic Polypeptide Poly(Γ-Glutamic Acid)-Functionalized Magnetic Fe3O4-GO-(o-MWCNTs) Hybrid Nanocomposite for High-Efficiency Removal of Cd(II), Cu(II) and Ni(II) Heavy Metal Ions. Chem. Eng. J. 2018, 346, 38–49. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Peng, Z.; Tan, F.; Wang, X.; Chen, J.; Qiao, X. Facile Fabrication of Fe@MgO Magnetic Nanocomposites for Efficient Removal of Heavy Metal Ion and Dye from Water. Powder Technol 2018, 326, 393–401. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, H.; Wu, L.; Yu, N.; Luo, Z.; Geng, W. Preparation of Magnetic Surface Ion-Imprinted Polymer Based on Functionalized Fe3O4 for Fast and Selective Adsorption of Cobalt Ions from Water. Water 2022, 14, 261. [Google Scholar] [CrossRef]
- Hu, J.; Sedki, M.; Shen, Y.; Mulchandani, A.; Gao, G. Chemiresistor Sensor Based on Ion-Imprinted Polymer (IIP)-Functionalized RGO for Cd(II) Ions in Water. Sens. Actuators B Chem. 2021, 346, 130474. [Google Scholar] [CrossRef]
- Hashami, Z.S.; Taheri, A.; Alikarami, M. Synthesis of a Magnetic SBA-15-NH2@Dual-Template Imprinted Polymer for Solid Phase Extraction and Determination of Pb and Cd in Vegetables; Box Behnken Design. Anal. Chim. Acta 2022, 1204, 339262. [Google Scholar] [CrossRef]
- Lee, H.K.; Choi, J.W.; Choi, S.J. Magnetic Ion-Imprinted Polymer Based on Mesoporous Silica for Selective Removal of Co(II) from Radioactive Wastewater. Sep. Sci. Technol. 2020, 56, 1842–1852. [Google Scholar] [CrossRef]
- Islam, A.; Javed, H.; Chauhan, A.; Ahmad, I.; Rais, S. Triethylenetetramine-Grafted Magnetite Graphene Oxide-Based Surface-Imprinted Polymer for the Adsorption of Ni(II) in Food Samples. J. Chem. Eng. Data 2021, 66, 456–465. [Google Scholar] [CrossRef]
- Huang, R.; Shao, N.; Hou, L.; Zhu, X. Fabrication of an Efficient Surface Ion-Imprinted Polymer Based on Sandwich-like Graphene Oxide Composite Materials for Fast and Selective Removal of Lead Ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 218–228. [Google Scholar] [CrossRef]
- Wang, H.; Shang, H.; Sun, X.; Hou, L.; Wen, M.; Qiao, Y. Preparation of Thermo-Sensitive Surface Ion-Imprinted Polymers Based on Multi-Walled Carbon Nanotube Composites for Selective Adsorption of Lead(II) Ion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124139. [Google Scholar] [CrossRef]
- Dickey, F.H. The Preparation of Specific Adsorbents. Proc. Natl. Acad. Sci. USA 1949, 35, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly Imprinted Polymer-Based Optical Sensors for Pesticides in Foods: Recent Advances and Future Trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent Advances and Applications of Molecularly Imprinted Polymers in Solid-Phase Extraction for Real Sample Analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Wang, R.; Pan, J.; Qin, M.; Guo, T. Molecularly Imprinted Nanocapsule Mimicking Phosphotriesterase for the Catalytic Hydrolysis of Organophosphorus Pesticides. Eur. Polym. J. 2019, 110, 1–8. [Google Scholar] [CrossRef]
- Parisi, O.I.; Dattilo, M.; Patitucci, F.; Malivindi, R.; Delbue, S.; Ferrante, P.; Parapini, S.; Galeazzi, R.; Cavarelli, M.; Cilurzo, F.; et al. Design and Development of Plastic Antibodies against SARS-CoV-2 RBD Based on Molecularly Imprinted Polymers That Inhibit in Vitro Virus Infection. Nanoscale 2021, 13, 16885–16899. [Google Scholar] [CrossRef]
- Guha, A.; Ahmad, O.S.; Guerreiro, A.; Karim, K.; Sandström, N.; Ostanin, V.P.; van der Wijngaart, W.; Piletsky, S.A.; Ghosh, S.K. Direct Detection of Small Molecules Using a Nano-Molecular Imprinted Polymer Receptor and a Quartz Crystal Resonator Driven at a Fixed Frequency and Amplitude. Biosens. Bioelectron. 2020, 158, 112176. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhang, M.; Jiao, J.; Zhang, H.; Du, J.; Zhang, B.; Ren, Z. Preparation of Highly Efficient Ion-Imprinted Polymers with Fe3O4 Nanoparticles as Carrier for Removal of Cr(VI) from Aqueous Solution. Sci. Total Environ. 2020, 699, 134334. [Google Scholar] [CrossRef]
- Nishide, H.; Deguchi, J.; Tsuchida, E. Selective Adsorption of Metal Ions on Crosslinked Poly (Vinylpyridine) Resin Prepared with a Metal Ion as a Template. Chem. Lett. 1976, 5, 169–174. [Google Scholar] [CrossRef]
- Yang, P.; Cao, H.; Mai, D.; Ye, T.; Wu, X.; Yuan, M.; Yu, J.; Xu, F. A Novel Morphological Ion Imprinted Polymers for Selective Solid Phase Extraction of Cd(II): Preparation, Adsorption Properties and Binding Mechanism to Cd(II). React. Funct. Polym. 2020, 151, 104569. [Google Scholar] [CrossRef]
- Lazar, M.M.; Ghiorghita, C.A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution. Molecules 2023, 28, 2798. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Huang, C.; Ma, S.; Gong, B.; Ou, J. Rapid Adsorption and Detection of Copper Ions in Water by Dual-Functional Ion-Imprinted Polymers Doping with Carbon Dots. Sep. Purif. Technol. 2023, 315, 123666. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, W.; Hu, X.; He, H.; Zhang, Y.; Li, W.; Hu, G.; Li, Y.; Deng, X. Selective Adsorption Behavior and Mechanism for Cd(II) in Aqueous Solution with a Recoverable Magnetie-Surface Ion-Imprinted Polymer. Polymers 2023, 15, 2416. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, Y.; Zhang, D.; Zhou, F.; Ning, H.; He, M.; Chi, R.; Yin, W. Highly Effective and Selective Recovery of Gd(III) from Wastewater by Defective MOFs-Based Ion-Imprinted Polymer: Performance and Mechanism. Chem. Eng. J. 2023, 474, 145782. [Google Scholar] [CrossRef]
- Nchoe, O.B.; Klink, M.J.; Mtunzi, F.M.; Pakade, V.E. Synthesis, Characterization, and Application of β-Cyclodextrin-Based Ion-Imprinted Polymer for Selective Sequestration of Cr(VI) Ions from Aqueous Media: Kinetics and Isotherm Studies. J. Mol. Liq. 2020, 298, 111991. [Google Scholar] [CrossRef]
- Rais, S.; Islam, A.; Ahmad, I.; Kumar, S.; Chauhan, A.; Javed, H. Preparation of a New Magnetic Ion-Imprinted Polymer and Optimization Using Box-Behnken Design for Selective Removal and Determination of Cu(II) in Food and Wastewater Samples. Food Chem. 2021, 334, 127563. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Balouch, A.; Mahesar, S.A.; Kumar, A.; Baloch, A.R.; Abdullah; Bhanger, M.I. Fabrication of Cadmium Tagged Novel Ion Imprinted Polymer for Detoxification of the Toxic Cd2+ ion from Aqueous Environment. Microchem. J. 2020, 158, 105247. [Google Scholar] [CrossRef]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Daňo, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, H.; Zhou, Z.; Ren, Z. Cadmium Ion-Imprinted Polymers for Adsorption and Detection of Cadmium Ions. J. Environ. Chem. Eng. 2023, 11, 110804. [Google Scholar] [CrossRef]
- Bivián-Castro, E.Y.; Zepeda-Navarro, A.; Guzmán-Mar, J.L.; Flores-Alamo, M.; Mata-Ortega, B. Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results. Polymers 2023, 15, 1186. [Google Scholar] [CrossRef]
- Giove, A.; El Ouardi, Y.; Sala, A.; Ibrahim, F.; Hietala, S.; Sievänen, E.; Branger, C.; Laatikainen, K. Highly Selective Recovery of Ni(II) in Neutral and Acidic Media Using a Novel Ni(II)-Ion Imprinted Polymer. J. Hazard. Mater. 2023, 444, 130453. [Google Scholar] [CrossRef]
- Chai, N.; Gao, L.; Li, S.; Cao, Y.; Ma, Z.; Li, L.; Hu, M. In-Suit Ion-Imprinted Bio-Sorbent with Superior Adsorption Performance for Gallium(III) Capture. J. Clean. Prod. 2023, 387, 135861. [Google Scholar] [CrossRef]
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. Multi-Ion Imprinted Polymers (MIIPs) for Simultaneous Extraction and Preconcentration of Sb(III), Te(IV), Pb(II) and Cd(II) Ions from Drinking Water Sources. J. Hazard. Mater. 2021, 416, 126175. [Google Scholar] [CrossRef]
- Xie, C.; Huang, X.; Wei, S.; Xiao, C.; Cao, J.; Wang, Z. Novel Dual-Template Magnetic Ion Imprinted Polymer for Separation and Analysis of Cd2+ and Pb2+ in Soil and Food. J. Clean. Prod. 2020, 262, 121387. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, M.; Yin, J.; Shui, R.; Yang, S.; Hua, D. Dual Ion-Imprinted Mesoporous Silica for Selective Adsorption of U(VI) and Cs(I) through Multiple Interactions. ACS Appl. Mater. Interfaces 2021, 13, 6322–6330. [Google Scholar] [CrossRef]
- Fu, J.; Wang, X.; Li, J.; Ding, Y.; Chen, L. Synthesis of Multi-Ion Imprinted Polymers Based on Dithizone Chelation for Simultaneous Removal of Hg2+, Cd2+, Ni2+ and Cu2+ from Aqueous Solutions. RSC Adv. 2016, 6, 44087–44095. [Google Scholar] [CrossRef]
- Abdullah; Balouch, A.; Alveroglu, E.; Ullah, R.; Shah, M.T.; Jagirani, M.S.; Mahar, A.M.; Chang, S.A. Synthesis of Amine-Functionalized Ultrasonic Assisted Dual Metal Imprinted Polymer: A Real Magnetic Sorbent for Simultaneous Removal of Pb2+ and Cd2+ from Water Samples. J. Polym. Res. 2023, 30, 1–13. [Google Scholar] [CrossRef]
- Ying, R.; Lu, H.; Xu, S. Ion Imprinted Dual Reference Ratiometric Fluorescence Probe for Respective and Simultaneous Detection of Fe3+ and Cu2+. New J. Chem. 2019, 43, 6404–6410. [Google Scholar] [CrossRef]
- Lu, H.; Yu, C.; Xu, S. A Dual Reference Ion-Imprinted Ratiometric Fluorescence Probe for Simultaneous Detection of Silver (I) and Lead (II). Sens. Actuators B Chem. 2019, 288, 691–698. [Google Scholar] [CrossRef]
- Rahangdale, D.; Kumar, A. Chitosan as a Substrate for Simultaneous Surface Imprinting of Salicylic Acid and Cadmium. Carbohydr. Polym. 2018, 202, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, J.; Ma, Y.; Liu, S.; Qiu, F.; Yan, Y. A Versatile Strategy to Fabricate Dual-Imprinted Porous Adsorbent for Efficient Treatment Co-Contamination of Λ-Cyhalothrin and Copper(II). Chem. Eng. J. 2018, 332, 517–527. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, T.; Tang, L.; Zeng, G.; Deng, Y.; Lu, Y.; Fang, S.; Wang, J.; Liu, Y.; Yu, J. Highly Efficient Extraction of Lead Ions from Smelting Wastewater, Slag and Contaminated Soil by Two-Dimensional Montmorillonite-Based Surface Ion Imprinted Polymer Absorbent. Chemosphere 2018, 209, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Deng, Y.; Merchant, A.; Su, J.; Zeng, G.; Long, X.; Zhong, M.E.; Yang, L.; Gong, D.; Bai, L.; et al. Insights into Surface Ion-Imprinted Materials for Heavy Metal Ion Treatment: Challenges and Opportunities. Sep. Purif. Rev. 2022, 52, 123–134. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Wu, M.; Yang, H.; Wu, X.; Hao, L.; Yu, J.; Wang, P.; Xu, F. “One-Pot” Synthesis of Mesoporous Ion Imprinted Polymer for Selective Adsorption and Detection of As(V) in Aqueous Phase via Cooperative Extraction Mechanism. Microchem. J. 2022, 177, 107272. [Google Scholar] [CrossRef]
- Yu, L.; Sun, L.; Zhang, Q.; Zhou, Y.; Zhang, J.; Yang, B.; Xu, B.; Xu, Q. Nanomaterials-Based Ion-Imprinted Electrochemical Sensors for Heavy Metal Ions Detection: A Review. Biosensors 2022, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Saedi, Z.; Hamdi, F.; Dizajdizi, B.Z. Preparation of an Electrochemical Sensor for Detection of Manganese (II) Ions Using Glassy Carbon Electrode Modified with Multi-Walled Carbon Nanotube-Chitosan-Ionic Liquid Nanocomposite Decorated with Ion Imprinted Polymer. J. Electroanal. Chem. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Chen, L.; Liang, H.; Xing, J. Synthesis of Multidentate Functional Monomer for Ion Imprinting. J. Sep. Sci. 2020, 43, 1356–1364. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, J.; Guo, X.; Wang, X.; Choo, J.; Chen, L. Green Multi-Functional Monomer Based Ion Imprinted Polymers for Selective Removal of Copper Ions from Aqueous Solution. J. Colloid Interface. Sci. 2019, 541, 376–386. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shang, H.; Zhang, X.; Sun, X. Synthesis and Application of Ion Imprinting Polymer Coated Magnetic Multi-Walled Carbon Nanotubes for Selective Adsorption of Nickel Ion. Appl. Surf. Sci. 2018, 428, 110–117. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, X.; Peng, J. Highly Selective Removal and Recovery of Ni(II) from Aqueous Solution Using Magnetic Ion-Imprinted Chitosan Nanoparticles. Carbohydr. Polym. 2021, 271, 118435. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wei, S.; Chen, D.; Lan, W.; Yan, Z.; Wang, Z. Preparation of Magnetic Ion Imprinted Polymer with Waste Beer Yeast as Functional Monomer for Cd(Ii) Adsorption and Detection. RSC Adv. 2019, 9, 23474–23483. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A. A Note about Crosslinking Density in Imprinting Polymerization. Molecules 2021, 26, 5139. [Google Scholar] [CrossRef]
- Wei, P.; Li, Z.; Zhao, X.; Song, R.; Zhu, Z. Fe3O4/SiO2/CS Surface Ion-Imprinted Polymer Modified Glassy Carbon Electrode for Highly Sensitivity and Selectivity Detection of Toxic Metal Ions. J. Taiwan Inst. Chem. Eng. 2020, 113, 107–113. [Google Scholar] [CrossRef]
- Wu, W.; Jia, M.; Zhang, Z.; Chen, X.; Zhang, Q.; Zhang, W.; Li, P.; Chen, L. Sensitive, Selective and Simultaneous Electrochemical Detection of Multiple Heavy Metals in Environment and Food Using a Lowcost Fe3O4 Nanoparticles/Fluorinated Multi-Walled Carbon Nanotubes Sensor. Ecotoxicol. Environ. Saf. 2019, 175, 243–250. [Google Scholar] [CrossRef]
- Islam, A.; Rais, S. A Facile Approach for Grafting Ion Imprinted Polymer onto Magnetic Multi-Walled Carbon Nanotubes for Selective Removal and Preconcentration of Cadmium in Food and Wastewater Samples Prior to Atomic Spectrometric Determination. Food Chem. 2023, 405, 134751. [Google Scholar] [CrossRef]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A.; Ghanei-Motlagh, M. Synthesis and Application of Novel Ion-Imprinted Polymer Coated Magnetic Multi-Walled Carbon Nanotubes for Selective Solid Phase Extraction of Lead(II) Ions. Mater. Sci. Eng. C 2016, 60, 365–373. [Google Scholar] [CrossRef]
- Kasiri, E.; Arabkhani, P.; Haddadi, H.; Asfaram, A.; Varma, R.S. A Silanized Magnetic Amino-Functionalized Carbon Nanotube-Based Multi-Ion Imprinted Polymer for the Selective Aqueous Decontamination of Heavy Metal Ions. New J. Chem. 2022, 46, 21704–21716. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhou, C.; Sun, C. Determination of Cesium Ions in Environmental Water Samples with a Magnetic Multi-Walled Carbon Nanotube Imprinted Potentiometric Sensor. RSC Adv. 2021, 11, 10075–10082. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Rahman, M.M.; Dhahi, T.S.; Kashif, M.; Sarkar, M.S.; Basirun, W.J.; Hamid, S.B.A.; Bhargava, S.K. Nanostructured Materials: Bioengineering Platforms for Sensing Nucleic Acids. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Huang, R.; Ma, X.; Li, X.; Guo, L.; Xie, X.; Zhang, M.; Li, J. A Novel Ion-Imprinted Polymer Based on Graphene Oxide-Mesoporous Silica Nanosheet for Fast and Efficient Removal of Chromium (VI) from Aqueous Solution. J. Colloid Interface. Sci. 2018, 514, 544–553. [Google Scholar] [CrossRef]
- Fang, P.; Xia, W.; Zhou, Y.; Ai, Z.; Yin, W.; Xia, M.; Yu, J.; Chi, R.A.; Yue, Q. Ion-Imprinted Mesoporous Silica/Magnetic Graphene Oxide Composites Functionalized with Schiff-Base for Selective Cu(II) Capture and Simultaneously Being Transformed as a Robust Heterogeneous Catalyst. Chem. Eng. J. 2020, 385, 123847. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Huang, R.F.; Ma, X.G.; Guo, L.H.; Wang, Y.; Fan, Y.M. Selective Fluorescence Sensor Based on Ion-Imprinted Polymer-Modified Quantum Dots for Trace Detection of Cr(VI) in Aqueous Solution. Anal. Bioanal. Chem. 2019, 411, 7165–7175. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.; Li, Y.; Dolgormaa, A.; Fang, H.; Guo, L.; Huang, J.; Yang, J. A Novel Magnetic Cd(II) Ion-Imprinted Polymer as a Selective Sorbent for the Removal of Cadmium Ions from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1874–1885. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Taghizadeh, M.; Hassanpour, S. Preparation of a Novel Magnetic Pd(II) Ion-Imprinted Polymer for the Fast and Selective Adsorption of Palladium Ions from Aqueous Solutions. Environ. Sci. Pollut. Res. 2019, 26, 18493–18508. [Google Scholar] [CrossRef]
- Kumar, S.; Alveroǧlu, E.; Balouch, A.; Talpur, F.N.; Jagirani, M.S.; Abdullah; Mahar, A.M.; Pato, A.H.; Mal, D.; Lal, S. Fabrication of Chromium-Imprinted Polymer: A Real Magneto-Selective Sorbent for the Removal of Cr(vi) Ions in Real Water Samples. New J. Chem. 2020, 44, 18668–18678. [Google Scholar] [CrossRef]
- Omidvar-Motlagh, M.; Es’haghi, Z. Magnetic Porous Ion Imprinted Polymer Based on Surface Polymerization and Nano-ZnO as Sacrificial Support for Selective Extraction and Determination of Pb (II) in Water Samples and Cosmetics. Water. Air. Soil Pollut. 2024, 235, 211. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An Ion-Imprinted Sensor Based on Chitosan-Graphene Oxide Composite Polymer Modified Glassy Carbon Electrode for Environmental Sensing Application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Y.; Wang, N.; Song, G.; Yin, X.; Zhang, L.; Ni, X.; Xu, W. A Sensitive Electrochemical Sensor Based on Ion Imprinted Polymers with Gold Nanoparticles for High Selective Detecting Cd (II) Ions in Real Samples. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2043–2053. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A Comprehensive Review of Applications of Magnetic Graphene Oxide Based Nanocomposites for Sustainable Water Purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.; Chen, C. Adsorption of Radionuclides on Carbon-Based Nanomaterials. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29. [Google Scholar]
- Chi, Z.; Zhu, Y.; Liu, W.; Huang, H.; Li, H. Selective Removal of As(III) Using Magnetic Graphene Oxide Ion-Imprinted Polymer in Porous Media: Potential Effect of External Magnetic Field. J. Environ. Chem. Eng. 2021, 9, 105671. [Google Scholar] [CrossRef]
- Hou, L.; Yang, C.; Rao, X.; Hu, L.; Bao, Y.; Gao, Y.; Zhu, X. Fabrication of Recoverable Magnetic Surface Ion-Imprinted Polymer Based on Graphene Oxide for Fast and Selective Removal of Lead Ions from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126949. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, Q.; Yang, Y.; Liu, W.; Liu, X. Magnetic Graphene Oxide Surface Lithium Ion-Imprinted Material towards Lithium Extraction from Salt Lake. Sep. Purif. Technol. 2021, 265, 118513. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Ye, S.Q.; Yang, X.T.; Zhu, B.S.; Li, W.L.; He, H.X.; Deng, X.J. A Recoverable Magnetic Surface Ion-Imprinted Polymer Based on Graphene Oxide for Fast and Selective Adsorption of Ni(Ii) from Aqueous Solution: Experimental and DFT Calculations. New J. Chem. 2022, 47, 1197–1208. [Google Scholar] [CrossRef]
- Topcu, C.; Lacin, G.; Yilmaz, V.; Coldur, F.; Caglar, B.; Cubuk, O.; Isildak, I. Electrochemical Determination of Copper(II) in Water Samples Using a Novel Ion-Selective Electrode Based on a Graphite Oxide–Imprinted Polymer Composite. Anal. Lett. 2018, 51, 1890–1910. [Google Scholar] [CrossRef]
- Shi, M.; Lu, T.; Li, X.; Yang, Y. Preparation and Properties of GO-Based Lanthanum Ion-Imprinted Polymer, La-IIP-MAA/Fe3O4-GO. J. Rare Earths 2022, 40, 135–142. [Google Scholar] [CrossRef]
- Abdollahi, F.; Taheri, A.; Shahmari, M. Application of Selective Solid-Phase Extraction Using a New Core-Shell-Shell Magnetic Ion-Imprinted Polymer for the Analysis of Ultra-Trace Mercury in Serum of Gallstone Patients. Sep. Sci. Technol. 2020, 55, 2758–2771. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an Environment-Friendly Biomaterial—A Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent Advances of Chitosan-Based Polymers in Biomedical Applications and Environmental Protection. J. Polym. Res. 2022, 29, 1–19. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, E487. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. A Novel Ion-Imprinted Polymer Induced by the Glycylglycine Modified Metal-Organic Framework for the Selective Removal of Co(II) from Aqueous Solutions. Chem. Eng. J. 2018, 333, 280–288. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Y.; Zhang, J.; Zhao, M.; Zhu, K. Study on the Adsorption Behavior of Glutaric Acid Modified Pb(II)Imprinted Chitosan-Based Composite Membrane to Pb(II)in Aqueous Solution. Mater. Lett. 2019, 251, 172–175. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, L.; Yang, S.; Liu, D.; Wang, Y.; Wang, S.; Han, X.; Zhang, X. Adsorption of Ni(II) Ion on Ni(II) Ion-Imprinted Magnetic Chitosan/Poly(Vinyl Alcohol) Composite. Colloid Polym. Sci. 2015, 293, 2497–2506. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Q. Selective Adsorption Behavior of Ion-Imprinted Magnetic Chitosan Beads for Removal of Cu(II) Ions from Aqueous Solution. Chin. J. Chem. Eng. 2021, 39, 103–111. [Google Scholar] [CrossRef]
- Hu, Q.; Tang, D.; Xiang, Y.; Chen, X.; Lin, J.; Zhou, Q. Magnetic Ion-Imprinted Polyacrylonitrile-Chitosan Electro-Spun Nanofibrous Membrane as Recyclable Adsorbent with Selective Heavy Metal Removal and Antibacterial Fouling in Water Treatment. Int. J. Biol. Macromol. 2023, 241, 124620. [Google Scholar] [CrossRef]
- Kazemi, E.; Dadfarnia, S.; Haji Shabani, A.M.; Ranjbar, M. Synthesis, Characterization, and Application of a Zn (II)-Imprinted Polymer Grafted on Graphene Oxide/Magnetic Chitosan Nanocomposite for Selective Extraction of Zinc Ions from Different Food Samples. Food Chem. 2017, 237, 921–928. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, S.; Shao, N.; Tian, Z.; Zhu, X. Synthesis of a Novel Magnetic Chitosan-Mediated GO Dual-Template Imprinted Polymer for the Simultaneous and Selective Removal of Cd(II) and Ni(II) from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132266. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Cui, K.; Li, H.; Feng, J.; Pu, X.; Xiong, W.; Liu, N.; Yuan, G. Novel MOFs-Based Ion-Imprinted Polymer for Selective Separation of Cobalt Ions from Waste Battery Leaching Solution. Inorganica Chim. Acta 2022, 536, 120922. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Novel Ion Imprinted Polymer Electrochemical Sensor for the Selective Detection of Lead(II). Food Chem. 2020, 303, 125374. [Google Scholar] [CrossRef]
- Marfà, J.; Pupin, R.R.; Sotomayor, M.; Pividori, M.I. Magnetic-Molecularly Imprinted Polymers in Electrochemical Sensors and Biosensors. Anal. Bioanal. Chem. 2021, 413, 6141–6157. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A. Magnetic Silver(I) Ion-Imprinted Polymeric Nanoparticles on a Carbon Paste Electrode for Voltammetric Determination of Silver(I). Microchim. Acta 2017, 184, 1691–1699. [Google Scholar] [CrossRef]
- Torkashvand, M.; Gholivand, M.B.; Azizi, R. Synthesis, Characterization and Application of a Novel Ion-Imprinted Polymer Based Voltammetric Sensor for Selective Extraction and Trace Determination of Cobalt (II) Ions. Sens. Actuators B Chem. 2017, 243, 283–291. [Google Scholar] [CrossRef]
- Prasad, B.B.; Jauhari, D. Double-Ion Imprinted Polymer @magnetic Nanoparticles Modified Screen Printed Carbon Electrode for Simultaneous Analysis of Cerium and Gadolinium Ions. Anal. Chim. Acta 2015, 875, 83–91. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Alatawi, A.; Monier, M. Diacetylmonoxine Modified Chitosan Derived Ion-Imprinted Polymer for Selective Solid-Phase Extraction of Nickel (II) Ions. React. Funct. Polym. 2020, 151, 104570. [Google Scholar] [CrossRef]
- Hua, Y.; Li, J.Y.; Min, H.; Wu, X.H.; Cui, X.B.; Chen, Y.J.; Lian, H.Z.; Sheng, D. Hybrid Monolith Assisted Magnetic Ion-Imprinted Polymer Extraction Coupled with ICP-MS for Determination of Trace Au(III) in Environmental and Mineral Samples. Microchem. J. 2020, 158, 105210. [Google Scholar] [CrossRef]
- Taheri, Z.; Afkhami, A.; Madrakian, T.; Kamalabadi, M. Application of Magnetic Ion Imprinted Polymers for Simultaneous Quantification of Al3+ and Be2+ Ions Using the Mean Centering of Ratio Spectra Method. Talanta 2021, 225, 122003. [Google Scholar] [CrossRef]
- Landarani, M.; Ebrahimzadeh, H.; Asgharinezhad, A.A. A Magnetic Ion-Imprinted Polymer Composed of Silica-Coated Magnetic Nanoparticles and Polymerized 4-Vinyl Pyridine and 2,6-Diaminopyridine for Selective Extraction and Determination of Lead Ions. New J. Chem. 2020, 44, 7561–7568. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Determination of Cadmium(II) Using a Glassy Carbon Electrode Modified with a Cd-Ion Imprinted Polymer. J. Electroanal. Chem. 2018, 810, 185–190. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; AlJarrah, M.; Alrebaki, M.; Mayyas, M. Nanoionic Exchanger with Unprecedented Loading Capacity of Uranium. Sep. Purif. Technol. 2020, 238, 116423. [Google Scholar] [CrossRef]
- Kumar, S.; Balouch, A.; Alveroğlu, E.; Jagirani, M.S.; Abdullah; Mughal, M.A.; Mal, D. Fabrication of Nickel-Tagged Magnetic Imprinted Polymeric Network for the Selective Extraction of Ni(II) from the Real Aqueous Samples. Environ. Sci. Pollut. Res. 2021, 28, 40022–40034. [Google Scholar] [CrossRef]
- Adibmehr, Z.; Faghihian, H. Preparation of Highly Selective Magnetic Cobalt Ion-Imprinted Polymer Based on Functionalized SBA-15 for Removal Co2+ from Aqueous Solutions. J. Environ. Health Sci. Eng. 2019, 17, 1213–1225. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, Y.; Qin, G.; Wang, J.; Li, K.; Zhu, X. Histidine-Mediated Dendritic Mesoporous Magnetic Ion-Imprinted Polymer toward Effective and Recoverable Cadmium Removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130365. [Google Scholar] [CrossRef]
- Amini, M.H.; Beyki, M.H. Construction of 1, 10-Phenanthroline Functionalized Magnetic Starch as a Lead (II) Tagged Surface Imprinted Biopolymer for Highly Selective Targeting of Toxic Lead Ions. Int. J. Biol. Macromol. 2023, 242, 124996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Deng, X.; Ye, S.; Xia, Y.; Li, L.; Li, W.; He, H. Selective Removal and Recovery of Ni(Ii) Using a Sulfonic Acid-Based Magnetic Rattle-Type Ion-Imprinted Polymer: Adsorption Performance and Mechanisms. RSC Adv. 2022, 12, 34571–34583. [Google Scholar] [CrossRef]
- Islam, A.; Chauhan, A. Tailored-Designed Material for the Preconcentration of Cd(II) on Glycidyl Methacrylate-Based Ion–Imprinted Polymer for Flame Atomic Absorption for Trace Determination in Real Samples: Multivariate Optimization. Environ. Sci. Pollut. Res. 2022, 29, 69068–69081. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Luo, S.; Zhan, Y.; Shu, H.; Huang, Y.; Tu, X. Novel Cu (II) Magnetic Ion Imprinted Materials Prepared by Surface Imprinted Technique Combined with a Sol-Gel Process. J. Hazard. Mater. 2011, 192, 949–955. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).