Characterization and Comparison of Anammox Immobilization in Polyvinyl Alcohol, Polyethylene Glycol and Water-Borne Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Immobilized Particles
2.2. Physicochemical Characterization of Embedded Pellets and Particles
2.2.1. Measurement of Sedimentation Rate
2.2.2. Measurement of Mechanical Strength
2.2.3. Measurement of the Expansion Coefficient
2.2.4. The Elasticity Is Measured as Follows
2.2.5. Determination of Mass Transfer Coefficient
2.3. Microbial Activity Batch Test under Various Salinity Levels
2.4. Operation of the Reactor Filled with Immobilized Anammox Pellets
2.5. Morphological Observation of Immobilized Anammox Pellets by Scanning Electron Microscope
3. Results and Discussion
3.1. Comparison of Immobilized Anammox Pellets of PVA, PEG, and WPU
3.1.1. Determination and Analysis of Sedimentation Rate
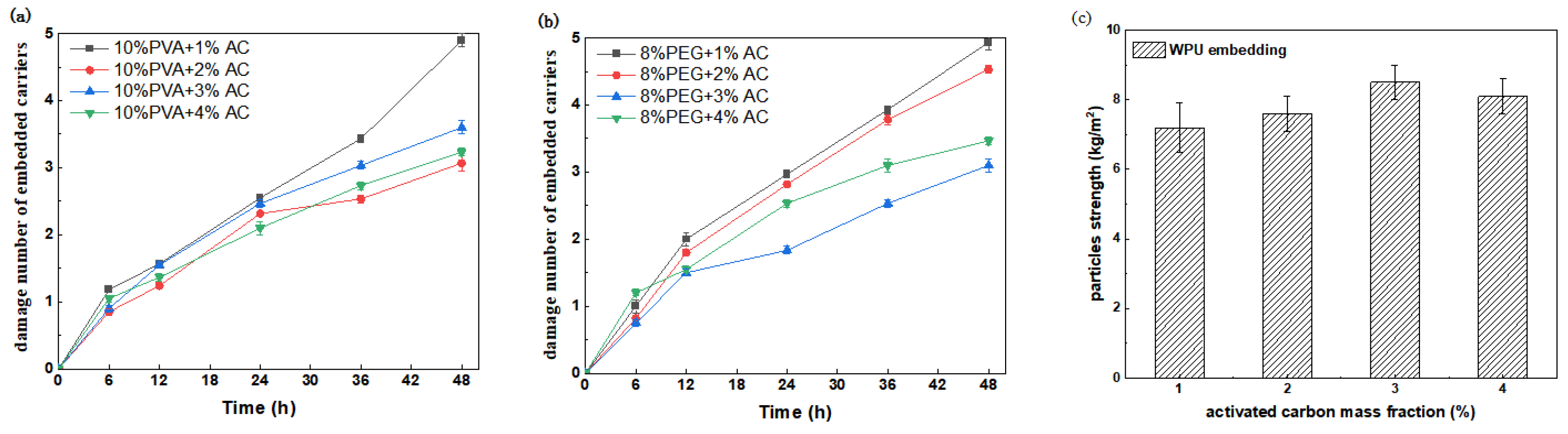
3.1.2. Mechanical Strength Analysis
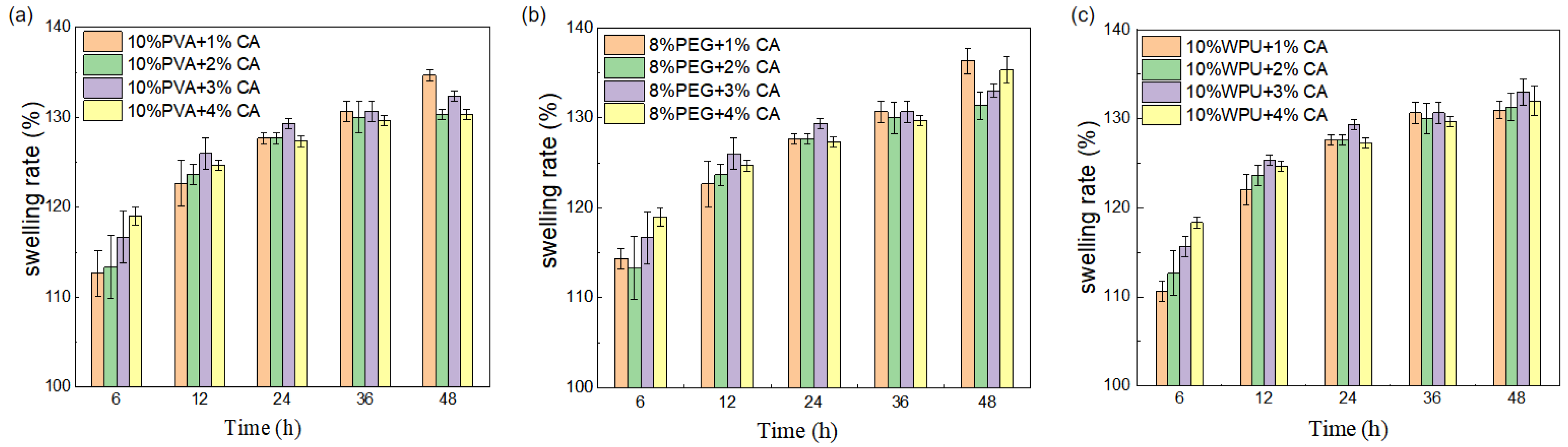
3.1.3. The Coefficient of Expansion Is Measured and Analyzed
3.1.4. Elasticity Result Analysis
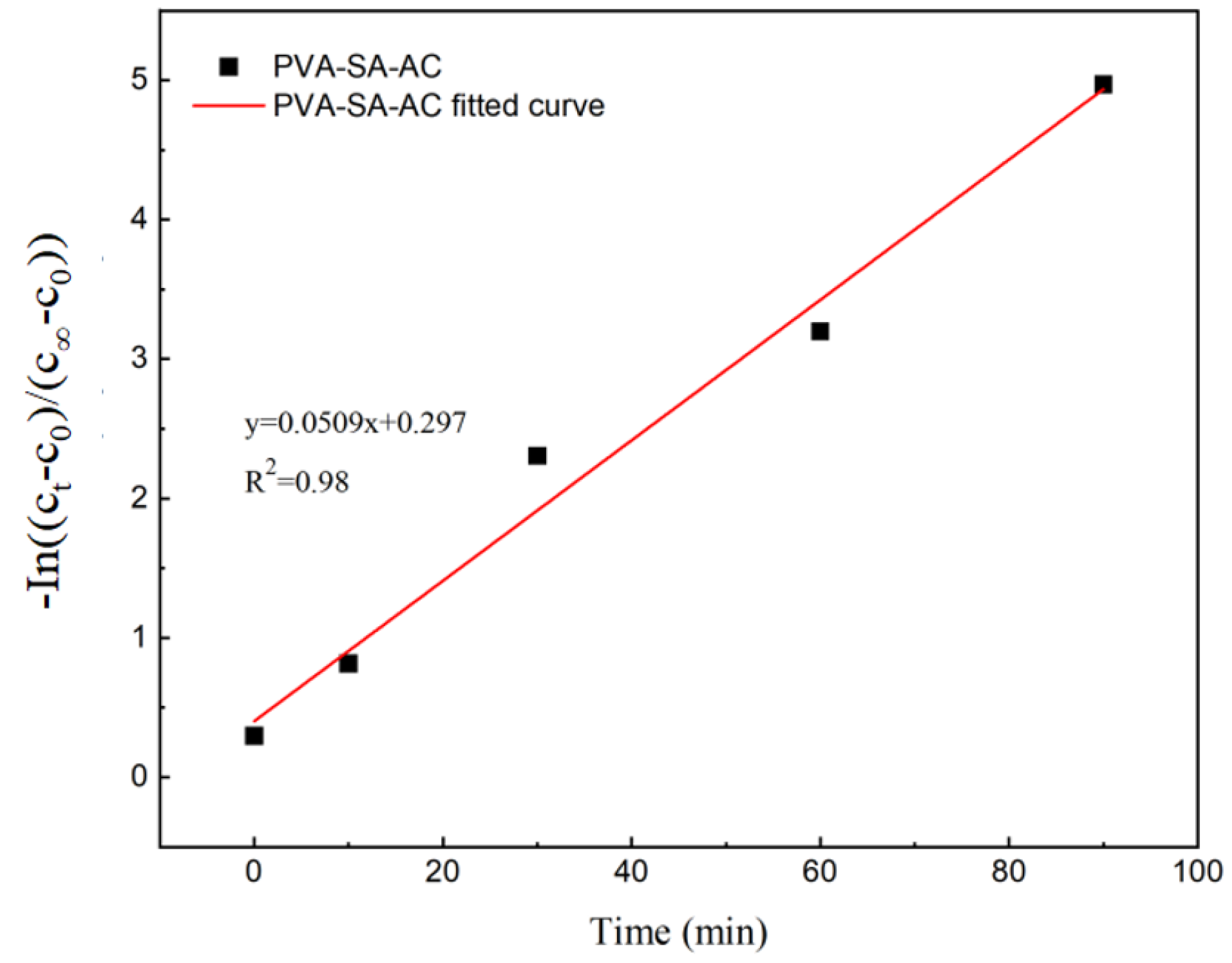
3.1.5. Ammonia Nitrogen Mass Transfer Properties of Embedded Pellets and Particles
3.2. Batch Experimental Results with Salinity Addition
3.3. Performances of Anammox Reactor with Anammox Immobilization Pellets
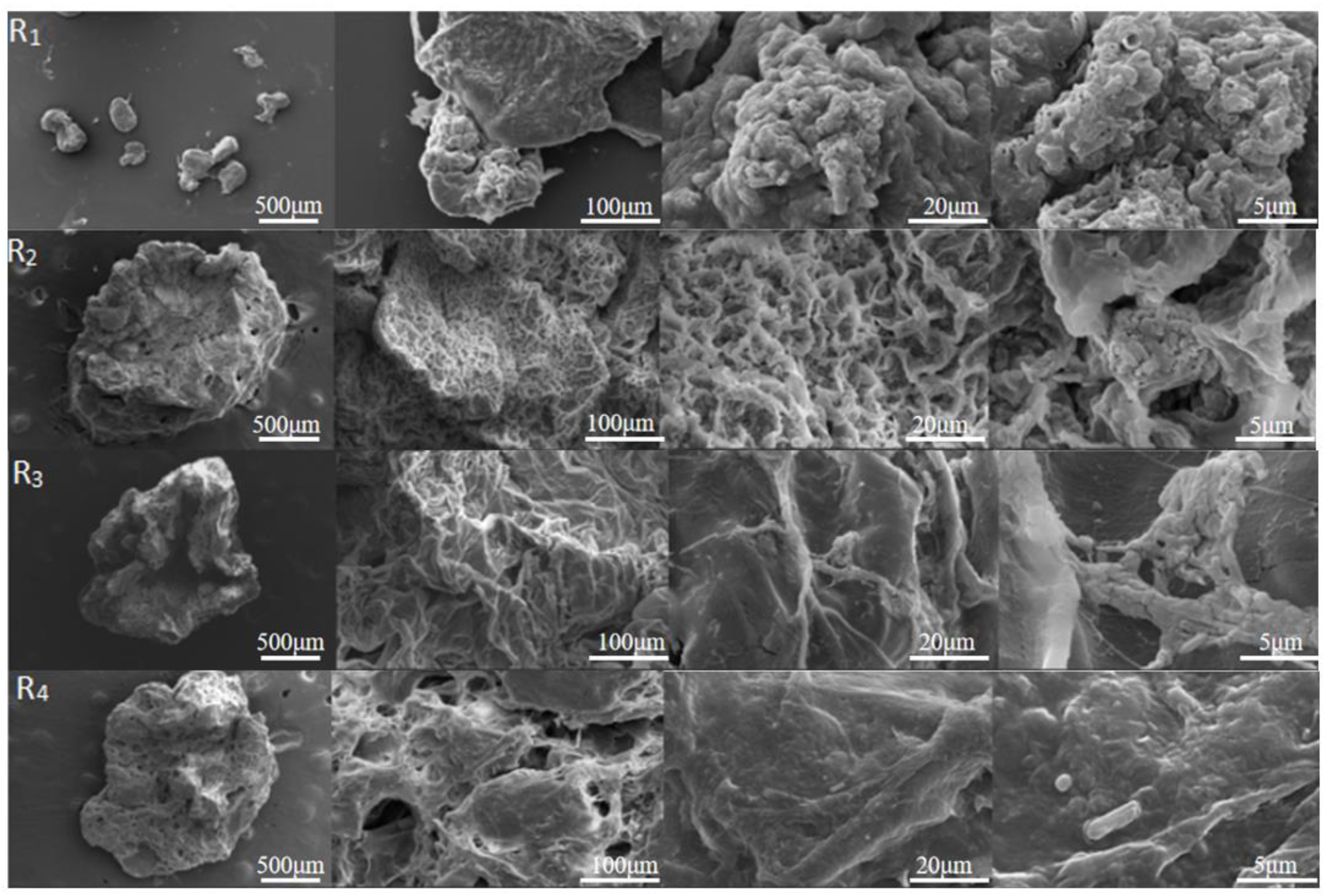
3.4. Scanning Electron Microscopy Image Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuyen, N.V.; Ryu, J.H.; Kim, H.G.; Ahn, D.H. Anammox bacteria immobilization using polyvinyl alcohol/sodium alginate crosslinked with sodium sulfate. J. Environ. Eng. 2020, 146, 04020020. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.C.; Chen, Y.P. Nitrogen removal performance and characteristics of gel beads immobilized anammox bacteria under different PVA: SA ratios. Water Environ. Res. 2021, 93, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ji, J.; Chen, Y.; Takemura, Y.; Liu, Y.; Kubota, K.; Ma, H.; Li, Y.Y. Successful operation performance and syntrophic micro-granule in partial nitritation and anammox reactor treating low-strength ammonia wastewater. Water Res. 2019, 155, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Vlaeminck, S.E.; Morgenroth, E.; Udert, K.M. Successful application of nitritation/anammox to wastewater with elevated organic carbon to ammonia ratios. Water Res. 2014, 49, 316–326. [Google Scholar] [CrossRef]
- Laureni, M.; Falas, P.; Robin, O.; Wick, A.; Weissbrodt, D.G.; Nielsen, J.L.; Ternes, T.A.; Morgenroth, E.; Joss, A. Mainstream partial nitritation and anammox: Long-term process stability and effluent quality at low temperatures. Water Res. 2016, 101, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; He, S.; Zhang, Y.; Ma, H.; Liu, Y.; Li, Y.-Y. Process stability and the recovery control associated with inhibition factors in a UASB-anammox reactor with a long-term operation. Bioresour. Technol. 2016, 203, 132–141. [Google Scholar] [CrossRef]
- Wang, X.; Gao, D. In-situ restoration of one-stage partial nitritation-anammox process deteriorated by nitrate build-up via elevated substrate levels. Sci. Rep. 2016, 6, 37500. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Zhang, S.; Zhang, Y.; Li, W.; Kan, R.; Wang, W.; Zheng, Z.; Li, J. Achieving nitritation in a continuous moving bed biofilm reactor at different temperatures through ratio control. Bioresour. Technol. 2017, 226, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; Geilvoet, S.P.; Hendrickx, T.L.G.; Kip, C.S.v.E.T.; Kleerebezem, R.; van Loosdrecht, M.C.M. Towards mainstream anammox: Lessons learned from pilot-scale research at WWTP Dokhaven. Environ. Technol. 2018, 40, 1721–1733. [Google Scholar] [CrossRef]
- Chandran, K.; Smets, B.F. Applicability of two-step models in estimating nitrification kinetics from batch respirograms under different relative dynamics of ammonia and nitrite oxidation. Biotechnol. Bioeng. 2000, 70, 54–64. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.P.; Dai, X.H. Operation of pilot-scale nitrification-anammox reactors for the treatment of reject-water produced from the anaerobic digestion of thermal hydrolysis-treated sludge. Bioresour. Technol. 2020, 314, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Krause, S.; Tecklenburg, C.; Binley, A. Reducing monitoring gaps at the aquifer-river interface by modelling groundwater-surface water exchange flow patterns. Hydrol. Process. 2011, 25, 3547–3562. [Google Scholar] [CrossRef]
- Wett, B.; Hell, M.; Nyhuis, G.; Puempel, T.; Takacs, I.; Murthy, S. Syntrophy of aerobic and anaerobic ammonia oxidisers. Water Sci. Technol. 2010, 61, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C. Full-scale partial nitritation/anammox experiences-an application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Piculell, M.; Suarez, C.; Li, C.; Christensson, M.; Persson, F.; Wagner, M.; Hermansson, M.; Jönsson, K.; Welander, T. The inhibitory effects of reject water on nitrifying populations grown at different biofilm thickness. Water Res. 2016, 104, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, G.H.; Ma, Y.; Xu, X.; Chen, J.; Yang, Y.; Liu, X.; Wang, H. A pilot-scale study on start-up and stable operation of mainstream partial nitrification-anammox biofilter process based on online pH-DO linkage control. Chem. Eng. J. 2018, 350, 1035–1042. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, Y.; Zhou, L.; Jia, X. Biosorption behaviors of biosorbents based on microorganisms immobilized by Ca-alginate for removing lead (II) from aqueous solution. Biotechnol. Bioprocess Eng. 2011, 16, 808–820. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; Kirchgessner, J.; Gerö, L.; Kunz, L.A.; Beyer, J.; Mueller-Klieser, W. Effect of microencapsulation on oxygen distribution in islets organs. Transplantation 1994, 57, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Heidebach, T.; Foerst, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Olguin, E.J. Dual purpose microalgae-bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol. Adv. 2012, 30, 1031–1046. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Calvet, D.; Wong, J.Y.; Giasson, S. Rheological monitoring of polyacrylamide gelation: Importance of cross-link density and temperature. Macromolecules 2004, 37, 7762–7771. [Google Scholar] [CrossRef]
- Loh, K.C.; Chung, T.S.; Ang, W.F. Immobilized-cell membrane bioreactor for high-strength phenol wastewater. J. Environ. Eng. 2000, 126, 75–79. [Google Scholar] [CrossRef]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Mollaei, M.; Abdollahpour, S.; Atashgahi, S.; Abbasi, H.; Masoomi, F.; Rad, I.; Lotfi, A.S.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Enhanced phenol degradation by Pseudomonas sp. SA01: Gaining insight into the novel single and hybrid immobilizations. J. Hazard. Mater. 2010, 175, 284–292. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudencio, E.S.; Amboni RD, M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Pedroso, D.L.; Thomazini, M.; Barrozo Heinemann, R.J.; Favaro-Trindade, C.S. Protection of Bifidobacterium lactis and Lactobacillus acidophilus by microencapsulation using spray-chilling. Int. Dairy J. 2012, 26, 127–132. [Google Scholar] [CrossRef]
- Cui, J.; Van Koeverden, M.P.; Müllner, M.; Kempe, K.; Caruso, F. Emerging methods for the fabrication of polymer capsules. Adv. Colloid Interface Sci. 2014, 207, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Amigoni, S.; de Givenchy, E.T.; Dufay, M.; Guittard, F. Covalent layer-by-layer assembled superhydrophobic organic-inorganic hybrid films. Langmuir 2009, 25, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical applications of layer-by-layer self assembly for cell encapsulation: Current status and future perspectives. Adv. Healthc. Mater. 2019, 8, e1800939. [Google Scholar] [CrossRef]
- Tanner, P.; Onaca, O.; Balasubramanian, V.; Meier, W.; Palivan, C.G. Enzymatic cascade reactions inside polymeric nanocontainers: A means to combat oxidative stress. Chem.-A Eur. J. 2011, 17, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016, 28, 9486. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kang, S.M.; Lee, K.B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef] [PubMed]



| Components | Concentration | Components | Concentration |
|---|---|---|---|
| NH4Cl | 50 mgN/L | NaNO2 | 65 mgN/L |
| KH2PO4 | 5 mg/L | CaCl2 | 0.5 g/L |
| trace element I | 1 mL/L | trace element II | 1 mL/L |
| Component | Concentration, mg/L | Component | Concentration, mg/L |
|---|---|---|---|
| NH4Cl | on-demand configuration | KH2PO4 | 10 |
| NaNO2 | on-demand configuration | KHCO3 | 1250 |
| CaCl2·2H2O | 3.6 | trace element I | 1 mL/L |
| MgSO4·7H2O | 300 | trace element II | 1 mL/L |
| Number | Immobilized Granules | Elasticity |
|---|---|---|
| 1 | 10%PVA-2%SA-2%AC | ✔✔✔ |
| 2 | 8%PEG-2%SA-2%AC | ✔ |
| 3 | 8%PEG-2%SA-3%AC | ✔✔ |
| 4 | 10%WPU-1%AC | ✔ |
| 5 | 10%WPU-2%AC | ✔✔ |
| 6 | 10%WPU-3%AC | ✔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Gong, H.; Zhou, Z.; Dai, X. Characterization and Comparison of Anammox Immobilization in Polyvinyl Alcohol, Polyethylene Glycol and Water-Borne Polyurethane. Processes 2024, 12, 1442. https://doi.org/10.3390/pr12071442
Yang Y, Gong H, Zhou Z, Dai X. Characterization and Comparison of Anammox Immobilization in Polyvinyl Alcohol, Polyethylene Glycol and Water-Borne Polyurethane. Processes. 2024; 12(7):1442. https://doi.org/10.3390/pr12071442
Chicago/Turabian StyleYang, Yi, Hui Gong, Zhen Zhou, and Xiaohu Dai. 2024. "Characterization and Comparison of Anammox Immobilization in Polyvinyl Alcohol, Polyethylene Glycol and Water-Borne Polyurethane" Processes 12, no. 7: 1442. https://doi.org/10.3390/pr12071442
APA StyleYang, Y., Gong, H., Zhou, Z., & Dai, X. (2024). Characterization and Comparison of Anammox Immobilization in Polyvinyl Alcohol, Polyethylene Glycol and Water-Borne Polyurethane. Processes, 12(7), 1442. https://doi.org/10.3390/pr12071442







