Exopolysaccharides Synthesized by Lacticaseibacillus rhamnosus ŁOCK 0943: Structural Characteristics and Evaluation of Biological and Technological Properties
Abstract
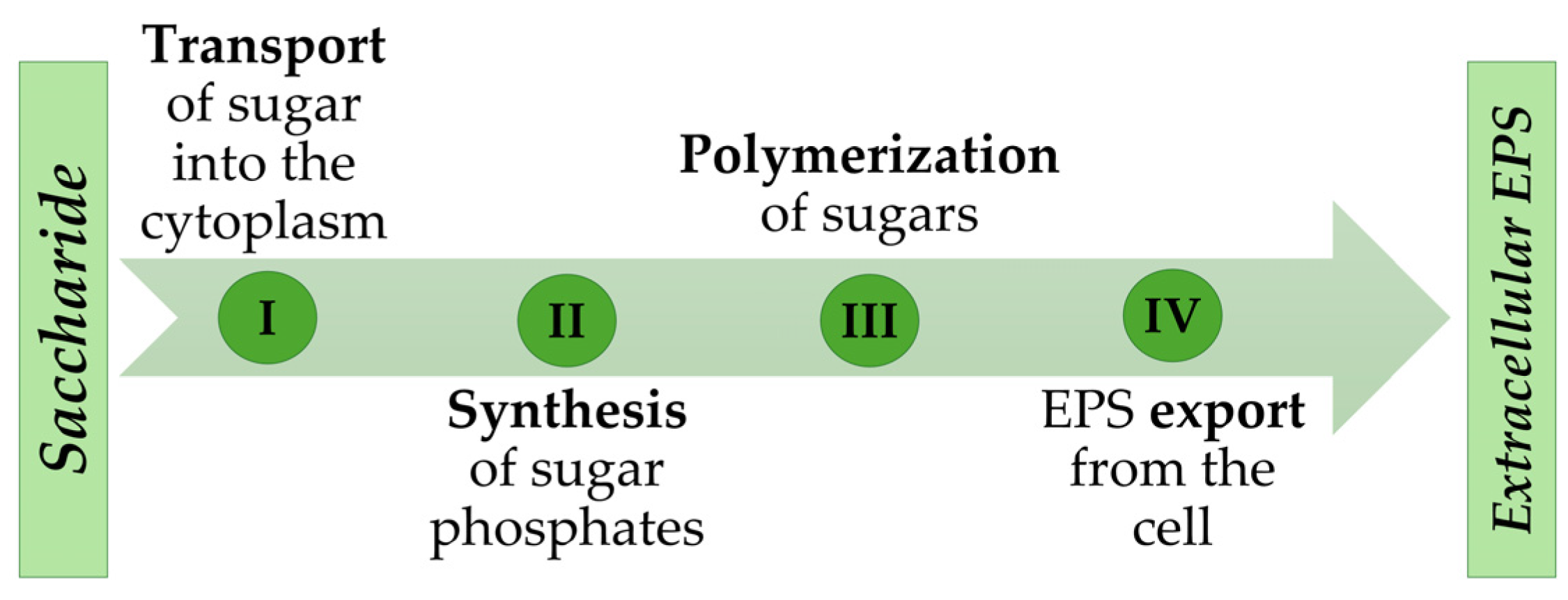
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Isolation of Exopolysaccharides
2.2. Purification of Exopolysaccharides for Structural Analysis
2.3. Determination of Monosaccharide Composition
2.4. Methylation Analysis
2.5. NMR Spectroscopy
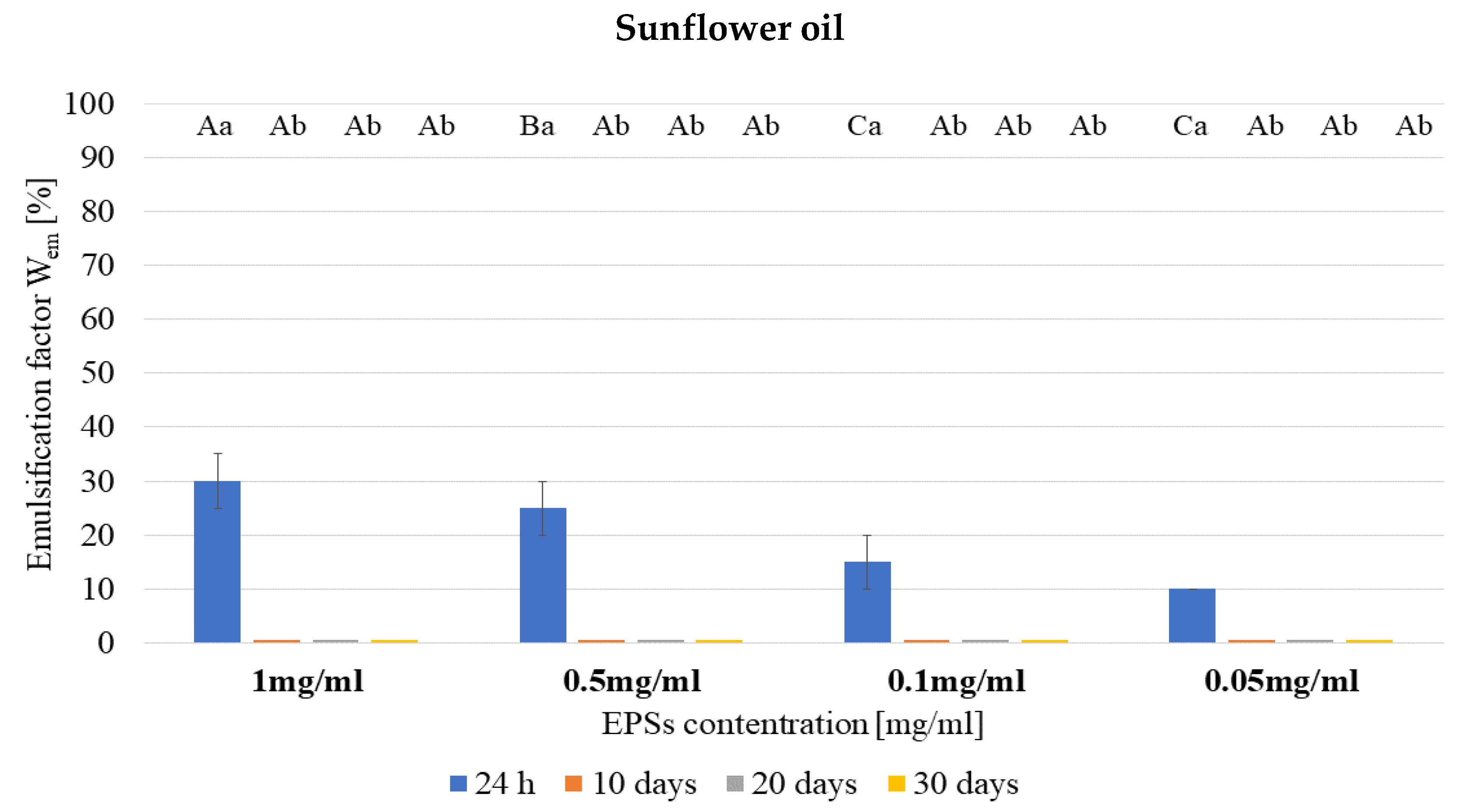
2.6. Emulsifying Properties
2.7. Antagonistic Activity
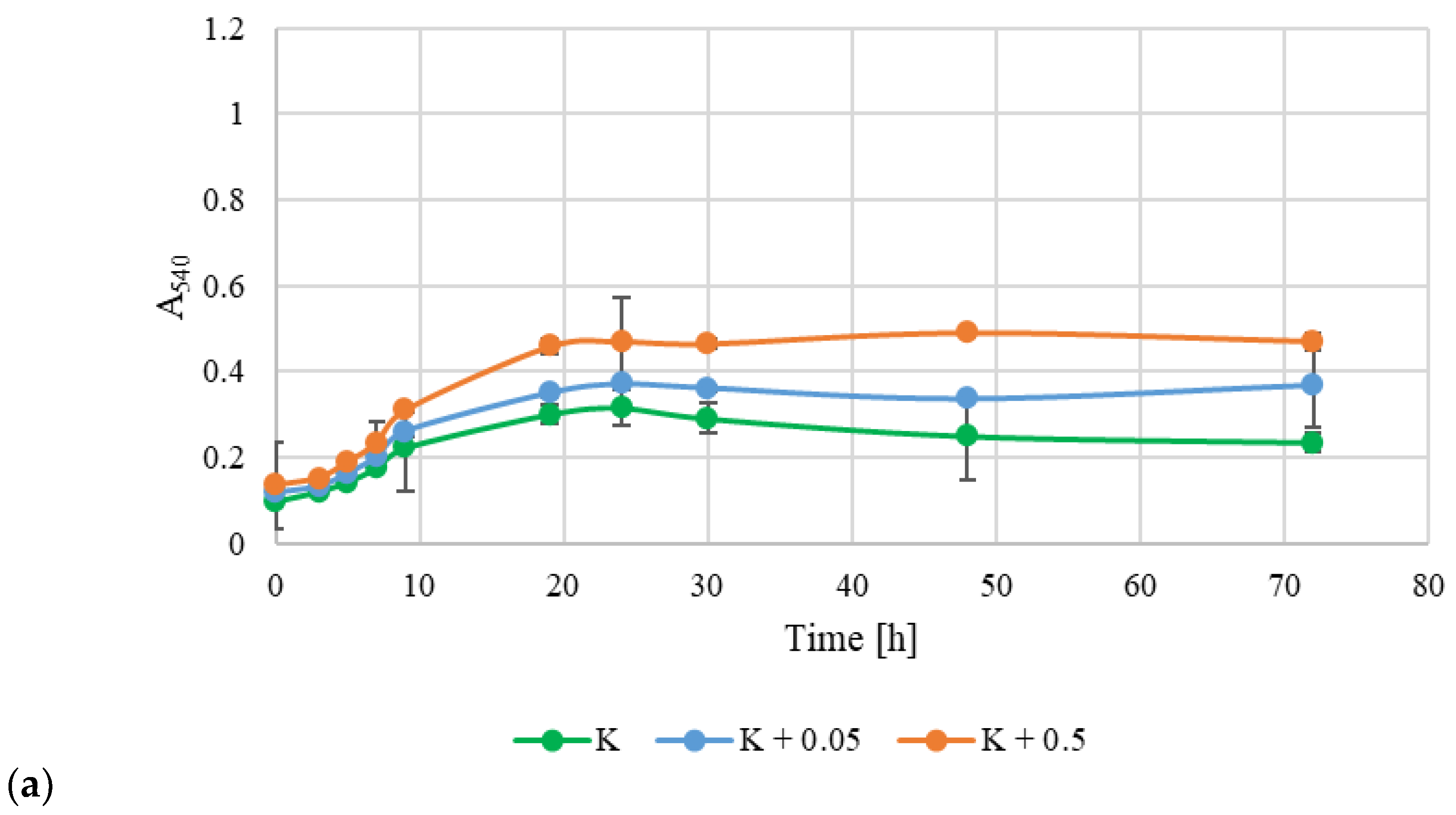
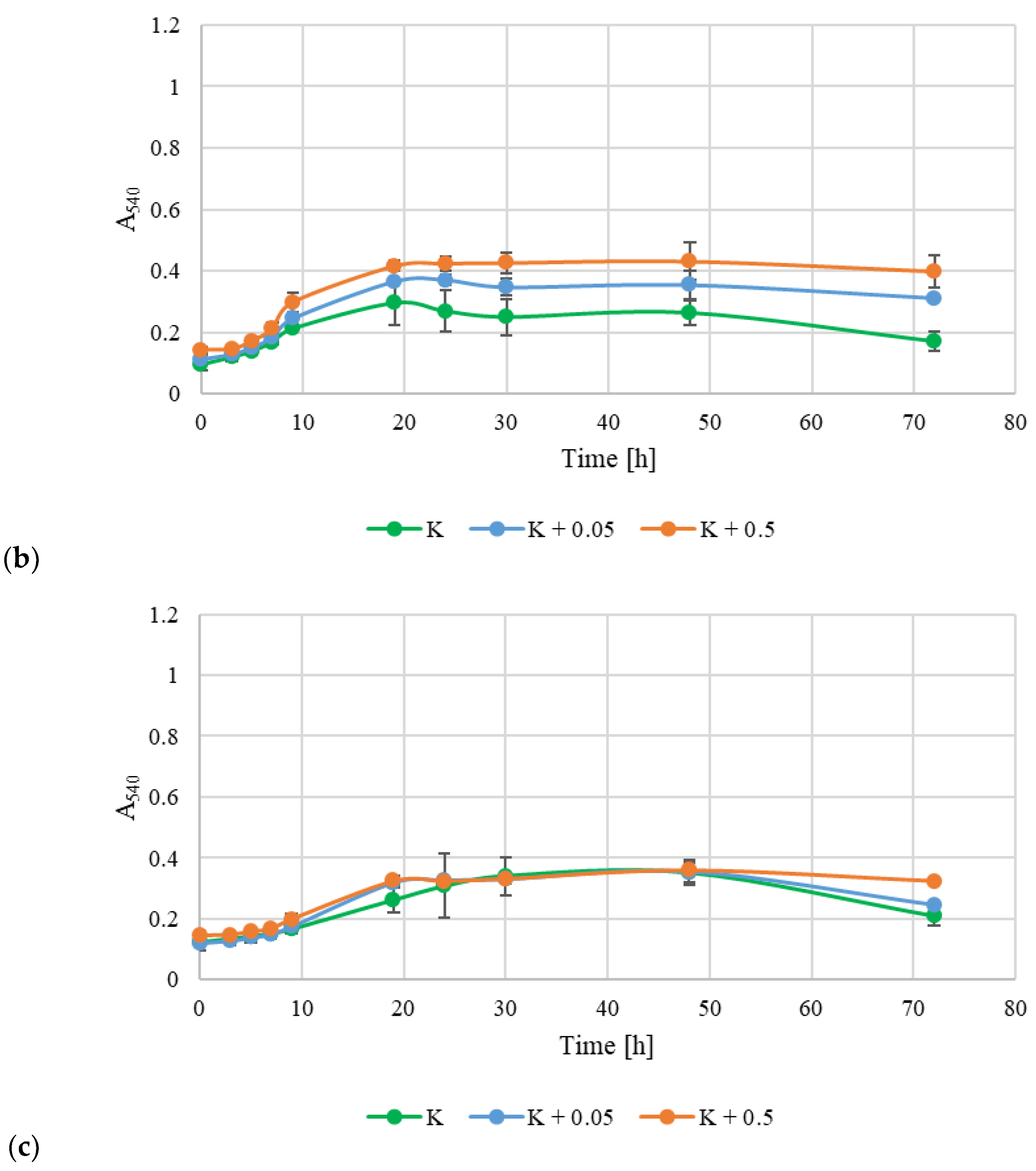
2.8. Prebiotic Potential
2.9. Antioxidant Activity
2.10. Evaluation of the Effectiveness of EPSs as a Preservative in Cosmetic Products
3. Results
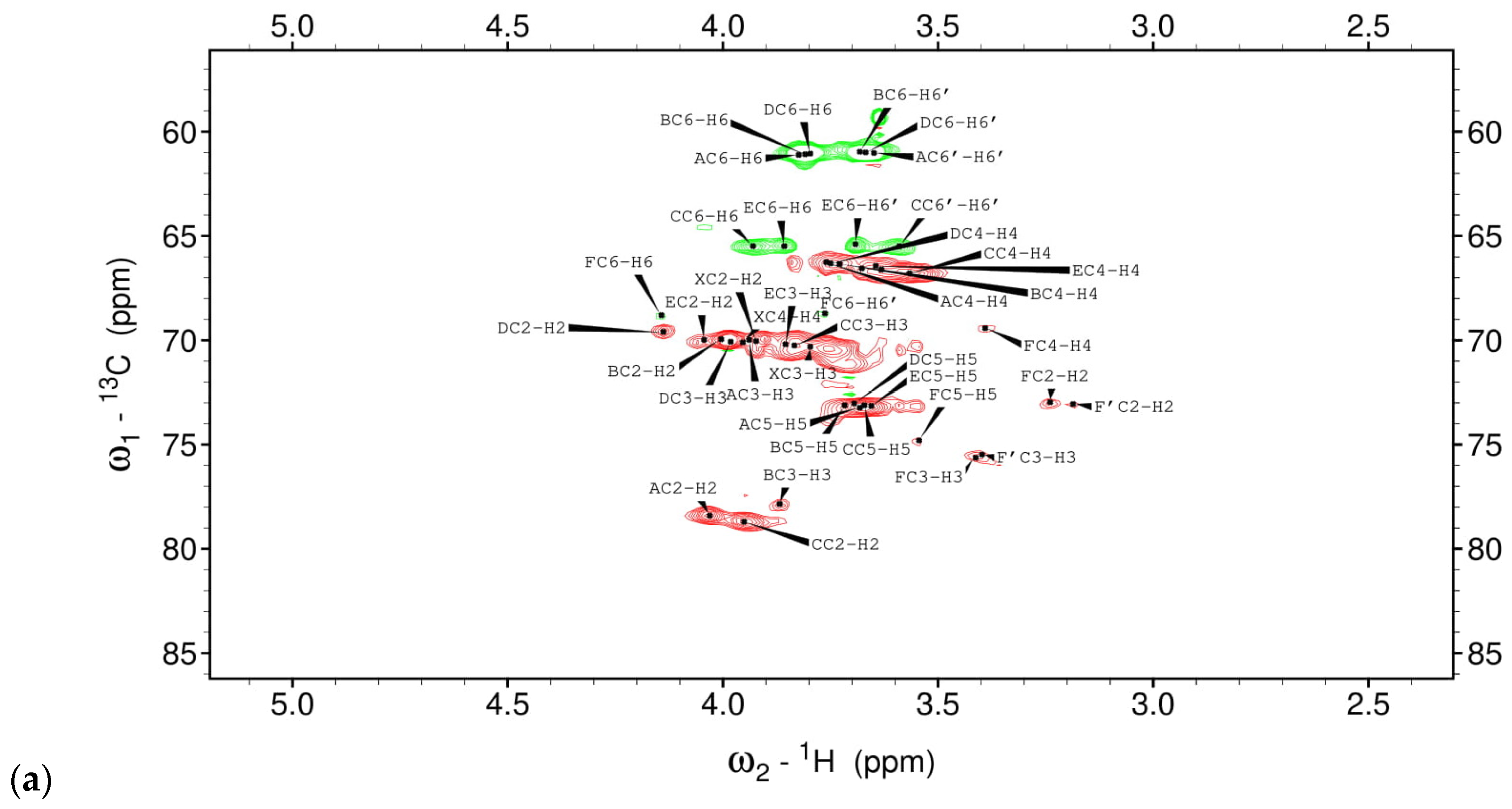
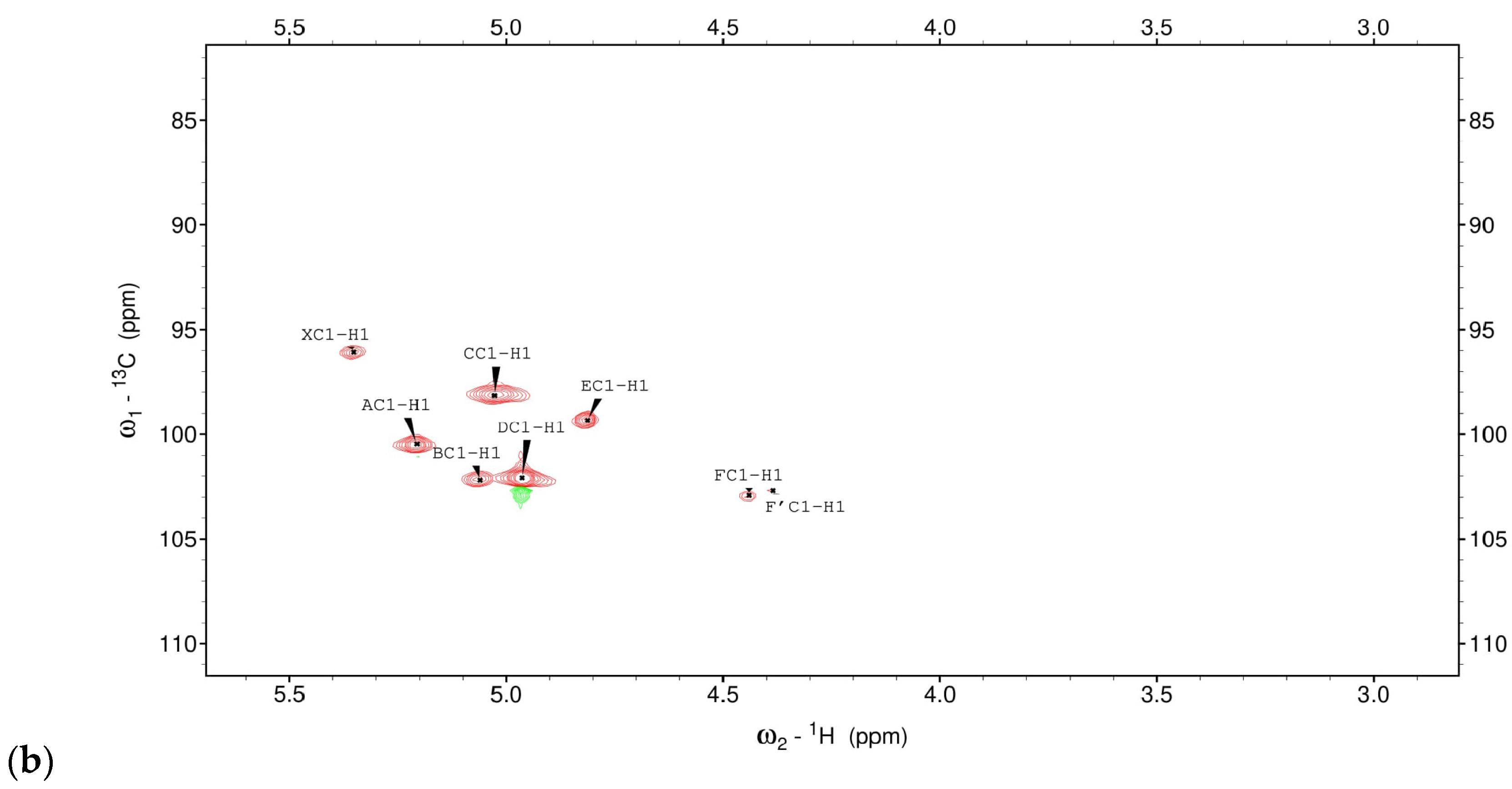
3.1. Structural Studies of EPSs Isolated from L. rhamnosus ŁOCK 0943
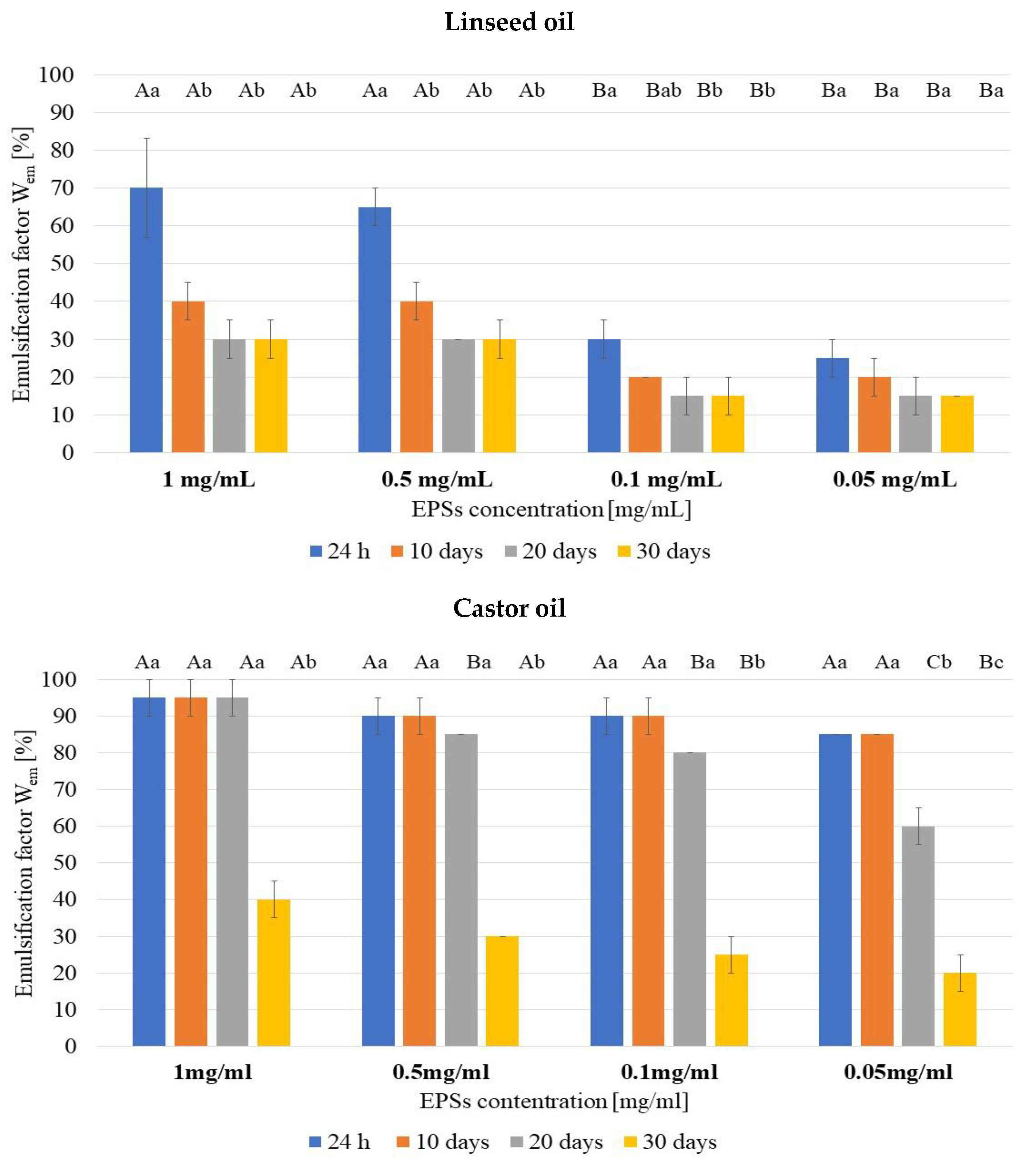
3.2. Emulsifying Properties
3.3. Antimicrobial Activity of EPSs
3.4. Prebiotic Potential of Exopolysaccharides
3.5. Antioxidant Activity
3.6. Assessment of the Technological Suitability of EPSs as a Preservative in the Cosmetics Industry
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Trabelsi, I.; Slima, B.S.; Chaabane, H.; Riadh, S.B. Purification and characterization of a novel exopolysaccharides produced by Lactobacillus sp. Ca6. Int. J. Biol. Macromol. 2015, 74, 541–546. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Jin, M.; Haobin, Z.; Li, Q.; Shao, D.; Jiang, C.; Huang, Q.; Yang, H.; Shi, J.; Hussain, N. Functional characterization and biotechnological potential of exopolysaccharide produced by Lactobacillus rhamnosus strains isolated from human breast milk. LWT—Food Sci. Technol. 2018, 89, 638–647. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Dertli, E.; Toker, O.S.; Tatlisu, N.B.; Sagdic, O.; Arici, M. Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink Aryan: An optimization study based on fermentation kinetics. J. Diary Sci. 2015, 98, 1604–1624. [Google Scholar] [CrossRef]
- De Vuyst, L.; De Vin, F.; Vaningelgem, F.; Degeest, B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- Madhuri, K.V.; Prabhakar, K.V. Microbial exopolysaccharides: Biosynthesis and potential applications. Orient. J. Chem. 2014, 30, 1401–1410. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Grobben, G.J.; Smith, M.R.; Sikkema, J.; Bont, J.A.M. Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. Appl. Microbiol. Biotechnol. 1996, 46, 279–284. [Google Scholar]
- Górska, S.; Grycko, P.; Rybka, J.; Gamian, A. Egzopolisacharydy bakterii kwasu mlekowego–biosynteza I struktura. Postępy Hig. I Med. Dośw. 2007, 61, 805–818. [Google Scholar]
- Kim, D.; Robyt, J.F. Production and selection of mutants of Leuconostoc mesenteroides constitutive for glucansucrases. Enzym. Microb. Technol. 1994, 16, 659–664. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rather, I.A.; Majumder, R.; Shukla, S.; Aeron, A.; Kim, K.; Park, Y.H. Exopolysaccharide and lactic acid bacteria: Perception, functionality and prospects. Bangladesh J. Pharmacol. 2016, 11, 1–23. [Google Scholar] [CrossRef]
- Oleksy-Sobczak, M.; Klewicka, E.; Piekarska-Radzik, L. Exopolysaccharides production by Lactobacillus rhamnosus strains–Optimization of synthesis and extraction conditions. LWT—Food Sci. Technol. 2020, 122, 109055. [Google Scholar] [CrossRef]
- Oleksy-Sobczak, M.; Klewicka, E. Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics Antimicrob. Proteins 2020, 12, 774–783. [Google Scholar] [CrossRef]
- Dubois, M.; Giller, K.A.; Rebers, P.A.; Smith, F.A. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bock, K.; Pedersen, C. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv. Carbohydr. Chem. Biochem. 1983, 41, 27–66. [Google Scholar]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Determination of the absolute configuration of monosaccharides in complex carbohydrates by capillary G.L.C. Carbohydr. Res. 1979, 77, 10–17. [Google Scholar] [CrossRef]
- Gorin, P.A.J.; Mazurek, M. Further studies on the assignment of signals in 13C magnetic resonance spectra of aldoses and derived methyl glycosides. Can. J. Chem. 1975, 53, 1212–1223. [Google Scholar] [CrossRef]
- Sauvageau, J.; Ryan, J.; Lagutin, K.; Sims, I.M.; Stocker, B.L.; Timmer, M.S. Isolation and structural characterisation of the major glycolipids from Lactobacillus plantarum. Carbohydr. Res. 2012, 357, 151–156. [Google Scholar] [CrossRef]
- Katzenellenbogen, E.; Kocharova, N.A.; Toukach, P.V.; Górska, S.; Korzeniowska-Kowal, A.; Bogulska, M.; Gamian, A.; Knirel, Y.A. Structure of an abequose-containing O-polysaccharide from Citrobacter freundii O22 strain PCM 1555. Carbohydr. Res. 2009, 344, 1724–1728. [Google Scholar] [CrossRef]
- Górska-Frączek, S.; Sandström, C.; Kenne, L.; Rybka, J.; Strus, M.; Heczko, P.; Gamian, A. Structural studies of the exopolysaccharide consisting of a nonasaccharide repeating unit isolated from Lactobacillus rhamnosus KL37B. Carbohydr. Res. 2011, 346, 2926–2932. [Google Scholar] [CrossRef]
- Molinaro, A.; Piscopo, V.; Lanzetta, R.; Parrilli, M. Structural determination of the complex exopolysaccharide from the virulent strain of Cryphonectria parasitica. Carbohydr. Res. 2002, 337, 1707–1713. [Google Scholar] [CrossRef]
- Sandal, I.; Inzana, T.J.; Molinaro, A.; De Castro, C.; Shao, J.Q.; Apicella, M.A.; Cox, A.D.; St Michael, F.; Berg, G. Identification, structure, and characterization of an exopolysaccharide produced by Histophilus somni during biofilm formation. BMC Microbiol. 2011, 11, 186. [Google Scholar] [CrossRef]
- Long, X.; Hou, X.; Li, S.; Chen, A.; Zhang, Z.; Shen, G. Fermentation Optimization and In-Vitro Antioxidant Activity of Exopolysaccharides Produced by Leuconostoc suionicum LSBM1 Using Sugar Beet Molasses. Sugar Tech 2024, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, B.; Liu, C.-J.; Yang, E. Optimization of biosynthesis conditions for the production of exopolysaccharides by Lactobacillus plantarum SP8 and the exopolysaccharides antioxidant activity test. Indian J. Microbiol. 2020, 60, 334–345. [Google Scholar] [CrossRef]
- Yan, Z.X.; Li, M.; Wei, H.Y.; Peng, S.Y.; Xu, D.J.; Zhang, B.; Cheng, X. Characterization and antioxidant activity of the polysaccharide hydrolysate from Lactobacillus plantarum LPC-1 and their effect on spinach (Spinach oleracea L.) growth. Appl. Biochem. Biotechnol. 2024, 1–23. [Google Scholar] [CrossRef]
- Donnarumma, G.; Molinaro, A.; Cimini, D.; De Castro, C.; Valli, V.; De Gregorio, V.; De Rosa, M.; Schiraldi, C. Lactobacillus crispatus L1: High cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol. 2014, 14, 137. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Yuan, X.; Liu, Y.; Zou, J.; Wang, Q.; Zhang, H. Study on the re-emulsification process of water in heavy oil emulsion with addition of water-soluble viscosity reducer solution. Energy Fuels 2019, 33, 10852–10860. [Google Scholar] [CrossRef]
- Chen, S.; AlSofi, A.M.; Wang, J.; Alotaibi, M.B. A Polycyclic–Aromatic Hydrocarbon-Based Water-Soluble Formulation for Heavy Oil Viscosity Reduction and Oil Displacement. Energy Fuels 2023, 37, 11864–11880. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Kavitake, D.; Balyan, S.; Devi, P.B.; Shetty, P.H. Evaluation of oil-in-water (O/W) emulsifying properties of galactan exopolysaccharide from Weissella confusa KR780676. J. Food Sci. Technol. 2020, 57, 1579–1585. [Google Scholar] [CrossRef]
- Kavitake, D.; Marchawala, F.Z.; Delattre, C.; Shetty, P.H.; Pathak, H.; Andhare, P. Biotechnological potential of exopolysaccharide as a bioemulsifier produced by Rhizobium radiobacter CAS isolated from curd. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100202. [Google Scholar] [CrossRef]
- Han, Y.; Liu, E.; Liu, L.; Zhang, B.; Wang, Y.; Gui, M.; Wu, R.; Li, P. Rheological, emulsifying and thermostability properties of two exopolysaccharides produced by Bacillus amyloliquefaciens LPL061. Carbohydr. Polym. 2015, 115, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashald, Y.; Obaide, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.H.; Kang, I.B.; Kim, H.; Song, K.Y.; Kim, H.S.; Seo, K.H. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanson, C. Sugarcoated: Exopolysaccharides producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Das, S.; Mishra, B.K.; Hati, S. Techno-functional characterization of indigenous Lactobacillus isolates from the traditional fermented foods of Meghalaya, India. Curr. Res. Food Sci. 2020, 3, 9–18. [Google Scholar] [CrossRef]
- Sarikaya, H.; Aslim, B.; Yuksekdag, Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int. J. Food Prop. 2017, 20, 362–371. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ktari, N.; Slima, S.B.; Triki, M.; Bardaa, S.; Mnif, H.; Salah, R.B. Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp. Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Ravindran, A.D. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef]
- Bai, L.J.; Wang, L.; Ji, S.J. Structural Elucidation and Antioxidant Activities of Exopolysaccharide from L. helveticus SMN2-1. Chem. Eng. Trans. 2016, 55, 61–66. [Google Scholar]
- Srikanth, R.; Reddy, C.H.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Thollas, B.; Gonzalez, R.D.; Puche, J.C.; Astals, A.S. Exopolysaccharide for Treatment or Care of Skin, Mucous Membranes, Hair or Nails. U.S. Patent No. 9,770,400, 26 September 2017. [Google Scholar]


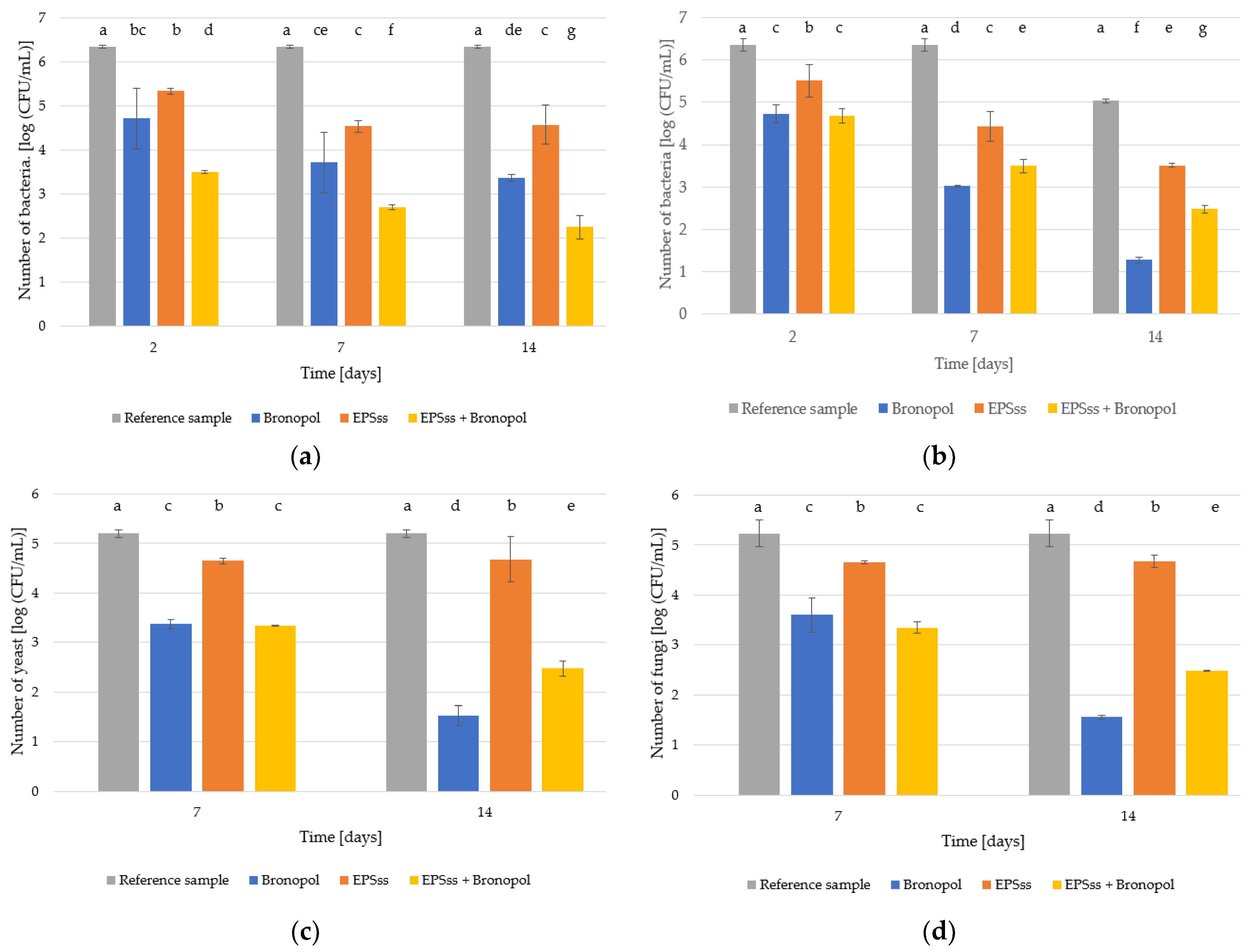
| The Value of Microbial Reduction * | ||||
|---|---|---|---|---|
| 2 Days | 7 Days | 14 Days | 28 Days | |
| Bacteria | 2 | 3 | - | NI |
| Fungi | - | - | 2 | NI |
| Chemical Shifts 1H, 13C (ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Sugar Residue | H1 | H2 | H3 | H4 | H5 | H6 | H6’ |
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| X α-D-Galp-(1→ | 5.35 | 3.90 | 3.80 | 3.95 | n.d | n.d | |
| 96.07 | 70.2 | 70.3 | 70.4 | n.d | n.d | ||
| A →2)-α-D-Manp-(1→ | 5.21 | 4.03 | 3.94 | 3.76 | 3.68 | 3.82/3.65 | |
| 100.9 | 78.5 | 70.0 | 66.3 | 73.3 | 61.1 | ||
| B →3)-α-D-Manp-(1→ | 5.06 | 4.01 | 3.87 | 3.73 | 3.72 | 3.81/3.68 | |
| 102.2 | 70.2 | 77.8 | 66.5 | 73.2 | 61.1 | ||
| C →2,6)-α-D-Manp-(1→ | 5.03 | 3.95 | 3.83 | 3.57 | 3.67 | 3.93/3.59 | |
| 98.3 | 78.7 | 70.3 | 66.8 | 73.2 | 65.5 | ||
| D α-D-Manp-(1→ | 4.96 | 4.14 | 3.98 | 3.75 | 3.70 | 3.79/3.66 | |
| 102.1 | 69.6 | 70.1 | 66.3 | 73.1 | 61.1 | ||
| E →6)-α-D-Manp-(1→ | 4.82 | 3.91 | 3.85 | 3.64 | 3.66 | 3.86/3.70 | |
| 99.4 | 69.9 | 70.3 | 66.4 | 73.2 | 65.6 | ||
| F/F’ →6)-β-D-Glcp-(1→ | 4.44/4.39 | 3.24/3.19 | 3.41/3.39 | 3.40 | 3.54 | 4.14/3.76 | |
| 102.9/102.7 | 72.9/73.1 | 75.6/75.5 | 69.5 | 74.8 | 68.72 | ||
| LAB | EPS Concentration (mg/mL) | DPPH Inhibition (%) | References |
|---|---|---|---|
| Lacticaseibacillus rhamnosus ŁOCK 0943 | 0.05 | 8.09 ± 0.12 a | Our data |
| 0.5 | 8.71 ± 0.03 b | ||
| Lactiplantibacillus plantarum SP8 | 0.05 | <2.00 | Zhang et al., 2020 [24] |
| 0.2 | 8.98 | ||
| Lactiplantibacillus plantarum LPC-1 | 1.0–9.0 | <20 | Yan et al., 2024 [25] |
| Leuconostoc suionicum LSBM1 | 8.0 | 67.41 ± 0.33 | Long et al., 2024 [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksy-Sobczak, M.; Górska, S.; Piekarska-Radzik, L.; Ścieszka, S.; Klewicka, E. Exopolysaccharides Synthesized by Lacticaseibacillus rhamnosus ŁOCK 0943: Structural Characteristics and Evaluation of Biological and Technological Properties. Processes 2024, 12, 1192. https://doi.org/10.3390/pr12061192
Oleksy-Sobczak M, Górska S, Piekarska-Radzik L, Ścieszka S, Klewicka E. Exopolysaccharides Synthesized by Lacticaseibacillus rhamnosus ŁOCK 0943: Structural Characteristics and Evaluation of Biological and Technological Properties. Processes. 2024; 12(6):1192. https://doi.org/10.3390/pr12061192
Chicago/Turabian StyleOleksy-Sobczak, Magdalena, Sabina Górska, Lidia Piekarska-Radzik, Sylwia Ścieszka, and Elżbieta Klewicka. 2024. "Exopolysaccharides Synthesized by Lacticaseibacillus rhamnosus ŁOCK 0943: Structural Characteristics and Evaluation of Biological and Technological Properties" Processes 12, no. 6: 1192. https://doi.org/10.3390/pr12061192
APA StyleOleksy-Sobczak, M., Górska, S., Piekarska-Radzik, L., Ścieszka, S., & Klewicka, E. (2024). Exopolysaccharides Synthesized by Lacticaseibacillus rhamnosus ŁOCK 0943: Structural Characteristics and Evaluation of Biological and Technological Properties. Processes, 12(6), 1192. https://doi.org/10.3390/pr12061192








