Supercritical CO2-Based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Extraction
2.4. Liquid Chromatography
2.5. Mass Spectrometry
2.6. Statistical Analysis
3. Results
3.1. SC-CO2 Extraction of Aerial Parts of M. varia
- –
- Pressure: 150 Bar, extraction temperature: 50 °C, extraction time: 1 h; the global yield of biologically active substances was 3.1 mg/100 mg of plant sample; the share of the EtOH modifier was 2%;
- –
- Pressure: 150 Bar, extraction temperature: 55 °C, extraction time: 1 h; the global yield of biologically active substances was 3.1 mg/100 mg of plant sample; the share of the EtOH modifier was 2%.
- –
- Pressure: 150 Bar, extraction temperature: 50 °C, extraction time: 1 h; the global yield of biologically active substances was 2.7 mg/100 mg of plant sample; the share of the EtOH modifier was 2%;
- –
- Pressure: 150 Bar, extraction temperature: 55 °C, extraction time: 1 h; the global yield of biologically active substances was 2.8 mg/100 mg of plant sample; the share of the EtOH modifier was 2%.
- –
- Pressure: 150 Bar, extraction temperature: 50 °C, extraction time: 1 h; the global yield of biologically active substances was 3.2 mg/100 mg of plant sample; the share of the EtOH modifier was 2%.
3.2. Global Metabolome Profile of M. varia
3.2.1. Flavones
Hydroxy(iso)flavones
Dihydroxyflavones
Trihydroxyflavones
3.2.2. Flavan-3-ols
3.2.3. Anthocyanins
3.3. Newly Detected Chemical Compounds in M. varia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; De Geyter, N.; Pollier, J.; Goormachtig, S.; Goossens, A. Natural product biosynthesis in Medicago species. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Tepe, H.D. Qualitative analysis of alfalfa seed methanol extract by GC–MS and determination of antioxidant properties. Celal Bayar Univ. J. Sci. 2019, 15, 175–180. [Google Scholar]
- Molgaard, J.; Von Schenck, H.; Olsson, A.G. Alfalfa seeds lower low density lipoprotein cholesterol and apolipoprotein B concentrations in patients with type II hyperlipoproteinemia. Atherosclerosis 1987, 65, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.A.; El-Shamy, S.; Farag, M.A. Comparative GC–MS based nutrients profiling of less explored legume seeds of Melilotus, Medicago, Trifolium, and Ononis analysed using chemometric tools. Sci. Rep. 2023, 13, 18221. [Google Scholar] [CrossRef]
- Echeverria, A.; Larrainzar, E.; Li, W.; Watanabe, Y.; Sato, M.; Tran, C.D.; Moler, J.A.; Hirai, M.Y.; Sawada, Y.; Phan Tran, L.-S.; et al. Medicago sativa and Medicago truncatula Show Contrasting Root Metabolic Responses to Drought. Front. Plant Sci. 2021, 12, 652143. [Google Scholar] [CrossRef] [PubMed]
- Mielmann, A. The utilization of lucerne (Medicago sativa): A review. Br. Food J. 2013, 115, 590–600. [Google Scholar] [CrossRef]
- Barna, D.; Alshaal, T.; Toth, I.O.; Cziaky, Z.; Fari, M.G.; Domokos-Szabolcsy, E.; Bakonyi, N. Bioactive metabolite profile and antioxidant properties of brown juice, a processed alfalfa (Medicago sativa) by-product. Heliyon 2022, 8, e11655. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, H.; Fu, X.; Li, X.; Gao, H. Development of simple sequence repeat markers and diversity analysis in alfalfa (Medicago sativa L.). Mol. Biol. Rep. 2013, 40, 3291–3298. [Google Scholar] [CrossRef] [PubMed]
- Gainullina, K.P.; Nizaeva, A.A.; Kuluev, B.R. Assessment of genetic diversity of Medicago varia Mart. populations selected for breeding in the conditions of the Republic of Bashkortostan using SSR-analysis. Biomics 2023, 5, 159–166. [Google Scholar] [CrossRef]
- Hou, W.; Liu, C.; Xia, J.; Niu, H.; Li, S. Rapid screening and purification of potential inhibitors from Medicago sativa by ultrafiltration-liquid chromatography combined with stepwise flow rate counter-current chromatography. Phytochem. Anal. 2021, 32, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Leoni, F.; Hazrati, H.; Fomsgaard, I.S.; Moonen, A.C.; Kudsk, P. Determination of the Effect of Co-cultivation on the Production and Root Exudation of Flavonoids in Four Legume Species Using LC-MS/MS Analysis. J. Agric. Food Chem. 2021, 69, 9208–9219. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xu, H.; Sun, N.; Jiang, L.; Tian, P.; Yong, Y.; Yang, W.; Cai, H.; Cui, G. Metabolomic Analysis of Alfalfa (Medicago sativa L.) Root-Symbiotic Rhizobia Responses under Alkali Stress. Front. Plant Sci. 2017, 8, 1208. [Google Scholar] [CrossRef] [PubMed]
- Glagoleva, L.E.; Zatsepilina, N.P.; Nesterenko, I.P.; Pevneva, D.M. About the use of plant-based complex of alfalfa in production of dairy products. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Volgograd, Russia, 17–18 June 2021; Volume 848, p. 012049. [Google Scholar] [CrossRef]
- De Geyter, E.; Swevers, L.; Soin, T.; Geelen, D.; Smagghe, G. Saponins do not affect the ecdysteroid receptor complex but cause membrane permeation in insect culture cell lines. J. Insect Physiol. 2012, 58, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Golawska, S. Deterrence and Toxicity of Plant Saponins for the Pea Aphid Acyrthosiphon pisum Harris. J. Chem. Ecol. 2007, 33, 1598–1606. [Google Scholar] [CrossRef]
- Golawska, S.; Lukasik, I.; Leszczynski, B. Effect of alfalfa saponins and flavonoids on pea aphid. Entomol. Exp. Appl. 2008, 128, 147–153. [Google Scholar] [CrossRef]
- Saniewska, A.; Jarecka, A.; Bialy, Z.; Jurzysta, M. Antifungal activity of saponins originated from Medicago hybrida against some ornamental plant pathogens. Acta Agrobot. 2006, 59, 51–58. [Google Scholar] [CrossRef]
- Jarecka, A.; Saniewska, A.; Bialy, Z.; Jurzysta, M. The effect of Medicago arabica, M. hybrida and M. sativa saponins on the grown and development of Fusarium oxysporum Schlecht f. sp. tulipae apt. Acta Agrobot. 2008, 61, 147–155. [Google Scholar] [CrossRef]
- Pharmacopoeia of the Eurasian Economic Union. Approved by Decision of the Board of Eurasian Economic Commission No. 100 Dated 11 August 2020. Available online: https://eec.eaeunion.org/upload/medialibrary/37c/PHARMACOPOEIA-of-the-Eurasian-Economic-Union.pdf (accessed on 1 January 2021).
- Razgonova, M.; Zakharenko, A.; Kalenik, T.; Nosyrev, A.; Stratidakis, A.; Mezhuev, Y.; Burykina, T.; Nicolae, A.; Arsene, A.; Tsatsakis, A.; et al. Supercritical fluid technology and supercritical fluid chromatography for application in ginseng extracts. Farmacia 2019, 67, 2. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Ercisli, S.; Grudev, V.; Golokhvast, K. Comparative Analysis of Far East Sikhotinsky Rhododendron (Rh. sichotense) and East Siberian Rhododendron (Rh. adamsii) Using Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Molecules 2020, 25, 3774. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K.S. Zostera marina L. Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- Chung, N.C.; Miasojedow, B.; Startek, M.; Gambin, A. Jaccard/Tanimoto similarity test and estimation methods for biological presence-absence data. BMC Bioinform. 2019, 20, 644. [Google Scholar] [CrossRef] [PubMed]
- Lipkus, A.H. A proof of the triangle inequality for the Tanimoto distance. J. Math. Chem. 1999, 26, 263–265. [Google Scholar] [CrossRef]
- Levandowsky, M.; Winter, D. Distance between sets. Nature 1971, 234, 34–35. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.-J.; Xu, W.; Huang, J.; Zhu, D.; Qiu, X.-H. Rapid Characterization and Identification of Flavonoids in Radix Astragali by Ultra-High-Pressure Liquid Chromatography Coupled with Linear Ion Trap-Orbitrap Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 945–952. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, K.; Wei, L.; Chen, D.; Chen, Q.; Jiao, M.; Li, X.; Huang, J.; Gong, Z.; Kang, N.; et al. The Molecular Mechanism of Antioxidation of Huolisu Oral Liquid Based on Serum Analysis and Network Analysis. Front. Pharmacol. 2021, 12, 710976. [Google Scholar] [CrossRef]
- Okhlopkova, Z.M.; Razgonova, M.P.; Rozhina, Z.G.; Egorova, P.S.; Golokhvast, K.S. Dracocephalum jacutense Peschkova from Yakutia: Extraction and Mass Spectrometric Characterization of 128 Chemical Compounds. Molecules 2023, 28, 4402. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Cherevach, E.I.; Tekutyeva, L.A.; Fedoreev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Demidova, E.N.; Kirilenko, N.S.; Golokhvast, K.S. Maackia amurensis Rupr. et Maxim.: Supercritical CO2-extraction and Mass Spectrometric Characterization of Chemical Constituents. Molecules 2023, 28, 2026. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Chen, Q.; Hu, Z.; Li, Z.; Zheng, P.; Liu, X.; Li, S.; Zhang, S.; Chen, J. Chemical characterisation and quantification of the major constituents in the Chinese herbal formula Jian-Pi-Yi-Shen pill by UPLC-Q-TOF-MS/MS and HPLC-QQQ-MS/MS. Phytochem. Anal. 2020, 31, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Relat. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Belmehdi, O.; Bouyahya, A.; Jeko, J.; Cziaky, Z.; Zengin, G.; Sotkó, G.; El Baaboua, A.; Skali-Senhaji, N.; Abrini, J. Synergistic interaction between propolis extract, essential oils, and antibiotics against Staphylococcus epidermidis and methicillin resistant Staphylococcus aureus. Int. J. Second Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Xu, L.L.; Xu, J.J.; Zhong, K.R.; Shang, Z.P.; Wang, F.; Wang, R.F.; Liu, B. Analysis of non-volatile chemical constituents of Menthae Haplocalycis herba by ultra-high performance liquid chromatography—High resolution mass spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Piasecka, A.; Garcia-Lopez, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, L.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochem. 2013, 92, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Teles, Y.C.E.; Rebello Horta, C.C.; de Fatima Agra, M.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; de Fatima Vanderlei de Souza, M. New Sulphated Flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Wozniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Silinsin, M.; Bursal, E. UHPLC-MS/MS phenolic profiling and in vitro antioxidant activities of Inula graveolens (L.) Desf. Nat. Prod. Res. 2018, 31, 1467–1471. [Google Scholar] [CrossRef]
- Aghakhani, F.; Kharazin, N.; Gooini, Z.L. Flavonoid Constituents of Phlomis (Lamiaceae) Species Using Liquid Chromatography Mass Spectrometry. Phytochem. Anal. 2018, 29, 180–195. [Google Scholar] [CrossRef]
- Jaiswal, R.; Muller, H.; Muller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF-MS/MS and UHPLC-MS/MS. J. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shaari, K. LC–MS metabolomics analysis of Stevia rebaudiana Bertoni leaves cultivated in Malaysia in relation to different developmental stages. Phytochem. Anal. 2021, 33, 249–261. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Etienne, O.K.; Sharmeen, J.B.; Brunetti, L.; Leone, S.; Di Simone, S.C.; Recinella, L.; et al. Chemical composition and biological properties of two Jatropha species: Different parts and different extraction methods. Antioxidants 2021, 10, 792. [Google Scholar] [CrossRef]
- Huo, J.-H.; Du, X.-W.; Sun, G.-D.; Dong, W.-T.; Wang, W.-M. Identification and characterization of major constituents in Juglans mandshurica using ultra performance liquid chromatography coupled with time-of-flight mass spectrometry (UPLC-ESI-Q-TOF/MS). Chin. J. Nat. Med. 2018, 16, 0525–0545. [Google Scholar] [CrossRef] [PubMed]
- Mosic, M.; Trifkovic, J.; Vovk, I.; Gasic, U.; Tesic, Z.; Sikoparija, B.; Milojkovic-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Felegyi-Toth, C.A.; Garadi, Z.; Darcsi, A.; Csernak, O.; Boldizsar, I.; Beni, S.; Alberti, A. Isolation and quantification of diarylheptanoids from European hornbeam (Carpinus betulus L.) and HPLC-ESI-MS/MS characterization of its antioxidative phenolics. J. Pharm. Biomed. Anal. 2022, 210, 114554. [Google Scholar] [CrossRef]
- Yasir, M.; Sultana, B.; Anwar, F. LC–ESI–MS/MS based characterization of phenolic components in fruits of two species of Solanaceae. J. Food Sci. Technol. 2018, 55, 2370–2376. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS–Bioassay Guided Approach. J. Chromatogr. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; Bento da Silva, A.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Wang, C.; Guo, N.; Song, Z.; Wang, C.; Xia, L.; Lu, A. Comparative Analyses of Chromatographic Fingerprints of the Roots of Polygonum multiflorum Thunb. and Their Processed Products Using RRLC/DAD/ESI-MSn. Planta Med. 2011, 77, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Shin, J.-S.; Park, S.-K.; Kang, S.; Jeong, S.-C.; Moon, J.-K.; Choi, Y. Differences in the metabolic profiles and antioxidant activities of wild and cultivated black soybeans evaluated by correlation analysis. Food Res. Int. 2017, 100, 166–174. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wang, J.; Chen, G.; Tao, X.; Xu, S. Metabolomic Analysis Reveals Domestication-Driven Reshaping of Polyphenolic Antioxidants in Soybean Seeds. Antioxidants 2023, 12, 912. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfild, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. Agric. Food Chem. 2010, 51, 706–713. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; Abbas, F.A.; Refaat, S.; El-Shafae, A.M.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea ‘Scarlett O’Hara’ Cultivated in Egypt. Egypt. J. Chem. 2021, 64, 22. [Google Scholar] [CrossRef]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols; Hindawi Publishing Corp.: New York, NY, USA, 2013; p. 813563. [Google Scholar]
- Fermo, P.; Comite, V.; Sredojevic, M.; Ciric, I.; Gasic, U.; Mutic, J.; Baosic, R.; Tesic, Z. Elemental Analysis and Phenolic Profiles of Selected Italian Wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jimenez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G. Colonic fermentation of polyphenols from Chilean currants (Ribes spp.) and its effect on antioxidant capacity and metabolic syndrome-associated enzymes. Food Chem. 2018, 258, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arraes-Roman, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Abdelaziz, S.; Yousef, H. Chemical Composition and Biological Activities of the Aqueous Fraction of Parkinsonea aculeata L. Growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In Vitro Inhibition of the Transcription Factor NF-kB and Cyclooxygenase by Bamboo Extracts. Phytother. Res. 2013, 28, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Lv, Z.; Dong, J.; Zhang, B. Rapid identification and detection of flavonoid compounds from Bamboo leaves by Lc-(ESI)-IT-TOF/FM. BioResources 2011, 7, 1405–1418. [Google Scholar]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Minute Res. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; de Chaves, D.S.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Braz. J. Pharmacol. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Yamazaki, M.; Nakajima, J.-I.; Yamanashi, M.; Sugiyama, M.; Makita, Y.; Springob, K.; Awazuhara, M.; Saito, K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 2003, 62, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, J.; Schweiger, R.; Pons, C.A.; Müller, C. Wheat growth, applied water use efficiency and flag leaf metabolome under continuous and pulsed deficit irrigation. Sci. Rep. 2020, 10, 10112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Chao, I.-C.; Hu, D.-J.; Shakerian, F. Comparison of Antioxidant Activity and Main Active Compounds Among Different Parts of Alpinia officinarum Hance Using High-Performance Thin Layer Chromatography-Bioautography. J. AOAC Int. 2019, 102, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Navaz, M.A.; Sabitov, A.S.; Zinchenko, Y.N.; Rusakova, E.A.; Petrusha, E.N.; Golokhvast, K.S.; Tikhonova, N.G. The Global metabolome profiles of four varieties of Lonicera caerulea, established via tandem mass spectrometry. Horticulturae 2023, 9, 1188. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef] [PubMed]
- Said, R.B.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes; UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera Royle isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitisidaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography-Mass Spectrometry. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Acquavia, M.A.; Cataldi, T.R.I.; Onzo, A.; Coviello, D.; Bufo, S.A.; Scrano, L.; Ciriello, R.; Guerrieri, A.; Bianco, G. Profiling of quercetin glycosides and acyl glycosides in sun-dried peperoni di Senise peppers (Capsicum annuum L.) by a combination of LC-ESI(-)-MS/MS and polarity prediction in reversed-phase separations. Anal. Bioanal. Chem. 2020, 412, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Rai, D.K.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Prinzo, F.; Milella, L. Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd. Molecules 2018, 23, 2497. [Google Scholar] [CrossRef]
- Justesen, U. Negative atmospheric pressure chemical ionisation low-energy collision activation mass spectrometry for the characterisation of flavonoids in extracts of fresh herbs. J. Chromatogr. A 2000, 902, 369–379. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Gomez-Caravaca, A.M.; Guerra-Hernandez, E.; Cerretani, L.; Garcia-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves by HPLC-ESI-QTOF-MS. Molecules 2018, 112, 390–399. [Google Scholar] [CrossRef]
- Yin, N.-W.; Wang, S.-X.; Jia, L.-D.; Zhu, M.-C.; Yang, J.; Zhou, B.-J.; Yin, J.-M.; Lu, K.; Wang, R.; Li, J.-N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC−HESI-MS/MS. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Chen, H.-J.; Inbaraj, B.S.; Chen, B.-H. Determination of Phenolic Acids and Flavonoids in Taraxacum formosanum Kitam by Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Post-Column Derivatization Technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, J.-L.; Zhou, Q.; Wang, L.; Huang, W.; Wang, R.-D. Effect of ultrasonic and ball-milling treatment on cell wall, nutrients, and antioxidant capacity of rose (Rosa rugosa) bee pollen, and identification of bioactive components. J. Sci. Food Agric. 2019, 99, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous Determination of 78 Compounds of Rhodiola rosea Extract by Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Hindawi Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef] [PubMed]
- Okhlopkova, Z.M.; Ercisli, S.; Razgonova, M.P.; Ivanova, N.S.; Antonova, E.E.; Egorov, Y.A.; Kucharova, E.V.; Golokhvast, K.S. Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia. Horticulturae 2023, 9, 1329. [Google Scholar] [CrossRef]
- Cukelj, N.; Jakasa, I.; Sarajlija, H.; Novotni, D.; Curic, D. Identification and quantification of lignans in wheat bran by gas chromatography-electron capture detection. Talanta 2011, 84, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Chang, F.-R.; Chen, S.-L.; Wu, C.-C.; Lee, K.-H.; Wu, Y.-C. Novel cytotoxic monotetrahydrofuranic Annonaceous acetogenins from Annona montana. Bioorg. Med. Chem. 2005, 13, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Dokwal, D.; Cocuron, J.-C.; Alonso, A.P.; Dickstein, R. Metabolite shift in Medicago truncatula occurs in phosphorus deprivation. J. Exp. Bot. 2022, 73, 2093–2111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Xu, J.; Liu, X.; Wang, S.; Shi, L. Physiological and metabolomics analyses of young and old leaves from wild and cultivated soybean seedlings under low-nitrogen conditions. BMC Plant Biol. 2019, 19, 589. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Boiko, A.P.; Zinchenko, Y.; Tikhonova, N.G.; Sabitov, A.S.; Zakharenko, A.; Golokhvast, K.S. Actinidia deliciosa: A high-resolution mass spectrometric approach for the comprehensive characterization of bioactive compounds. Turk. J. Agric. For. 2023, 47, 155–169. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Okhlopkova, Z.M.; Razgonova, M.P.; Kucharova, E.V.; Egorova, P.S.; Golokhvast, K.S. Rare Plant of Central Yakutia Polygala sibirica L.: Phytochemical Profile and In Vitro Morphogenic Culture. Russ. J. Plant Physiol. 2023, 70, 176. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Bazhenova, B.B.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Tree Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S. Investigation of the Supercritical CO2 Extracts of Wild Ledum palustre, L. (Rhododendron tomentosum Harmaja) and Identification of Its Metabolites by Tandem Mass Spectrometry. Russ. J. Bioorg. Chem. 2023, 49, 1645–1657. [Google Scholar] [CrossRef]
- Lei, Z.; Watson, B.S.; Huhman, D.; Yang, D.S.; Sumner, L.W. Large-Scale Profiling of Saponins in Different Ecotypes of Medicago truncatula. Front. Plant Sci. 2019, 10, 850. [Google Scholar] [CrossRef]
- Lei, Z.-H.; Zhu, H.; Cai, X.-P.; He, D.-D.; Hua, J.-L.; Ju, J.-M.; Lv, H.; Li, W.-L. Simultaneous determination of five triterpene acids in rat plasma by liquid chromatography–mass spectrometry and its application in pharmacokinetic study after oral administration of Folium Eriobotryae effective fraction. Biomed. Chromatogr. 2015, 29, 1791–1797. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Canjura, F.L.; Schwartz, S.J. Identification of Chlorophyll Derivatives by Mass Spectrometry. J. Agric. Food Chem. 1991, 39, 1452–1456. [Google Scholar] [CrossRef]
- Milenkovic, S.M.; Zvezdanovic, J.B.; Andelkovic, T.D.; Markovic, D.Z. The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, Visible and spectroscopy studies. Adv. Technol. 2012, 1, 16–24. [Google Scholar]
- Ha, T.J.; Lee, B.W.; Park, K.H.; Jeong, S.H.; Kim, H.-T.; Ko, J.-M.; Baek, I.-Y.; Lee, J.H. Rapid characterisation and comparison of saponin profiles in the seeds of Korean Leguminous species using ultra performance liquid chromatography with photodiode array detector and electrospray ionisation/mass spectrometry (UPLC–PDA–ESI/MS) analysis. Food Chem. 2014, 146, 270–277. [Google Scholar] [CrossRef]
- Kinjo, J.; Hatakeyama, M.; Udayama, M.; Tsutanaga, Y.; Yamashita, M.; Nohara, T.; Yoshiki, Y.; Okubo, K. HPLC Profile Analysis of Oleane-Glucuronides in Several Edible Beans. Biosci. Biotechnol. Biochem. 1998, 62, 429–433. [Google Scholar] [CrossRef][Green Version]
- Shakya, R.; Navarre, D.A. LC-MS Analysis of Solanidane Glycoalkaloid Diversity among Tubers of Four Wild Potato Species and Three Cultivars (Solanum tuberosum). J. Agric. Food Chem. 2008, 56, 6949–6958. [Google Scholar] [CrossRef]
- Huang, W.; Serra, O.; Dastmalchi, K.; Jin, L.; Yang, L.; Stark, R.E. Comprehensive MS and Solid-state NMR Metabolomic Profiling Reveals Molecular Variations in Native Periderms from Four Solanum tuberosum Potato Cultivars. J. Agric. Food Chem. 2017, 65, 2258–2274. [Google Scholar] [CrossRef]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DADAPCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef]
- Penagos-Calvete, D.; Guauque-Medina, J.; Villegas-Torres, M.F.; Montoya, G. Analysis of triacylglycerides, carotenoids and capsaicinoids as disposable molecules from Capsicum agroindustry. Hortic. Environ. Biotechnol. 2019, 60, 227–238. [Google Scholar] [CrossRef]
- Decroos, K.; Vincken, J.-P.; Heng, L.; Bakker, R.; Gruppen, H.; Verstraete, W. Simultaneous quantification of differently glycosylated, acetylated, and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one-conjugated soyasaponins using reversed-phase high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. A 2005, 1072, 185–193. [Google Scholar] [CrossRef]
- Pollier, J.; Morreel, K.; Geelen, D.; Goossens, A. Metabolite Profiling of Triterpene Saponins in Medicago truncatula Hairy Roots by Liquid Chromatography Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Nat. Prod. 2011, 74, 1462–1476. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Kupnik, K.; Leitgeb, M.; Primozic, M.; Postruznik, V.; Kotnik, P.; Kucuk, N.; Knez, Z.; Marevci, M.K. Supercritical Fluid and Conventional Extractions of High Value-Added Compounds from Pomegranate Peels Waste: Production, Quantification and Antimicrobial Activity of Bioactive Constituents. Plants 2022, 11, 928. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Challenges in the production of pharmaceutical and food related compounds by SC-CO2 processing of vegetable matter. J. Supercrit. Fluids 2018, 134, 269–273. [Google Scholar] [CrossRef]
- Popova, A.S.; Ivahnov, A.D.; Skrebets, T.E.; Bogolitsyn, K.G. Supercritical fluid extraction of carotenoids and chlorophyll from Ledum palustre. Chem. Plant Mater. 2018, 1, 61–66. [Google Scholar] [CrossRef][Green Version]
- Baananou, S.; Bagdonaite, E.; Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Boughattas, N.A. Supercritical CO2 extract and essential oil of aerial part of Ledum palustre L.—Chemical composition and anti-inflammatory activity. Nat. Prod. Res. 2015, 29, 999–1005. [Google Scholar] [CrossRef]
- Aliev, A.M.; Radzhabov, G.K.; Vagabova, F.A.; Islamova, F.I.; Goriainov, S.V.; Hajjar, F.; Hammami, S. Relationship of the Component Composition of Supercritical CO2 Extracts of Wild Carrots with Growing Conditions. Russ. J. Phys. Chem. 2023, 17, 1619–1627. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chitescu, C.L.; Borda, D.; Lupoae, M.; Gird, C.E.; Geana, E.-I.; Blaga, G.-V.; Boscencu, R. Comparison of the Polyphenolic Profile of Medicago sativa L. and Trifolium pratense L. Sprouts in Different Germination Stages Using the UHPLC-Q Exactive Hybrid Quadrupole Orbitrap High-Resolution Mass Spectrometry. Molecules 2020, 25, 2321. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Malencic, D.; Cvejic, J.; Miladinovic, J. Polyphenol Content and Antioxidant Properties of Colored Soybean Seeds from Central Europe. J. Med. Food 2012, 15, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Rossi, Y.E.; Luciana, P.B.; Vanden Braber, N.L.; Ballatore, M.B.; Escobar, F.M.; Bodoira, R.; Maestri, D.M.; Porporatto, C.; Cavaglieri, L.R.; Montenegro, M.A. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interaction with ROS. J. Funct. Foods 2020, 67, 103862. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from Edible Legumes: Chemistry, Processing, and Health Benefits. J. Med. Food 2004, 7, 67–78. [Google Scholar] [CrossRef]
- Sharma, K.; Kaur, R.; Kumar, S.; Kumar Saini, R.; Sharma, S.; Pawde, S.V.; Kumar, V. Saponins: A concise review on food related aspects, applications and health implications. Food Chem. Advances 2023, 2, 100191. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Tsukamoto, C.; Ishimoto, M. Reconstruction of the Evolutionary Histories of UGT Gene Superfamily in Legumes Clarifies the Functional Divergence of Duplicates in Specialized Metabolism. Int. J. Mol. Sci. 2020, 21, 1855. [Google Scholar] [CrossRef]
- Hou, B.; Yuan, X.; Li, S.; Li, Y.; Li, Z.; Li, J. Genome-Wide Identification of CYP72A Gene Family and Expression Patterns Related to Jasmonic Acid Treatment and Steroidal Saponin Accumulation in Dioscorea zingiberensis. Int. J. Mol. Sci. 2021, 22, 10953. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, J.; Park, G.T.; Komagamine, K.; Tsukamoto, C.; Chang, J.H.; Lee, J.-D.; Kim, J.H.; Seo, H.S.; Song, J.T. Biosynthesis of DDMP saponins in soybean is regulated by a distinct UDP-glycosyltransferase. New Phytol. 2019, 222, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Characterization of saponins from the leaves and stem bark of Jatropha curcas L. for surface-active properties. Heliyon 2023, 9, e15807. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Miyakoshi, M.; Isoda, S.; Ida, Y.; Shoji, J. Saponins from leaves of Acanthopanax sieboldianus. Phytochemistry 1993, 34, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.E.M.; de Carvalho, A.C.; Yendo, A.C.A.; Magedans, Y.V.S.; Zachert, E.; Fett-Neto, A.G. Phytotoxicity of Quillaja lancifolia Leaf Saponins and Their Bioherbicide Potential. Plants 2023, 12, 663. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, W. Alfalfa saponins: Structure, biological activity and chemotaxonomy. In Saponins Used in Food and Agriculture; Waller, G.R., Yamasaki, K., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 155–170. [Google Scholar]
- Jurzysta, M.; Bialy, Z. Antifungal and hemolytic activity of roots of alfalfa (Medicago app.) in relation to saponin composition. In Modern Fungicides and Antifungal Compounds II; Lyr, H., Russell, P.E., Dehne, H.W., Sisler, H.D., Eds.; Intercept: Andover, UK, 1999; pp. 445–451. [Google Scholar]
- Martyniuk, S.; Wroblewska, B.; Jurzysta, M.; Bialy, Z. Saponins as inhibitors of cereal pathogens: Gaeumannomyces graminis v. tritici and Cephalosporium grami nem. In Modern Fungicides and Antifungal Compounds; Lyr, H., Russel, P.E., Sisler, H.D., Eds.; Intercept: Andover, UK, 1996; pp. 193–197. [Google Scholar]
- Saniewska, A.; Bialy, Z.; Jurzysta, M. The effect of alfalfa (Medicago sativa) saponins on Botrytis tulipae and Phoma narcissi growth. Phytopathol. Pol. 2003, 27, 15–27. [Google Scholar]
- Saniewska, A.; Jarecka, A.; Bialy, Z.; Jurzysta, M. Antifungal activity of saponins from Medicago arabica L. shoots against some pathogens. Allelopath. J. 2005, 16, 105–112. [Google Scholar]
- Tava, A.; Mella, M.; Avato, P.; Argentieri, M.P.; Bialy, Z.; Jurzysta, M. Triterpenoid glycosides from leaves of Medicago arborea L. J. Agric. Food Chem. 2005, 53, 9954–9965. [Google Scholar] [CrossRef] [PubMed]
- Bialy, Z.; Jurzysta, M.; Mella, M.; Tava, A. Triterpene saponins from the roots of Medicago hybrida. J. Agric. Food Chem. 2006, 54, 2520–2526. [Google Scholar] [CrossRef]
- Szczepanik, M.; Bialy, Z.; Jurzysta, M. The insecticidal activity of saponins from various Medicago spp. against Colorado potato beetle, Leptinotarsa decemlineata Say. Allelopath. J. 2004, 14, 177–186. [Google Scholar]
- Martyniuk, S.; Bialy, Z.; Jurzysta, M. Inhibitory effect of Medicago arabica and Medicago murex constituents on the growth and pathogenicity of Gaeumannomyces graminis var. tritici. In Modern Fungicides and Antifungal Compounds; Dehne, H.W., Gisi, U., Kuck, K.H., Russell, P.E., Lyr, H., Eds.; Agro Concept GmbH: Bonn, Germany, 2002; pp. 395–400. [Google Scholar]
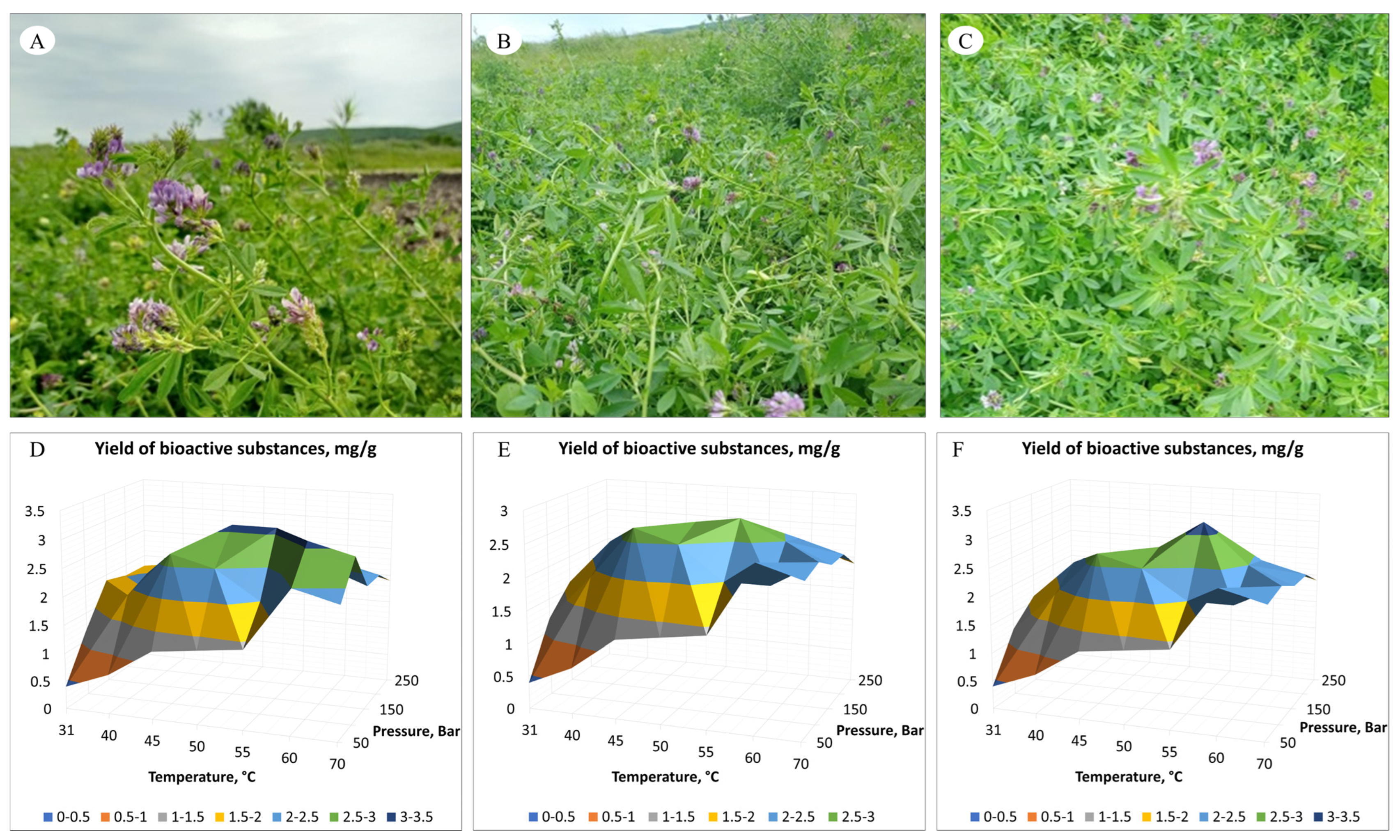
| Temperature (°C) | Pressure (Bar) | ||||
|---|---|---|---|---|---|
| 50 Bar | 100 Bar | 150 Bar | 200 Bar | 250 Bar | |
| 31 °C | 0.4 | 1.2 | 1.9 | 1.8 | 1.8 |
| 40 °C | 0.7 | 1.9 | 2.1 | 1.9 | 1.9 |
| 45 °C | 1.2 | 2.7 | 2 | 2 | 2.1 |
| 50 °C | 1.3 | 2.5 | 3.1 | 2.5 | 2.2 |
| 55 °C | 1.4 | 2 | 3.1 | 2.6 | 2.1 |
| 60 °C | 2.5 | 2.9 | 2.5 | 2.4 | 2.1 |
| 70 °C | 2.3 | 2.9 | 2.2 | 2.2 | 1.9 |
| Pressure | 50 Bar | 100 Bar | 150 Bar | 200 Bar | 250 Bar |
|---|---|---|---|---|---|
| 31 °C | 0.4 | 1.2 | 1.6 | 1.7 | 1.7 |
| 40 °C | 0.7 | 1.9 | 2.3 | 1.9 | 1.9 |
| 45 °C | 1.2 | 2.7 | 2 | 2 | 2.1 |
| 50 °C | 1.3 | 2.5 | 2.7 | 2.2 | 2.2 |
| 55 °C | 1.4 | 2 | 2.8 | 2.4 | 2.1 |
| 60 °C | 2.5 | 2 | 2.5 | 2.4 | 2.1 |
| 70 °C | 2.3 | 2.4 | 2.2 | 2.2 | 1.9 |
| Pressure | 50 Bar | 100 Bar | 150 Bar | 200 Bar | 250 Bar |
|---|---|---|---|---|---|
| 31 °C | 0.4 | 1.2 | 1.6 | 1.7 | 1.7 |
| 40 °C | 0.7 | 1.9 | 2.3 | 1.9 | 1.9 |
| 45 °C | 1.2 | 2.7 | 2 | 2 | 2.1 |
| 50 °C | 1.3 | 2.5 | 2.7 | 2.2 | 2.2 |
| 55 °C | 1.4 | 2 | 3.2 | 2.4 | 2.1 |
| 60 °C | 2.5 | 2 | 2.5 | 2.4 | 2.1 |
| 70 °C | 2.3 | 2.4 | 2.2 | 2.2 | 1.9 |
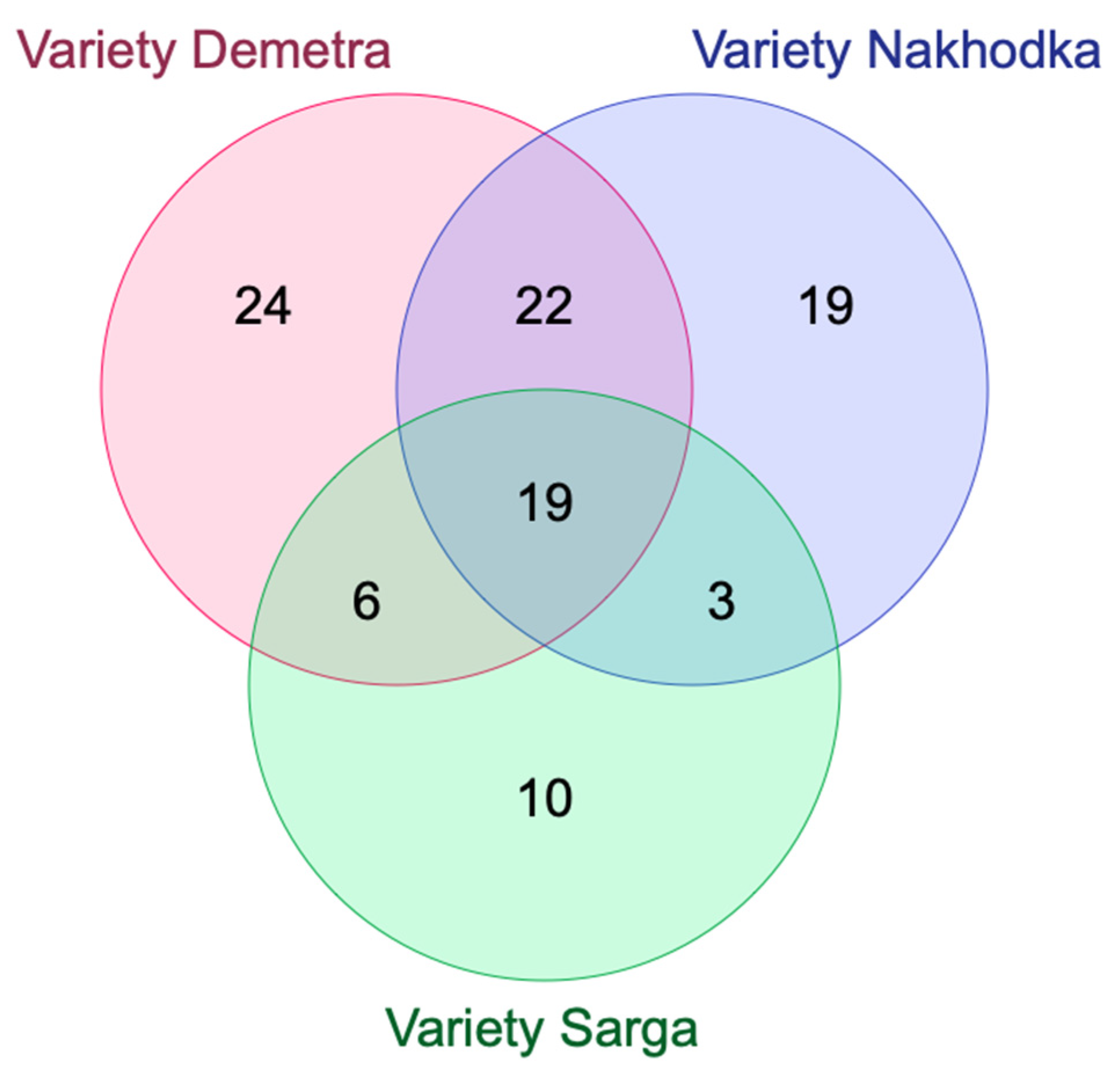
| Variety Demetra (71) | Variety Nakhodka (63) | Variety Sarga (38) | |
| Variety Demetra (71) | -- | 41 0.4409 | 25 0.2976 |
| Variety Nakhodka (63) | 41 0.4409 | -- | 22 0.2785 |
| Variety Sarga (38) | 25 0.2976 | 22 0.2785 | -- |
| Class of Compound | Identification | Formula | Calculated Mass | Observed Mass [M − H]− | Observed Mass [M + H]+ | MS/MS Stage 1 Fragmentation | MS/MS Stage 2 Fragmentation | MS/MS Stage 3 Fragmentation | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic compounds | ||||||||||
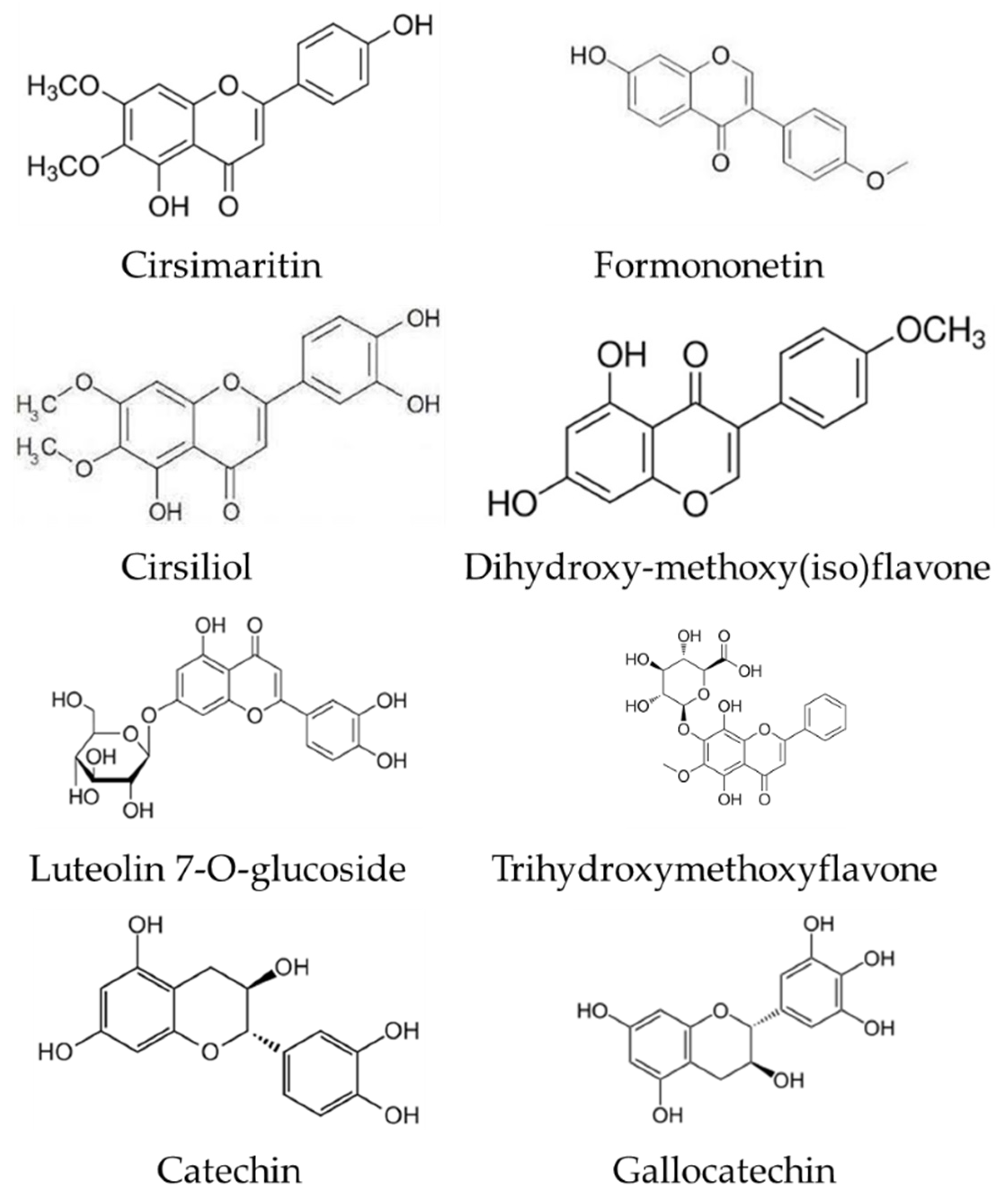
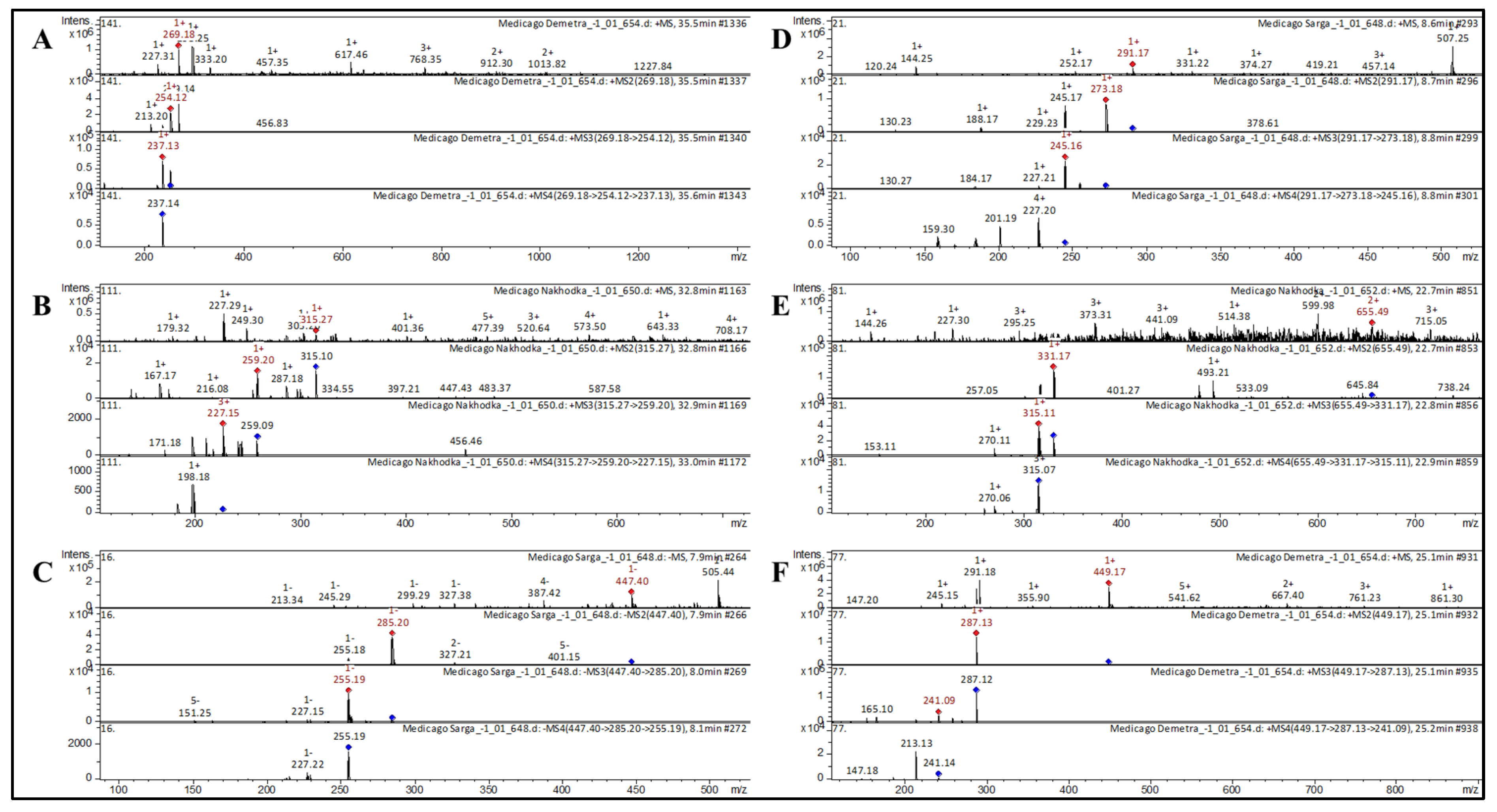
| 1 | 7-Hydroxyisoflavone | Formononetin [Biochanin B; Formononetol] * | C16H12O4 | 268.2641 | 269 | 254; 213 | 237 | 237 | Astragali Radix [29]; Huolisu oral liquid [30]; Dracocephalum jacutense [31]; Maackia amurense [32]; Chinese herbal formula, Jian-Pi-Yi-Shen pill [33] | |
| 2 | Flavone | Apigenin [5,7-Dixydroxy-2-(40Hydroxyphenyl)-4H-Chromen-4-One] | C15H10O5 | 270.2369 | 271 | 271; 153 | Propolis [35]; Inula gaveolens [41]; Phlomis (Lamiaceae) [42]; Lonicera henryi [43]; Ribes meyeri [44]; Lonicera japonica [45]; Stevia rebaudiana [46]; Jatropha [47] | |||
| 3 | Flavone | Acacetin [Linarigenin; Buddleoflavonol] | C16H12O5 | 284.2635 | 285 | 270 | 269; 242 | 213; 185 | Propolis [35]; Mentha [36]; Mexican lupine species [37]; Wissadula periplocifolia [38] | |
| 4 | Flavone | Dihydroxy-methoxy(iso)flavone | C16H12O5 | 284.2635 | 285 | 270 | 269 | 227 | Propolis [35] | |
| 5 | Flavone | Luteolin | C15H10O6 | 286.2363 | 287 | 213; 165 | 157 | Propolis [35]; Artemisia absinthium [39]; Inula gaveolens [41]; Lonicera henryl [43]; Ribes meyeri [44]; Lonicera japonica [45]; Jatropha [47]; potato [50] | ||
| 6 | Flavone | Apigenin-7, 4′-dimethyl ether | C17H14O5 | 298.2901 | 299 | 284 | 256; 169 | 255; 132 | Ocimum [34]; propolis [35] | |
| 7 | Flavone | Trihydroxymethoxyflavone | C16H12O6 | 300.2629 | 301 | 286 | 258 | Artemisia absinthium [39] | ||
| 8 | Flavone | Chrysoeriol [Chryseriol] * | C16H12O6 | 300.2629 | 301 | 286 | 258 | Propolis [35]; Mentha [36]; Mexican lupine species [37]; Rhus coriaria [66] | ||
| 9 | Flavone | Cirsimaritin [Scrophulein; 4′,5-Dihydroxy-6,7-Dimethoxyflavone] * | C17H14O6 | 314.2895 | 315 | 287; 259; 216 | 227; 171 | 198 | Ocimum [34]; Artemisia annua [39]; Rosmarinus officinalis [40] | |
| 10 | Flavone | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 314.2895 | 315 | 287; 259; 216 | 227; 171 | 198 | Astragali radix [29]; propolis [35]; Rosmarinus officinalis [50] | |
| 11 | Flavone | Cirsiliol * | C17H14O7 | 330.2889 | 332 | 315; 271 | Ocimum [34]; Juglans mandshurica [48]; Inula viscosa [55] | |||
| 12 | Flavone | Nevadensin | C18H16O7 | 344.3154 | 345 | 312; 222; 181 | 284; 256 | 283; 269; 255 | Ocimum [34]; Mentha [36] | |
| 13 | Flavone | Cirsilineol [Eupatrin; Fastigenin] * | C18H16O7 | 344.3154 | 345 | 312; 222; 181 | 284; 256 | 283; 269; 255 | Ocimum [34] | |
| 14 | Flavone | Tetrahydroxy-dimethoxyflavone | C17H14O8 | 346.2883 | 345 | 330 | 315 | 287 | Artemisia absinthium [39] | |
| 15 | Flavone | 5,6-Dihydroxy-7,8,3’,4’-tetramethoxyflavone | C19H18O8 | 374.3414 | 375 | 368; 348; 325; 304 | 358; 301; 226 | Mentha [36] | ||
| 16 | Flavone | Luteolin 7-O-glucoside [Cynaroside; Luteoloside] | C21H20O11 | 448.3769 | 449 | 287 | 241; 165 | 213; 147 | Mexican lupine species [37]; Lonicera henryi [43]; Lonicera japonica [45] | |
| 17 | Flavone | Luteolin 8-C-Glucoside [Orientin; Orientin (Flavone); Lutexin] | C21H20O11 | 448.3769 | 449 | 430; 373; 328; 285; 215 | Lemon, passion fruit [67]; P. aculeata [68]; Phyllostachys nigra [69]; Aspalathus linearis [70]; bamboo [71] | |||
| 18 | Flavone | Tricin O-hexoside * | C23H24O12 | 492.4295 | 493 | 331 | 315 | Triticum aestivum L. [72]; bamboo [71] | ||
| 19 | Flavone | Dihydroxy-trimethoxyflavone-O-hexoside | C23H22O14 | 506.414 | 507 | 331 | 315; 270 | 270 | Citrus species [73] | |
| 20 | Flavone | Apigenin O-pentosyl hexoside | C26H28O14 | 564.4921 | 565 | 433; 288 | 415; 334; 271; 163 | 127 | F. glaucescens [53] | |
| 21 | Flavone | Apigenin C-glucose C-deoxyhexoside * | C27H30O14 | 578.5187 | 579 | 547; 488; 403; 365 | Passiflora incarnata [74] | |||
| 22 | Flavone | Apigenin 7-O-diglucuronide * | C27H26O17 | 622.4851 | 623 | 447 | 271 | 153 | Perilla frutescens [75] | |
| 23 | Flavone | Tricin di-O,O-hexoside | C29H34O17 | 654.5701 | 655 | 331; 493 | 315; 270; 153 | 270 | Triticum aestivum L. [72,76] | |
| 24 | Flavone | 2′-Hydroxygenistein 4′, 7-O-diglucoside malonylated * | C30H32O19 | 696.5637 | 697 | 287 | 241; 165; 121 | 185 | Mexican lupine species [37] | |
| 25 | Flavonol | Kaempferol | C15H10O6 | 286.2363 | 287 | 241 | 165 | Ribes meyeri [44]; Lonicera japonicum [45]; Juglans mandshurica [48]; Rhus coriaria [66] | ||
| 26 | Flavonol | Kaempferide [4′-O-Methylkaempferol] | C16H12O6 | 300.2629 | 301 | 286 | 258 | Ribes meyeri [44]; Alpinia officinarum [77]; Brazilian propolis [78] | ||
| 27 | Flavonol | Rhamnocitrin | C16H12O6 | 300.2629 | 301 | 273; 163 | 243 | 227 | Astragali radix [29]; Lonicera caerulea [79] | |
| 28 | Flavonol | Quercetin | C15H10O7 | 302.2357 | 303 | 285; 167 | 257; 197 | 257 | Inula gaveolens [41]; Juglans mandshurica [48]; Inula viscosa [55]; Black soja [57] | |
| 29 | Flavonol | Dihydroquercetin (Taxifolin; Taxifoliol) | C15H12O7 | 304.2516 | 305 | 285; 211; 175 | 268; 185; 124 | 168 | Juglans mandshurica [48]; Glycine soja [58]; Camellia kucha [80] | |
| 30 | Flavonol | Isorhamnetin [Isorhamnetol; Quercetin 3′-Methyl ether; 3-Methylquercetin] | C16H12O7 | 316.2623 | 317 | 302 | 274; 153 | 229; 153 | Propolis [35]; Rosmarinus officinalis [40]; Stevia rebaudiana [46]; Inula viscosa [55]; Lonicera caerulea [79]; Phoenix dactylifera [81] | |
| 31 | Flavonol | Myricetin | C15H10O8 | 318.2351 | 317 | 273; 295 | 260 | 238 | Juglans mandshurica [48]; F. glaucescens [53]; Taraxacum officinale [82] | |
| 32 | Flavonol | Ampelopsin [Dihydromyricetin; Ampeloptin] * | C15H12O8 | 320.251 | 321 | 301; 201 | 267; 201 | Juglans mandshurica [48]; Rnus coriaria [66]; Impatients glandulifera Royle [83] | ||
| 33 | Flavonol | Kaempferol-3-O-α-L-rhamnoside | C21H20O10 | 432.3775 | 433 | 427; 340; 287 | Carpinus betulus [51]; C. edulis; F. glaucescens [53]; Taraxacum officinale [82]; Cassia abbreviata [84] | |||
| 34 | Flavonol | Astragalin [Kaempferol 3-O-glucoside] * | C21H20O11 | 448.3769 | 447 | 285; 255 | 255; 227; 151 | 227 | Juglans mandshurica [48]; bee pollen [49]; Lonicera japonica [45]; Ribes meyeri [44]; potato [50] | |
| 35 | Flavonol | Quercetin 3-O-glucoside [Isoquercetin; Isoquercitrin; Hirsutrin; Quercetin-3-O-Glucopyranoside] | C21H20O12 | 464.3763 | 465 | 303; 258; 164 | 243; 179 | Juglans mandshurica [48]; Black soja [57]; Ribes meyeri [44]; Lonicera henryi [43]; Lonicera japonica [45]; Vaccinium myrtillus [85]; Solanaceae [52]; Rhus coriaria [66]; Embelia [54] | ||
| 36 | Flavonol | Isorhamnetin 3-O-glucoside | C22H22O12 | 478.4029 | 479 | 317 | 301; 257; 177 | 274; 218 | Artemisia annua [86]; Eucalyptus [87]; Capsicum annuum [88]; Senecio clivicolus [89] | |
| 37 | Flavonol | Isorhamnetin 3-O-glucoronide | C22H20O13 | 492.3864 | 493 | 317 | 302 | 274 | Anethum graveolens [90]; Strawberry [91]; | |
| 38 | Flavonol | Kaempferol 3-(6″-malonylglucoside) * | C24H22O14 | 534.4231 | 535 | 287 | 241; 165 | 213 | A. cordifolia [53]; Strawberry [91] | |
| 39 | Flavonol | Rhamnosylhexosyl-methyl-quercetin * | C26H28O17 | 612.4903 | 613 | 595; 540; 489 | 521; 337; 241 | 503; 349; 239 | Phoenix dactylifera [81] | |
| 40 | Flavonol | Quercetin 3,4’-di-O-beta-glucopyranoside [Quercetin diglucoside] * | C27H30O17 | 626.5169 | 627 | 465; 393; 303 | 303 | 257; 165 | Potato leaves [92]; potato [50]; rapeseed petals [93] | |
| 41 | Flavonol | Quercetin-O-dihexoside | C27H30O17 | 626.5169 | 627 | 465; 393; 303 | 303 | 257; 165 | Inula viscosa [55]; Artemisia absinthium [39]; Chilean currants [64]; Phoenix dactylifera [81]; Taraxacum formosanum [94] | |
| 42 | Flavonol | Isorhamnetin-di-O-hexoside [Methyl quercetin-O-dihexoside] * | C28H32O17 | 640.5435 | 641 | 317 | 302 | 285; 228; 169 | Artemisia absinthium [39]; passion fruit [67]; Phoenix dactylifera [81] | |
| 43 | Flavonol | Quercetin-7-O-(acetyl-hexoside)-3-O-rhamnoside * | C29H32O17 | 652.5542 | 653 | 301 | 286; 153 | 258 | Capsicum annuum [88] | |
| 44 | Flavonol | Isorhamnetin-3-O-6-O-acetyl-beta-D-glucopyranosyl * | C30H34O18 | 682.5802 | 683 | 331 | 315; 270 | Rosa rugosa [95] | ||
| 45 | Flavan-3-ol | Catechin | C15H14O6 | 290.2681 | 291 | 273; 188 | 245 | 227; 201 | Inula viscosa [55]; Juglans mandshurica [48]; Black soja [57]; Glycine soja [58] | |
| 46 | Flavan-3-ol | (epi)-Catechin | C15H14O6 | 290.2681 | 291 | 261; 173 | 173 | Black soja [57]; Glycine soja [58]; Jatropha [47] | ||
| 47 | Flavan-3-ol | Gallocatechin [+(-)Gallocatechin] * | C15H14O7 | 306.2675 | 307 | 289 | 260; 175 | 244; 171 | Carpinus betulus [51]; Solanaceae [52]; G. linguiforme [53]; Ribes meyeri [44]; Embelia [54] | |
| 48 | Anthocyanin | Cyanidin-3-O-glucoside [Cyanidin 3-O-beta-D-Glucoside; Kuromarin] | C21H21O11+ | 449.3848 | 449 | 287 | 241; 165 | 213; 147 | Black soybean [57]; Glycine soja [58]; Ribes magellanicum [64]; Rubus ulmifolius [65]; B. ilicifolia; B. empetrifolia; R. maellanicum; R. cucullatum; M. nummalaria; G. mucronata; G. antarctica; Fuchsia magellanica [59]; B. microphylla [60] | |
| 49 | Anthocyanin | Malvidin 3-O-glucoside [Oenin] | C23H25O12 | 493.4374 | 493 | 331 | 315; 270 | 315; 270 | G. mucronata; G. antarctica [59]; Berberis microphylla [60] | |
| 50 | Anthocyanin | Cyanidin 3-(6″-malonylglucoside) | C24H23O14 | 535.4310 | 535 | 287 | 241; 165 | 213 | Strawberry [91]; Zostera marina [25] | |
| 51 | Anthocyanin | Cyanidin-3-O-dioxayl-glucoside | C31H28O12 | 592.5468 | 592 | 287 | 241 | 227; 209; 144 | Rubus ulmifolius [65] | |
| 52 | Anthocyanin | Peonidin 3-O-(6-O-p-coumaroyl) glucoside | C31H29O13 | 609.554 | 609 | 303 | 257; 153 | 229 | Grape [62]; vines [63] | |
| 53 | Anthocyanin | Malvidin 3-O-(6-O-p-caffeoyl) glucoside | C32H31O15 | 655.5795 | 655 | 331; 493 | 315; 270; 153 | 270 | Grape [62]; Bougainvillea [61] | |
| 54 | Phenolic acid | Coniferyl aldehyde [4-Hydroxy-3-methoxycinnamaldehyde; Coniferaldehyde; Ferulaldehyde] | C10H10O3 | 178.1846 | 179 | 161; 133; 119 | 119 | Juglans mandshurica [48]; potato [50]; A. cordifolia [53] | ||
| 55 | Phenolic acid | Caffeic acid derivative | C16H18O9Na | 377.2985 | 377 | 341 | 215 | Bougainvillea [61]; Embelia [54] | ||
| 56 | Hydroxybenzoic acid (Phenolic acid) | Ellagic acid [Benzoaric acid; Elagostasine; Lagistase; Eleagic acid] | C14H6O8 | 302.1926 | 303 | 257; 229; 165 | 229; 201 | 201 | Juglans mandshurica [48]; Rhus coriaria [66]; Eucalyptus [87] | |
| 57 | Phenylpropanoid (cinnamic alcohol glycoside) | Vimalin * | C16H22O7 | 326.3417 | 327 | 309; 195 | 241; 195 | Rhodiola rosea [96] | ||
| 58 | Coumarin | Fraxetin * | C10H8O5 | 208.1675 | 209 | 167 | Embelia [54]; Jatropha [47]; Artemisia martjanovii [97] | |||
| 59 | Lignan | Syringaresinol * | C22H26O8 | 418.4436 | 419 | 326; 253; 184 | 298; 254; 174 | 252; 226; 182 | Wheat [98]; Annona montana [99]; Lonicera caerulea [79] | |
| Others | ||||||||||
| 60 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one [DDMP] | C6H8O4 | 144.1253 | 145 | 127 | Radix polygoni multiflori [56] | ||||
| 61 | Aliphatic amino acid | L-Glutamic acid [L-Glutamate] | C5H7NO4 | 145.1134 | 146 | 144; 118 | Medicago truncatula [100]; soybean leaves [101]; Lonicera japonica [45] | |||
| 62 | Amino acid | L-Histidine | C6H9N3O2 | 155.1546 | 156 | 110 | Medicago truncatula [100]; Lonicera japonica [45]; Camellia kucha [80]; Actinidia deliciosa [102]; Lonicera caerulea [79] | |||
| 63 | Amino acid | Phenylalanine [L-Phenylalanine] | C9H11NO2 | 165.1891 | 166 | 120 | Medicago truncatula [100]; Juglans mandshurica [48]; soybean [58]; soybean leaves [101]; Lonicera japonica [45]; potato leaves [92]; Camellia kucha [80] | |||
| 64 | Cyclohexenecarboxylic acid | Shikimic acid [L-Schikimic acid] | C7H10O5 | 174.1513 | 175 | 157 | Medicago truncatula [100]; soybean [58]; Camellia kucha [80]; Ribes meyeri [44] | |||
| 65 | Tricarboxylic acid | cis-Aconitic acid | C6H6O6 | 174.1082 | 175 | 157 | Medicago truncatula [100] | |||
| 66 | Tricarboxylic acid | Trans-Aconitic acid [trans-Aconitate] | C6H6O6 | 174.1082 | 175 | 157 | Medicago truncatula [100]; Inula graveolens [41] | |||
| 67 | Amino acid | L-theanine [Theanine; Theanin; N-Ethyl-L-glutamine] | C7H14N2O3 | 174.1977 | 175 | 157 | Camellia kucha [80] | |||
| 68 | Aromatic amino acid | Tyrosine [(2S)-2-Amino-3-(4-Hydroxyphnyl)Propanoic acid] | C9H11NO3 | 181.1885 | 182 | 177; 165 | 123 | Medicago truncatula [100]; soybean leaves [101]; Hylocereus polyrhizus [103]; Polygala sibirica [104] | ||
| 69 | Essential amino acid | L-Tryptophan [Tryptophan; (S)-Tryptophan] | C11H12N2O2 | 204.2252 | 205 | 187 | 121 | Camellia kucha [80]; Rosa acicularis [105] | ||
| 70 | Carboxylic acid | Myristoleic acid [Cis-9-Tetradecanoic acid] | C14H26O2 | 226.3550 | 227 | 209; 165 | 121 | F. glaucescens [53]; Maackia amurensis [32]; | ||
| 71 | Ribonucleoside composite of adenine (purine) | Adenosine | C10H13N5O4 | 267.2413 | 268 | 136 | 121 | Lonicera japonica [45]; Huolisu oral liquid [30]; L. palustre [106]; Rosa acicularis [105] | ||
| 72 | Ribonucleoside composite of adenine (purine) | Inosine | C10H12N4O5 | 268.2261 | 269 | 136 | Lonicera japonica [45] | |||
| 73 | Monosaccharides | 6-Phosphogluconic acid | C6H13O10P | 276.1352 | 277 | 259; 205; 188; 130 | Medicago truncatula [100] | |||
| 74 | Omega-3 fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) | C18H30O2 | 278.4296 | 279 | 219; 154 | 159 | Soybean [58]; soybean leaves [101]; Maackia amurense [32]; Polygala sibirica [104] | ||
| 75 | Polyunsaturated long-chain fatty acid | Hydroxy eicosatetraenoic acid | C20H32O3 | 320.4663 | 321 | 312; 256; 228; 193 | 224; 176; 143 | F. glaucescens; F herrerae [53] | ||
| 76 | Polysaccharides | Adenosine 5′-monophosphate [5′-adenylic acid] | C10H14N5O7P | 347.2212 | 348 | 341; 273; 233 | 205 | Medicago truncatula [100] | ||
| 77 | Phytosterol | Ergosterol [Provitamin D2; Ergosterin] | C28H44O | 396.6484 | 397 | 392; 311; 183; 129 | 361; 311; 226; 183; 130 | F. glaucescens [53] | ||
| 78 | Pentacyclic triterpenoid | Sophoradiol | C30H50O2 | 442.7168 | 443 | 425; 175 | 175 | 115 | Medicago truncatula [107] | |
| 79 | Polysaccharides | Guanosine 5′-diphosphate | C10H15N5O11P2 | 443.2005 | 444 | 359; 323; 297; 274 | 189; 154 | 172 | Medicago truncatula [100] | |
| 80 | Anabolic steroid | Vebonol | C30H44O3 | 452.6686 | 453 | 435; 336; 209 | 336; 226 | 209 | Rhus coriaria [66]; Hylosereus polyrhizus [103] | |
| 81 | Triterpenic acid | Ursolic acid | C30H48O3 | 456.7003 | 457 | 411; 203 | 393; 283; 201 | 201 | Juglans mandshurica [48]; Ocimum [34]; Mentha [36] | |
| 82 | Triterpenic acid | Oleanolic acid | C30H48O3 | 456.7004 | 457 | 411; 263; 203 | 393; 309; 177 | 375; 203; 145 | Medicago truncatula [107]; C. edulis [53]; Folium Eriobotryae [108] | |
| 83 | Saponin | Soyasapogenol B [24-Hydroxysophoradiol; Soyasapogenin B] | C30H50O3 | 458.7162 | 459 | 452; 317; 279; 212 | 445; 298; 216 | Medicago truncatula [107] | ||
| 84 | Saponin | Soyasapogenol A | C30H50O4 | 474.5434 | 475 | 437; 343; 249 | 168; 127 | Medicago truncatula [107] | ||
| 85 | Polysaccharides | UTP [Uridine 5′-triphosphate] | C9H15N2O15P3 | 484.1411 | 485 | 478; 365; 321; 261 | Medicago truncatula [100] | |||
| 86 | Polysaccharides | Guanosine-5′-triphosphate [GTP] | C10H16N5O14P3 | 523.1804 | 524 | 494; 386; 303; 165 | Medicago truncatula [100] | |||
| 87 | Polysaccharides | UDP-arabinose [Uridine 5′-diphosphate arabinose] | C14H20N2O16P2 | 534.2599 | 535 | 275; 217; 159 | 220 | Medicago truncatula [100] | ||
| 88 | Polysaccharides | Uridine diphosphate-xylose [UDP-xylose] | C14H22N2O16P2 | 536.2758 | 537 | 277; 187 | 249; 136 | Medicago truncatula [100] | ||
| 89 | Polysaccharides | UDP-glucose [Uridine diphosphate glucose] | C15H24N2O17P2 | 566.3018 | 567 | 423; 385; 324; 283 | 405; 367; 297; 241 | Medicago truncatula [100] | ||
| 90 | Product of chlorophyll degradation | Chlorophyllide a | C35H34MgN4O5 | 614.9733 | 615 | 583 | 565; 458; 269 | 520; 441 | [109,110] | |
| 91 | Medicagenic acid -3-O-beta-D-glucopyranoside | C36H56O11 | 664.8232 | 665 | 635; 510; 452; 401 | 337 | 319 | Pubchem | ||
| 92 | Saponin | Azukisaponin II | C42H68O14 | 796.9809 | 797 | 519 | 429; 357; 243 | Leguminosae [111]; Glycine max [112] | ||
| 93 | Saponin | Soyasaponin Bb’ [Soyasaponin III] * | C42H68O14 | 796.9809 | 797 | 519 | 429; 357; 243 | Black soja [57] | ||
| 94 | Product of chlorophyll degradation | Pyropheophytin a | C53H72N4O3 | 813.1638 | 813 | 535 | 435; 329 | [110] | ||
| 95 | Steroidal alkaloid | Alpha-chaconine * | C45H73NO14 | 852.0594 | 852 | 706; 560; 398 | 560 | 398 | Potato [113,114] | |
| 96 | Product of chlorophyll degradation | Pheophytin A | C55H74N4O5 | 871.1999 | 871 | 533 | 461 | Physalis peruviana [115]; Capsicum [116]; [109, 110] | ||
| 97 | Saponin | Soyasaponin II [Soyasaponin II (SH); Soyasaponin Bc] * | C47H76O17 | 913.0961 | 912 | 501 | 483; 425 | 425 | Black soja [57]; Leguminosae [111]; soya [117] | |
| 98 | Saponin | Soyasaponin gamma g * | C48H74O17 | 923.0910 | 924 | 581; 423; 321 | 213 | Black soja [57]; Leguminosae [111]; soya [117] | ||
| 99 | Saponin | 3-Rhamnose-galactose-glucuronic acid-soyasapogenol B | C48H78O18 | 943.1221 | 944 | 598; 423; 295 | 581; 419; 215 | 572 | Medicago truncatula [100]; Rhus coriaria [66] | |
| 100 | Saponin | Soyasaponin I [Soyasaponin Bb] * | C48H78O18 | 943.1221 | 944 | 598; 423; 365; 281 | 205 | Leguminosae [111]; soya [117]; Black soja [57]; | ||
| 101 | Saponin | Soyasaponin Bd * | C48H76O19 | 957.1056 | 958 | 456; 595; 718; 812 | 409; 247 | 271 | Black soja [57]; Leguminosae [111]; soya [117] | |
| 102 | Saponin | 6-deoxyhexose-hexoside-uronic acid-aglycone D | C48H78O20 | 975.1209 | 975 | 799; 715; 529; 477 | 301 | 286; 259; 201 | Medicago truncatula [118] | |
| 103 | Saponin | Soyasaponin beta g * | C54H84O21 | 1069.2322 | 1068 | 967; 879; 741; 584 | 659; 483 | Black soja [57]; Leguminosae [111]; soya [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgonova, M.P.; Nawaz, M.A.; Ivanova, E.P.; Cherevach, E.I.; Golokhvast, K.S. Supercritical CO2-Based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry. Processes 2024, 12, 1041. https://doi.org/10.3390/pr12051041
Razgonova MP, Nawaz MA, Ivanova EP, Cherevach EI, Golokhvast KS. Supercritical CO2-Based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry. Processes. 2024; 12(5):1041. https://doi.org/10.3390/pr12051041
Chicago/Turabian StyleRazgonova, Mayya P., Muhammad Amjad Nawaz, Elena P. Ivanova, Elena I. Cherevach, and Kirill S. Golokhvast. 2024. "Supercritical CO2-Based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry" Processes 12, no. 5: 1041. https://doi.org/10.3390/pr12051041
APA StyleRazgonova, M. P., Nawaz, M. A., Ivanova, E. P., Cherevach, E. I., & Golokhvast, K. S. (2024). Supercritical CO2-Based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry. Processes, 12(5), 1041. https://doi.org/10.3390/pr12051041









