Adsorption of Zinc(II) Ion by Spent and Raw Agaricus bisporus in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Biosorbent Preparation
2.2. Zn2+ Solution Preparation
2.3. Batch Experiments
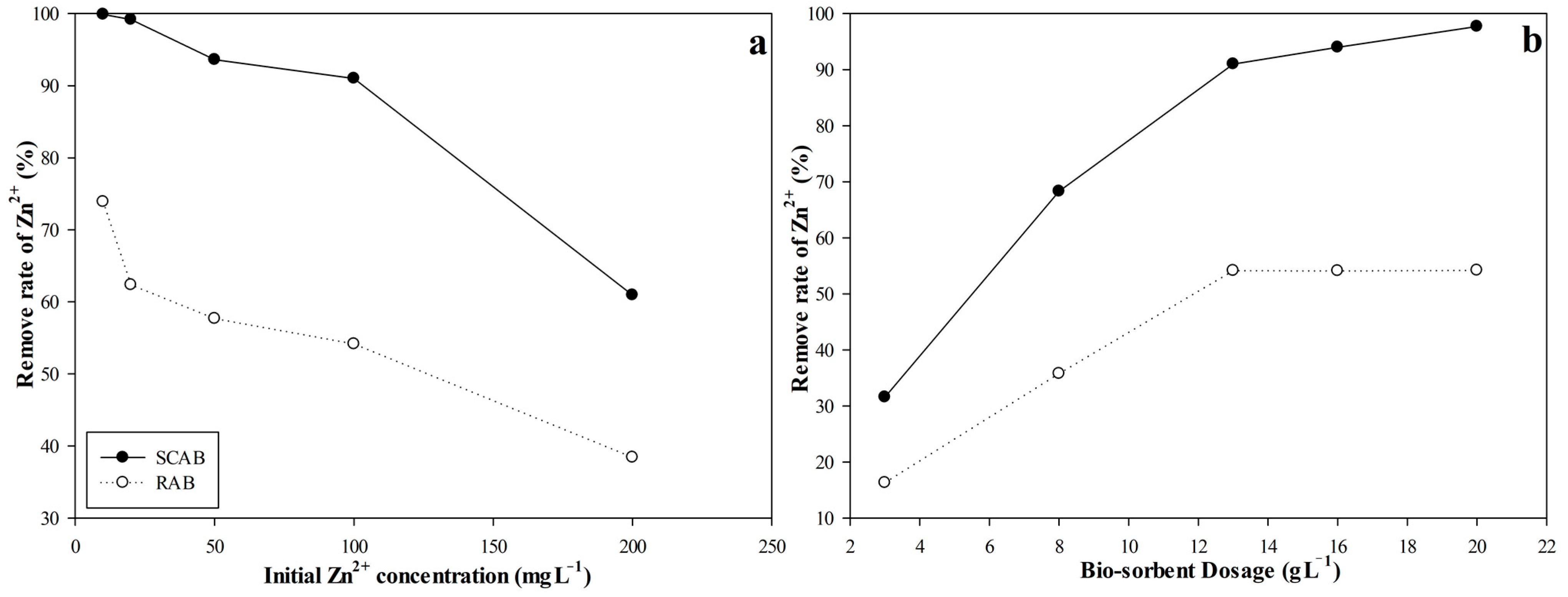
2.3.1. Effect of Biosorbent on Zn2+ Biosorption by RAB and SCAB
2.3.2. Effect of Zn2+ Concentration on Zn2+ Biosorption by RAB and SCAB
2.3.3. Effect of Adsorption Time on Zn2+ Biosorption by SCAB
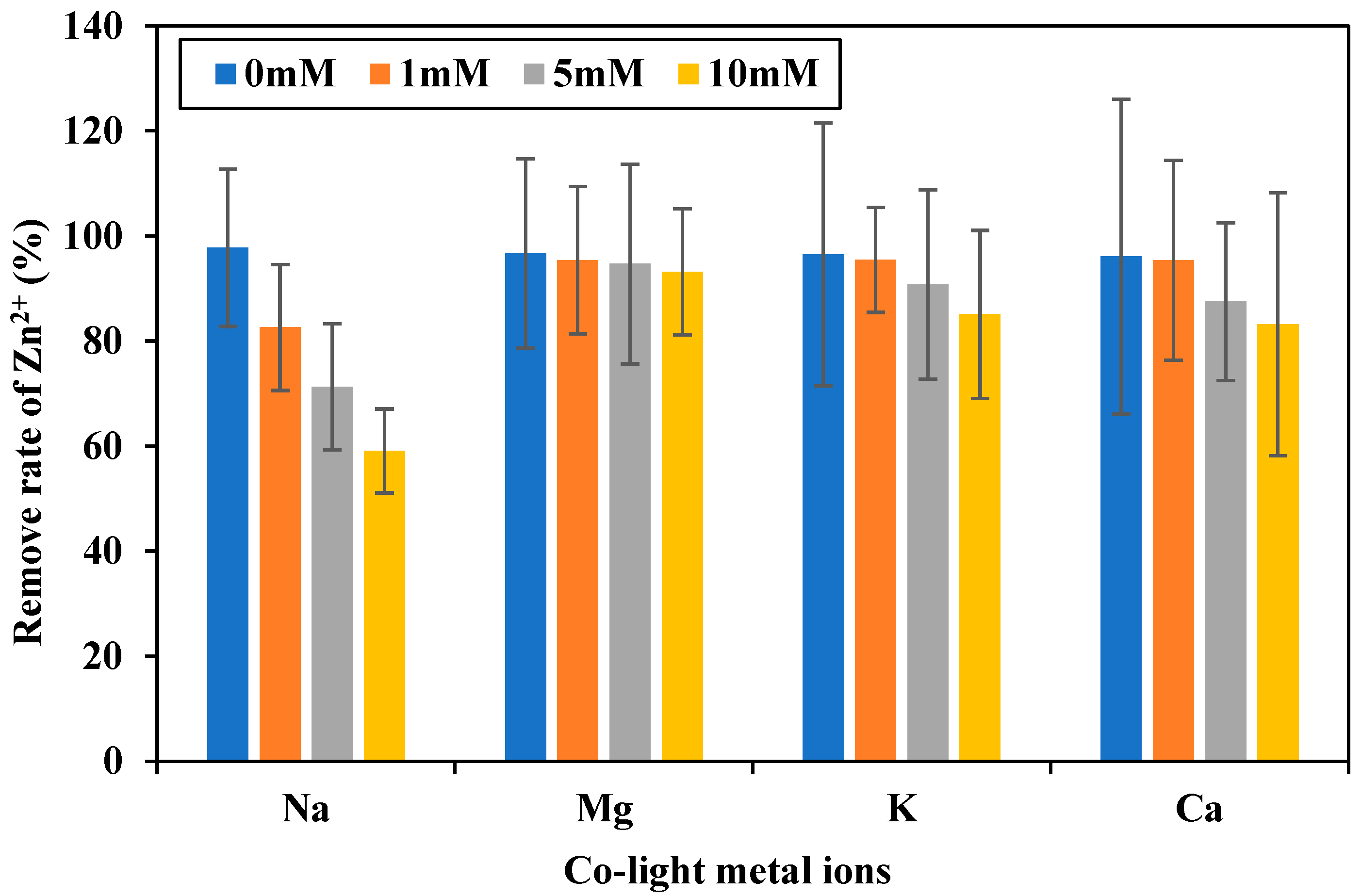
2.3.4. Effect of Co-Ions on Zn2+ Biosorption by SCAB
2.3.5. Specific Surface Area Detection of Bioadsorbent Materials
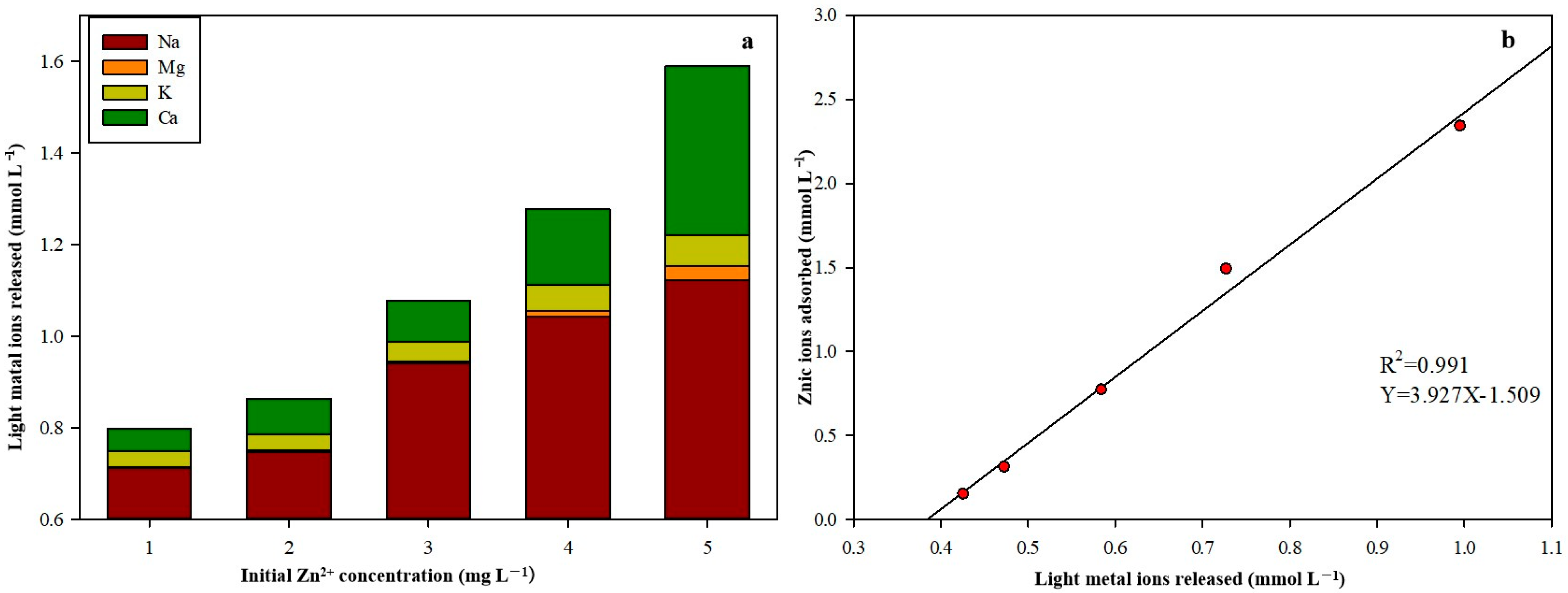
2.4. Light Metal Ion Release
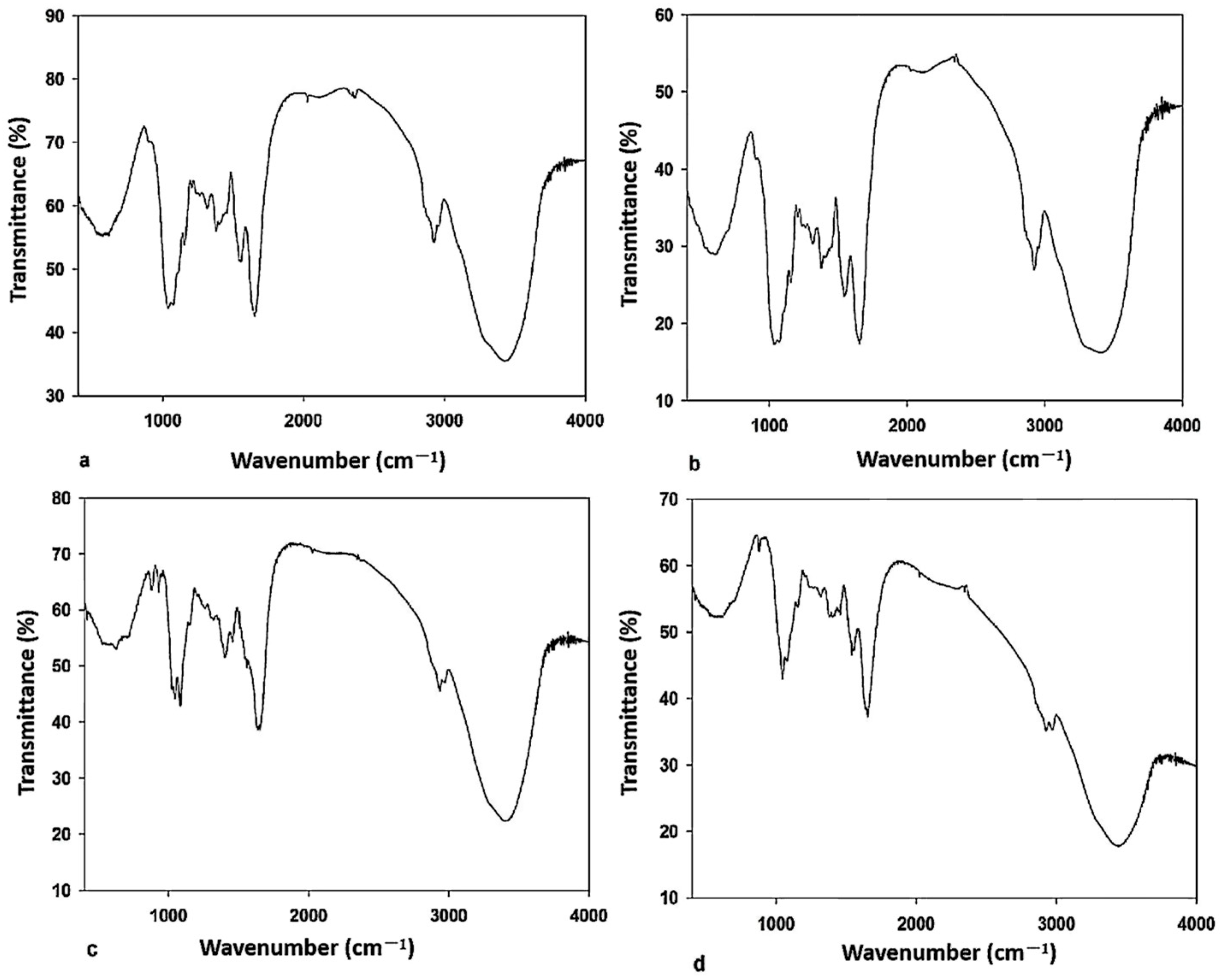
2.5. Results of Fourier Transform Infrared Spectroscopy
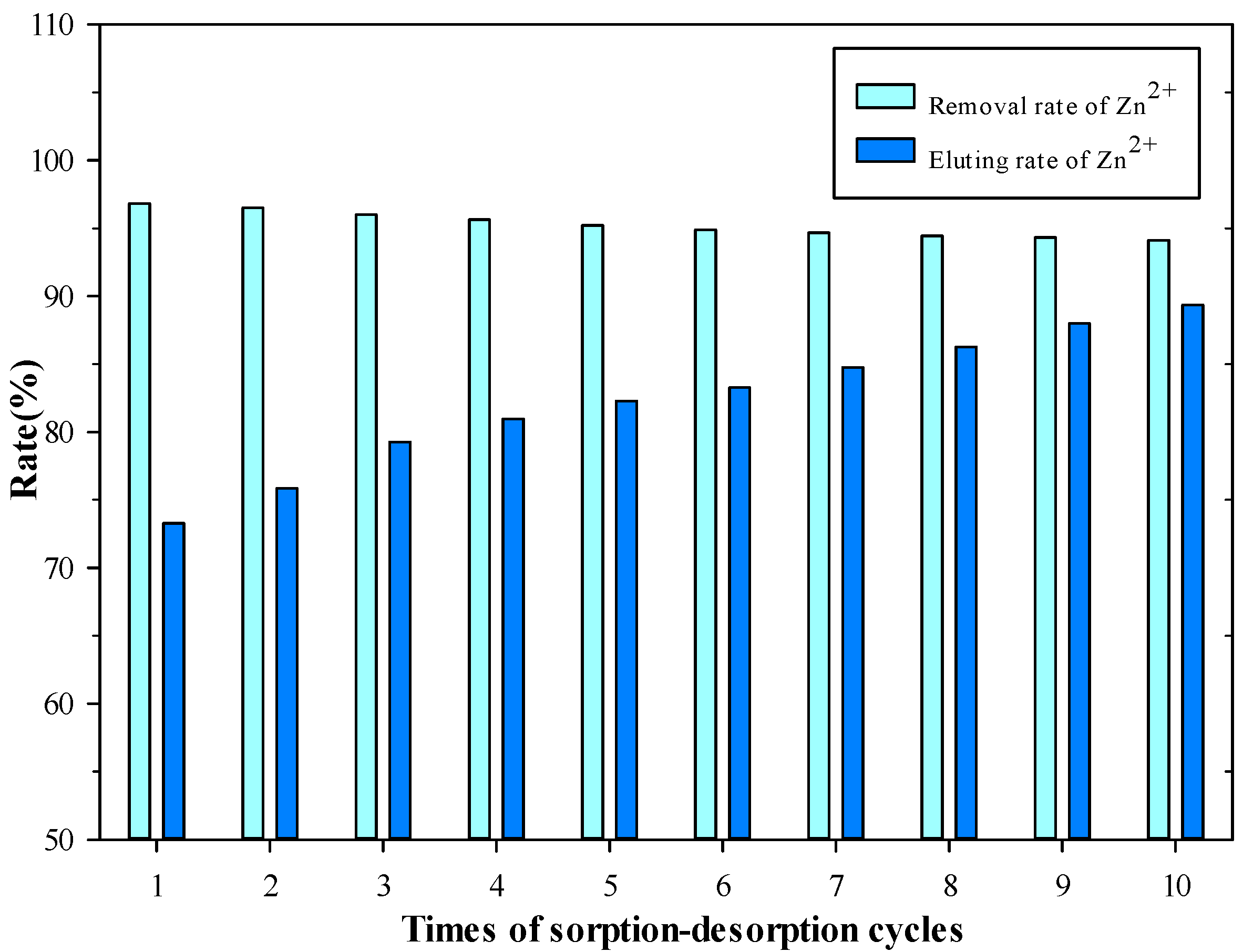
2.6. Results of Sorption–Desorption Cycles
2.7. Experimental Data Analysis
2.7.1. Capacity of Biosorption
2.7.2. Equilibrium Isotherms
2.7.3. Biosorption Kinetics
2.7.4. Biosorption Thermodynamics
3. Discussion of Results
3.1. Effect of A. bisporus Pretreatment
3.2. Effect of Co-Ions on Zn2+ Biosorption by SCAB
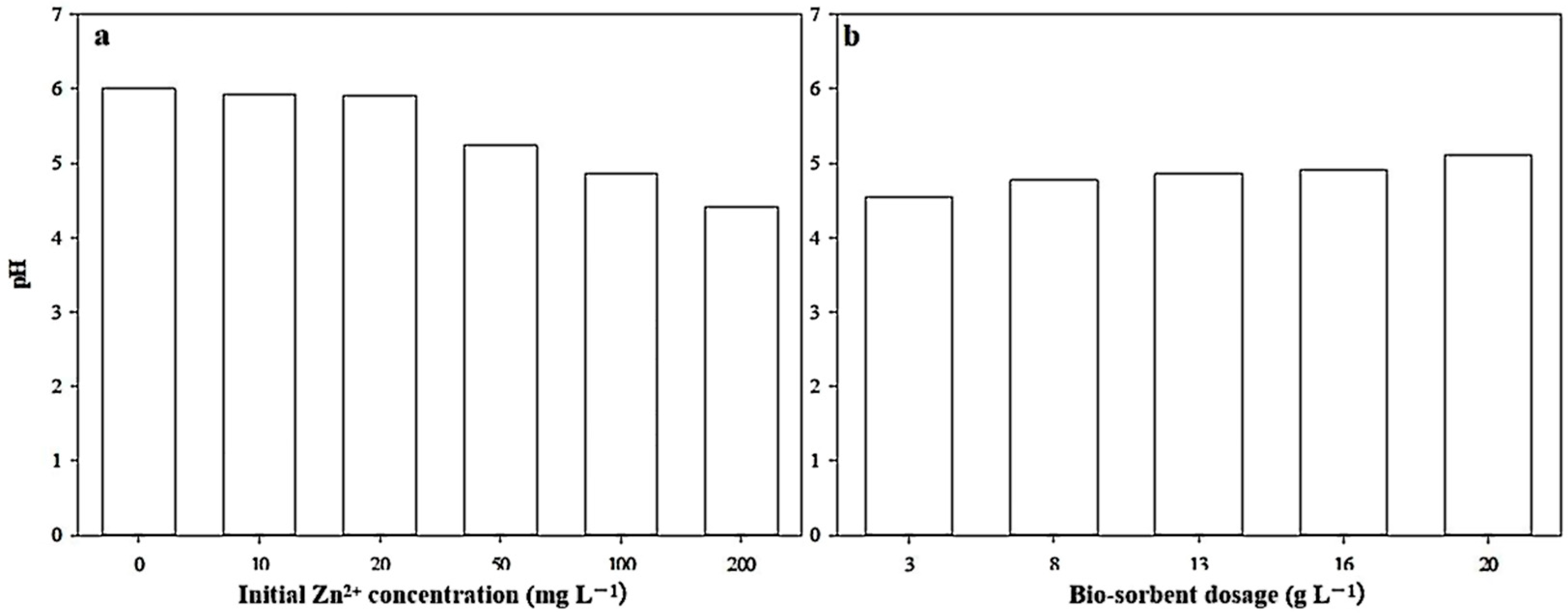
3.3. Effect of Biosorption on Solution pH
3.4. Ion Exchange during Biosorption
- (1)
- During biosorption, a similar exchange occurs between Ca2+ ions and heavy metal ions (represented as M2+), as shown in Equation (13):
- (2)
- The driving force behind the equilibrium on the right side of the equation was the stronger affinity of M2+ for R2−. As demonstrated in Equation (12), when the Ca2+ in the solution exchanged the H+ in the biosorbent, the solution’s pH increased.
- (3)
- During the uptake of Zn2+, ion exchange was essential; however, it appeared that the nonstoichiometric exchange of ions meant that the ion exchange mechanism was not solely responsible for the biosorption of Zn2+ by SCAB.
3.5. Functional Groups in Zn2+ Biosorption
3.6. SCAB Regeneration and Reuse
3.7. Equilibrium Isotherms
3.8. Kinetic Studies
3.9. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elahi, A.; Arooj, I.; Bukhari, D.A.; Rehman, A. Successive use of microorganisms to remove chromium from wastewater. Appl. Microbiol. Biotechnol. 2020, 104, 3729–3743. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2022, 29, 41909–41922. [Google Scholar] [CrossRef] [PubMed]
- Berkani, M.; Smaali, A.; Kadmi, Y.; Almomani, F.; Vasseghian, Y.; Lakhdari, N.; Alyane, M. Photocatalytic degradation of Penicillin G in aqueous solutions: Kinetic, degradation pathway, and microbioassays assessment. Hazard. Mater. 2022, 421, 126719. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gu, S.; Rana, D.; Matsuura, T.; Lan, C.Q. Chemical precipitation enabled UF and MF filtration for lead removal. J. Water Process Eng. 2021, 41, 101987. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Nokman, W.; Benluvankar, V.; Packiam, S.M.; Vincent, S. Screening and molecular identification of heavy metal resistant Pseudomonas putida S4 in tannery effluent wastewater. Biocatal. Agric. Biotechnol. 2019, 18, 101052. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, Y.; Huang, C.; Huang, M.; Chen, J.; Rao, T.; Ran, X. Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide. Hazard. Mater. 2019, 373, 131–140. [Google Scholar] [CrossRef]
- Şentürk, İ.; Yıldız, M.R. Highly efficient removal from aqueous solution by adsorption of Maxilon Red GRL dye using activated pine sawdust. Korean J. Chem. Eng. 2020, 37, 985–999. [Google Scholar] [CrossRef]
- Bano, A.; Hussain, J.; Akbar, A.; Mehmood, K.; Anwar, M.; Hasni, M.S.; Ullah, S.; Sajid, S.; Ali, I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 2018, 199, 218–222. [Google Scholar] [CrossRef]
- Hameed, A.H.A.E.; Eweda, W.E.; Abou-Taleb, K.A.A.; Mira, H.I. Biosorption of uranium and heavy metals using some local fungi isolated from phosphatic fertilizers. Ann. Agr. Sci. 2015, 60, 345–351. [Google Scholar] [CrossRef]
- Choma, A.; Nowak, K.; Komaniecka, I.; Waśko, A.; Pleszczyńska, M.; Siwulski, M.; Wiater, A. Chemical characterization of alkali-soluble polysaccharides isolated from a Boletus edulis (Bull.) fruiting body and their potential for heavy metal biosorption. Food Chem. 2018, 266, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, S.; Balaria, A. Biosorption of Pb2+ by original and protonated citrus peels: Equilibrium, kinetics, and mechanism. Chem. Eng. J. 2009, 146, 211–219. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Al-Zaban, M.I.; Albarakaty, F.M.; Abdelwahab, S.F.; Hassan, S.H.; Fawzy, M.A. Kinetic, isotherm and thermodynamic aspects of Zn2+ biosorption by Spirulina platensis: Optimization of process variables by response surface methodology. Life 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Zinicovscaia, I. Spirulina platensis as a model object for the environment bioremediation studies. In Handbook of Algal Science, Technology and Medicine; Academic Press: Cambridge, MA, USA, 2020; pp. 629–640. [Google Scholar]
- Saravanan, A.; Jeevanantham, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Karishma, S. Enhanced Zn (II) ion adsorption on surface modified mixed biomass-Borassus flabellifer and Aspergillus tamarii: Equilibrium, kinetics and thermodynamics study. Ind. Crop. Prod. 2020, 153, 112613. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Balamuralikrishnan, B.; Durairaj, K.; Vasudhevan, P.; Shivakumar, M.S.; Kaul, T.; Chang, S.W.; Ravindran, B.; Venkatesan, S. Isolation and characterization of heavy-metal-resistant bacteria and their applications in environmental bioremediation. Int. J. Environ. Sci. Technol. 2020, 17, 1455–1462. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Turco, R.F.; Aminikhoei, Z.; Bhatt, K.; Simsek, H. Algae in wastewater treatment, mechanism, and application of biomass for production of value-added product. Environ. Pollut. 2022, 309, 119688. [Google Scholar] [CrossRef] [PubMed]
- Matheickal, J.T.; Yu, Q. Biosorption of lead (II) and copper (II) from aqueous solutions by pre-treated biomass of Australian marine algae. Bioresour. Technol. 1999, 69, 223–229. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, L. Study of a heavy metal biosorption onto raw and chemically modified Sargassum sp. via spectroscopic and modeling analysis. Langmuir 2006, 22, 8906–8914. [Google Scholar] [CrossRef]
- Sudha, B.R.; Abraham, T.E. Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Res. 2002, 36, 1224–1236. [Google Scholar]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef]
- Regine, H.S.; Volesky, F.B. Biosorption: A solution to pollution. Int. Microbiol. 2000, 3, 17–24. [Google Scholar]
- Sarangi, N.V.; Rajkumar, R. Biosorption potential of Stoechospermum marginatum for removal of heavy metals from aqueous solution: Equilibrium, kinetic and thermodynamic study. Chem. Eng. Res. Des. 2024, 203, 207–218. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Mechanism of heavy metal ion biosorption by microalgal cells: A mathematic approach. J. Hazard. Mater. 2024, 463, 132875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Sathya, A.B.; Sivashankar, R.; Kanimozhi, J.; Devika, R.; Balaji, R. Biosorption and different native sources for preparation of biosorbents. Emerg. Contam. 2022, 67, 23–41. [Google Scholar]
- Xu, Y.; Chen, Y. Leaching heavy metals in municipal solid waste incinerator fly ash with chelator/biosurfactant mixed solution. Waste Manag. Res. 2015, 33, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Xiong, Y.; Wang, G.; Zheng, N. Effective solidification/stabilisation of mercury-contaminated wastes using zeolites and chemically bonded phosphate ceramics. Waste Manag. Res. 2015, 33, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Seid, L.; Lakhdari, D.; Berkani, M.; Belgherbi, O.; Chouder, D.; Vasseghian, Y.; Lakhdari, N. High-efficiency electrochemical degradation of phenol in aqueous solutions using Ni-PPy and Cu-PPy composite materials. Hazard. Mater. 2022, 423, 126986. [Google Scholar] [CrossRef]
- Joshi, H.K.; Vishwakarma, M.C.; Kumar, R.; Sharma, H.; Bhandari, N.S.; Joshi, S.K. The biosorption of Zn2+ by various biomasses from wastewater: A review. J. Water Process Eng. 2023, 56, 104389. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Shroff, K.A.; Vaidya, V.K. Kinetics and equilibrium studies on biosorption of nickel from aqueous solution by dead fungal biomass of Mucor hiemalis. Chem. Eng. J. 2011, 171, 1234–1245. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Khan, M.A.; Rehman, F. Utilization of Fennel biomass (Foeniculum vulgari) a medicinal herb for the biosorption of Cd(II) from aqueous phase. Chem. Eng. J. 2010, 156, 106–113. [Google Scholar] [CrossRef]
- Rudy, C.; Milhome, M.A.L.; Cavalcante, R.M.; Silveira, E.R.; Denis, D.K.; Nascimento, R.F. Removal of some polycyclic aromatic hydrocarbons from petrochemical wastewater using low-cost adsorbents of natural origin. Bioresour. Technol. 2008, 99, 4515–4519. [Google Scholar]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Kinetic and pseudo-second-order modeling of lead biosorption onto pine cone powder. Ind. Eng. Chem Res. 2010, 49, 2562–2572. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ofomaja, A.E. Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem. 2005, 40, 3455–3461. [Google Scholar] [CrossRef]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ. Technol. 1996, 17, 71–77. [Google Scholar] [CrossRef]
- Dinesh, M.; Kunwar, P.S. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse-an agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar]
- Jaafari, J.; Yaghmaeian, K. Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) using response surface methodology (RSM). Chemosphere 2019, 217, 447–455. [Google Scholar] [CrossRef]
- Vimala, R.; Das, N. Biosorption of cadmium (II) and lead (II) from aqueous solutions using mushrooms: A comparative study. J. Hazard. Mater. 2009, 168, 376–382. [Google Scholar] [CrossRef]
- Abia, D.; Nzali, S.; Acayanka, E.; Kamgang, G.Y.; Laminsi, S.; Ghogomu, P.M. Synergetic effect of gliding arc discharge treatment and biosorption for removal of nitrophene and glycine from aqueous solution. J. Ind. Eng. Chem. 2015, 29, 156–162. [Google Scholar] [CrossRef]
- Moyo, M.; Guyo, U.; Mawenyiyo, G.; Zinyama, N.P.; Nyamunda, B.C. Marula seed husk (Sclerocarya birrea) biomass as a low cost biosorbent for removal of Pb(II) and Cu(II) from aqueous solution. J. Ind. Eng. Chem. 2015, 27, 126–132. [Google Scholar] [CrossRef]
- Munoz, A.J.; Espínola, F.; Ruiz, E. Removal of Pb(II) in a packed-bed column by a Klebsiella sp. 3S1 biofilm supported on porous ceramic Raschig rings. J. Ind. Eng. Chem. 2016, 40, 118–127. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Ghezelbash, G.R.; Asfaram, A.; Purkait, M.K. Highly efficient simultaneous biosorption of Hg2+, Pb2+ and Cu2+ by live yeast Yarrowia lipolytica 70562 following response surface methodology optimization: Kinetic and isotherm study. J. Ind. Eng. Chem. 2017, 48, 162–172. [Google Scholar]
- Nadeem, R.; Manzoor, Q.; Iqbal, M.; Nisar, J. Biosorption of Pb(II) onto immobilized and native Mangifera indica waste biomass. J. Ind. Eng. Chem. 2016, 35, 185–194. [Google Scholar] [CrossRef]
- Gunasundari, E.; Kumar, P.S. Adsorption isotherm, kinetics and thermodynamic analysis of Cu (II) ions onto the dried algal biomass (Spirulina platensis). J. Ind. Eng. Chem. 2017, 56, 129–144. [Google Scholar]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Esposito, A.; Pagnanelli, F.; Lodi, A.; Solisio, C.; Vegliò, F. Biosorption of heavy metals by Sphaerotilus natans: An equilibrium study at different pH biomass concentrations. Hydrometallurgy 2001, 60, 129–141. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P. Biosorption of heavy metal ions (Pb, Cu, and Cd) from aqueous solutions by the marine alga Sargassum sp. in single- and multiple-metal systems. Ind. Eng. Chem. Res. 2007, 46, 2438–2444. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, Z.G.; Liu, Y.C.H.; Kong, X. Kinetics and equilibrium studies on biosorption of Pb (II) from aqueous solution by a novel biosorbent: Cyclosorus interruptus. J. Ind. Eng. Chem. 2015, 3, 2219–2228. [Google Scholar] [CrossRef]
- Masoumi, F.; Khadivinia, E.; Alidoust, L.; Mánourinejad, Z.; Shahryari, S.; Safaei, M.; Mousavi, A.; Salmanian, A.H.; Zahiri, H.S.; Vali, H.; et al. Nickel and lead biosorption by Curtobacterium sp. FM01, an indigenous bacterium isolated from farmland soils of northeast Iran. J. Ind. Eng. Chem. 2016, 4, 950–957. [Google Scholar] [CrossRef]
- Mohapatra, M.; Rout, K.; Mohapatra, B.K.; Anand, S. Sorption behavior of Pb(II) and Cd(II) on iron ore slime and characterization of metal ion loaded sorbent. Ind. Eng. Chem. Res. 2009, 166, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Wang, L.K.; Yang, L.; Lim, S.F. Emerging biosorption, adsorption, ion exchange, and membrane technologies. In Advanced Physicochemical Treatment Technologies; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Humana Press: Clifton, NJ, USA, 2007; pp. 367–390. [Google Scholar]
- Tran, H.T.; Vu, N.D.; Matsukawa, M.; Okajima, M.; Kaneko, T.; Ohki, K.; Yoshikawa, S. Heavy metal biosorption from aqueous solutions by algae inhabiting rice paddies in Vietnam. J. Environ. Chem. Eng. 2016, 4, 2529–2535. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef]
- Ayhan, Ş.; Mahmut, Ö. Competitive biosorption of Pb2+, Cu2+ and Zn2+ ions from aqueous solutions onto Valonia tannin resin. J. Hazard. Mater. 2009, 166, 1488–1494. [Google Scholar]
- Ertugay, N.; Bayhan, Y.K. The removal of copper (II) ion by using mushroom biomass (Agaricus bisporus) and kinetic modelling. Desalination 2010, 255, 137–142. [Google Scholar] [CrossRef]
- Mahmood, Z.; Zahra, S.; Iqbal, M.; Raza, M.A.; Nasir, S. Comparative study of natural and modified biomass of Sargassum sp. for removal of Cd2+ and Zn2+ from wastewater. Appl. Water Sci. 2017, 7, 3469–3481. [Google Scholar] [CrossRef]
- Tunali, S.; Akar, T. Zn(II) biosorption properties of Botrytis cinerea biomass. J. Hazard. Mater. 2006, 131, 137–145. [Google Scholar] [CrossRef]
- Luna, A.S.; Costa, A.L.H.; Costa, A.C.A.; Henriques, C.A. Competitive biosorption of cadmium(II) and zinc(II) ions from binary systems by Sargassum filipendula. Bioresour. Technol. 2010, 101, 5104–5111. [Google Scholar] [CrossRef]
- Miretzky, P.; Saralegui, A.; Fernandezcirelli, A. Simultaneous heavy metal removal mechanism by dead macrophytes. Chemosphere 2006, 62, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Sağ, Y.; Kaya, A.; Kutsal, T. The simultaneous biosorption of Cu and Zn on Rhizopus arrhizus: Application of the adsorption models. Hydrometallurgy 1998, 50, 297–314. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Dundar, M. Adsorption of chromium(VI) on pomace—An olive oil industry waste: Batch and column studies. J. Hazard. Mater. 2006, 138, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Tewari, N.; Vasudevan, P.; Guha, B. Study on biosorption of Cr(VI) by Mucor hiemalis. Biochem. Eng. J. 2005, 23, 185–192. [Google Scholar] [CrossRef]
- Tunali, S.; Akar, T.; Özcan, A.S.; Kiran, I. Equilibrium and kinetics of biosorption of lead(II) from aqueous solutions by Cephalosporium aphidicola. Sep. Purif. Technol. 2006, 47, 105–112. [Google Scholar] [CrossRef]
- Sprynskyy, M. Solid-liquid-solid extraction of heavy metals (Cr, Cu, Cd, Ni and Pb) in aqueous systems of zeolite–sewage sludge. J. Hazard. Mater. 2009, 161, 1377–1383. [Google Scholar] [CrossRef]
| Isotherm Equations | Parameters | R2 | |
|---|---|---|---|
| Langmuir | Q0 (mg g−1) | b (L mg−1) | 0.950 |
| 19.607 | 0.593 | ||
| Freundlich | Kf (mg g1−(1/n) L1/n) g−1) | 1/n | 0.952 |
| 4.518 | 0.268 | ||
| Dubinin–Radushkevich | qm (mg g−1) | β (mol2 kJ−2) | 0.664 |
| 50.816 | 7.0 × 10−9 | ||
| Temkin | AT (L g−1) | bT | 0.878 |
| 1.467 | 2370.882 | ||
| Biosorbents | Kf | References |
|---|---|---|
| Sargassum sp. | 1.44 | [60] |
| Botrytis cinerea | 1.13 | [61] |
| Sargassum | 0.41 | [62] |
| S. intermedia | 1.03 | [63] |
| L. minor | 1.06 | [64] |
| P. stratiots | 0.25 | [61] |
| Botrytis cinerea | 1.13 | [58] |
| Plain Ca-alginate bead | 0.51 | [65] |
| SCAB | 4.52 | This study |
| C0 (mg L−1) | qe,exp (mg g−1) | First-Order Kinetic Model | Second-Order Kinetic Model | |||||
|---|---|---|---|---|---|---|---|---|
| k1 (1 min−1) | qe,cal (mg g−1) | R2 | k2 (g mg−1 min−1) | qe,cal (mg g−1) | R2 | H (mg g−1 min−1) | ||
| 22 | 1.746 | 0.099 | 0.004 | 0.824 | 65.437 | 1.748 | 0.999 | 200.000 |
| 65 | 4.978 | 0.108 | 0.061 | 0.928 | 4.444 | 5.000 | 0.999 | 111.111 |
| 75 | 6.449 | 0.122 | 0.242 | 0.969 | 1.395 | 6.494 | 0.999 | 58.824 |
| 135 | 9.567 | 0.219 | 2.193 | 0.915 | 0.379 | 9.709 | 0.999 | 35.714 |
| Temperature (K) | KC | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|
| 298 | 29.348 | −8.376 | 5.739 a | 0.047 a |
| 303 | 30.696 | −8.630 | ||
| 313 | 32.837 | −9.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhao, C.; Xue, F.; Xia, B.; Lu, Y.; Ying, R.; Hu, Z. Adsorption of Zinc(II) Ion by Spent and Raw Agaricus bisporus in Aqueous Solution. Processes 2024, 12, 717. https://doi.org/10.3390/pr12040717
Zhang X, Zhao C, Xue F, Xia B, Lu Y, Ying R, Hu Z. Adsorption of Zinc(II) Ion by Spent and Raw Agaricus bisporus in Aqueous Solution. Processes. 2024; 12(4):717. https://doi.org/10.3390/pr12040717
Chicago/Turabian StyleZhang, Xiaoyu, Caiyi Zhao, Feng Xue, Beicheng Xia, Yuanyuan Lu, Rongrong Ying, and Zhewei Hu. 2024. "Adsorption of Zinc(II) Ion by Spent and Raw Agaricus bisporus in Aqueous Solution" Processes 12, no. 4: 717. https://doi.org/10.3390/pr12040717
APA StyleZhang, X., Zhao, C., Xue, F., Xia, B., Lu, Y., Ying, R., & Hu, Z. (2024). Adsorption of Zinc(II) Ion by Spent and Raw Agaricus bisporus in Aqueous Solution. Processes, 12(4), 717. https://doi.org/10.3390/pr12040717





