Assessing Phytoremediation Potential: Dominant Plants in Soils Impacted by Polymetal(loid)lic Mining
Abstract
1. Introduction
2. Experimental
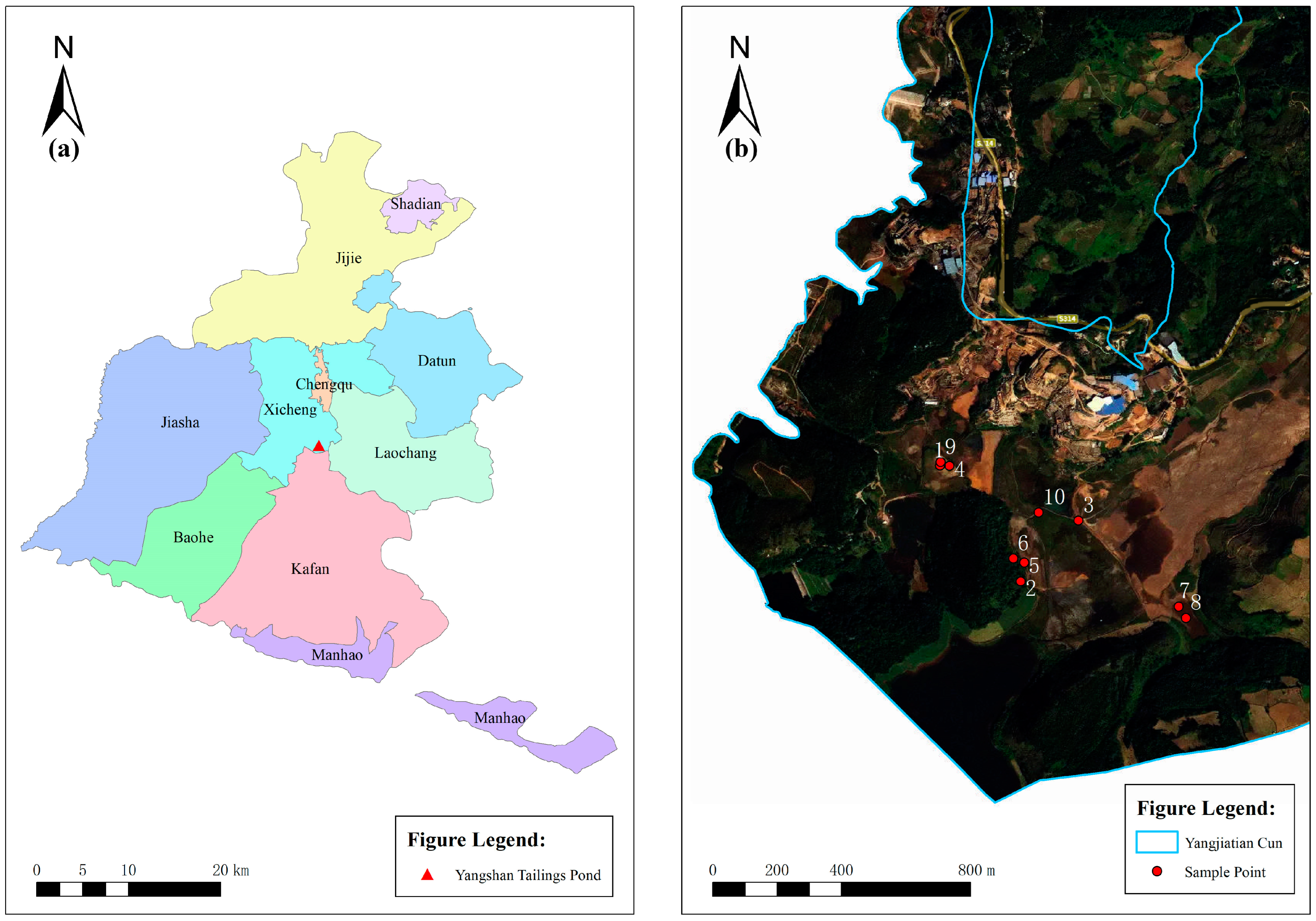
2.1. Sites and Sampling
2.2. Analysis Methods
2.3. Data Analyses
3. Results and Discussion
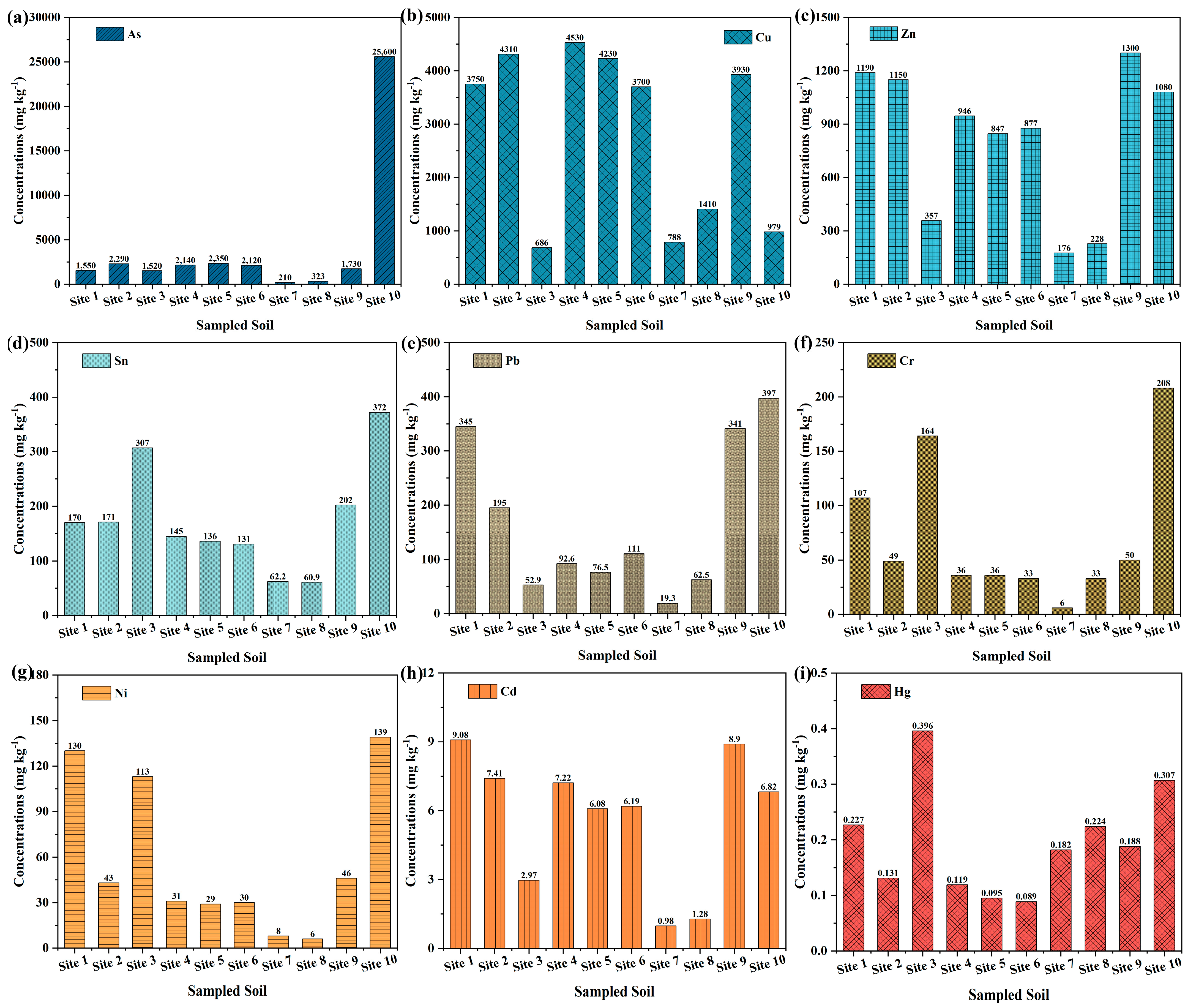
3.1. Total Concentration of Heavy Metal(loid)s in the Soil
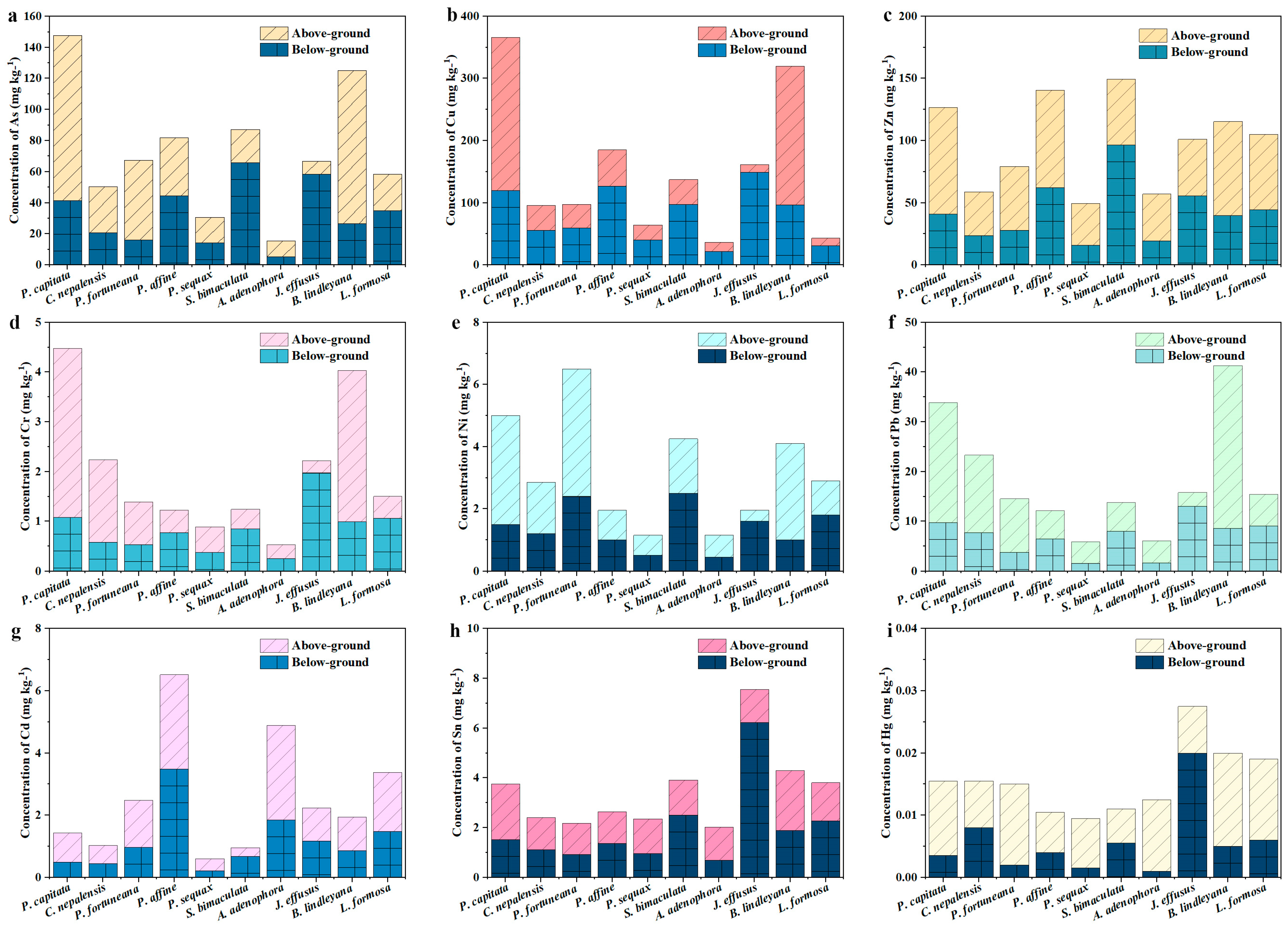
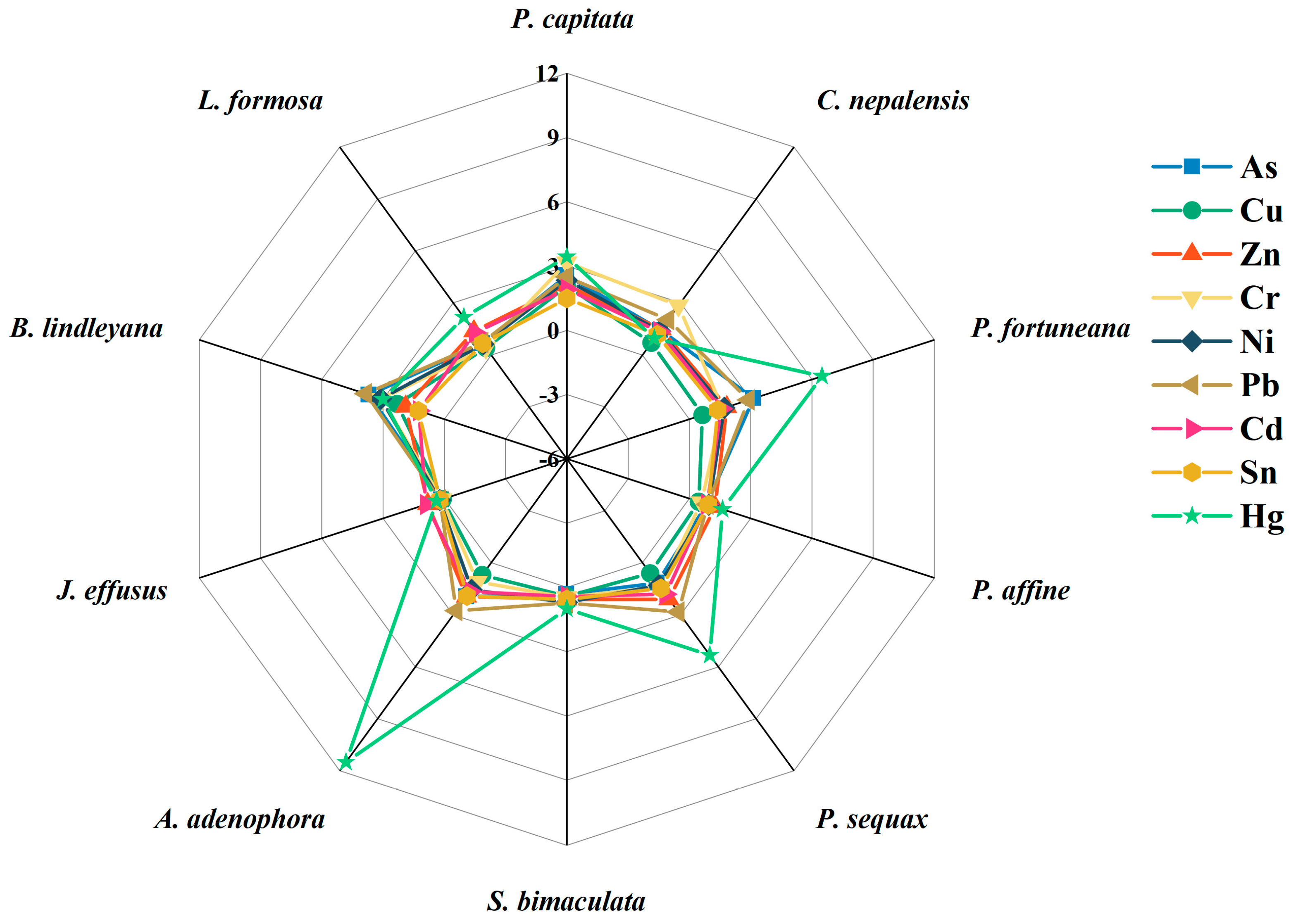
3.2. Concentration of Heavy Metal(loid)s in Plants
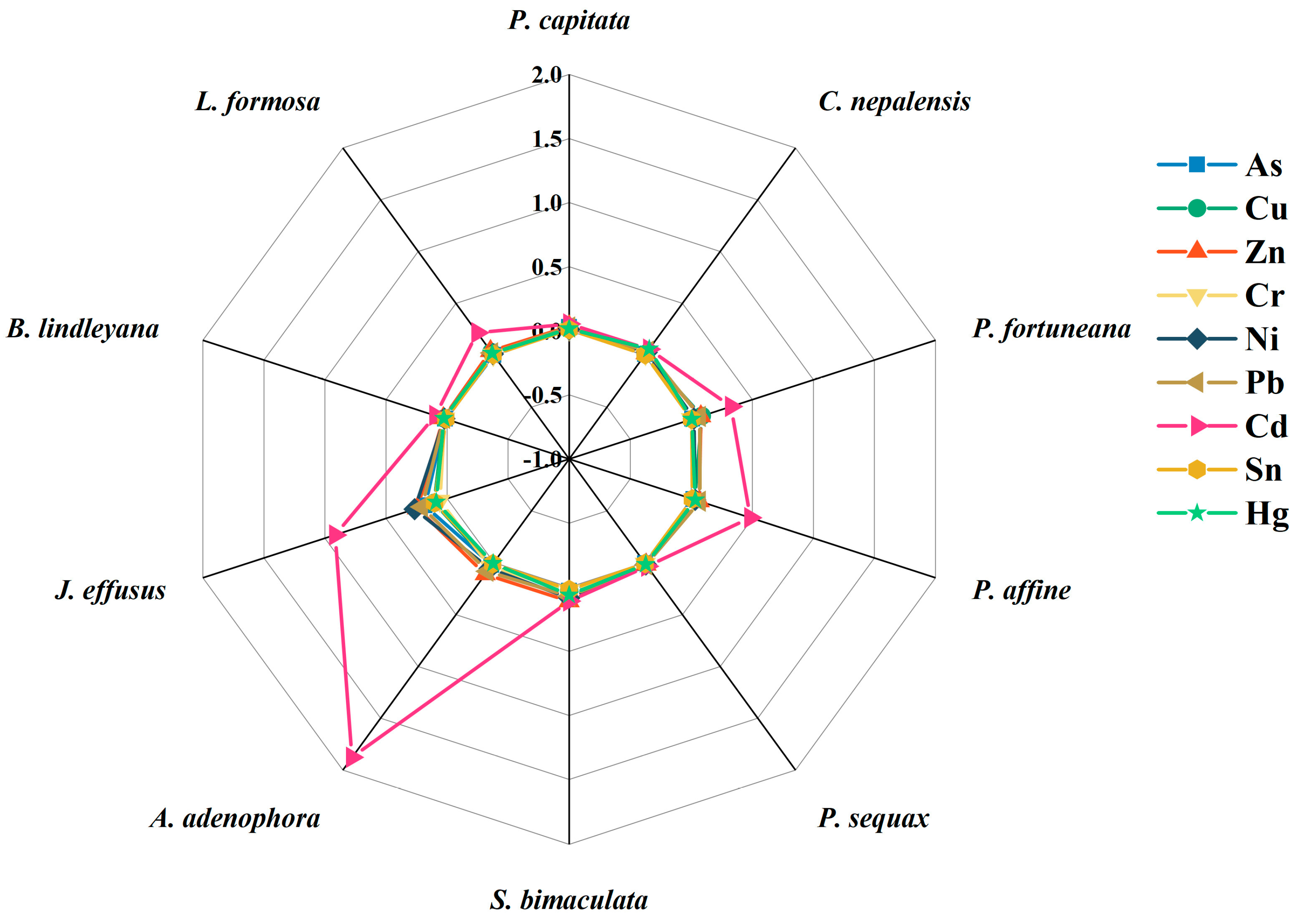
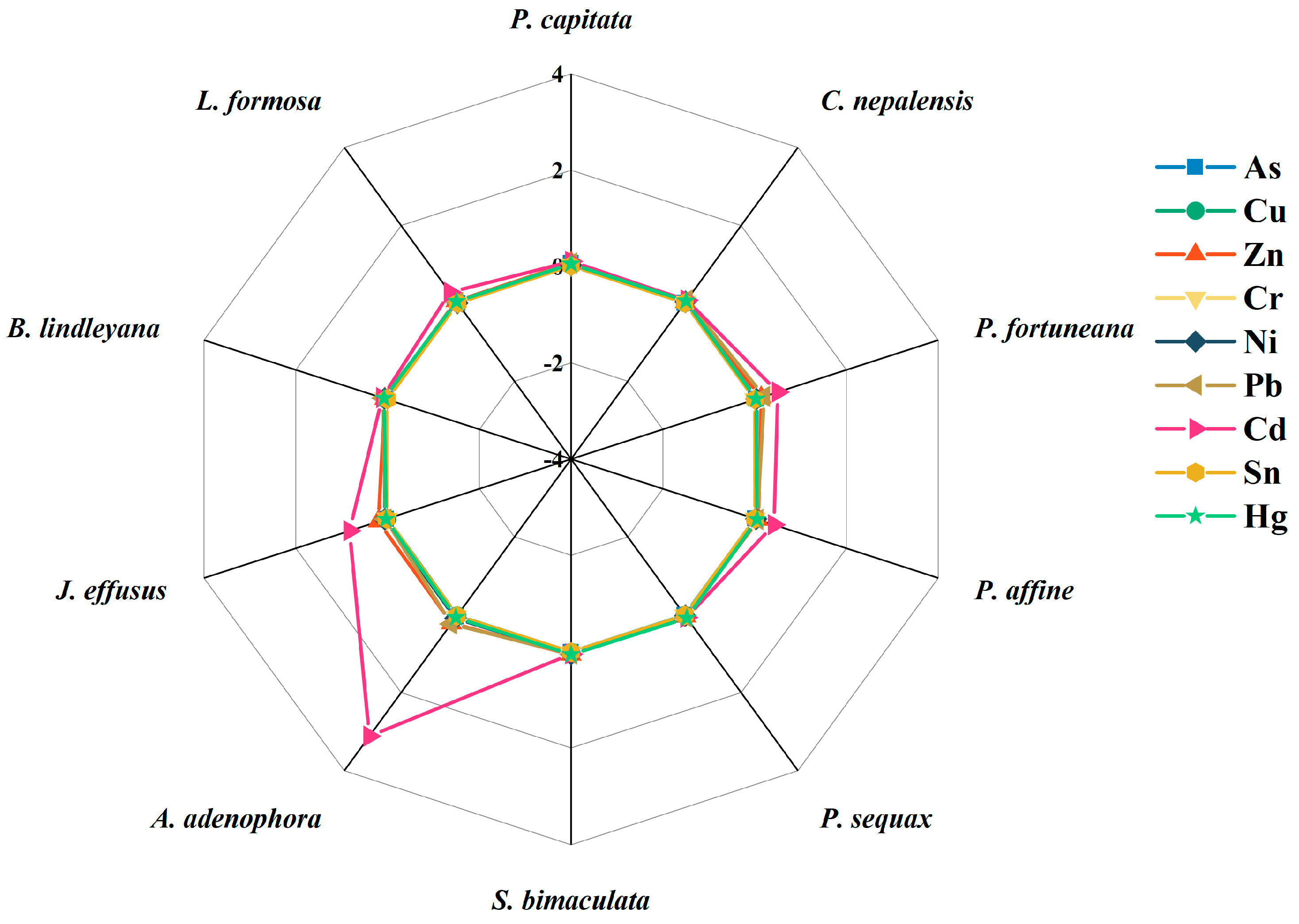
3.3. Bioconcentration and Translocation of Heavy Metal(loid)s in Plants
3.4. Potential Candidates for Phytoremediation
3.5. Prospects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mateos, P.; Alés-Álvarez, F.J.; García-Martín, J.F. Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V. Phytoremediation of Metal-Polluted Ecosystems: Hype for Commercialization. Russ. J. Plant Physiol. 2003, 50, 686–701. [Google Scholar] [CrossRef]
- Mench, M.; Schwitzguebel, J.P.; Schroeder, P.; Bert, V.; Gawronski, S.; Gupta, S. Assessment of successful experiments and limitations of phytotechnologies: Contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ. Sci. Pollut. Res. Int. 2009, 16, 876–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Aghajani Delavar, M. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metal elements. A review of their distribution, ecology, and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Mocek-Plociniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an Effective Remedy for Removing Trace Elements from Ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-t.; Gurajala, H.K.; Wu, L.-h.; Van Der Ent, A.; Qiu, R.L.; Baker, A.J.M.; Tang, Y.-t.; Yang, X.-E.; Shu, W.-S. Hyperaccumulator Plants from China: A Synthesis of the Current State of Knowledge. Environ. Sci. Technol. 2018, 52, 11980–11994. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Jia, Y.; Wu, X.; Wang, S.; Hou, J.; Zhang, W. Phytoremediation potential of indigenous plants growing in soils affected by mine activities in Gejiu City, Yunnan Province. Int. J. Phytoremediat. 2022, 25, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Schachtschneider, K.; Chamier, J.; Somerset, V. Phytostabilization of metals by indigenous riparian vegetation. Water SA 2017, 43, 177–185. [Google Scholar] [CrossRef]
- Zeidler, M.; Šipoš, J.; Banaš, M.; Černohorský, J. The successive trend of vegetation confirms the removal of non-indigenous woody species as an insufficient restoration action. Biodivers. Conserv. 2021, 30, 699–717. [Google Scholar] [CrossRef]
- Albert, K.M. Role of revegetation in restoring fertility of degraded mined soils in Ghana: A review. Int. J. Biodivers. Conserv. 2015, 7, 57–80. [Google Scholar] [CrossRef]
- Yang, X.e.; Long, X.; Ni, W.; Fu, C. Sedum alfredii H: A new Zn hyperaccumulating plant first found in China. Chin. Sci. Bull. 2002, 47, 1634–1637. [Google Scholar] [CrossRef]
- Xu, W.; Du, Q.; Yan, S.; Cao, Y.; Liu, X.; Guan, D.X.; Ma, L.Q. Geographical distribution of As-hyperaccumulator Pteris vittata in China: Environmental factors and climate changes. Sci. Total Environ. 2022, 803, 149864. [Google Scholar] [CrossRef] [PubMed]
- Visoottiviseth, P.; Francesconi, K.; Sridokchan, W. The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ. Pollut. 2002, 118, 453–461. [Google Scholar] [CrossRef]
- Oyuela Leguizamo, M.A.; Fernández Gómez, W.D.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tian, Z.; Zhao, Y.; Wang, X.; Ma, Z.; Yu, C. Exploring the accumulation capacity of dominant plants based on soil heavy metals forms and assessing heavy metals contamination characteristics near gold tailings ponds. J. Environ. Manag. 2024, 351, 119838. [Google Scholar] [CrossRef] [PubMed]
- Bothe, H. Plants in Heavy Metal Soils. In Detoxification of Heavy Metals; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 35–57. [Google Scholar]
- Singh, S.; Karwadiya, J.; Srivastava, S.; Patra, P.K.; Venugopalan, V.P. Potential of indigenous plant species for phytoremediation of arsenic contaminated water and soil. Ecol. Eng. 2022, 175, 106476. [Google Scholar] [CrossRef]
- Odoh, C.K.; Zabbey, N.; Sam, K.; Eze, C.N. Status, progress and challenges of phytoremediation—An African scenario. J. Environ. Manag. 2019, 237, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, H.; Kim, J.-G. Current Status of and Challenges for Phytoremediation as a Sustainable Environmental Management Plan for Abandoned Mine Areas in Korea. Sustainability 2023, 15, 2761. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhou, J.X.; Tan, S.C.; Li, H.M.; Hao, S.; Jiang, Y.G.; He, X.H. Genesis of the oxidized Sn ores in the Gejiu district, Yunnan Province, SW China. Ore Geol. Rev. 2020, 121, 103474. [Google Scholar] [CrossRef]
- Sun, S.; Dong, C.; Zhang, H.; Yang, H.; Zhang, N.; Bao, L. Heavy Metal Risk Assessment of Soils and Crops in the Intensive Mining Area of Gejiu, Yunnan Province. J. Geosci. Environ. Prot. 2022, 10, 128–139. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, C.; Hu, N.; Wang, B.; Zheng, K.; Zhao, Z.; Li, T. Contributions of ectomycorrhizal fungi in a reclaimed poplar forest (Populus yunnanensis) in an abandoned metal mine tailings pond, southwest China. J. Hazard. Mater. 2023, 448, 130962. [Google Scholar] [CrossRef]
- Li, C.; Dong, P.; Yan, J.; Gong, R.; Meng, Q.; Yao, J.; Yu, H.; Ma, Y.; Liu, B.; Xie, R. Analytical study on heavy metal output fluxes and source apportionment of a non-ferrous smelter in southwest China. Environ. Pollut. 2023, 331, 121867. [Google Scholar] [CrossRef]
- Ascar, L.; Ahumada, I.; Richter, P. Influence of redox potential (Eh) on the availability of arsenic species in soils and soils amended with biosolid. Chemosphere 2008, 72, 1548–1552. [Google Scholar] [CrossRef]
- Gil-Cardeza, M.L.; Ferri, A.; Cornejo, P.; Gomez, E. Distribution of chromium species in a Cr-polluted soil: Presence of Cr(III) in glomalin related protein fraction. Sci. Total Environ. 2014, 493, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhang, Y.; Gao, L.; Guo, J.; Xu, L.; Ding, Y. Clustering and Principal Component Analysis for the Heavy Metal Contents of Soil in Yunnan Province. In Proceedings of the 2012 Second International Conference on Electric Technology and Civil Engineering, Bangalore, India, 3–4 August 2012; IEEE Computer Society: Washington, DC, USA, 2012; pp. 657–660. [Google Scholar]
- Nedelkoska, T.V.; Doran, P.M. Characteristics of heavy metal uptake by plant species with potential for phytoremediation and phytomining. Miner. Eng. 2000, 13, 549–561. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, B.; Zhu, L.; Zhou, Z. Evaluation of the metal(loid)s phytoextraction potential of wild plants grown in three antimony mines in southern China. Int. J. Phytoremediat. 2020, 23, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yi, L.; Liu, C. Spatial distribution, chemical fractionation and risk assessment of Cr in soil from a typical industry smelting site in Hunan Province, China. Environ. Geochem. Health 2024, 46, 113. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, D.C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Lu, Y.; Ren, Y.; He, L.; Li, J. Efficient reduction of Cr(VI) by guava (Psidium guajava) leaf extract and its mitigation effect on Cr toxicity in rice seedlings. J. Environ. Sci. 2024, 141, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bouhadi, M.; Lahmidi, A.; Am, A.; Elhajjouji, H.; Elkouali, M.; Talbi, M.; Fougrach, H. Study of the Toxicity and Translocation of Chromium (VI) in Vicia faba Plant. Bull. Environ. Contam. Toxicol. 2024, 112, 40. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, X.; Yin, Z.; Feng, H.; Hu, C.; Guo, N.; Tang, Y.; Qiu, R.; Ma, L.Q.; Cao, Y. As-hyperaccumulator Pteris vittata and non-hyperaccumulator Pteris ensiformis under low As-exposure: Transcriptome analysis and implication for As hyperaccumulation. J. Hazard. Mater. 2023, 458, 132034. [Google Scholar] [CrossRef]
- Han, F.X.; Su, Y.; Sridhar, B.B.M.; Monts, D.L. Distribution, transformation and bioavailability of trivalent and hexavalent chromium in contaminated soil. Plant Soil. 2004, 265, 243–252. [Google Scholar] [CrossRef]
- Soubasakou, G.; Cavoura, O.; Damikouka, I. Phytoremediation of Cadmium-Contaminated Soils: A Review of New Cadmium Hyperaccumulators and Factors Affecting their Efficiency. Bull. Environ. Contam. Toxicol. 2022, 109, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Qin, Y. Cadmium phytoremediation potential of Gnaphalium affine D. Don. and physiological response in metal tolerance. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 042029. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-accumulation Metals in Plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Wang, Y.; Hao, W.; Zhou, Q.; Liu, J. Growth Responses and Accumulation Characteristics of Three Ornamental Plants to Sn Contamination in Soil. Agriculture 2021, 11, 205. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Assessment of phytoextraction efficiency of naturally grown plant species at the former tin mining catchment. Fresenius Environ. Bull. 2012, 21, 523–533. [Google Scholar]
- Ladislas, S.; Gérente, C.; Chazarenc, F.; Brisson, J.; Andrès, Y. Performances of Two Macrophytes Species in Floating Treatment Wetlands for Cadmium, Nickel, and Zinc Removal from Urban Stormwater Runoff. Water Air Soil Pollut. 2013, 224, 1408. [Google Scholar] [CrossRef]
- Singh, S.; Saha, L.; Kumar, M.; Bauddh, K. Chapter 12—Phytoremediation potential of invasive species growing in mining dumpsite. In Phytorestoration of Abandoned Mining and Oil Drilling Sites; Bauddh, K., Korstad, J., Sharma, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 287–305. [Google Scholar]
- Dai, Z.C.; Cai, H.H.; Qi, S.S.; Li, J.; Zhai, D.L.; Wan, J.S.H.; Du, D.L. Cadmium hyperaccumulation as an inexpensive metal armor against disease in Crofton weed. Environ. Pollut. 2020, 267, 115649. [Google Scholar] [CrossRef]
- Khan, I.U.; Qi, S.-S.; Gul, F.; Manan, S.; Rono, J.K.; Naz, M.; Shi, X.-N.; Zhang, H.; Dai, Z.-C.; Du, D.-L. A Green Approach Used for Heavy Metals ‘Phytoremediation’ Via Invasive Plant Species to Mitigate Environmental Pollution: A Review. Plants 2023, 12, 725. [Google Scholar] [CrossRef]
- Agostini, E.; Talano, M.A.; González, P.S.; Oller, A.L.W.; Medina, M.I. Application of hairy roots for phytoremediation: What makes them an interesting tool for this purpose? Appl. Microbiol. Biotechnol. 2013, 97, 1017–1030. [Google Scholar] [CrossRef]
| Pictures of Plants | Sites | Plant Name | Family | Location (Longitude, Latitude, and Altitude) | Average Size of Plants (cm) | |
|---|---|---|---|---|---|---|
| Above-Ground | Underground | |||||
 | 1 | Ageratina adenophora (Spreng.) R. M. King & H. Rob. | Asteraceae | 103°9′ E, 23°18′ N 1.90 × 103 m | 78.5 | 31.5 |
 | 2 | Buddleja lindleyana Fortune | Scrophulariaceae | 103°8′ E, 23°18′ N 1.91 × 103 m | 87.0 | 45.0 |
 | 3 | Coriaria nepalensis Wall. (Morus calva Coriaria kweichowensis Coriaria sinica) | Coriariaceae | 103°8′ E, 23°18′ N 1.92 × 103 m | 40.5 | 22.0 |
 | 4 | Juncus effusus L. | Juncaceae | 103°9′ E, 23°18′ N 1.91 × 103 m | 60.0 | 27.0 |
 | 5 | Leycesteria formosa Wall. | Caprifoliaceae | 103°8′ E, 23°18′ N 1.90 × 103 m | 101 | 30.0 |
 | 6 | Puhuaea sequax (Wall.) H. Ohashi & K. Ohashi | Fabaceae | 103°8′ E, 23°18′ N 1.92 × 103 m | 65.0 | 80.5 |
 | 7 | Pseudognaphalium affine (D. Don) Anderb. | Asteraceae | 103°8′ E, 23°18′ N 1.92 × 103 m | 35.0 | 13.5 |
 | 8 | Pyracantha fortuneana (Maxim.) H. L. Li | Rosaceae | 103°8′ E, 23°18′ N 1.91 × 103 m | 45.0 | 24.0 |
 | 9 | Persicaria capitata (Buch.-Ham. ex D. Don) H. Gross | Polygonaceae | 103° 8′ E, 23°18′ N 1.91 × 103 m | 34.5 | 51.0 |
 | 10 | Swertia bimaculata (Siebold & Zucc.) Hook. f. & Thomson ex C. B. Clarke | Gentianaceae | 103°8′ E, 23°18′ N 1.92 × 103 m | 36.0 | 12.0 |
| Heavy Metal(loid)s | Soil Sample Pre-Treatment and Analysis Method | Plant Sample Pre-Treatment and Analysis Method |
|---|---|---|
| Cu | HJ 491-2019 | GB 5009.268-2016 |
| Cr | HJ 491-2019 | GB 5009.268-2016 |
| Ni | HJ 491-2019 | GB 5009.268-2016 |
| Zn | HJ 491-2019 | GB 5009.268-2016 |
| Pb | GB/T 17141-1997 | GB 5009.268-2016 |
| Cd | GB/T 17141-1997 | GB 5009.268-2016 |
| As | GB/T 22105.2-2008 | GB 5009.268-2016 |
| Sn | USEPA 6020B-2014 | GB 5009.268-2016 |
| Hg | GB/T 22105.1-2008 | GB 5009.17-2021 |
| As | Cu | Zn | Sn | Pb | Cr | Ni | Cd | Hg | |
|---|---|---|---|---|---|---|---|---|---|
| Min. (mg kg−1) | 2.56 × 103 | 686 | 176 | 60.9 | 19.3 | 6.00 | 6.00 | 0.980 | 0.089 |
| Max. (mg kg−1) | 210 | 4.53 × 103 | 1300 | 372 | 397 | 208 | 139 | 9.08 | 0.396 |
| Average (mg kg−1) | 3.98 × 103 | 2.83 × 103 | 815 | 176 | 169 | 72.2 | 57.5 | 5.69 | 0.200 |
| Coefficient of variation | 182% | 54.8% | 48.2% | 53.1% | 78.9% | 86.7% | 82.8% | 49.0% | 47.4% |
| Average contents in Yunnan Province, China [34] | 11.0 | 34.0 | 77.7 | / | 37.9 | 68.1 | 36.2 | 0.120 | 0.0400 |
| Soil environmental quality–risk control standard for soil contamination of agricultural land (GB 15618-2018) * | 20.0 | 200 | 300 | / | 240 | 350 | 190 | 20.0 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Hou, J.; Wu, X.; Niu, X.; Zhou, F. Assessing Phytoremediation Potential: Dominant Plants in Soils Impacted by Polymetal(loid)lic Mining. Processes 2024, 12, 833. https://doi.org/10.3390/pr12040833
Wang B, Hou J, Wu X, Niu X, Zhou F. Assessing Phytoremediation Potential: Dominant Plants in Soils Impacted by Polymetal(loid)lic Mining. Processes. 2024; 12(4):833. https://doi.org/10.3390/pr12040833
Chicago/Turabian StyleWang, Boxin, Juan Hou, Xueyong Wu, Xuekui Niu, and Fengping Zhou. 2024. "Assessing Phytoremediation Potential: Dominant Plants in Soils Impacted by Polymetal(loid)lic Mining" Processes 12, no. 4: 833. https://doi.org/10.3390/pr12040833
APA StyleWang, B., Hou, J., Wu, X., Niu, X., & Zhou, F. (2024). Assessing Phytoremediation Potential: Dominant Plants in Soils Impacted by Polymetal(loid)lic Mining. Processes, 12(4), 833. https://doi.org/10.3390/pr12040833





