Comparing the Aging Processes of PLA and PE: The Impact of UV Irradiation and Water
Abstract
1. Introduction
2. Materials and Methods
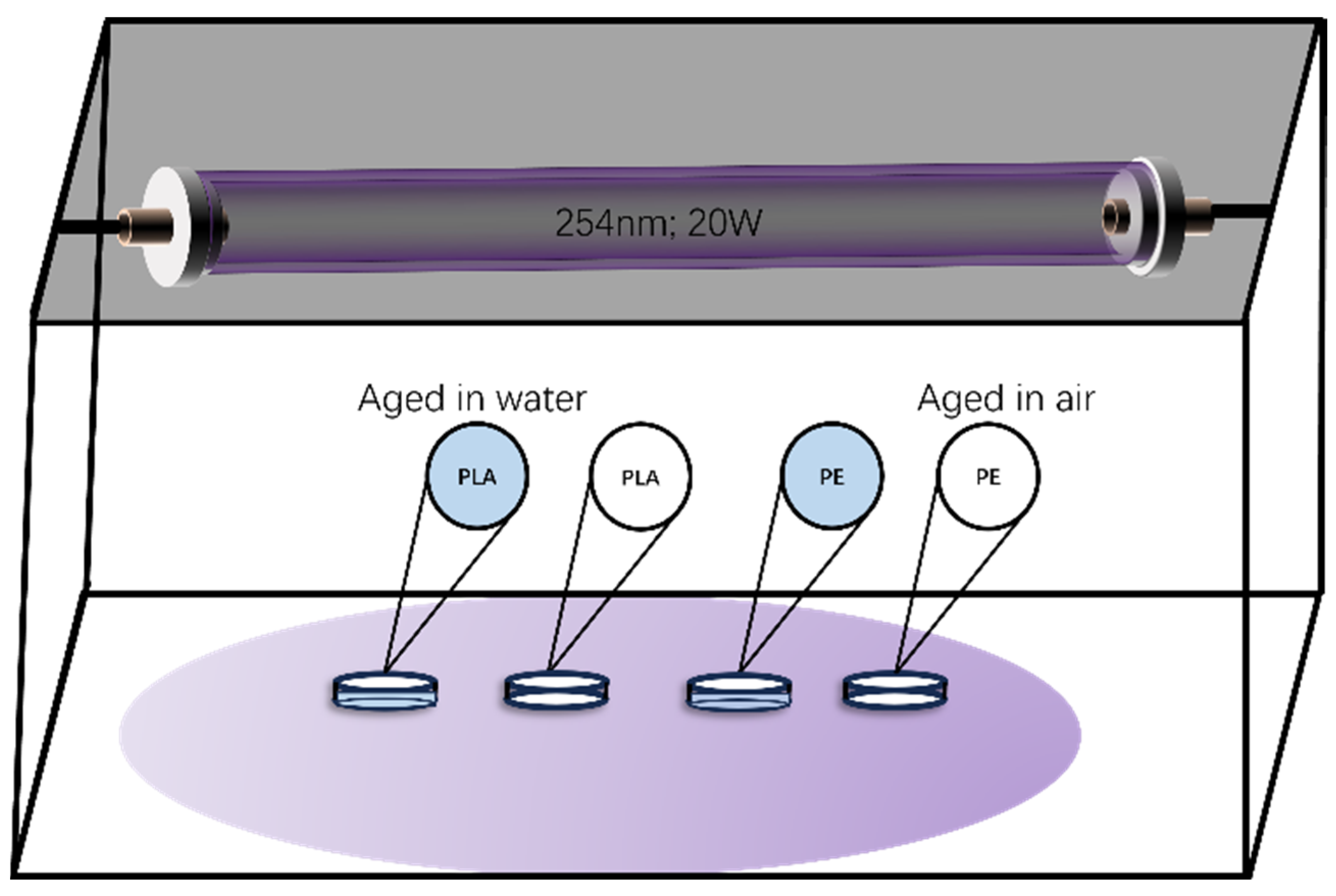
2.1. Accelerated Laboratory Aging of Plastic Particles
2.2. Characterization of PE and PLA MPs, before and after Aging
2.3. Data Analysis
3. Results and Discussion
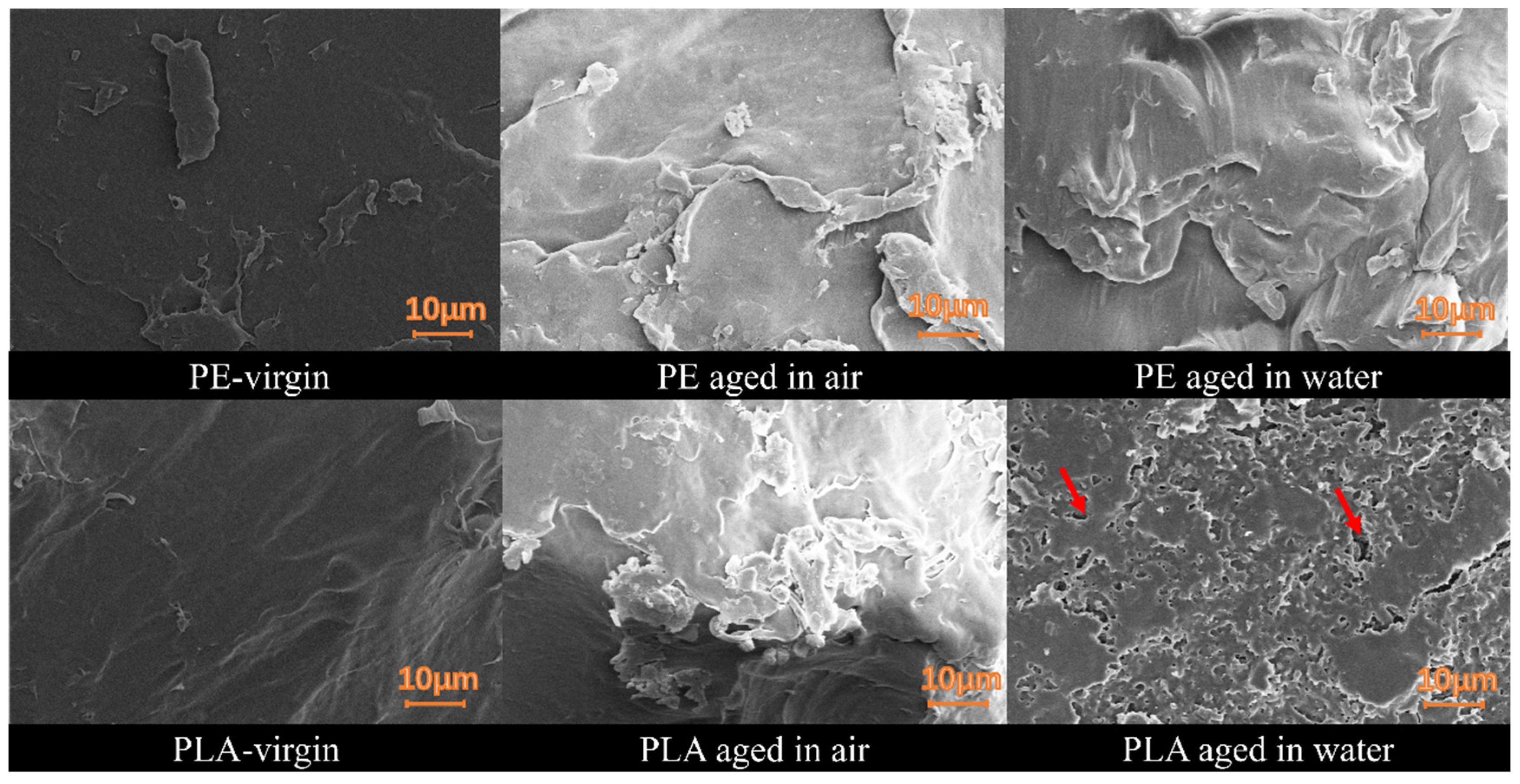
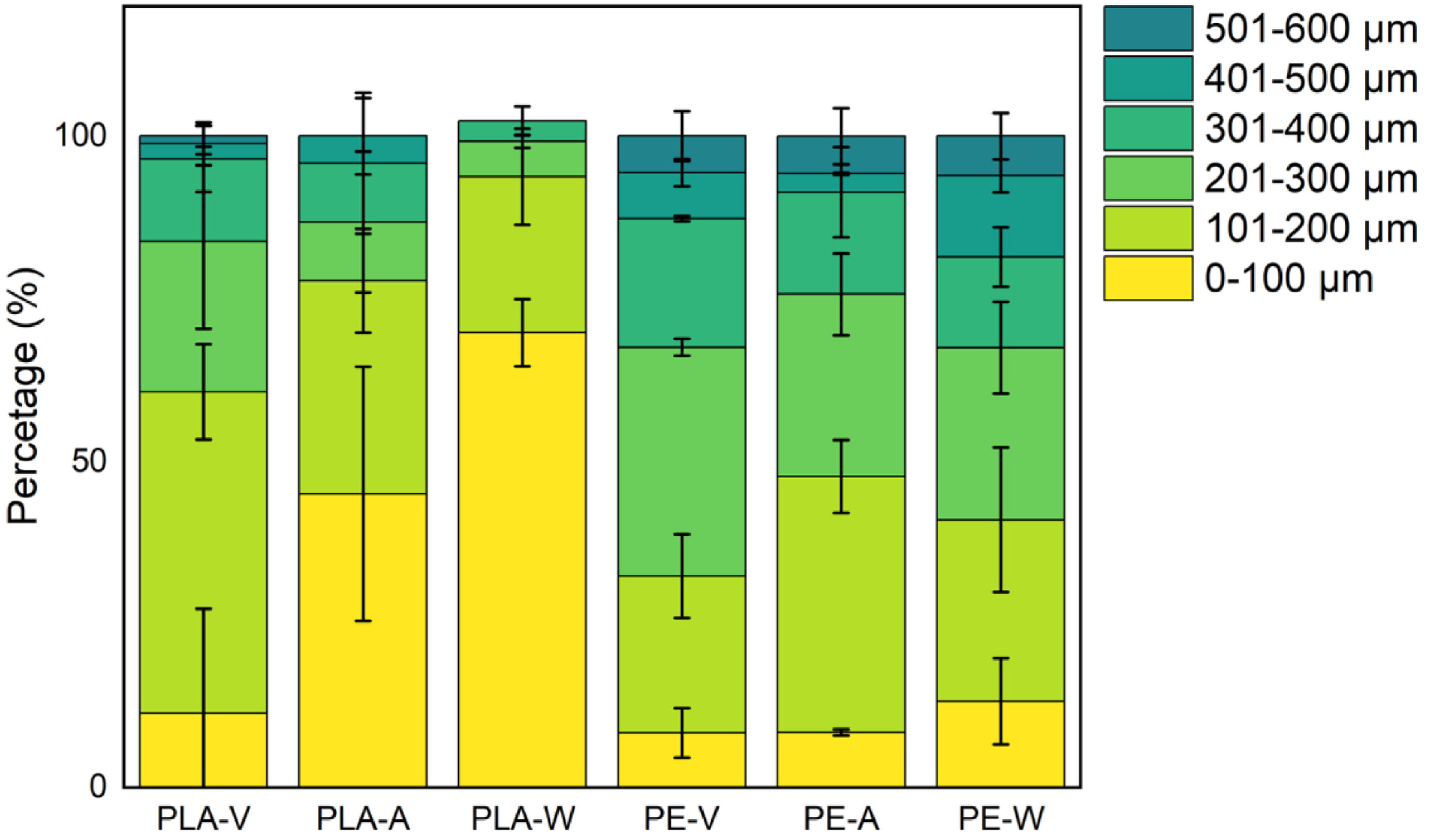
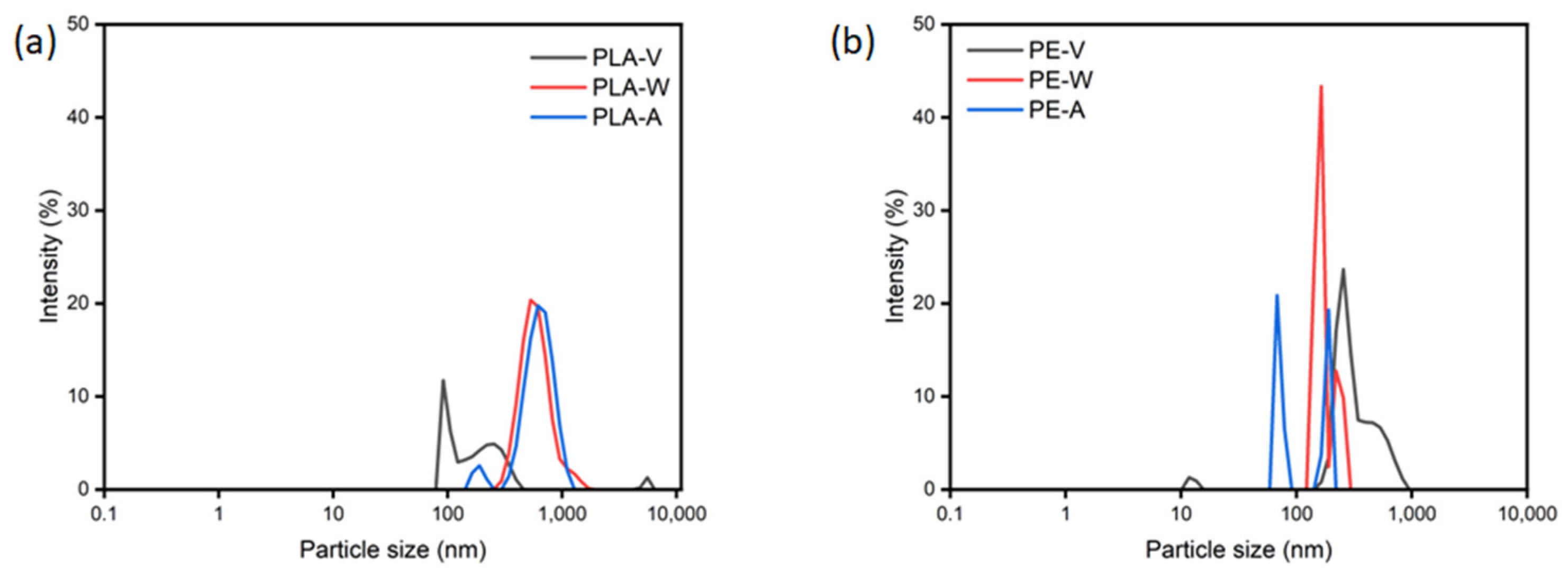
3.1. UV Aging Altered the Morphological Characteristics of the PE and PLA MPs Particles
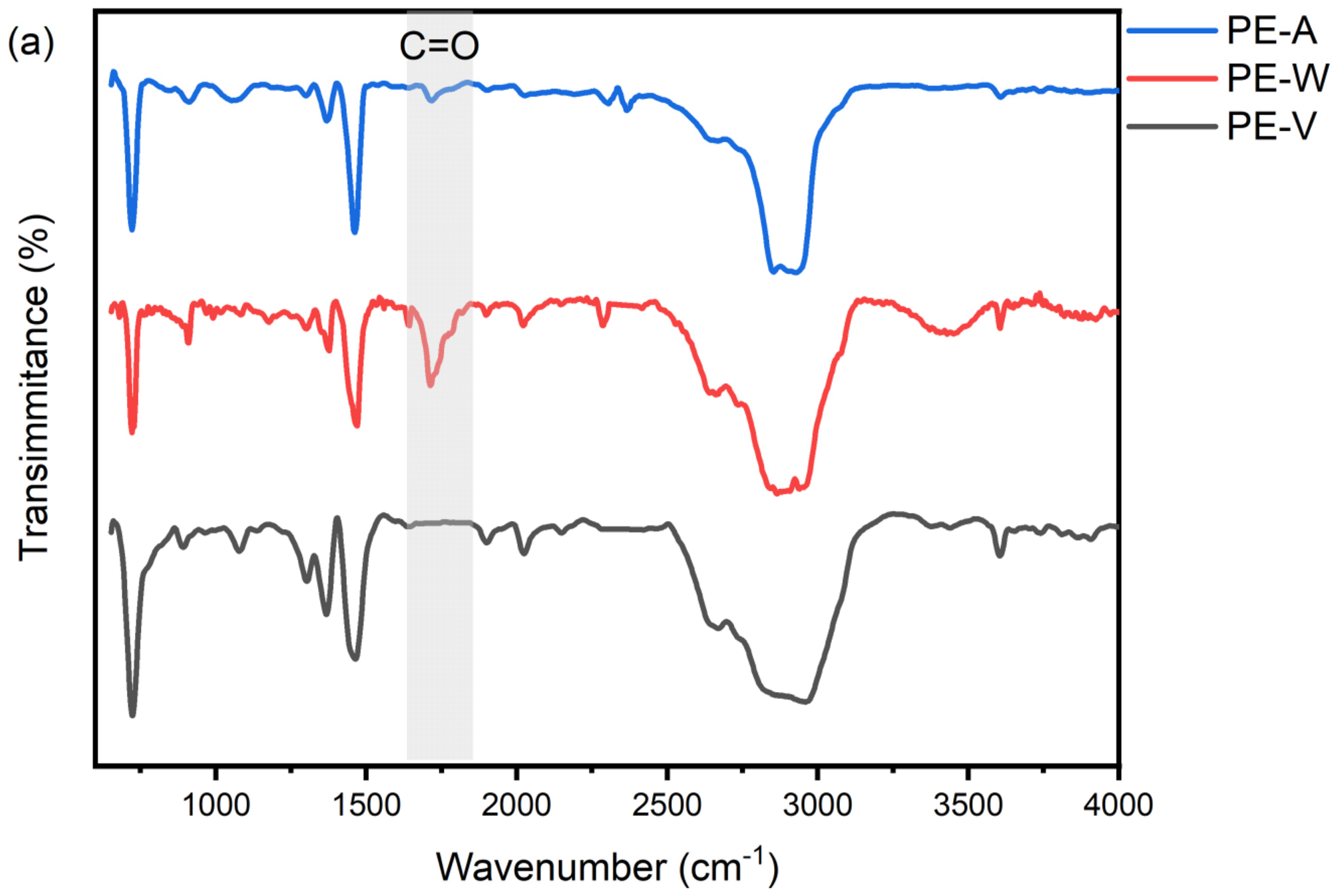
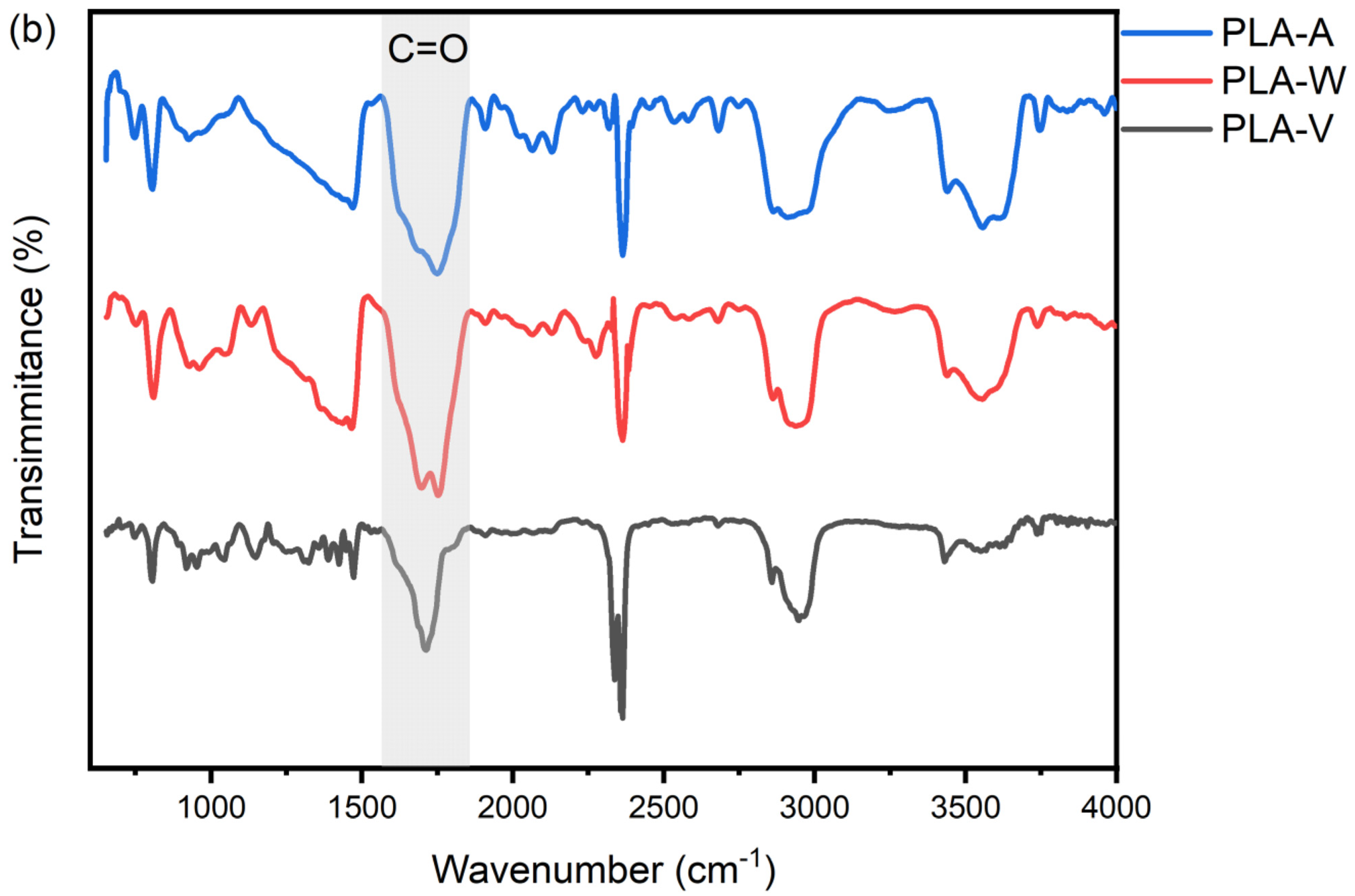
3.2. UV Aging Altered the Chemical Functional Groups of PE and PLA MPs Particles
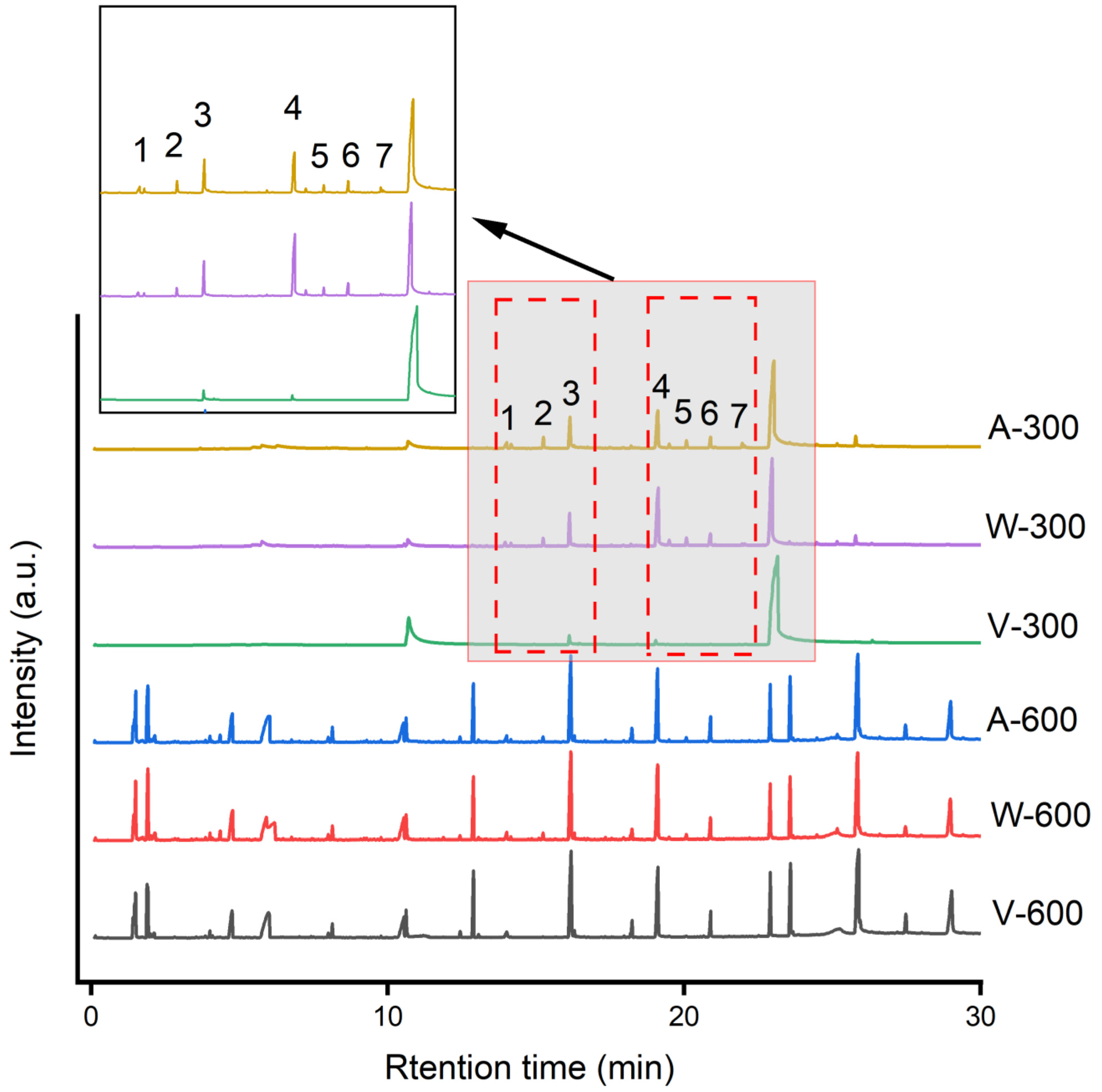
3.3. Characterization of MP Eluate after Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Zhao, X.; Wu, X.; Wang, J.; Wang, X.; Liang, W. Research progress on occurrence characteristics and source analysis of microfibers in the marine environment. Mar. Pollut. Bull. 2024, 198, 115834. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liang, J.; Zhu, M.; Zheng, S.; Zhao, Y.; Sun, X. Occurrence, characteristics, and factors influencing the atmospheric microplastics around Jiaozhou Bay, the Yellow Sea. Mar. Pollut. Bull. 2023, 196, 115568. [Google Scholar] [CrossRef]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Fok, L.; Yan, Y.; Li, H.X.; Xu, X.R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Oh, M.J.; Kim, P.G.; Kim, G.; Jeong, D.H.; Ju, B.K.; Lee, W.S.; Chung, H.M.; Kang, H.J.; Kwon, J.H. National Reconnaissance Survey of Microplastics in Municipal Wastewater Treatment Plants in Korea. Environ. Sci. Technol. 2020, 54, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806 Pt 3, 151312. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, N.; Qu, R.; Ge, F. High salinity promotes the photoaging of polystyrene microplastics with humic acid in seawater. Sci. Total Environ. 2023, 901, 165741. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhang, M.; Ding, L.; Qiu, X.; Guo, X. New sight of microplastics aging: Reducing agents promote rapid aging of microplastics under anoxic conditions. J. Hazard. Mater. 2023, 451, 131123. [Google Scholar] [CrossRef] [PubMed]
- Fueser, H.; Mueller, M.T.; Weiss, L.; Hoss, S.; Traunspurger, W. Ingestion of microplastics by nematodes depends on feeding strategy and buccal cavity size. Environ. Pollut. 2019, 255 Pt 2, 113227. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Asgari Lajayer, B.; Biglari Quchan Atigh, Z.; Nayeri, S.; Ahmadabadi, M.; Taghipour, L.; Senapathi, V.; Astatkie, T.; Price, G.W. Micro (nano) plastics uptake, toxicity and detoxification in plants: Challenges and prospects. Ecotoxicol. Environ. Saf. 2023, 268, 115676. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.-L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, Y.; Sui, Q.; Zhou, Y. Mechanism and characterization of microplastic aging process: A review. Front. Environ. Sci. Eng. 2023, 17, 100. [Google Scholar] [CrossRef]
- Liu, S.; Huang, W.; Yang, J.; Xiong, Y.; Huang, Z.; Wang, J.; Cai, T.; Dang, Z.; Yang, C. Formation of environmentally persistent free radicals on microplastics under UV irradiations. J. Hazard. Mater. 2023, 453, 131277. [Google Scholar] [CrossRef]
- Jiang, X.; Gallager, S.; Pedrosa Pàmies, R.; Ruff, S.E.; Liu, Z. Laboratory-Simulated Photoirradiation Reveals Strong Resistance of Primary Macroplastics to Weathering. Work. Pap. 2023; ahead of print. [Google Scholar] [CrossRef]
- Zhong, Y.; Ding, Q.; Huang, Z.; Xiao, X.; Han, X.; Su, Y.; Wang, D.; You, J. Influence of ultraviolet-aging and adsorbed pollutants on toxicological effects of polyvinyl chloride microplastics to zebrafish. Environ. Pollut. 2023, 316 Pt 2, 120617. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hernandez, J.C.; Capowiez, Y.; Ro, K.S. Potential Use of Earthworms to Enhance Decaying of Biodegradable Plastics. ACS Sustain. Chem. Eng. 2020, 8, 4292–4316. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Q.; Jiang, W.; Wang, L.; Xie, H.; Kalogerakis, N.; Ma, Y.; Ji, R. A carbon-14 radiotracer-based study on the phototransformation of polystyrene nanoplastics in water versus in air. Environ. Sci. Nano 2019, 6, 2907–2917. [Google Scholar] [CrossRef]
- De Jong, S.; Arias, E.R.; Rijkers, D.; Van Nostrum, C.; Kettenes-Van den Bosch, J.; Hennink, W. New insights into the hydrolytic degradation of poly (lactic acid): Participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Ohkita, T.; Lee, S.-H. Thermal degradation and biodegradability of poly (lactic acid)/corn starch biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, M.; Han, P.; Liang, G.; Zhang, T.; Liu, G. Comparative analysis on the sorption kinetics and isotherms of fipronil on nondegradable and biodegradable microplastics. Environ. Pollut. 2019, 254, 112927. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dombrowski, A.; Volker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Chang, Q.; Zhu, D.; Hu, L.; Kim, H.; Liu, Y.; Cai, L. Rapid photo aging of commercial conventional and biodegradable plastic bags. Sci. Total Environ. 2022, 822, 153235. [Google Scholar] [CrossRef]
- Porfyris, A.; Vasilakos, S.; Zotiadis, C.; Papaspyrides, C.; Moser, K.; Van der Schueren, L.; Buyle, G.; Pavlidou, S.; Vouyiouka, S. Accelerated ageing and hydrolytic stabilization of poly(lactic acid) (PLA) under humidity and temperature conditioning. Polym. Test. 2018, 68, 315–332. [Google Scholar] [CrossRef]
- Hoseini, M.; Stead, J.; Bond, T. Ranking the accelerated weathering of plastic polymers. Environ. Sci. Process Impacts 2023, 25, 2081–2091. [Google Scholar] [CrossRef]
- Venkataravanappa, R.Y.; Lakshmikanthan, A.; Kapilan, N.; Chandrashekarappa, M.P.G.; Der, O.; Ercetin, A. Physico-Mechanical Property Evaluation and Morphology Study of Moisture-Treated Hemp–Banana Natural-Fiber-Reinforced Green Composites. J. Compos. Sci. 2023, 7, 266. [Google Scholar] [CrossRef]
- Abaroa-Perez, B.; Ortiz-Montosa, S.; Hernandez-Brito, J.J.; Vega-Moreno, D. Yellowing, Weathering and Degradation of Marine Pellets and Their Influence on the Adsorption of Chemical Pollutants. Polymers 2022, 14, 1305. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, Z.; Kong, F.; Ma, X. The effect of metal catalyst on the discoloration of poly(ethylene terephthalate) in thermo-oxidative degradation. Polym. Degrad. Stab. 2010, 95, 53–58. [Google Scholar] [CrossRef]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Castelvetro, V.; Modugno, F. Seeping plastics: Potentially harmful molecular fragments leaching out from microplastics during accelerated ageing in seawater. Water Res. 2022, 219, 118521. [Google Scholar] [CrossRef]
- Su, J.; Ruan, J.; Luo, D.; Wang, J.; Huang, Z.; Yang, X.; Zhang, Y.; Zeng, Q.; Li, Y.; Huang, W.; et al. Differential Photoaging Effects on Colored Nanoplastics in Aquatic Environments: Physicochemical Properties and Aggregation Kinetics. Environ. Sci. Technol. 2023, 57, 15656–15666. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef]
- Isadounene, S.; Hammiche, D.; Boukerrou, A.; Rodrigue, D.; Djidjelli, H. Accelerated Ageing of Alkali Treated Olive Husk Flour Reinforced Polylactic Acid (PLA) Biocomposites Physico-Mechanical Properties. Polym. Polym. Compos. 2018, 26, 223–232. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, T.; Zhang, P. Fate and environmental behaviors of microplastics through the lens of free radical. J. Hazard. Mater. 2023, 453, 131401. [Google Scholar] [CrossRef]
- Joppien, M.; Westphal, H.; Stuhr, M.; Doo, S.S. Microplastics alter feeding strategies of a coral reef organism. Limnol. Oceanogr. Lett. 2022, 7, 131–139. [Google Scholar] [CrossRef]
- Jin, F.; Wei, M.; Liu, C.; Ma, Y. The mechanism for the formation of OH radicals in condensed-phase water under ultraviolet irradiation. Phys. Chem. Chem. Phys. 2017, 19, 21453–21460. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Reis, V.; Paco, A.; Martins, P.; Cruz, A.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Effects of spatial and seasonal factors on the characteristics and carbonyl index of (micro)plastics in a sandy beach in Aveiro, Portugal. Sci. Total Environ. 2020, 709, 135892. [Google Scholar] [CrossRef] [PubMed]
- Campanaro, A.L.; Simcik, M.F.; Maurer-Jones, M.A.; Penn, R.L. Sewage sludge induces changes in the surface chemistry and crystallinity of polylactic acid and polyethylene films. Sci. Total Environ. 2023, 890, 164313. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Orjuela, A.; Yanez, A.J.; Evans, J.; Hassan, A.M.; Miller, D.J.; Lira, C.T. Phase equilibria in binary mixtures with monoethyl succinate. Fluid. Phase Equilibria 2011, 309, 121–127. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, J.; Song, X.; Zheng, L.; Huang, J.; Zou, H.; Wang, Z. Aging of poly (lactic acid)/poly (butylene adipate-co-terephthalate) blends under different conditions: Environmental concerns on biodegradable plastic. Sci. Total Environ. 2023, 855, 158921. [Google Scholar] [CrossRef]
- Beltran-Sanahuja, A.; Casado-Coy, N.; Simo-Cabrera, L.; Sanz-Lazaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.S.; Li, G.S. Development of PLA/CS/ZnO nanocomposites and optimization its mechanical, thermal and water absorption properties. Polym. Test. 2018, 68, 302–308. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef]
- Sun, C.; Wei, S.; Tan, H.; Huang, Y.; Zhang, Y. Progress in upcycling polylactic acid waste as an alternative carbon source: A review. Chem. Eng. J. 2022, 446, 136881. [Google Scholar] [CrossRef]
- Usachev, S.V.; Lomakin, S.M.; Koverzanova, E.V.; Shilkina, N.G.; Levina, I.I.; Prut, E.V.; Rogovina, S.Z.; Berlin, A.A. Thermal degradation of various types of polylactides research. The effect of reduced graphite oxide on the composition of the PLA4042D pyrolysis products. Thermochim. Acta 2022, 712, 179227. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Parres, F.; López, J.; Jiménez, A. Development of a novel pyrolysis-gas chromatography/mass spectrometry method for the analysis of poly(lactic acid) thermal degradation products. J. Anal. Appl. Pyrolysis 2013, 101, 150–155. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Pinto, M.; Langer, T.M.; Alvarez-Salgado, X.A.; Herndl, G.J. Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat. Commun. 2018, 9, 1430. [Google Scholar] [CrossRef]
- Koh, K.Y.; Chen, Z.; Lin, S.; Chandra Mohan, K.; Luo, X.; Chen, J.P. Leaching of organic matters and formation of disinfection by-product as a result of presence of microplastics in natural freshwaters. Chemosphere 2022, 299, 134300. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Murphy, K.R.; Hur, J. Fluorescence Signatures of Dissolved Organic Matter Leached from Microplastics: Polymers and Additives. Environ. Sci. Technol. 2020, 54, 11905–11914. [Google Scholar] [CrossRef]
- Zhong, X.; Yi, X.; Cheng, F.; Tong, H.; Xu, W.; Yang, X. Leaching of di-2-ethylhexyl phthalate from biodegradable and conventional microplastics and the potential risks. Chemosphere 2023, 311, 137208. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, X.; Sui, Q.; Sun, X.; Zhu, L.; Booth, A.M.; Chen, B.; Qu, K.; Xia, B. Polystyrene nanoplastics affected the nutritional quality of Chlamys farreri through disturbing the function of gills and physiological metabolism: Comparison with microplastics. Sci. Total Environ. 2024, 910, 168457. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ma, X.; Lichtfouse, E.; Robert, D. Nanoplastics are potentially more dangerous than microplastics. Environ. Chem. Lett. 2022, 21, 1933–1936. [Google Scholar] [CrossRef]
- O’Connor, I.A.; Golsteijn, L.; Hendriks, A.J. Review of the partitioning of chemicals into different plastics: Consequences for the risk assessment of marine plastic debris. Mar. Pollut. Bull. 2016, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]




| MPs | C Atomic % | O Atomic % | O/C | CI |
|---|---|---|---|---|
| PE-V | 92.14 ± 2.39 | 7.86 ± 2.39 | 0.13 ± 0.03 | 0.01 ± 0.00 |
| PE-W | 86.98 ± 2.06 | 13.23 ± 2.34 | 0.15 ± 0.03 | 0.31 ± 0.05 |
| PE-A | 85.31 ± 1.97 | 14.71 ± 1.95 | 0.17 ± 0.03 | 0.09 ± 0.05 |
| PLA-V | 72.95 ± 1.34 | 27.06 ± 1.34 | 0.37 ± 0.03 | 19.66 ± 9.62 |
| PLA-W | 67.62 ± 1.16 | 32.38 ± 1.16 | 0.46 ± 0.01 | 14.90 ± 8.16 |
| PLA-A | 71.22 ± 0.29 | 28.79 ± 0.29 | 0.42 ± 0.02 | 12.13 ± 5.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, J.; Jia, W.; Huang, K.; Ma, Y. Comparing the Aging Processes of PLA and PE: The Impact of UV Irradiation and Water. Processes 2024, 12, 635. https://doi.org/10.3390/pr12040635
Wang X, Chen J, Jia W, Huang K, Ma Y. Comparing the Aging Processes of PLA and PE: The Impact of UV Irradiation and Water. Processes. 2024; 12(4):635. https://doi.org/10.3390/pr12040635
Chicago/Turabian StyleWang, Xucheng, Jinxin Chen, Wenhao Jia, Kaibo Huang, and Yini Ma. 2024. "Comparing the Aging Processes of PLA and PE: The Impact of UV Irradiation and Water" Processes 12, no. 4: 635. https://doi.org/10.3390/pr12040635
APA StyleWang, X., Chen, J., Jia, W., Huang, K., & Ma, Y. (2024). Comparing the Aging Processes of PLA and PE: The Impact of UV Irradiation and Water. Processes, 12(4), 635. https://doi.org/10.3390/pr12040635







