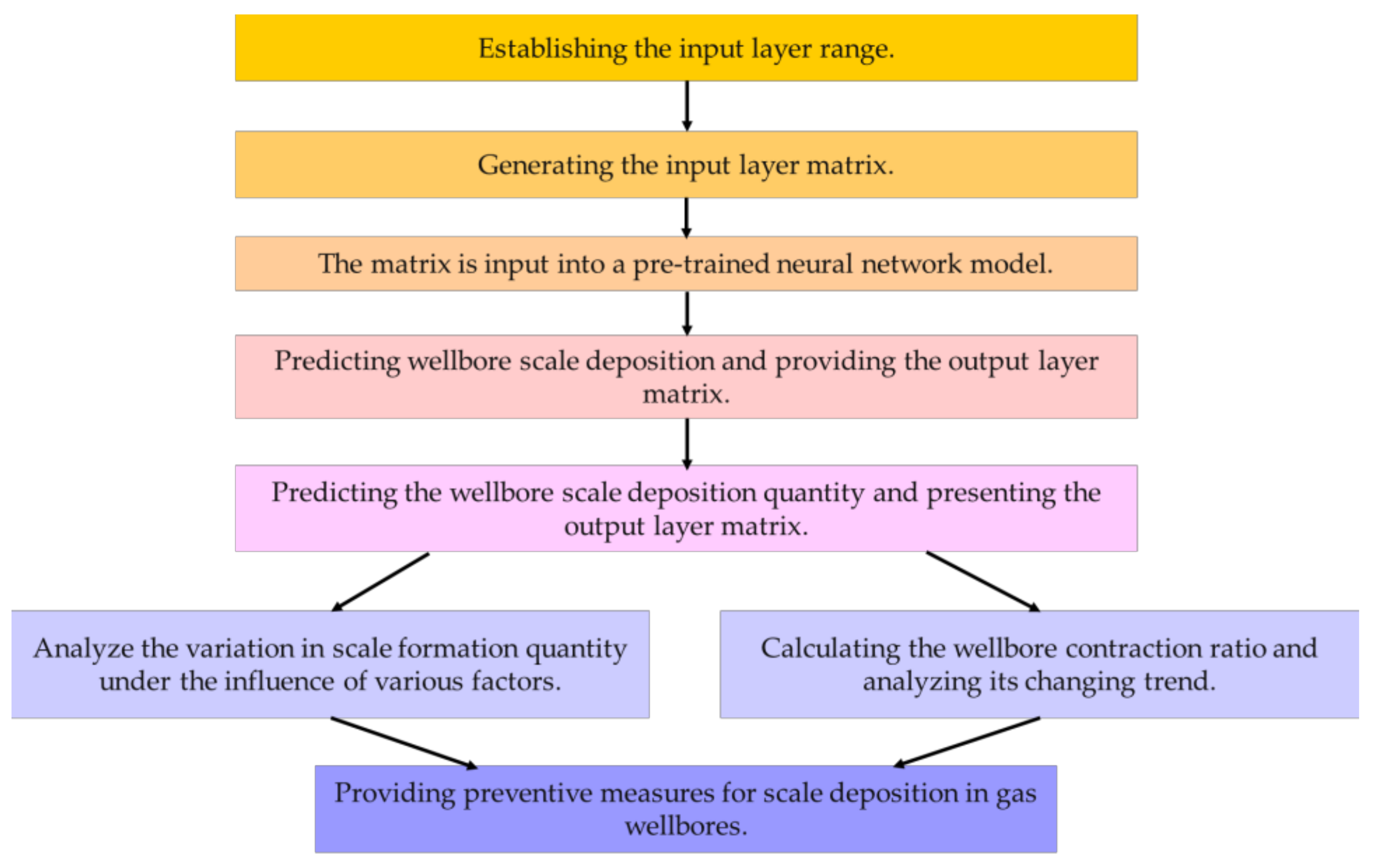
Utilizing the aforementioned algorithm enables the formulation of anti-scaling measures under various conditions.
4.1. Analysis of Changes in Scaling Amount
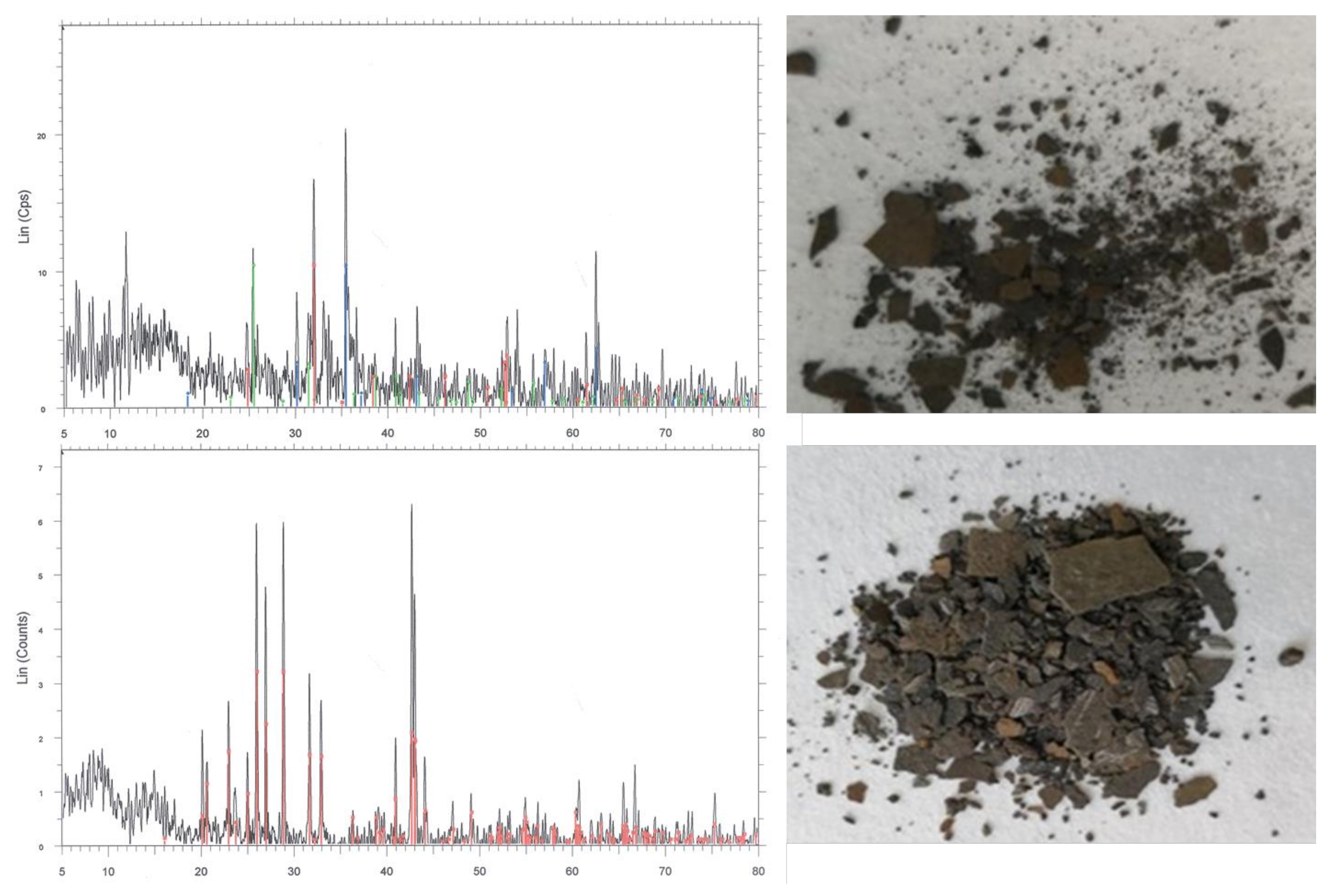
(1) Scale anions
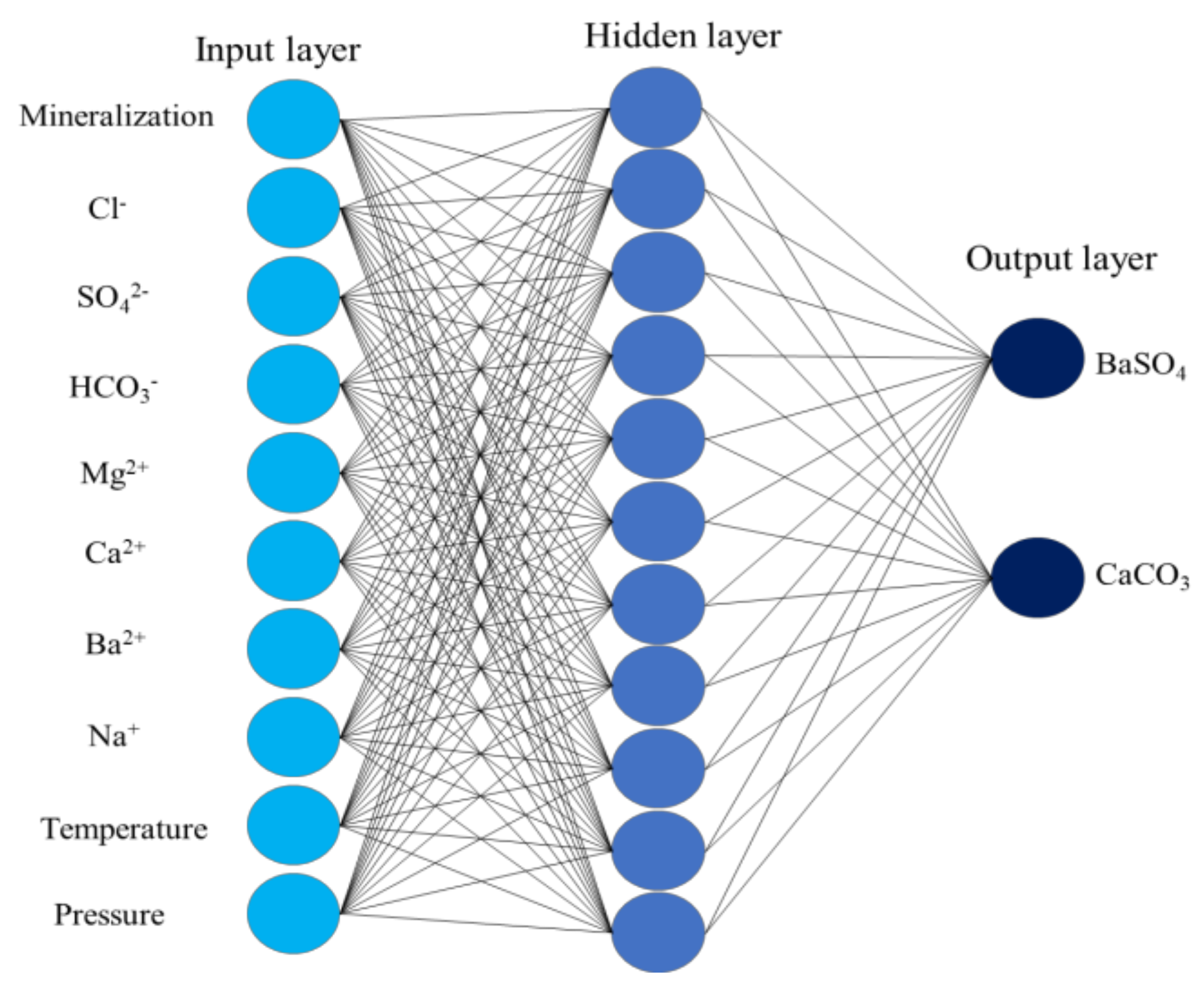
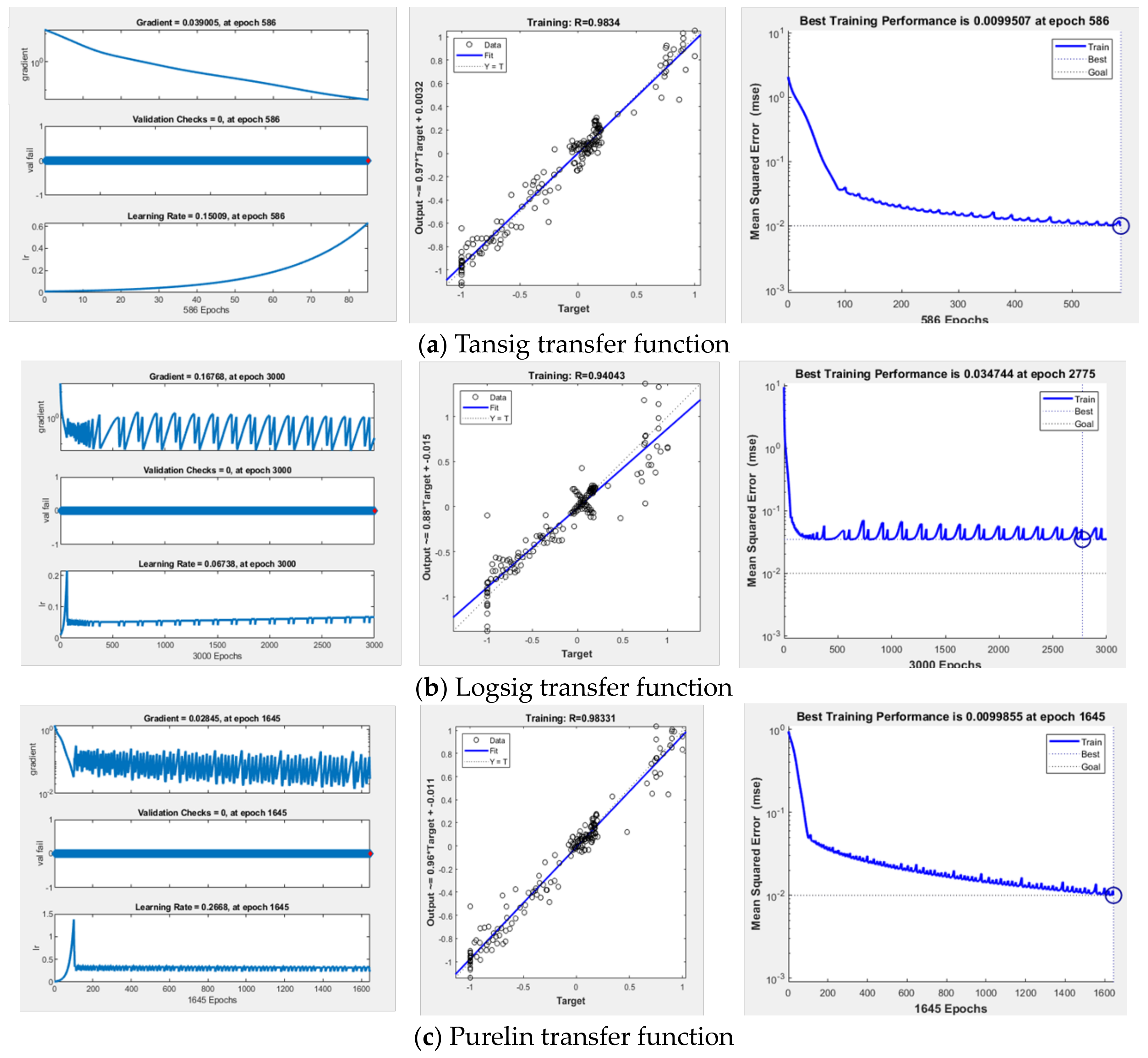
This study examines and forecasts scale formation patterns in the Sulige gas field wells across a range of input parameters, encompassing temperature, pressure, ion concentrations, and water salinity, through the development of algorithms. Here, temperature and pressure refer to the reservoir conditions, and scale formation quantity represents the scale concentration in unit volume of formation water at a specified pressure and temperature. The numerical ranges for temperature, pressure, and scale-forming ion concentrations are provided in
Table 6. Keeping other ion concentrations constant, the study separately investigates the effects of SO
42−, HCO
3−, Ba
2+, and Ca
2+ on scale formation quantity. The simulation results are depicted in
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8, where the colors on the scale bar correspond to the scale formation quantity.
Figure 5 displays the distribution of BaSO
4 scale quantities under different SO
42− ion concentrations (with an HCO
3− concentration at 300 mg/L, Ca
2+ concentration at 700 mg/L, and Ba
2+ concentration at 2000 mg/L). From
Figure 5, it is evident that the BaSO
4 scale quantities exhibit a “diagonal” distribution under varying SO
42− ion concentrations. In the high-pressure low-temperature region (top left corner), BaSO
4 scale formation is relatively low, whereas in the high-temperature low-pressure region (bottom right corner), BaSO
4 scale formation is higher. Nonetheless, in both the high-pressure low-temperature region and the low-pressure high-temperature region, the impact of temperature and pressure on BaSO
4 scale formation appears to be relatively minor. On the other hand, in the low-pressure low-temperature region (bottom left corner) and the high-pressure high-temperature region (top right corner), BaSO
4 scale formation is significantly affected by changes in temperature and pressure. Therefore, it can be observed that in gas wells with high temperatures and low pressures, BaSO
4 scale formation may be more severe.
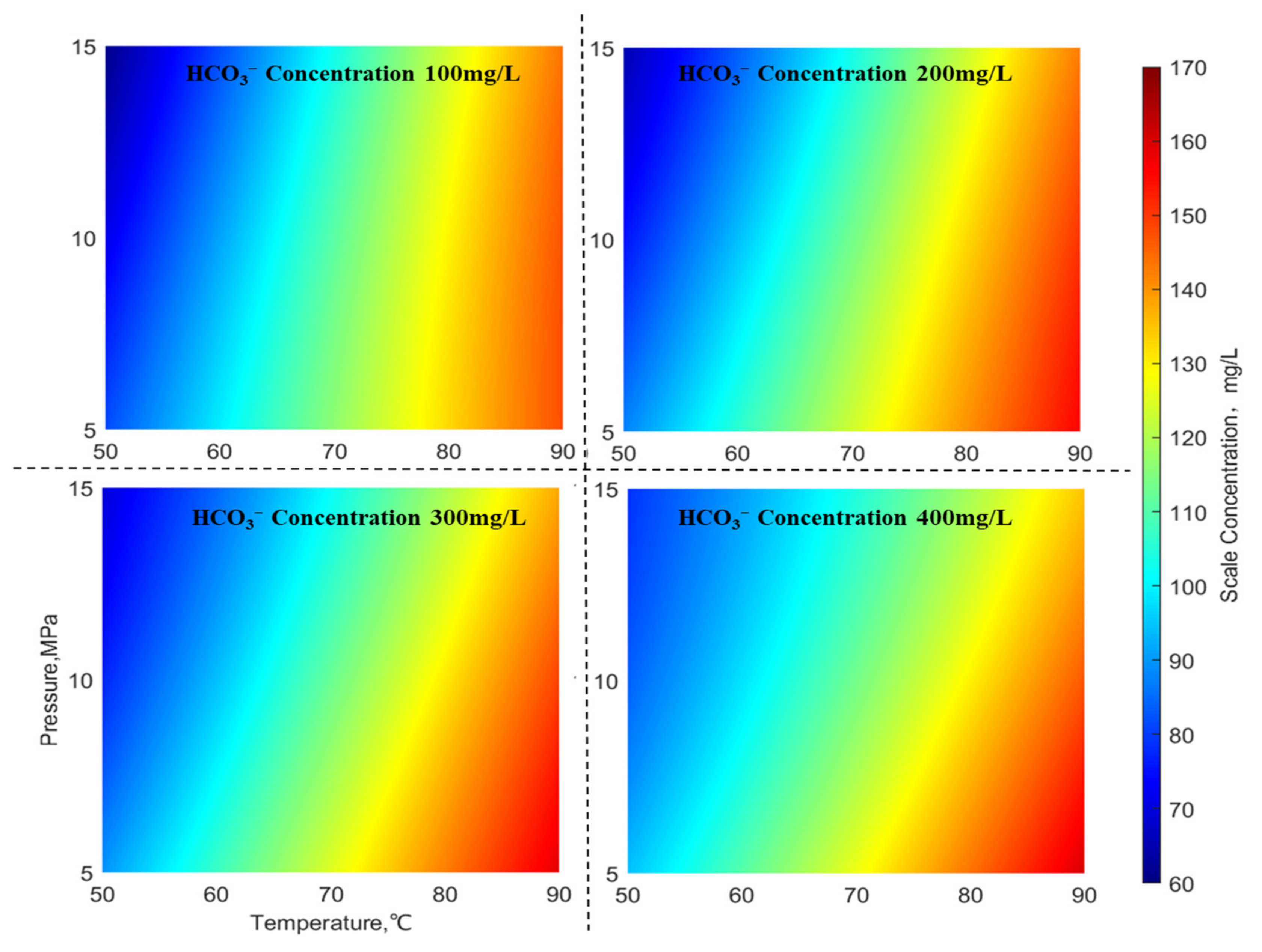
Figure 6 illustrates the distribution of CaCO
3 scale quantities under different HCO
3− ion concentrations (with an SO
42− concentration at 220 mg/L, Ca
2+ concentration at 700 mg/L, and Ba
2+ concentration at 2000 mg/L). From
Figure 6, it can be observed that the CaCO
3 scale quantities exhibit a “wave-like” distribution under varying HCO
3− ion concentrations. In the high-temperature region (on the right side), CaCO
3 scale formation is higher, while in the low-temperature region (on the left side), CaCO
3 scale formation is lower. Both high-temperature (right side) and low-temperature (left side) regions are relatively less influenced by temperature and pressure. However, as ion concentrations rise, the effect of temperature and pressure on BaSO4 scale formation becomes markedly more significant, observed in both the high-temperature high-pressure region (top right corner) and the low-temperature low-pressure region (bottom left corner). Therefore, it can be concluded that when ion concentrations are low, CaCO
3 scale formation is more severe in high-temperature wells. Conversely, when ion concentrations are high, CaCO
3 scale formation is more serious in high-temperature low-pressure wells.
(2) Scale cations
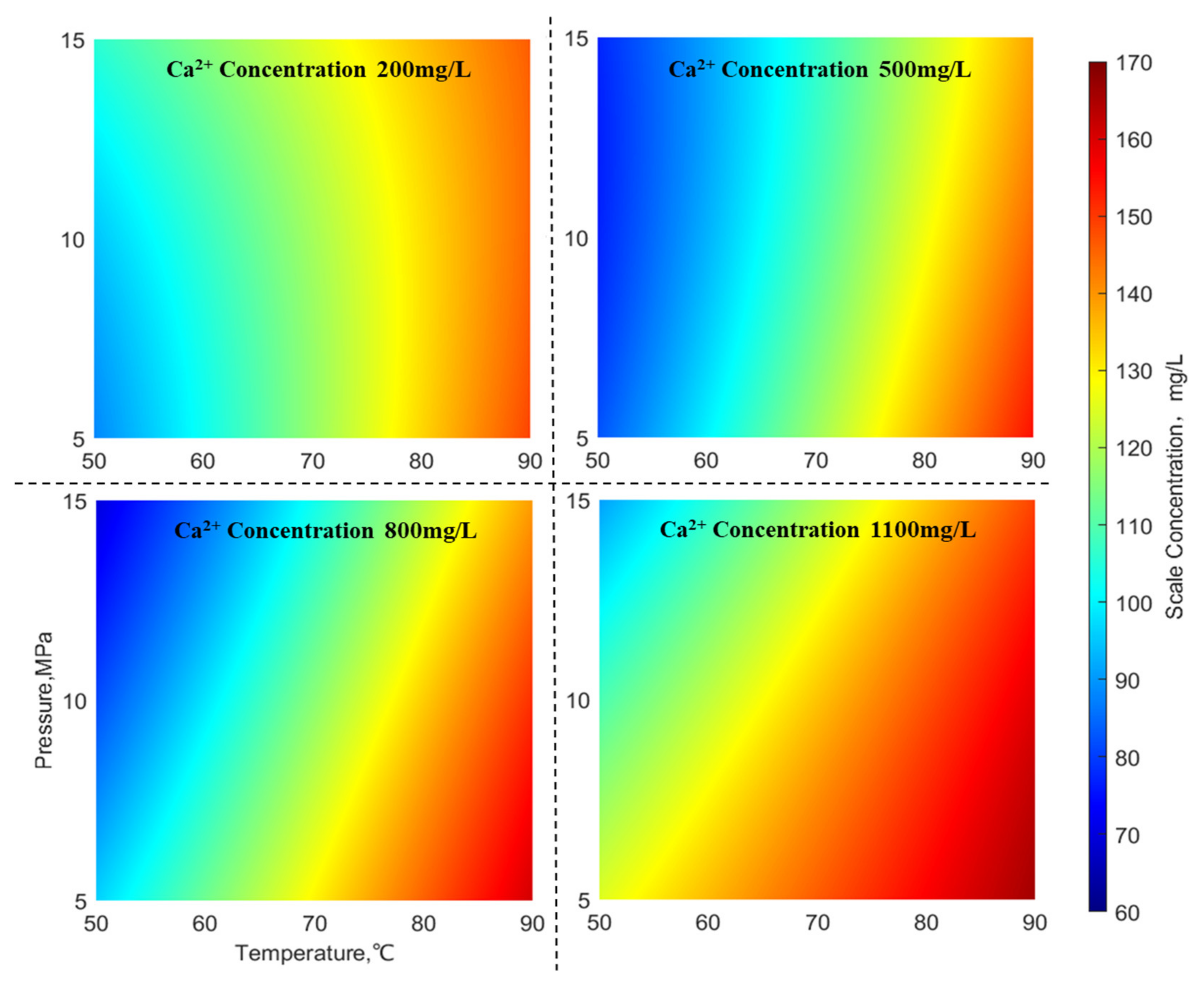
The figure depicted in
Figure 7 illustrates the distribution of CaCO
3 scaling amounts under different concentrations of Ca
2+ ions (with an HCO
3− concentration of 300 mg/L, SO
42− concentration of 220 mg/L, and Ba
2+ concentration of 2000 mg/L). From
Figure 7, it can be observed that the scaling amounts of CaCO
3 exhibit a “wave-like” pattern at varying concentrations of Ca
2+ ions. In the high-temperature region (on the right side), the scaling amount of CaCO
3 is relatively high, whereas in the low-temperature high-pressure region (bottom left corner), the scaling amount of CaCO
3 is comparatively low. Both the high-temperature region (on the right side) and the low-temperature high-pressure region (bottom left corner) show minor influences of temperature and pressure on CaCO
3 scaling. However, with an increase in ion concentration, the impact of temperature and pressure on CaCO
3 scaling in the high-temperature high-pressure region (top right corner) and the low-temperature low-pressure region (bottom left corner) gradually intensifies. Additionally, the scaling of CaCO3 in the low-temperature low-pressure region gradually increases, while in the low-temperature high-pressure region (top left corner), the CaCO
3 scaling gradually decreases. Therefore, it can be concluded that when the ion concentration is low, CaCO
3 scaling is more severe in high-temperature gas wells. Conversely, when the ion concentration is high, CaCO
3 scaling becomes even more pronounced in high-temperature low-pressure gas wells.
The graph presented in
Figure 8 illustrates the distribution of BaSO
4 scaling under different concentrations of Ba
2+ ions (with an HCO
3− concentration of 300 mg/L, SO
42− concentration of 220 mg/L, and Ca
2+ concentration of 700 mg/L). From
Figure 8, it can be observed that the BaSO
4 scaling amounts exhibit a “diagonal” pattern at varying Ba
2+ ion concentrations. In the high-pressure low-temperature region (top left corner), the BaSO
4 scaling is relatively small, whereas in the high-temperature low-pressure region (bottom right corner), the BaSO
4 scaling is significantly larger. However, in both the high-pressure low-temperature area and the low-pressure high-temperature area, BaSO
4 scaling is relatively unaffected by changes in temperature and pressure. Conversely, in the low-pressure low-temperature region (bottom left corner) and the high-pressure high-temperature region (top left corner), BaSO
4 scaling is significantly affected by fluctuations in temperature and pressure. Consequently, it can be inferred that in gas wells characterized by high temperatures and low pressures, the occurrence of BaSO
4 scaling might be more pronounced.
In summary, the formation of BaSO4 scaling under the influence of SO42− and Ba2+ ions follows a “diagonal” distribution pattern. In the high-pressure low-temperature region (top left corner), the BaSO4 scaling is relatively small, whereas in the high-temperature low-pressure region (bottom right corner), the BaSO4 scaling is more significant. However, in both the high-pressure low-temperature area and the low-pressure high-temperature area, BaSO4 scaling is relatively less affected by changes in temperature and pressure. Conversely, in the low-pressure low-temperature region (bottom left corner) and the high-pressure high-temperature region (top left corner), BaSO4 scaling is notably influenced by variations in temperature and pressure. Under the influence of Ca2+ and HCO3− ions, CaCO3 scaling exhibits a “wave-like” distribution pattern. In the high-temperature region (right side), there is a substantial buildup of CaCO3 scaling, whereas in the low-temperature region (left side), the scaling is minimal. Both the high-temperature region (right side) and the low-temperature region (left side) show relatively minor variations in CaCO3 scaling due to changes in temperature and pressure. However, with an increase in ion concentration, the impact of temperature and pressure on CaCO3 scaling becomes increasingly significant in the high-temperature high-pressure area (top right corner) and the low-temperature low-pressure area (bottom left corner). Overall, in gas wells with high temperatures and low pressures, BaSO4 scaling might be more severe. Additionally, when the ion concentration is low, there is severe CaCO3 scaling in high-temperature gas wells, whereas when the ion concentration is high, the scaling becomes even more severe, especially in high-temperature low-pressure gas wells.
4.2. Analysis of Changes in Diameter Reduction Ratio
According to recent research, wellbore scale can be classified into two main types: crystalline scale and particulate scale. Crystalline scale occurs when scale ions precipitate directly onto the wellbore surface from formation water. On the other hand, particulate scale forms when scale ions aggregate into particles within the formation water and then adhere to the wellbore surface as the fluid flows. Along with the deposition of scale particles, there is a process of particle detachment. The interaction between the deposition and detachment processes [
31] leads to wellbore scaling issues, gradually affecting the production of gas wells over time.
To simplify the problem, this study modeled the deposition and detachment processes of scale particles in the wellbore as the attachment of particulates from formation water, and a coefficient of attachment was assigned to describe the scaling process. The attachment of scale particles on the wellbore surface reduces the space available for fluid flow, potentially leading to wellbore blockages and impacting gas well production. Hence, to evaluate the potential risk of wellbore scaling and blockage, the Diameter Reduction Ratio (DRR) serves as a metric to gauge the extent of scaling. The DRR quantifies the ratio between the thickness of the scale layer and the internal diameter of the wellbore. Building upon the calculation of scaling quantity in the previous section, the total scaling within the predicted production lifespan is computed. This total scaling is converted into volume and averaged over the scaling intervals in the wellbore to determine the scale layer thickness. The specific calculation method is shown in Equations (1)–(3) [
32].
where
m—the total scale mass within a certain period of time, kg;
—daily water production of gas well, m3/d;
C—scale concentration, kg/L;
t—time, d.
where
d—scale layer thickness, m;
L—fouling section length, m;
—density of scale, kg/m
3.
where
D—wellbore inner diameter, m.
Based on the above analysis, and referring to the work of Zhang and colleagues [
22], an attachment ratio of 0.73 was adopted. Based on these principles, the variations in the Diameter Reduction Ratio (DRR) over a 3-year production period were calculated under different temperatures, pressures, and ion concentrations. The calculation process overlooks changes in ion concentration, reservoir temperature and pressure, and wellbore internal diameter, and assumes scaling occurs throughout the entire wellbore segment. The selected parameters for the calculation are detailed in
Table 7.
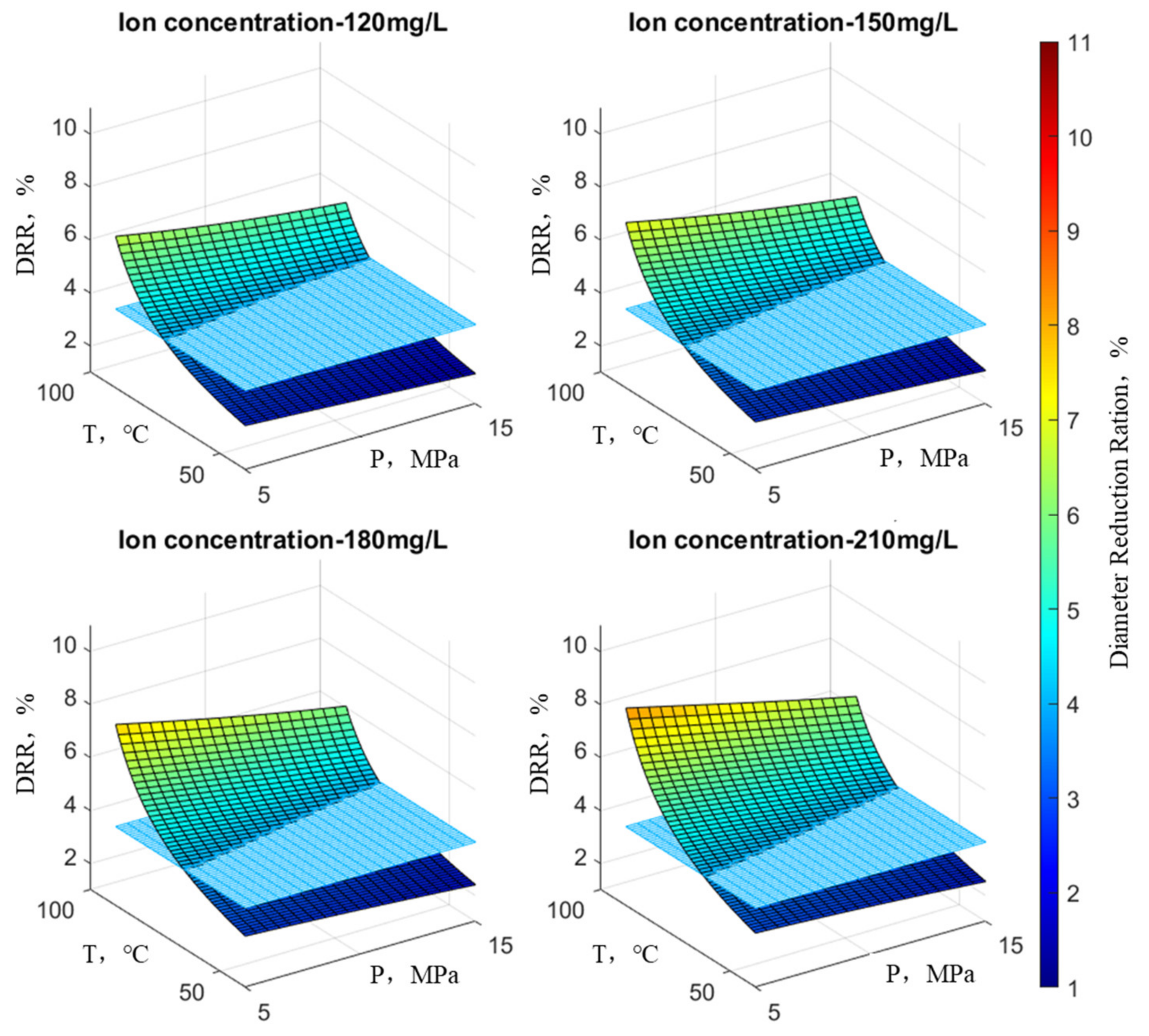
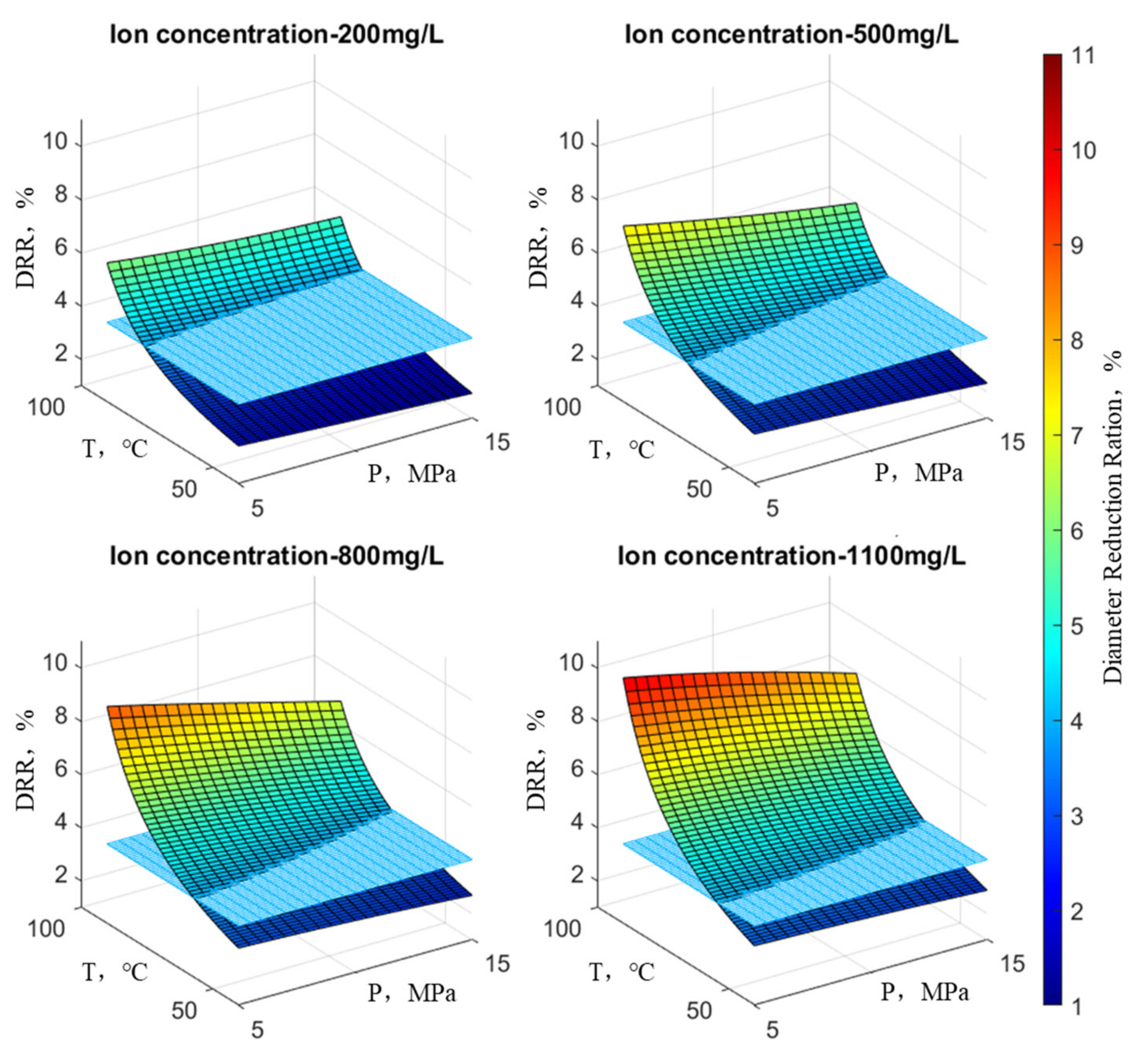
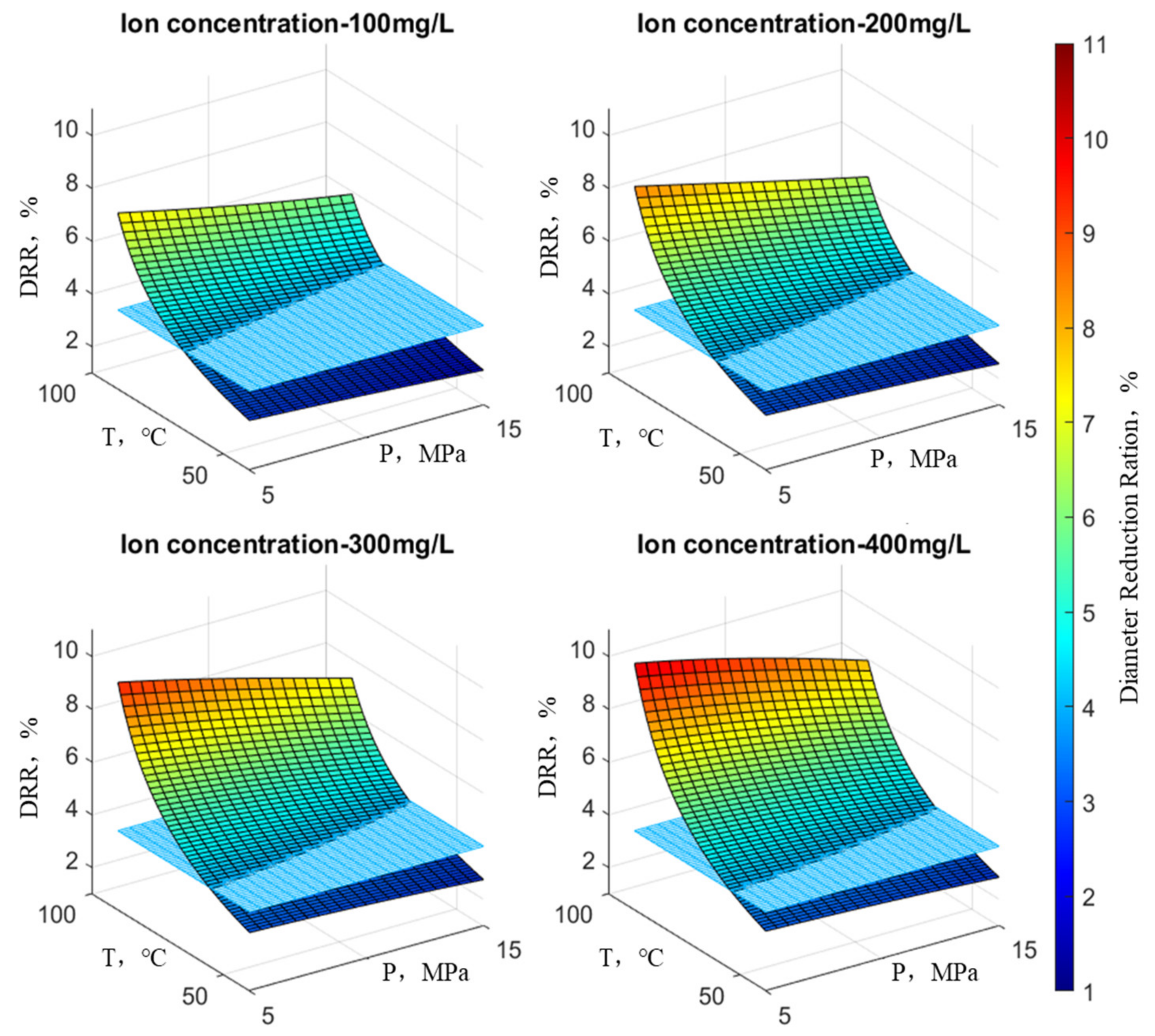
Following the principles outlined above, an examination was conducted on the reduction rate of wellbore diameter concerning scale ion concentration, temperature, and pressure. In the graph, the X-axis and Y-axis denote temperature and pressure, respectively, while the Z-axis illustrates the wellbore’s reduction rate. At any point on the surface of the graph, the height indicates the variation in the reduction rate under the current temperature, pressure, and ion concentration, with the color scale corresponding to the magnitude of the reduction rate. Practical observations on-site suggest that when the reduction rate is below 4%, there is considered to be no scaling risk, representing the position on the graph’s plane. This plane divides the surface representing the reduction rate variation into two parts and is parallel to the plane of temperature and pressure. At any point on this plane, the reduction rate is 4%.
Figure 9 illustrates the variation in the wellbore reduction rate when the concentration of SO
42− changes (with an HCO
3− concentration at 300 mg/L, Ca
2+ concentration at 700 mg/L, and Ba
2+ concentration at 2000 mg/L). Similarly,
Figure 10 depicts the variation in the wellbore reduction rate when the concentration of HCO
3− changes (with SO
42− concentration at 220 mg/L, Ca
2+ concentration at 700 mg/L, and Ba
2+ concentration at 2000 mg/L).
Figure 9 illustrates the trend in wellbore reduction rates under the influence of SO
42−. It is evident that when the SO
42− concentration remains constant, pressure has a minor impact on the reduction rate at low temperatures but a significant impact at high temperatures. Furthermore, at low pressure, temperature exerts a significant impact on the reduction rate, while at high pressure, the influence of temperature diminishes. With increasing ion concentration, the maximum reduction rate extends from the high-temperature, low-pressure region to encompass the entire surface, indicating variations in reduction rate. Overall, the reduction rate continuously increases, and the surface representing the reduction rate shifts upward. In other words, the surface above the plane where the reduction rate equals 4% becomes increasingly larger. In general, the influence of temperature on the reduction rate is greater than that of pressure.
Figure 10 illustrates the variation in wellbore reduction rates under the influence of HCO3-. It can be observed that the overall trend is similar to the reduction rate variation observed under the influence of SO
42−. Once again, temperature has a more significant impact on the reduction rate compared to pressure.
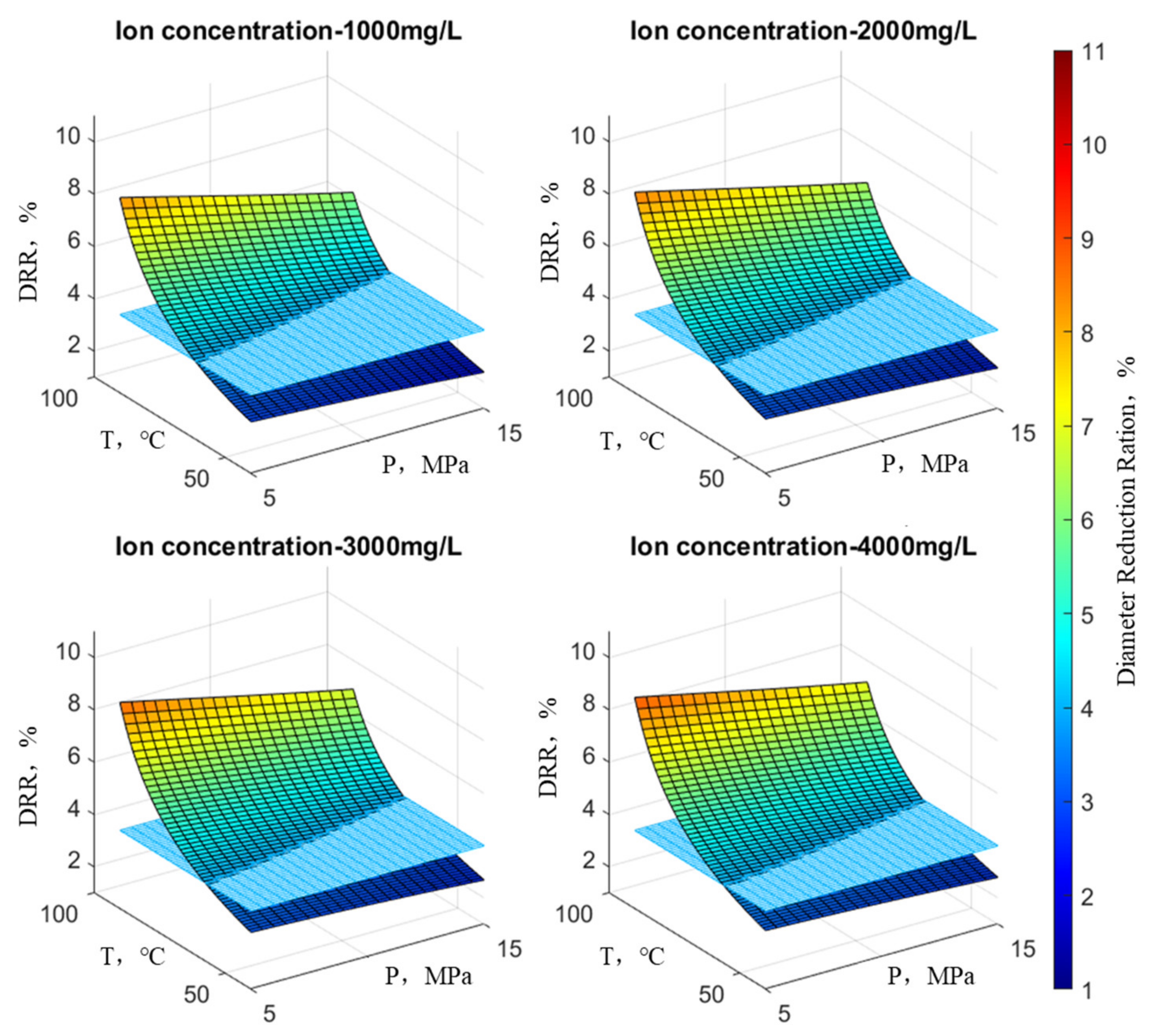
Figure 11 depicts the variation in the wellbore reduction rate when the concentration of Ca
2+ changes (with SO
42− concentration at 220 mg/L, HCO
3− concentration at 300 mg/L, and Ba
2+ concentration at 2000 mg/L). Similarly,
Figure 12 shows the variation in the wellbore reduction rate when the concentration of Ba
2+ changes (with SO
42− concentration at 220 mg/L, HCO
3− concentration at 300 mg/L, and Ca
2+ concentration at 700 mg/L).
Figure 11 represents the variation in wellbore reduction rates under the influence of Ca
2+. It is evident that at an ion concentration of 200 mg/L temperature has a far greater impact on the reduction rate than pressure. This is discernible from the spatial extent of the surface’s changes; the surface representing reduction rate variations is nearly perpendicular to the plane defined by reduction rate and temperature. As the ion concentration increases, the reduction rate surface starts to tilt.
This suggests that pressure exerts a lesser influence on the reduction rate at low temperatures but a more substantial impact at high temperatures. Conversely, at low pressure, temperature significantly affects the reduction rate, whereas at high pressure, the influence of temperature is comparatively minor. The wellbore reduction rate reaches its minimum at high temperatures and low pressures. In summary, the effect of temperature on the reduction rate surpasses that of pressure. Additionally, it can be observed that with increasing ion concentration, the reduction rate surface consistently shifts upward, and the portion of the surface above the 4% reduction rate plane becomes larger.
Figure 12 illustrates the variation in wellbore reduction rates under the influence of Ba
2+. The trend aligns with the variation observed under the influence of Ca
2+, emphasizing once again that temperature’s impact on the reduction rate outweighs that of pressure.
Based on the above analysis, it can be observed that under certain conditions in the research area, controlling the temperature, pressure, and ion concentration of the produced water within specific ranges can maintain the borehole contraction rate below 4% over a three-year production period. Therefore, during on-site production, focusing on parameters such as temperature, pressure, and ion concentration can effectively prevent scaling issues. However, in reality, the temperature of the reservoir and the ion concentration of the produced water are uncontrollable factors determined by geological conditions. On the other hand, the bottom-hole pressure of gas wells is related to the production system, which means that the bottom-hole pressure of gas wells is controllable. Production systems for gas wells include fixed production rate systems and fixed pressure systems. The latter involves fixed wellhead (bottom-hole) pressure systems and fixed pressure difference systems. The formulation of the production system depends on geological conditions and production requirements. Thus, in the gas well production process, mitigating scaling and minimizing associated risks can be achieved by manipulating the production system to regulate bottom-hole pressure, provided certain conditions like fixed temperature and ion concentration. However, due to economic considerations and reservoir constraints, bottom-hole pressure cannot be increased indefinitely. In practice, determining the optimal magnitude of bottom-hole pressure becomes a multi-objective optimization challenge, balancing economic benefits, reservoir conditions, technical costs, and other relevant factors. In conclusion, considering the temperature and ion concentration conditions in the research area, the selected bottom-hole pressure should be adjusted to achieve a borehole contraction rate as close to 4% as possible, striking a balance between scaling prevention and economic efficiency in the given reservoir context.