Thermochemical Conversion of Animal-Derived Waste: A Mini-Review with a Focus on Chicken Bone Waste
Abstract
1. Introduction

2. Methodology
2.1. Food Waste Definitions
- Herein, food is any substance or product (solid or liquid) intended for human consumption, regardless of its processed status (processed, partially processed, or unprocessed).
- Food waste refers to any “food” or inedible parts of food removed from the supply chain that can be recovered and disposed of.
- Inedible parts refer to any parts associated with food that that humans cannot consume (e.g., bones, pits, peels, seeds, and hooves).
2.2. Literature Review Method
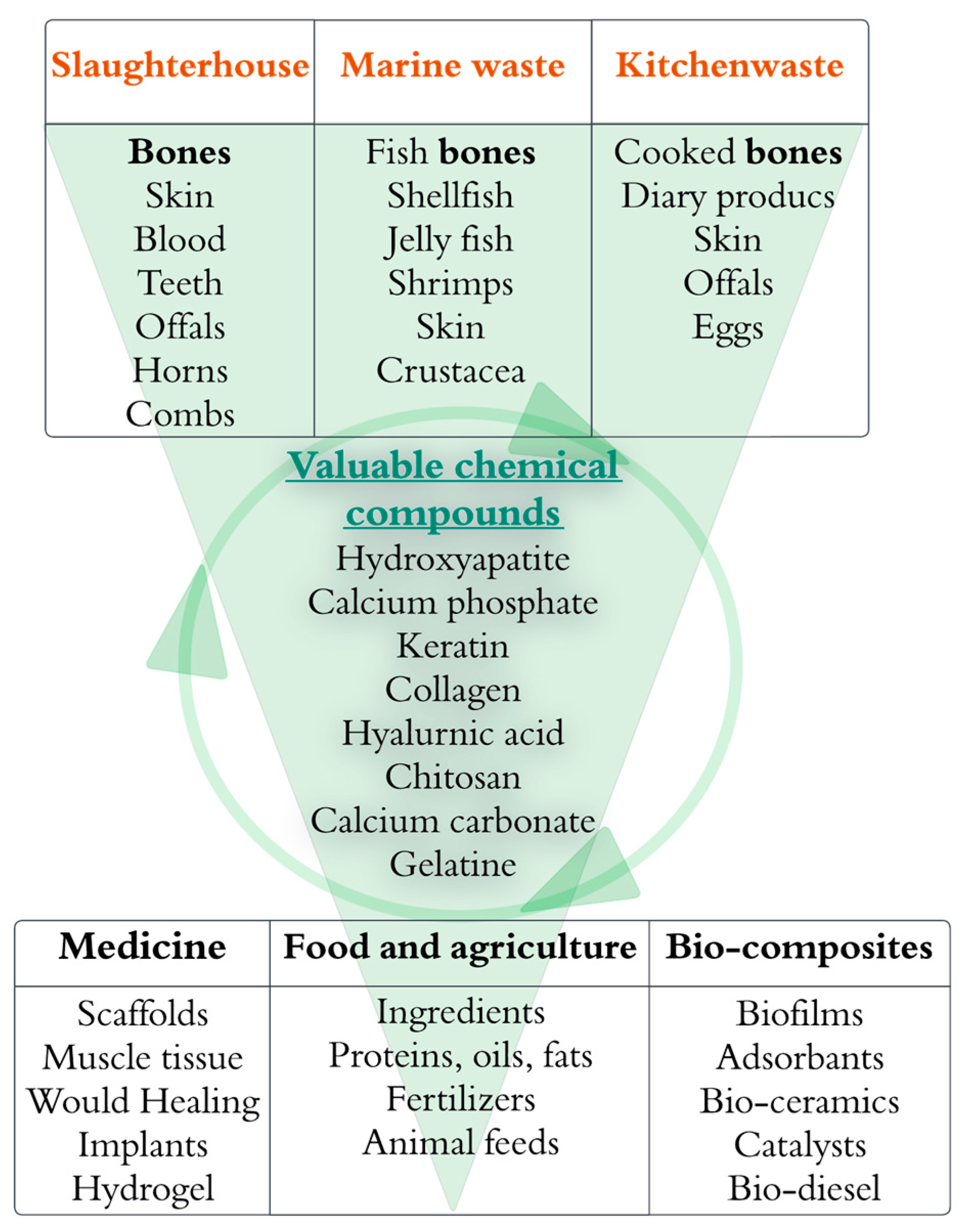
3. Characteristics of Animal-Derived Waste
- Animal-derived waste from slaughterhouses
- Meat production in a slaughterhouse involves various steps [21] whereby a specific type of animal-derived waste is produced. After the monetizable meat has been removed, multiple parts such as bones (which can account for 18% of the living animal’s weight) [6] and other animal-derived products (e.g., skin, blood, offal, horns, teeth, feathers, combs, hooves, feet, and shells) are left as waste. The estimated annual global slaughterhouse production of bone waste exceeds 130 billion kg, making it one of the most significant contributors to animal-derived wastes [37]. This number is reflected in the estimated livestock population, consisting of 4.89 billion bovines, caprine and ovine animals, and swine and 27.88 billion poultry animals [38]. Given these numbers, chicken farming is one of the most prominent animal industries, producing more than 100,000 tonnes of meat yearly [39].
- Animal-derived waste from fishing and aquaculture
- Marine waste consists of various waste-derived components, such as jellyfish, bones, shellfish, crustacea, shrimps, and skin [40]. The global production of seafood from aquaculture and fisheries reached 214 million tonnes in 2020 [41]. In the EU, up to 10 million tonnes of fish is estimated to be discarded each year [42]. These materials offer the opportunity to extract valuable chemical compounds through different chemical, biological, and thermochemical processes. HAp, calcium carbonate, chitin, collagen, omega-3 fatty acids [43], and polysaccharides [40] are the main chemical compounds that can be obtained. For example, shrimp shells are an essential source of protein (48%), chitin (38%), and other minerals (14%) [44].
- Animal-derived waste from kitchens
- This study discusses kitchen waste generated in residential households and restaurant kitchens. This waste, which includes leftovers and cooking residue, stems from food preparation and consists of the waste from cooking and leftovers. Kitchen waste provides a complex mixture of animal-derived waste, dairy products, various plant components (cereals, fruits, vegetables, roots, etc.) [33], and seafood obtained from households and restaurants. This composition creates an organic environment rich in proteins, carbohydrates, and lipids. [45].
3.1. Composition of Animal-Derived Waste
3.2. Extractable Valuable Chemical Compounds from Animal-Derived Waste
3.2.1. Hydroxyapatite (HAp)
3.2.2. Keratin
3.2.3. Hyaluronic Acid
4. Thermochemical Conversion of Chicken Bone Waste into Energy or Valuable Products
4.1. Pyrolysis of Chicken Bone Waste
4.1.1. Pyrolysis Process and Conditions
4.1.2. Product Yield
4.1.3. Catalyst Production from Chicken Bones
4.2. Gasification of Chicken Bone Waste
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morini, S.; Gustavsson, J.; Cederberg, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- United Nations Environment Programme. Food Waste Index Report 2021. 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 5 January 2024).
- Meat and Dairy Production-Our World in Data. Available online: https://ourworldindata.org/meat-production (accessed on 5 January 2024).
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2023–2032; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2023. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Hussain, F.; Alshahrani, S.; Abbas, M.M.; Khan, H.M.; Jamil, A.; Yaqoob, H.; Soudagar, M.E.M.; Imran, M.; Ahmad, M.; Munir, M. Waste Animal Bones as Catalysts for Biodiesel Production; A Mini Review. Catalysts 2021, 11, 630. [Google Scholar] [CrossRef]
- Statistics|Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_wasfw__custom_9431225/default/table?lang=en&page=time:2021 (accessed on 20 January 2024).
- Food and Agriculture Organization of the United Nations. Food Wastage Footprint: Impacts on Natural Resources: Summary Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- European Comission. Commission Staff Working Document Impact Assessment Report Accompanying the Document Directive of the European Parliament and of the Council Amending Directive 2008/98/EC on Waste; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- The State of Food Waste: Statistics, Trends, and Actionable Insights|GreenMatch.co.uk. Available online: https://www.greenmatch.co.uk/food-waste (accessed on 20 January 2024).
- ReFED. Roadmap to 2030: Reducing U.S. Food Waste by 50% and the ReFED Insights Engine; ReFED: Berkeley, CA, USA, 2021. [Google Scholar]
- Awasthi, A.K.; Cheela, V.S.; D’adamo, I.; Iacovidou, E.; Islam, M.R.; Johnson, M.; Miller, T.R.; Parajuly, K.; Parchomenko, A.; Radhakrishan, L.; et al. Zero waste approach towards a sustainable waste management. Resour. Environ. Sustain. 2021, 3, 100014. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; US Preventive Services Task Force; et al. Interventions to prevent falls in community-dwelling older adults. Us Preventive Services Task Force recommendation statement. JAMA 2018, 319, 1696–1704. [Google Scholar] [CrossRef]
- Paz, I.A.; Bruno, L. Bone mineral density: Review. Braz. J. Poult. Sci. 2006, 8, 69–73. [Google Scholar] [CrossRef]
- Hart, A.; Ebiundu, K.; Peretomode, E.; Onyeaka, H.; Nwabor, O.F.; Obileke, K. Value-added materials recovered from waste bone biomass: Technologies and applications. RSC Adv. 2022, 12, 22302–22330. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, A.P.; Henchion, M.M. Bovine and ovine meat co-products valorisation opportunities: A systematic literature review. Trends Food Sci. Technol. 2021, 118, 57–70. [Google Scholar] [CrossRef]
- Ahmed, M.; Nigussie, A.; Addisu, S.; Belay, B.; Lehmann, J.; Sato, S. Valorization of animal bone waste for agricultural use through biomass co-pyrolysis and bio-augmentation. Biomass Convers. Biorefinery 2023, 13, 12823–12832. [Google Scholar] [CrossRef]
- Muriana, C. A focus on the state of the art of food waste/losses issue and suggestions for future researches. Waste Manag. 2017, 68, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Boavida-Dias, R.; Matos, H.A.; Azevedo, J. Analysis of the Food Loss and Waste Valorisation of Animal By-Products from the Retail Sector. Sustainability 2022, 14, 2830. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, J.; Bai, W. A Review of Poultry Waste-to-Wealth: Technological Progress, Modeling and Simulation Studies, and Economic-Environmental and Social Sustainability. Sustainability 2023, 15, 5620. [Google Scholar] [CrossRef]
- Ragasri, S.; Sabumon, P. A critical review on slaughterhouse waste management and framing sustainable practices in managing slaughterhouse waste in India. J. Environ. Manag. 2023, 327, 116823. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Limeneh, D.Y.; Tesfaye, T.; Ayele, M.; Husien, N.M.; Ferede, E.; Haile, A.; Mengie, W.; Abuhay, A.; Gelebo, G.G.; Gibril, M.; et al. A Comprehensive Review on Utilization of Slaughterhouse By-Product: Current Status and Prospect. Sustainability 2022, 14, 6469. [Google Scholar] [CrossRef]
- Yadav, M.K.; Shukla, R.H.; Prashanth, K. A comprehensive review on development of waste derived hydroxyapatite (HAp) for tissue engineering application. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Schneider, F. Review of food waste prevention on an international level. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2013, 166, 187–203. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement David Moher and colleagues introduce PRISMA, an update of the QUOROM guidelines for reporting systematic reviews and meta-analyses. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; Wang, M.; Feng, L.; Liao, Y.; Wang, Z.; Yong, Z.; Qin, Z. Current advances on waste biomass transformation into value-added products. Appl. Microbiol. Biotechnol. 2020, 104, 4757–4770. [Google Scholar] [CrossRef]
- Arancon, R.A.D.; Lin, C.S.K.; Chan, K.M.; Kwan, T.H.; Luque, R. Advances on waste valorization: New horizons for a more sustainable society. Energy Sci. Eng. 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Fattah, I.M.R.; Ok, Y.S.; Jang, J.-H.; Wang, C.-T. State-of-the-art of the pyrolysis and co-pyrolysis of food waste: Progress and challenges. Sci. Total. Environ. 2022, 809, 151170. [Google Scholar] [CrossRef] [PubMed]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.; Heaven, S.; Zhang, Y.; Baier, U.; IEA Bioenergy Programme. Food Waste Digestion: Anaerobic Digestion of Food Waste for A Circular Economy; IEA Bioenergy: Lyon, France, 2018. [Google Scholar]
- Sharma, A.; Kuthiala, T.; Thakur, K.; Thatai, K.S.; Singh, G.; Kumar, P.; Arya, S.K. Kitchen waste: Sustainable bioconversion to value-added product and economic challenges. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Miskah, S.; Aprianti, T.; Agustien, M.; Utama, Y.; Said, M. Biodiesel synthesis from used cooking oil using calcium oxide (cao) catalyst from chicken bones. AIP Conf. Proc. 2020, 2248, 060004. [Google Scholar] [CrossRef]
- Genisel, M.; Erdal, S.; Turk, H.; Dumlupinar, R. The availability of bone powder as inorganic element source on growth and development in wheat seedlings. Toxicol. Ind. Health 2012, 28, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.L.; Khraibi, A.A.; Dumée, L.F.; Corridon, P.R. From waste to wealth: Repurposing slaughterhouse waste for xenotransplantation. Front. Bioeng. Biotechnol. 2023, 11, 1091554. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.N.; Ismail, A.R.; El-Salamony, R.A.; Aboelazayem, O.; Abu Amr, S.A.; El-Gendy, N.S. Animal bone affluence in environmental reclamation: Biodiesel production, petro-diesel biodesulfurization and wastewater photo-treatment. Biofuels Bioprod. Biorefining 2021, 15, 770–792. [Google Scholar] [CrossRef]
- Tarafdar, A.; Gaur, V.K.; Rawat, N.; Wankhade, P.R.; Gaur, G.K.; Awasthi, M.K.; Sagar, N.A.; Sirohi, R. Advances in biomaterial production from animal derived waste. Bioengineered 2021, 12, 8247–8258. [Google Scholar] [CrossRef]
- Ibarz-Blanch, N.; Alcaide-Hidalgo, J.M.; Cortés-Espinar, A.J.; Albi-Puig, J.; Suárez, M.; Mulero, M.; Morales, D.; Bravo, F.I. Chicken slaughterhouse by-products: A source of protein hydrolysates to manage non-communicable diseases. Trends Food Sci. Technol. 2023, 139, 104125. [Google Scholar] [CrossRef]
- Zamri, M.F.M.A.; Bahru, R.; Amin, R.; Khan, M.U.A.; Razak, S.I.A.; Abu Hassan, S.; Kadir, M.R.A.; Nayan, N.H.M. Waste to health: A review of waste derived materials for tissue engineering. J. Clean. Prod. 2021, 290, 125792. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- European Commission; European Climate; Infrastructure and Environment Executive Agency; Heinrich, J. Synthesis of the Landing Obligation Measures and Discard Rates; Publications Office: Luxembourg, Luxembourg, 2021. [Google Scholar] [CrossRef]
- Mbatia, B.; Adlercreutz, D.; Adlercreutz, P.; Mahadhy, A.; Mulaa, F.; Mattiasson, B. Enzymatic oil extraction and positional analysis of ω-3 fatty acids in Nile perch and salmon heads. Process. Biochem. 2010, 45, 815–819. [Google Scholar] [CrossRef]
- Arya, P.S.; Yagnik, S.M.; Rajput, K.N.; Panchal, R.R.; Raval, V.H. Valorization of agro-food wastes: Ease of concomitant-enzymes production with application in food and biofuel industries. Bioresour. Technol. 2022, 361, 127738. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Yan, J. Influence of pork and bone on product characteristics during the fast pyrolysis of pig carcasses. Waste Manag. 2018, 75, 352–360. [Google Scholar] [CrossRef]
- Ming, X.; Xu, F.; Jiang, Y.; Zong, P.; Wang, B.; Li, J.; Qiao, Y.; Tian, Y. Thermal degradation of food waste by TG-FTIR and Py-GC/MS: Pyrolysis behaviors, products, kinetic and thermodynamic analysis. J. Clean. Prod. 2020, 244, 118713. [Google Scholar] [CrossRef]
- Purevsuren, B.; Avid, B.; Gerelmaa, T.; Davaajav, Y.; Morgan, T.; Herod, A.; Kandiyoti, R. The characterisation of tar from the pyrolysis of animal bones. Fuel 2004, 83, 799–805. [Google Scholar] [CrossRef]
- Okopi, S.I.; Wang, J.; Kong, W.; Yu, Z.; Ndudi, E.A.; Che, L.; Gu, Z.; Xu, F. Valorization of food waste impurities by catalytic co-pyrolysis for production of pyrolysis oil with high energy potential. J. Anal. Appl. Pyrolysis 2023, 170, 105918. [Google Scholar] [CrossRef]
- Xue, S.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Zhang, Y.; Ma, C. Food waste based biochars for ammonia nitrogen removal from aqueous solutions. Bioresour. Technol. 2019, 292, 121927. [Google Scholar] [CrossRef] [PubMed]
- Kantorek, M.; Jesionek, K.; Polesek-Karczewska, S.; Ziółkowski, P.; Badur, J. Thermal utilization of meat and bone meals. Performance analysis in terms of drying process, pyrolysis and kinetics of volatiles combustion. Fuel 2019, 254, 115548. [Google Scholar] [CrossRef]
- Cascarosa, E.; Becker, J.; Ferrante, L.; Briens, C.; Berruti, F.; Arauzo, J. Pyrolysis of meat-meal and bone-meal blends in a mechanically fluidized reactor. J. Anal. Appl. Pyrolysis 2011, 91, 359–367. [Google Scholar] [CrossRef]
- Ayllón, M.; Aznar, M.; Sánchez, J.; Gea, G.; Arauzo, J. Influence of temperature and heating rate on the fixed bed pyrolysis of meat and bone meal. Chem. Eng. J. 2006, 121, 85–96. [Google Scholar] [CrossRef]
- Soni, C.; Wang, Z.; Dalai, A.; Pugsley, T.; Fonstad, T. Hydrogen production via gasification of meat and bone meal in two-stage fixed bed reactor system. Fuel 2009, 88, 920–925. [Google Scholar] [CrossRef]
- Ionescu, G.; Stan, C.; Mărculescu, C.; Ciută, S. Energetic Analysis of Meat Processing Industry Waste. 2013. Available online: https://www.researchgate.net/publication/259215145 (accessed on 25 December 2023).
- Masiá, A.A.T.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising ash of biomass and waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Y.; Fang, Y.; Wang, Y. Co-gasification behavior of meat and bone meal char and coal char. Fuel Process. Technol. 2011, 92, 298–307. [Google Scholar] [CrossRef]
- Hussin, M.S.F.; Abdullah, H.Z.; Idris, M.I.; Wahap, M.A.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef]
- Alam, R.; Shahid, A.; Alimuzzaman, S.; Khan, A.N. Sources, extractions and applications of bio-maker collagen–A review. Biomed. Eng. Adv. 2022, 4, 100064. [Google Scholar] [CrossRef]
- Timorshina, S.; Popova, E.; Osmolovskiy, A. Sustainable Applications of Animal Waste Proteins. Polymers 2022, 14, 1601. [Google Scholar] [CrossRef]
- Graciela, C.-Q.; Juan, E.-C.J.; Gieraldin, C.-L.; Alejandra, P.-M.X.; Gabriel, A. Hyaluronic Acid—Extraction Methods, Sources and Applications. Polymers 2023, 15, 3473. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Yadav, N.; Sultana, M.; Ahmaruzzaman, M. A comprehensive review on low-cost waste-derived catalysts for environmental remediation. Mater. Res. Bull. 2023, 164, 112261. [Google Scholar] [CrossRef]
- Boyetey, M.-J.B.; Torgbo, S.; Sukyai, P. Bio-scaffold for bone tissue engineering with focus on bacterial cellulose, biological materials for hydroxyapatite synthesis and growth factors. Eur. Polym. J. 2023, 194, 112168. [Google Scholar] [CrossRef]
- Bee, S.-L.; Mariatti, M.; Ahmad, N.; Yahaya, B.; Hamid, Z.A. Effect of the Calcination Temperature on the Properties of Natural Hydroxyapatite Derived from Chicken Bone Wastes. 2019. Available online: https://www.sciencedirect.comwww.materialstoday.com/proceedings2214-7853 (accessed on 28 December 2023).
- Khoo, W.; Nor, F.; Ardhyananta, H.; Kurniawan, D. Preparation of Natural Hydroxyapatite from Bovine Femur Bones Using Calcination at Various Temperatures. Procedia Manuf. 2015, 2, 196–201. [Google Scholar] [CrossRef]
- Sricharoen, P.; Kongsri, S.; Kukusamude, C.; Areerob, Y.; Nuengmatcha, P.; Chanthai, S.; Limchoowong, N. Ultrasound-irradiated synthesis of 3-mercaptopropyl trimethoxysilane-modified hydroxyapatite derived from fish-scale residues followed by ultrasound-assisted organic dyes removal. Sci. Rep. 2021, 11, 5560. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater. Sci. Eng. C 2020, 117, 111313. [Google Scholar] [CrossRef]
- Swamiappan, S.; Ganesan, S.; Sekar, V.; Devaraj, S.; Subramanian, A.; Ponnusamy, V.K.; Kathirvel, P. Effective removal of cationic methylene blue dye using nano-hydroxyapatite synthesized from fish scale bio-waste. Int. J. Appl. Ceram. Technol. 2021, 18, 902–912. [Google Scholar] [CrossRef]
- Obadiah, A.; Swaroopa, G.A.; Kumar, S.V.; Jeganathan, K.R.; Ramasubbu, A. Biodiesel production from Palm oil using calcined waste animal bone as catalyst. Bioresour. Technol. 2012, 116, 512–516. [Google Scholar] [CrossRef]
- Nisar Abdulla, A.; Suseel, N.A.; Munees Muhammed, C.; Shyja, S.M. Extraction of Keratin from Different Sources: A Comparative Study; SSRN: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Chen, H.; Gao, S.; Li, Y.; Xu, H.-J.; Li, W.; Wang, J.; Zhang, Y. Valorization of Livestock Keratin Waste: Application in Agricultural Fields. Int. J. Environ. Res. Public Health 2022, 19, 6681. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Madhan, B.; Shanmugam, G. Extraction and characterization of keratin from bovine hoof: A potential material for biomedical applications. SpringerPlus 2014, 3, 596. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.C. Hyaluronic Acid and Collagen Extraction from Chicken Combs. Master’s Thesis, Universidade Católica Portuguesa, Lisboa, Portugal, 2017. [Google Scholar]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Manna, F.; Dentini, M.; Desideri, P.; De Pità, O.; Mortilla, E.; Maras, B. Comparative chemical evaluation of two commercially available derivatives of hyaluronic acid (Hylaform® from rooster combs and Restylane® from streptococcus) used for soft tissue augmentation. J. Eur. Acad. Dermatol. Venereol. 1999, 13, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Someus, E.; Pugliese, M. Concentrated Phosphorus Recovery from Food Grade Animal Bones. Sustainability 2018, 10, 2349. [Google Scholar] [CrossRef]
- Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (European Commission); Pennington, D.; Tzimas, E.; Baranzelli, C.; Jo, D.; Simone, M.; Philip, N.; Grohol, M.; Van Maercke, A.; Kayam, Y.; et al. Methodology for Establishing the EU List of Critical Raw Materials—Guidelines; Publications Office of the European Union: Luxembourg, 2017; Available online: https://data.europa.eu/doi/10.2873/769526 (accessed on 5 January 2024).
- Smith, D.; Zoetis, N.M.; Zaki, M. Internet of Animal Health Things (IoAHT) Opportunities and Challenges; University of Cambridge: Cambridge, UK, 2015. [Google Scholar]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef]
- Xu, F.; Ming, X.; Jia, R.; Zhao, M.; Wang, B.; Qiao, Y.; Tian, Y. Effects of operating parameters on products yield and volatiles composition during fast pyrolysis of food waste in the presence of hydrogen. Fuel Process. Technol. 2020, 210, 106558. [Google Scholar] [CrossRef]
- Jo, J.-H.; Kim, S.-S.; Shim, J.-W.; Lee, Y.-E.; Yoo, Y.-S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191. [Google Scholar] [CrossRef]
- Nie, Y.; Hou, Q.; Qian, H.; Bai, X.; Xia, T.; Lai, R.; Yu, G.; Rehman, M.L.U.; Ju, M. Synthesis of mesoporous sulfonated carbon from chicken bones to boost rapid conversion of 5-hydroxymethylfurfural and carbohydrates to 5-ethoxymethylfurfural. Renew. Energy 2022, 192, 279–288. [Google Scholar] [CrossRef]
- Khan, A.M.; Usmani, M.A.; Yasmeen, K.; Ahmed, M.N. Conversion of waste animal bones to biofertilizer and adsorbent for wastewater treatment: An innovative approach to develop zero-waste technology. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Piash, M.I.; Uemura, K.; Itoh, T.; Iwabuchi, K. Meat and bone meal biochar can effectively reduce chemical fertilizer requirements for crop production and impart competitive advantages to soil. J. Environ. Manag. 2023, 336, 117612. [Google Scholar] [CrossRef]
- Côrtes, L.N.; Druzian, S.P.; Streit, A.F.M.; Junior, T.R.S.C.; Collazzo, G.C.; Dotto, G.L. Preparation of carbonaceous materials from pyrolysis of chicken bones and its application for fuchsine adsorption. Environ. Sci. Pollut. Res. 2019, 26, 28574–28583. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, H.; Wang, H.; Key, J.; Ji, S.; Mao, X.; Wang, R. Chicken bone-derived N-doped porous carbon materials as an oxygen reduction electrocatalyst. Electrochim. Acta 2014, 147, 520–526. [Google Scholar] [CrossRef]
- Daabo, A.M.; Saeed, L.I.; Altamer, M.H.; Fadhil, A.B.; Badawy, T. The production of bio-based fuels and carbon catalysts from chicken waste. Renew. Energy 2022, 201, 21–34. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: Two-stage adsorber analysis. J. Environ. Manag. 2018, 209, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Huyen, D.T.; Phat, L.N.; Tien, D.X.; Thu, D.P.G.; Thoai, D.Q. Bone-char from various food-waste: Synthesis, characterization, and removal of fluoride in groundwater. Environ. Technol. Innov. 2023, 32, 103342. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A. Characterization of chicken bone waste-derived hydroxyapatite and its functionality on chitosan membrane for guided bone regeneration. Compos. Part B Eng. 2019, 163, 562–573. [Google Scholar] [CrossRef]
- Niu, C.; Li, S.; Zhou, G.; Wang, Y.; Dong, X.; Cao, X. Preparation and characterization of magnetic modified bone charcoal for removing Cu2+ ions from industrial and mining wastewater. J. Environ. Manag. 2021, 297, 113221. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Shin, G.; Choi, Y. Effect of pyrolysis conditions on characteristics and fluoride adsorptive performance of bone char derived from bone residue. J. Water Process. Eng. 2020, 37, 101499. [Google Scholar] [CrossRef]
- Mărculescu, C.; Alexe, F.; Bacalum, F.; Doncea, S. Alternative fuels production and properties characterization using food industry waste to energy conversion. In Proceedings of the IEEE International Conference on Emerging Technologies and Innovative Business Practices for the Transformation of Societies (EmergiTech), Balaclava, Mauritius, 3–6 August 2016. [Google Scholar]
- Piccirillo, C. Preparation, characterisation and applications of bone char, a food waste-derived sustainable material: A review. J. Environ. Manag. 2023, 339, 117896. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Guan, Y.; Feng, Y. Characteristic of the production of hydrogen-rich combustible gas by pyrolysis of high-ash sewage sludge. J. Clean. Prod. 2021, 334, 130224. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Ayllón, M.; Gea, G.; Murillo, M.; Sánchez, J.; Arauzo, J. Kinetic study of meat and bone meal pyrolysis: An evaluation and comparison of different possible kinetic models. J. Anal. Appl. Pyrolysis 2005, 74, 445–453. [Google Scholar] [CrossRef]
- Skodras, G.; Grammelis, P.; Basinas, P.; Kaldis, S.; Kakaras, E.; Sakellaropoulos, G. A kinetic study on the devolatilisation of animal derived byproducts. Fuel Process. Technol. 2007, 88, 787–794. [Google Scholar] [CrossRef]
- Chaala, A.; Roy, C. Recycling of Meat and Bone Meal Animal Feed by Vacuum Pyrolysis. Environ. Sci. Technol. 2003, 37, 4517–4522. [Google Scholar] [CrossRef]
- Junqueira’s Basic Histology Text and Atlas, 16e|AccessMedicine|McGraw Hill Medical. Available online: https://accessmedicine.mhmedical.com/book.aspx?bookID=3047 (accessed on 3 January 2024).
- Oh, J.-I.; Lee, J.; Lee, T.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Strategic CO 2 utilization for shifting carbon distribution from pyrolytic oil to syngas in pyrolysis of food waste. J. CO2 Util. 2017, 20, 150–155. [Google Scholar] [CrossRef]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; McKnight, S. Effect of pyrolysis conditions on bone char characterization and its ability for arsenic and fluoride removal. Environ. Pollut. 2020, 262, 114221. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of food waste based on its composition through the concept of biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Jia, T.; Zhou, F.; Ma, H.; Zhang, Y. A highly stable waste animal bone-based catalyst for selective nitriles production from biomass via catalytic fast pyrolysis in NH3. J. Anal. Appl. Pyrolysis 2021, 157, 105217. [Google Scholar] [CrossRef]
- Mărculescu, C.; Minca, I.I.; Bacalum, F. Pyrolysis Processing of Organic Residues and Operating Parameters Influence on Products Distribution. 2015. Available online: https://www.researchgate.net/publication/284498496 (accessed on 2 January 2024).
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Dudyński, M.; Kwiatkowski, K.; Bajer, K. From feathers to syngas – Technologies and devices. Waste Manag. 2012, 32, 685–691. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Krzysztoforski, J.; Bajer, K.; Dudyński, M. Gasification of feathers for energy production-a case study. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 28–21 June 2012. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kumpiene, J.; Janiszewska, S.; Kasiński, S.; Pecio, M.; Piec, R.; Radziemska, M. A Mineral By-Product from Gasification of Poultry Feathers for Removing Cd from Highly Contaminated Synthetic Wastewater. Minerals 2020, 10, 1048. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Abdulkareem, S.A.; Adeyanju, C.A.; Iwuozor, K.O.; Ogunniyi, S.; Kawu, K.Y.; Emenike, E.C. Recovery of metallic oxide rich biochar from waste chicken feather. Low-Carbon Mater. Green Constr. 2023, 1, 7. [Google Scholar] [CrossRef]
- Keller, F.; Voss, R.L.; Lee, R.P.; Meyer, B. Life cycle assessment of global warming potential of feedstock recycling technologies: Case study of waste gasification and pyrolysis in an integrated inventory model for waste treatment and chemical production in Germany. Resour. Conserv. Recycl. 2022, 179, 106106. [Google Scholar] [CrossRef]
- Adil, A.; Brijesh; Rao, L. Integrative approach to kinetic modeling and verification of a downdraft gasification model. Bioresour. Technol. Rep. 2024, 25, 101701. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, H.; Yao, D.; Zhang, J.; Zhu, Z.; Wang, Y.; Cui, P. Modeling and comprehensive analysis for novel food waste gasification-based hydrogen production process. SSRN Electron. 2021, 258, 115509. [Google Scholar] [CrossRef]
- Xiong, S.; He, J.; Yang, Z.; Guo, M.; Yan, Y.; Ran, J. Thermodynamic analysis of CaO enhanced steam gasification process of food waste with high moisture and low moisture. Energy 2019, 194, 116831. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.-G.; Kim, Y.T.; Kim, K.-H.; Lee, J. Effect of Pt catalyst on the condensable hydrocarbon content generated via food waste pyrolysis. Chemosphere 2020, 248, 126043. [Google Scholar] [CrossRef]
- Fredriksson, H.O.; Lancee, R.J.; Thüne, P.C.; Veringa, H.J.; Niemantsverdriet, J. Olivine as tar removal catalyst in biomass gasification: Catalyst dynamics under model conditions. Appl. Catal. B Environ. 2013, 130–131, 168–177. [Google Scholar] [CrossRef]
- Varjani, S. Efficient removal of tar employing dolomite catalyst in gasification: Challenges and opportunities. Sci. Total. Environ. 2022, 836, 155721. [Google Scholar] [CrossRef] [PubMed]
- Narnaware, S.L.; Panwar, N.L. Catalysts and their role in biomass gasification and tar abetment: A review. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Review of Catalysts for Tar Elimination in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Wang, L.; Hisada, Y.; Koike, M.; Li, D.; Watanabe, H.; Nakagawa, Y.; Tomishige, K. Catalyst property of Co–Fe alloy particles in the steam reforming of biomass tar and toluene. Appl. Catal. B Environ. 2012, 121–122, 95–104. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Su, H.; Kanchanatip, E.; Wang, D.; Zhang, H.; Antoni; Mubeen, I.; Huang, Z.; Yan, M. Catalytic gasification of food waste in supercritical water over La promoted Ni/Al2O3 catalysts for enhancing H2 production. Int. J. Hydrogen Energy 2019, 45, 553–564. [Google Scholar] [CrossRef]
- Soni, C.G.; Dalai, A.K.; Pugsley, T.; Fonstad, T. Steam gasification of meat and bone meal in a two-stage fixed-bed reactor system. Asia-Pacific J. Chem. Eng. 2011, 6, 71–77. [Google Scholar] [CrossRef]
- Marculescu, C.; Cenuşă, V.; Alexe, F. Analysis of biomass and waste gasification lean syngases combustion for power generation using spark ignition engines. Waste Manag. 2016, 47, 133–140. [Google Scholar] [CrossRef]
- Cascarosa, E.; Boldrin, A.; Astrup, T. Pyrolysis and gasification of meat-and-bone-meal: Energy balance and GHG accounting. Waste Manag. 2013, 33, 2501–2508. [Google Scholar] [CrossRef]
- Cascarosa, E.; Gasco, L.; García, G.; Gea, G.; Arauzo, J. Meat and bone meal and coal co-gasification: Environmental advantages. Resour. Conserv. Recycl. 2012, 59, 32–37. [Google Scholar] [CrossRef]



| Production | Distribution | Consumption | Total | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Primary Production | Processing | Retail | Food Services | Households | |||||
| EU-27, 2021 | Food Waste | (million tonnes) | 5.1 | 12.4 | 4.2 | 5.4 | 31.3 | 58.4 | [7,9] |
| (kg/capita) | 11.0 | 28.0 | 9.0 | 12.0 | 70.0 | 130.0 | |||
| Estimated costs | (EUR billion) | - | - | - | - | - | 132.0 | ||
| USA, 2019 | Food Waste | (million tonnes) | 17 | 11 | 10 | 13 | 30 | 81.0 | [11] |
| (kg/capita) * | 55.0 | 35.6 | 32.3 | 42.0 | 97.0 | 261.9 | |||
| Estimated costs | (EUR billion) | 14 | 35 | 37 | 164 | 37 | 287.0 | ||
| Global, 2019 | Food Waste | (million tonnes) | - | - | 118 | 244 | 569 | 931 | [2,10] |
| (kg/capita) | - | - | 15 | 32 | 74 | 121 | |||
| Estimated costs | (EUR billion) | - | - | - | - | - | 896.5 | ||
| Feedstock | d.b/ a.r/ d.a.f | Elemental Analysis (%) | Proximate Analysis (%) | HHV (MJ/kg) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | VM | W | FC | Ash | ||||
| Pork | d.b | 63.2 | 9.8 | 16.6 | 4.2 | 0.6 | 90.9 | 4.1 | 3.5 | 1.5 | - | [46] |
| Pig bones | d.b | 37.9 | 5.4 | 9.3 | 7.0 | 0.2 | 57.3 | 0.9 | 2.6 | 39.3 | - | |
| Pork | d.b. | 43.6 | 8.1 | 33.9 | 9.8 | 1.0 | 83.6 | - | 12.6 | 3.8 | 19.6 | [47] |
| Cattle bones | d.b. | 32.9 | 8.0 | 55.9 | 3.2 | - | 28.9 | 4.1 | - | 64.7 | - | [48] |
| Mix bones | d.b. | 37.3 | 5.6 | 24.4 | 6.1 | 0.3 | 54.1 | 3.7 | 2.9 | 39.3 | 16.9 | [49] |
| Mixed bones | d.b. | 29.2 | 4.1 | 20.2 | 3.3 | 0.3 | 52.9 | 4.8 | 3.4 | 39.0 | 11.9 | |
| Mixed meat and bones | d.b. | 37.1 | 5.5 | 32.9 | 5.7 | - | 70.5 | - | 10.7 | 18.8 | - | [50] |
| Meat Meal | a.r d.a.f. | 57.2 | 10.3 | 23.8 | 8.1 | 0.4 | 84.3 | 8.1 | 5.5 | 2.2 | 25.2 | [51] |
| Meat Meal | m.a.b | 42.5 | 6.6 | - | 9.1 | 0.0 | 71.7 | 1.0 | 5.4 | 21.5 | 18.1 | [52] |
| Bone Meal | m.a.b | 27.0 | 4.0 | - | 4.6 | 0.0 | 65.3 | 1.0 | 3.7 | 30.0 | 9.5 | |
| Bone Meal | a.r d.a.f. | 58.8 | 9.0 | 22.0 | 9.7 | 0.3 | 49.1 | 4.2 | 7.3 | 39.5 | 5.8 | [51] |
| MBM | a.r d.a.f. | 57.8 | 9.8 | 23.1 | 8.7 | 0.3 | 54.1 | 5.5 | 15.3 | 25.1 | 4.3 | |
| MBM | d.b | 45.9 | 6.4 | 38.4 | 8.9 | 0.4 | 71.5 | 1.4 | 9.7 | 17.5 | - | [53] |
| MBM | d.b. | 46.3 | 6.6 | 36.4 | 9.7 | 1.0 | 73.8 | 4.5 | 7.8 | 18.3 | 17.1 | [54] |
| MBM | d.b. | 41.5 | 6.5 | 20.9 | 4.3 | 0 | 67 | 7 | 28 | [55] | ||
| MBM | d.b. | 43.1 | 6.0 | 15.6 | 9.2 | 1.3 | 63.3 | 2.5 | 12.7 | 23.9 | 18.6 | [56] |
| MBM | d.a.f. | 60.7 | 5.5 | 24.9 | 7.5 | 1.4 | 58.4 | 2.5 | 30.1 | 9.0 | - | [57] |
| MBM char | d.a.f. | 64.1 | 6.1 | 24.0 | 5.1 | 0.7 | 2.3 | 1.3 | 77.3 | 20.1 | - | |
| Feedstock | Temperature | Reactor Type | Yield Distribution (%) | Products | References | |
|---|---|---|---|---|---|---|
| Solid | Liquid | |||||
| Chicken bones | 800 °C | Quartz tube furnace | - | - | N-doped porous carbon | [88] |
| Chicken bones | 300 °C | Steel container placed in an electric oven | 84.8 | - | P Fertilizers | [84] |
| 500 °C | 55.0 | - | ||||
| 700 °C | 55.0 | - | ||||
| 900 °C | 48.0 | - | ||||
| Sheep bones | 300 °C | 71.3 | - | |||
| 500 °C | 58.7 | - | ||||
| 700 °C | 54.0 | - | ||||
| 900 °C | 52.0 | - | ||||
| Pig bones | 300 °C | 78.6 | - | |||
| 500 °C | 75.0 | - | ||||
| 700 °C | 72.0 | - | ||||
| 900 °C | 58.0 | - | ||||
| Chicken skin, fat, and bones | 400 °C | Cylindrical stainless steel pyrolizer enveloped by an electrical furnace | - | 45.33 | A new carbon-based catalyst (CBC) | [89] |
| 500 °C | - | 61.60 | ||||
| 600 °C | - | - | ||||
| Chicken bones | 800 °C | Bench reactor, operating in a batch system. | 64.62 | - | Activated carbon and biochar, which were used to remove basic fuchsine from aqueous solutions. | [87] |
| Chicken bones | 500 °C | - | - | - | Mesoporous sulfonated carbon materials | [84] |
| 700 °C | ||||||
| 900 °C | ||||||
| Chicken bones | 500 °C | Nabertherm; LE 1/11, Germany | - | - | Magnetite-modified chicken bone biochar (MCB) | [90] |
| Chicken bones | 200 °C | Muffle furnace | - | - | Biofertilizer Nano-bio-adsorbent | [85] |
| 400 °C | ||||||
| 600 °C | ||||||
| 800 °C | ||||||
| 1000 °C | ||||||
| Chicken bones | 30 °C to 600 °C | Muffle furnace (Nabertherm, model LE2/11/R7, Germany) | - | - | Chicken bones biochar for fluoride elimination | [91] |
| Chicken bones | 600 °C | - | - | - | Asymmetric resorbable membrane based on a hybrid of chitosan and natural HAp for guided bone regeneration | [92] |
| 700 °C | ||||||
| 800 °C | ||||||
| 900 °C | ||||||
| 1000 °C | ||||||
| Chicken bones | 500 °C | Muffle furnace | - | - | Adsorbent material with high efficiency in removing Cu2+ from wastewater. | [93] |
| Chicken bones | 300 °C | Muffle furnace (Model KT44-13B, Kastech, Korea) | - | - | Adsorbents used for a real application in groundwater | [94] |
| 400 °C | ||||||
| Cattle bones | 300 °C | |||||
| 400 °C | ||||||
| Cattle bones | 350 °C | Tube furnace (HTF-Q70, Hantech, Korea) | ||||
| 400 °C | ||||||
| 500 °C | ||||||
| 600 °C | ||||||
| 700 °C | ||||||
| MBM | 300 °C | Electrically heated tubular batch reactor | - | - | Liquid hydrocarbon-based fuel | [95] |
| 350 °C | ||||||
| 400 °C | ||||||
| 450 °C | ||||||
| 500 °C | ||||||
| 550 °C | ||||||
| 600 °C | ||||||
| MBM | 500 °C | Muffle furnace (FO810, Yamato Scientific, Japan) | 48.40 | - | Organic fertilizer | [86] |
| 800 °C | 42.99 | - | ||||
| 1000 °C | 42.67 | - | ||||
| Feedstock | Temperature | Reactor Type | Agent | Yield Distribution (%) | Results | References | ||
|---|---|---|---|---|---|---|---|---|
| Solid | Liquid | Gas | ||||||
| MBM | 800 °C | Thermogravimetric analyzer | 60% H2O 40% N2 | - | - | - | MBM char has high gasification selectivity | [57] |
| MBM | 650–850 °C | Fixed-bed reactor | N2 and O2 | 25–19 | 50–40 | 25–30 | Two-stage system increases H2 and gas yield | [54] |
| Fixed-bed reactor couple with tar-cracking reactor two-stage fixed-bed reaction | 18–19 | 25–19 | 42–55 | |||||
| MBM | 650–850 °C | Two-stage steam gasification | steam/MBM (wt/wt) 0.4–0.8 | 14.1–21.7 | 52.2–57.9 | 8.7–18.1 | With the increase in steam/MBM ratio, the amounts of H2 and gas increase | [125] |
| Pork bones and meat residues | 650–800 °C | Tubular batch | Air | - | - | - | - | [126] |
| Coal and MBM (99:1 weight) | 800 °C/ Silica bed | Fluidized bed | Air | - | - | - | - | [127] |
| Coal and MBM | 800–900 °C | Fluidized bed | Air | 70 | - | - | The addition of 1%wt of MBM to coal has no effect on CO and H2 production | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macavei, M.G.; Gheorghe, V.-C.; Ionescu, G.; Volceanov, A.; Pătrașcu, R.; Mărculescu, C.; Magdziarz, A. Thermochemical Conversion of Animal-Derived Waste: A Mini-Review with a Focus on Chicken Bone Waste. Processes 2024, 12, 358. https://doi.org/10.3390/pr12020358
Macavei MG, Gheorghe V-C, Ionescu G, Volceanov A, Pătrașcu R, Mărculescu C, Magdziarz A. Thermochemical Conversion of Animal-Derived Waste: A Mini-Review with a Focus on Chicken Bone Waste. Processes. 2024; 12(2):358. https://doi.org/10.3390/pr12020358
Chicago/Turabian StyleMacavei, Mircea Gabriel, Virginia-Cora Gheorghe, Gabriela Ionescu, Adrian Volceanov, Roxana Pătrașcu, Cosmin Mărculescu, and Aneta Magdziarz. 2024. "Thermochemical Conversion of Animal-Derived Waste: A Mini-Review with a Focus on Chicken Bone Waste" Processes 12, no. 2: 358. https://doi.org/10.3390/pr12020358
APA StyleMacavei, M. G., Gheorghe, V.-C., Ionescu, G., Volceanov, A., Pătrașcu, R., Mărculescu, C., & Magdziarz, A. (2024). Thermochemical Conversion of Animal-Derived Waste: A Mini-Review with a Focus on Chicken Bone Waste. Processes, 12(2), 358. https://doi.org/10.3390/pr12020358










