Characteristics of Molten Salt Gasification of Waste PVC
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
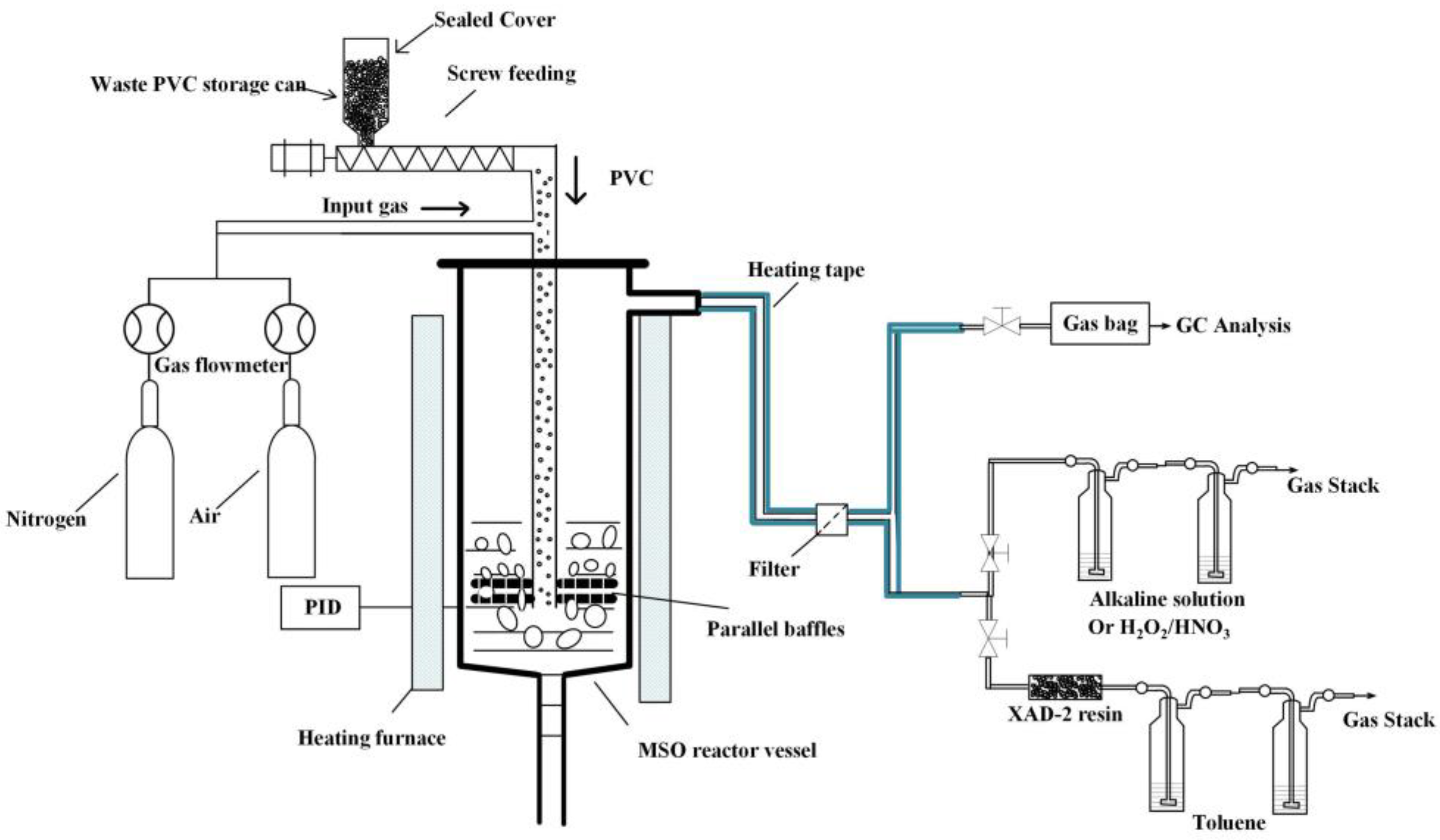
2.2. Experiment Setup and Procedure
2.3. Methods of Sampling and Analysis
3. Results
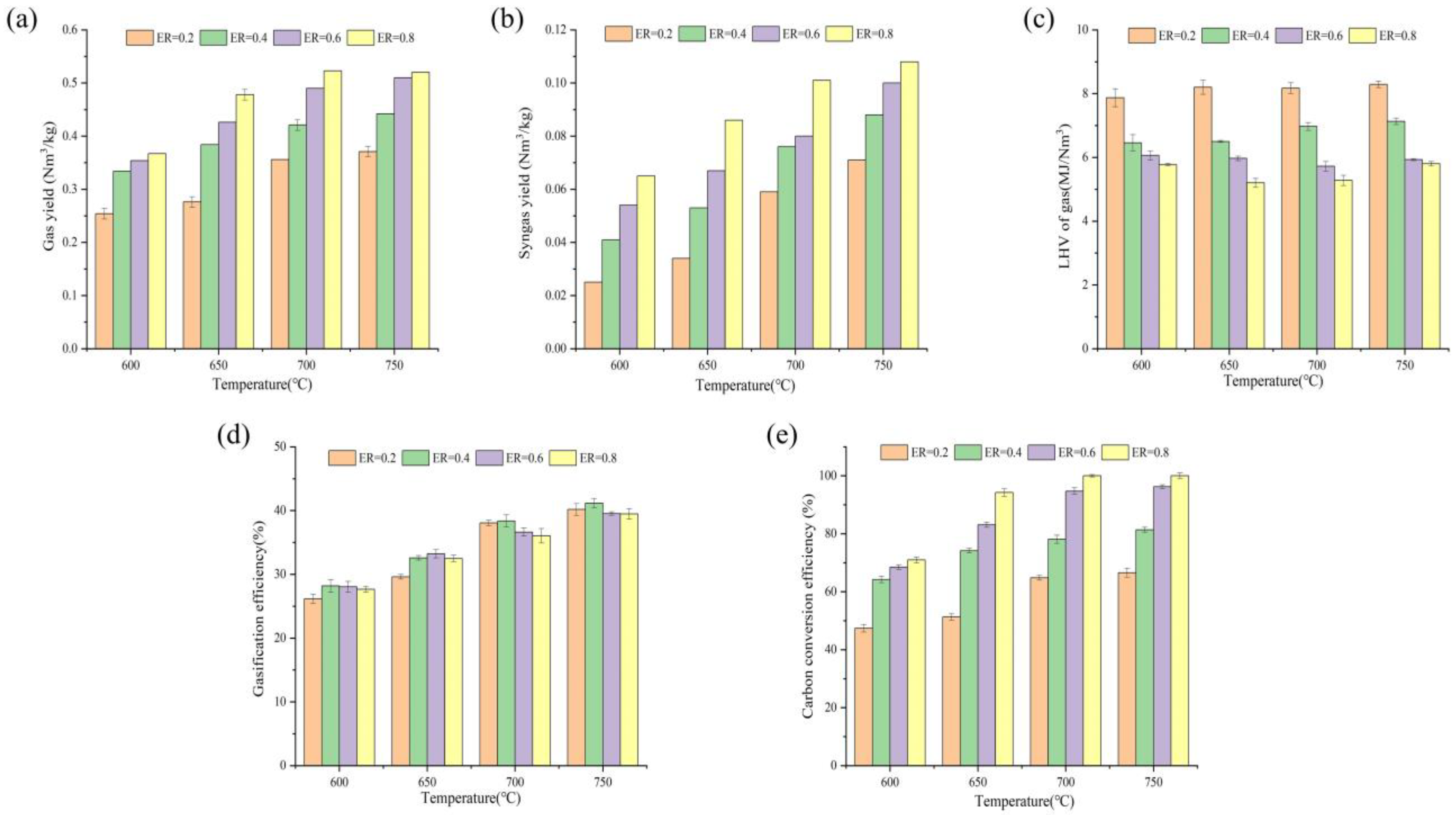
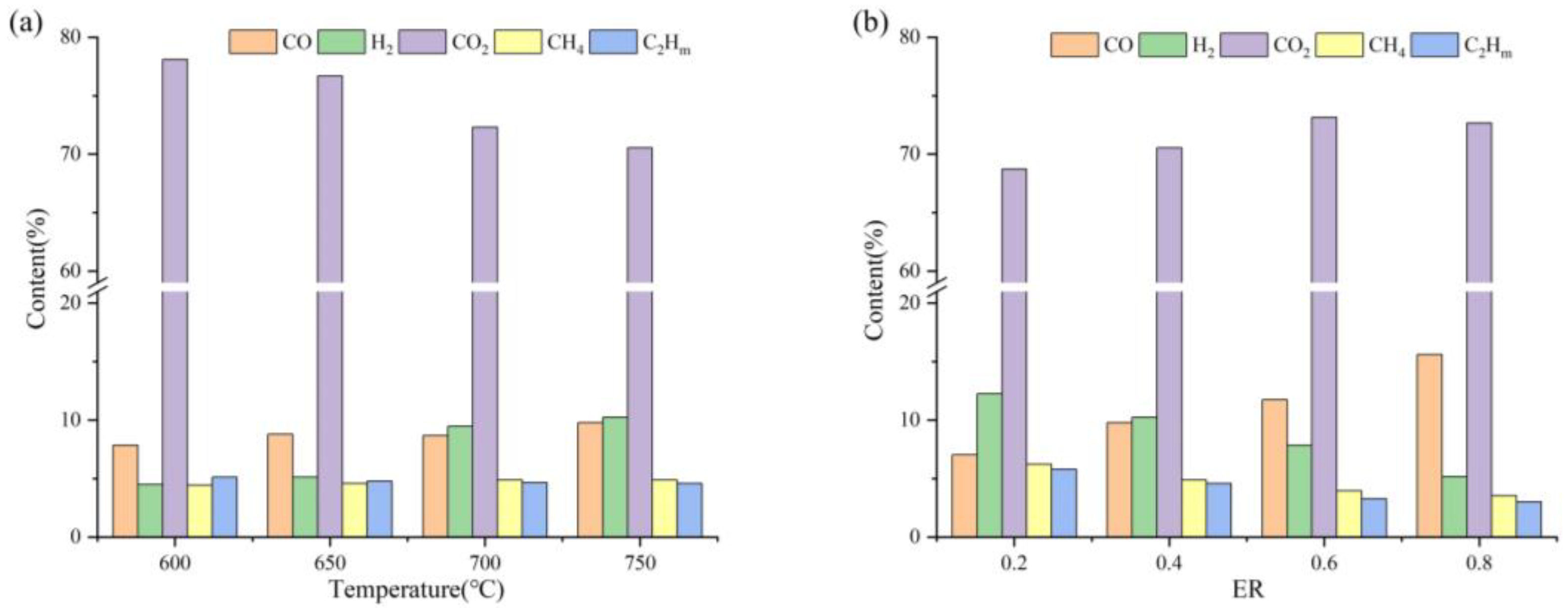
3.1. Gasification Characteristics of PVC during MSG
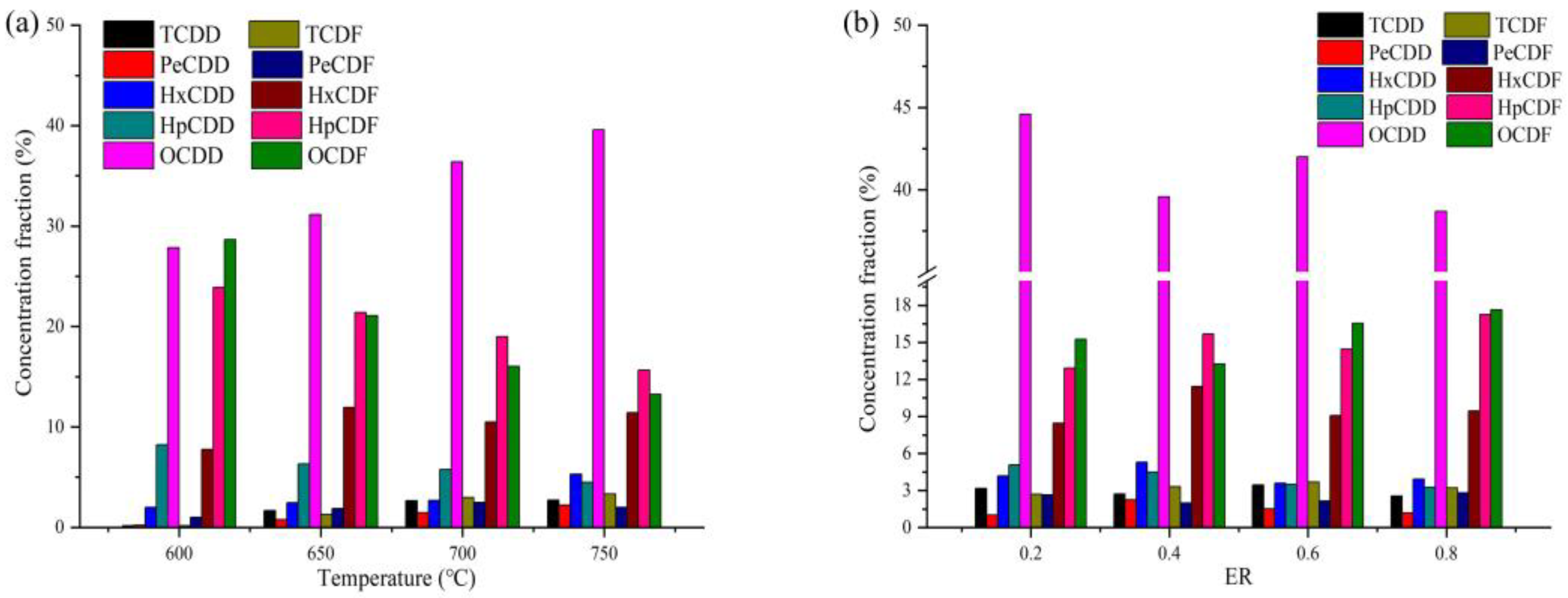
3.2. Chlorine Retention and PCDD/F Generation
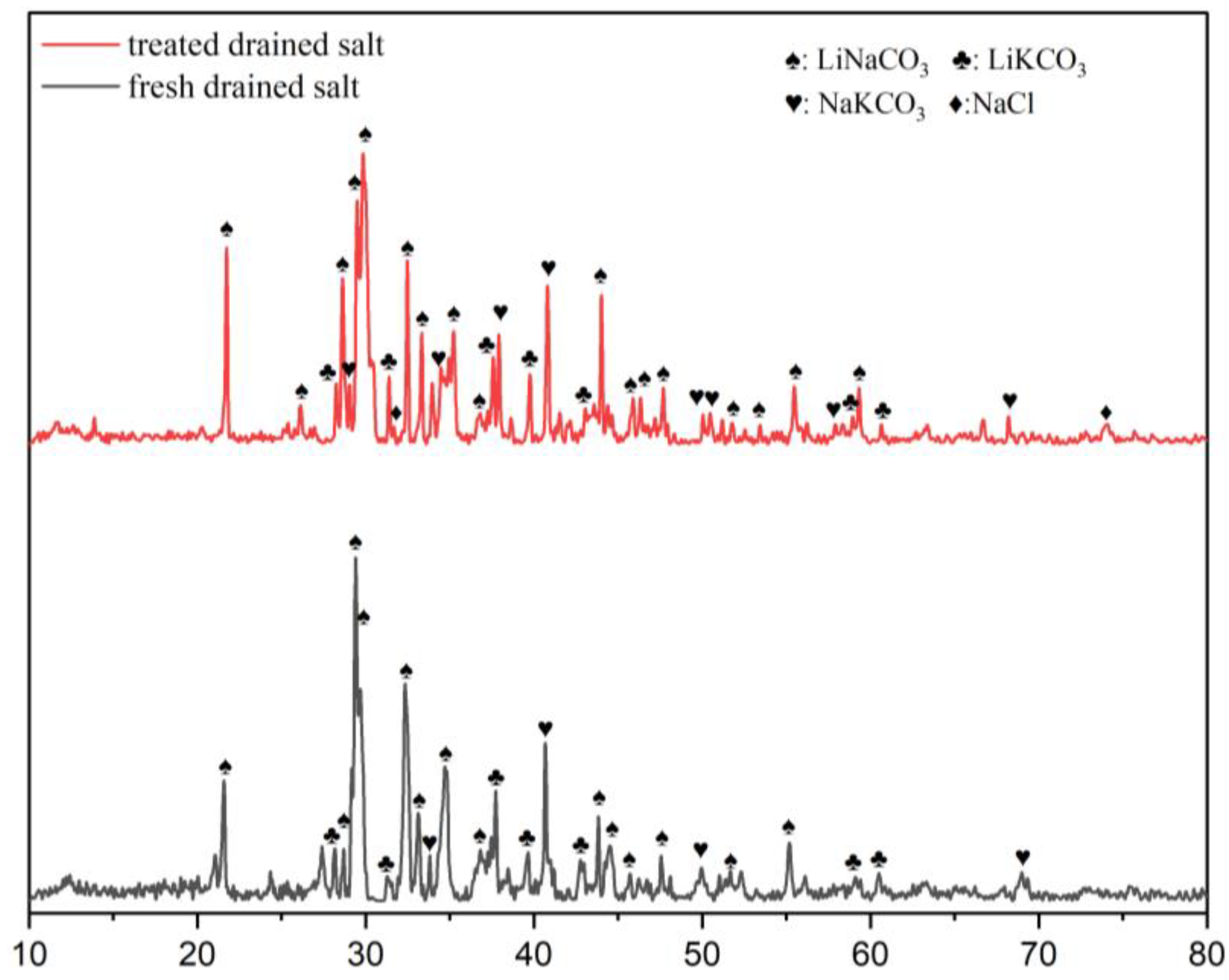
3.3. Heavy Metals Entrainment and Drained Salt
3.4. Reaction Mechanism Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, B.; Zhu, M. An overview on the recycling of waste poly(vinyl chloride). Green. Chem. 2023, 25, 6971–7025. [Google Scholar] [CrossRef]
- Hapipi, A.M.; Suda, H.; Uddin, M.A.; Kato, Y. Dechlorination of Polyvinyl Chloride under Superheated Steam with Catalysts and Adsorbents. Energy Fuels 2018, 32, 7792–7799. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Manag. 2023, 166, 245–258. [Google Scholar] [CrossRef]
- Castro, A.; Soares, D.; Vilarinho, C.; Castro, F. Kinetics of thermal de-chlorination of PVC under pyrolytic conditions. Waste Manag. 2012, 32, 847–851. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J.Y. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas–liquid fluidized bed reactor. Waste Manag. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Han, X.; Zhang, X.; Yi, M.; Yang, S.; Yu, D.; Liu, W. Catalytic Dechlorination and Charring Reaction of Polyvinyl Chloride by CuAl Layered Double Hydroxide. Energy Fuels 2018, 32, 2407–2413. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Angyal, A. Pyrolysis of Polyvinyl Chloride (PVC)-Containing Mixed Plastic Wastes for Recovery of Hydrocarbons. Energy Fuels 2009, 23, 2743–2749. [Google Scholar] [CrossRef]
- Slapak, M.J.P.; van Kasteren, J.M.N.; Drinkenburg, A.A.H. Design of a process for steam gasification of PVC waste. Resour. Conserv. Recycl. 2000, 30, 81–93. [Google Scholar] [CrossRef]
- Blazsó, M.; Zelei, B.; Jakab, E. Thermal decomposition of low-density polyethylene in the presence of chlorine-containing polymers. J. Anal. Appl. Pyrol 1995, 35, 221–235. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water. Processes 2022, 10, 1940. [Google Scholar] [CrossRef]
- Glas, D.; Hulsbosch, J.; Dubois, P.; Binnemans, K.; De Vos, D.E. End-of-Life Treatment of Poly(Vinyl Chloride) and Chlorinated Polyethylene by Dehydrochlorination in Ionic Liquids. Chemsuschem 2014, 7, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.; Yamaye, M.; Kito, T.; Ichiki, T.; Ogata, M.; Chen, J.; Fujino, H.; Tanimura, T.; Yamanobe, T. Alkaline dechlorination of poly(vinyl chloride) in organic solvents under mild conditions. Polym. Degrad. Stabil. 2004, 86, 541–547. [Google Scholar] [CrossRef]
- Tongamp, W.; Kano, J.; Zhang, Q.; Saito, F. Simultaneous treatment of PVC and oyster-shell wastes by mechanochemical means. Waste Manag. 2008, 28, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Zabłocka-Malicka, M.; Rutkowski, P.; Szczepaniak, W. Recovery of copper from PVC multiwire cable waste by steam gasification. Waste Manag. 2015, 46, 488–496. [Google Scholar] [CrossRef]
- Cho, M.; Mun, T.; Kim, J. Air gasification of mixed plastic wastes using calcined dolomite and activated carbon in a two-stage gasifier to reduce tar. Energy 2013, 53, 299–305. [Google Scholar] [CrossRef]
- Cho, M.; Choi, Y.; Kim, J. Air gasification of PVC (polyvinyl chloride)-containing plastic waste in a two-stage gasifier using Ca-based additives and Ni-loaded activated carbon for the production of clean and hydrogen-rich producer gas. Energy 2015, 87, 586–593. [Google Scholar] [CrossRef]
- Borgianni, C.; De Filippis, P.; Pochetti, F.; Paolucci, M. Gasification process of wastes containing PVC. Fuel 2002, 81, 1827–1833. [Google Scholar] [CrossRef]
- Sugiura, K.; Minami, K.; Yamauchi, M.; Morimitsu, S.; Tanimoto, K. Gasification characteristics of organic waste by molten salt. J. Power Sources 2007, 171, 228–236. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Honda, M.; Kittelson, D.B.; Davidson, J.H. Steam gasification of plant biomass using molten carbonate salts. Energy 2013, 49, 211–217. [Google Scholar] [CrossRef]
- Flandinet, L.; Tedjar, F.; Ghetta, V.; Fouletier, J. Metals recovering from waste printed circuit boards (WPCBs) using molten salts. J. Hazard. Mater. 2012, 213–214, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Zhao, X. Molten salt oxidation: A versatile and promising technology for the destruction of organic-containing wastes. Chemosphere 2011, 84, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Volkovich, V.A.; Griffiths, T.R.; Fray, D.J.; Thied, R.C. A new method for determining oxygen solubility in molten carbonates and carbonate–chloride mixtures using the oxidation of UO2 to uranate reaction. J. Nucl. Mater. 2000, 282, 152–158. [Google Scholar] [CrossRef]
- Yang, H.; Cho, Y.; Eun, H.; Yoo, J.; Kim, J. Behavior of Toxic Metals and Radionuclides During Molten Salt Oxidation of Chlorinated Plastics. J. Environ. Sci. Health Part A 2004, 39, 1601–1616. [Google Scholar] [CrossRef]
- Hsu, P.C.; Foster, K.G.; Ford, T.D.; Wallman, P.H.; Watkins, B.E.; Pruneda, C.O.; Adamson, M.G. Treatment of solid wastes with molten salt oxidation. Waste Manag. 2000, 20, 363–368. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Takeda, O.; Hoshi, M.; Yamamura, T.; Sato, Y. Decomposition of Carbon Tetrachloride and Mono-Chlorobenzen by Using Basic Molten Salts. Electrochemistry 2012, 80, 974–979. [Google Scholar] [CrossRef][Green Version]
- Yao, Z.; Zhao, X.; Li, J. Study on 1,2,3-trichlorobenzene destruction in a binary (Na,K)2CO3 molten salt oxidation system. Environ. Prog. Sustain. 2014, 33, 65–69. [Google Scholar] [CrossRef]
- Yang, H.; Cho, Y.; Eun, H.; Kim, E. Destruction of chlorobenzene and carbon tetrachloride in a two-stage molten salt oxidation reactor system. Chemosphere 2008, 73 (Suppl. 1), S311–S315. [Google Scholar] [CrossRef]
- Pandeti, S.; Buckley, S.G. Molten salt oxidation of chlorobenzene. Combust. Sci. Technol. 2004, 176, 257–276. [Google Scholar] [CrossRef]
- Yang, H.; Cho, Y.; Yun, J.; Kim, J. Destruction of Halogenated Plastics in a Molten Salt Oxidation Reactor. Can. J. Chem. Eng. 2003, 81, 713–718. [Google Scholar] [CrossRef]
- Lin, C.; Chi, Y.; Jin, Y. Experimental Study on Treating Waste Printed Circuit Boards by Molten Salt Oxidation. Waste Biomass Valori 2017, 8, 2523–2533. [Google Scholar] [CrossRef]
- Lin, C.; Chi, Y.; Jin, Y.; Jiang, X.; Buekens, A.; Zhang, Q.; Chen, J. Molten salt oxidation of organic hazardous waste with high salt content. Waste Manag. Res. 2018, 36, 140–148. [Google Scholar] [CrossRef] [PubMed]
- GB/T 212-2008; Proximate Analysis of Coal. Standardization Administration of the P.R.C.: Beijing, China, 2008.
- GB/T 476-2008; Determination of Carbon and Hydrogen in Coal. Standardization Administration of the P.R.C.: Beijing, China, 2008.
- GB/T 19227-2008; Determination of Nitrogen in Coal. Standardization Administration of the P.R.C.: Beijing, China, 2008.
- GB/T 214-2007; Determination of Total Sulfur in Coal. Standardization Administration of the P.R.C.: Beijing, China, 2007.
- Yao, Z.; Li, J.; Zhao, X. Destruction of decabromodiphenyl ether (BDE-209) in a ternary carbonate molten salt reactor. J. Environ. Manag. 2013, 127, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chi, Y.; Tang, Y.; Ni, M.; Nzihou, A.; Weiss-Hortala, E.; Huang, Q. Effect of Operating Parameters and Moisture Content on Municipal Solid Waste Pyrolysis and Gasification. Energy Fuels 2016, 30, 3994–4001. [Google Scholar] [CrossRef]
- Lin, C.; Wang, J.; Huang, Q.; Wang, S.; Jin, J.; Jin, Y.; Chi, Y. Chemical looping gasification of biomass using rare earth oxides doped ferric oxide oxygen carrier for hydrogen-rich syngas production. Can. J. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Buekens, A.; Olie, K.; Li, X. PCDD/F catalysis by metal chlorides and oxides. Chemosphere 2016, 159, 536–544. [Google Scholar] [CrossRef]
- Xiao, G.; Chi, Y.; Nie, M.; Zhang, J.; Miao, Q.; Shu, W.; Cen, K. Experiments of PVC gasification in a fluidized bed. J. Fuel Chem. Technol. 2005, 33, 708–713. [Google Scholar]
- Zhang, M.; Fujimori, T.; Shiota, K.; Li, X.; Takaoka, M. Formation pathways of polychlorinated dibenzo-p-dioxins and dibenzofurans from burning simulated PVC-coated cable wires. Chemosphere 2021, 264, 128542. [Google Scholar] [CrossRef]
- Katami, T.; Yasuhara, A.; Okuda, T.; Shibamoto, T. Formation of PCDDs, PCDFs, and Coplanar PCBs from Polyvinyl Chloride during Combustion in an Incinerator. Environ. Sci. Technol. 2002, 36, 1320–1324. [Google Scholar] [CrossRef]
- Yasuhara, A.; Katami, T.; Okuda, T.; Ohno, N.; Shibamoto, T. Formation of Dioxins during the Combustion of Newspapers in the Presence of Sodium Chloride and Poly(vinyl chloride). Environ. Sci. Technol. 2001, 35, 1373–1378. [Google Scholar] [CrossRef] [PubMed]


| Proximate analysis (wt.%) | Moisture | Ash | Volatiles | Fixed carbon | Total |
| 0.43 | 37.85 | 49.90 | 11.82 | 100 | |
| Ultimate analysis (wt.%) | C | H | N | S | O |
| 27.64 | 2.22 | 0.79 | 0.35 | 19.11 | |
| High heating value (J/g) | Cl (wt. %) | Cu (mg/g) | Pb (mg/g) | Zn (mg/g) | |
| 7651 | 11.61 | 0.39 | 2.48 | 12.69 |
| Temperature/ER | 600 °C | 650 °C | 700 °C | 750 °C |
|---|---|---|---|---|
| ER = 0.2 | 99.65 ± 0.08 | 99.78 ± 0.10 | 99.81 ± 0.05 | 99.65 ± 0.11 |
| ER = 0.4 | 99.75 ± 0.06 | 99.92 ± 0.05 | 99.71 ± 0.08 | 99.77 ± 0.17 |
| ER = 0.6 | 99.84 ± 0.07 | 99.70 ± 0.14 | 99.72 ± 0.11 | 99.78 ± 0.10 |
| ER = 0.8 | 99.71 ± 0.15 | 99.79 ± 0.06 | 99.83 ± 0.12 | 99.61 ± 0.13 |
| Conditions | PCDD/F (pg/g PVC) | I-TEQ (pg/g PVC) | PCDF/ PCDD | Cl-PCDD | Cl-PCDF | Cl-PCDD/F |
|---|---|---|---|---|---|---|
| 600 °C, ER = 0.4 | 632.83 ± 15.21 | 11.98 ± 0.64 | 1.60 ± 0.13 | 7.65 ± 0.12 | 7.30 ± 0.21 | 7.43 ± 0.19 |
| 650 °C, ER = 0.4 | 42.38 ± 1.82 | 1.95 ± 0.08 | 1.36 ± 0.11 | 7.52 ± 0.19 | 7.02 ± 0.10 | 7.23 ± 0.16 |
| 700 °C, ER = 0.4 | 20.94 ± 1.77 | 1.35 ± 0.13 | 1.04 ± 0.19 | 7.47 ± 0.20 | 6.85 ± 0.19 | 7.15 ± 0.19 |
| 750 °C, ER = 0.4 | 18.41 ± 0.86 | 1.38 ± 0.17 | 0.84 ± 0.29 | 7.40 ± 0.17 | 6.75 ± 0.12 | 7.10 ± 0.14 |
| 750 °C, ER = 0.2 | 18.25 ± 1.03 | 1.16 ± 0.09 | 0.72 ± 0.10 | 7.50 ± 0.20 | 6.84 ± 0.11 | 7.22 ± 0.15 |
| 750 °C, ER = 0.6 | 16.75 ± 1.37 | 1.20 ± 0.11 | 0.85 ± 0.13 | 7.46 ± 0.16 | 6.83 ± 0.21 | 7.17 ± 0.17 |
| 750 °C, ER = 0.8 | 18.50 ± 1.82 | 1.13 ± 0.08 | 1.02 ± 0.09 | 7.50 ± 0.29 | 6.86 ± 0.23 | 7.18 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Yang, T.; Chen, S.; Li, M.; Wang, S.; Huang, Q. Characteristics of Molten Salt Gasification of Waste PVC. Processes 2024, 12, 306. https://doi.org/10.3390/pr12020306
Lin C, Yang T, Chen S, Li M, Wang S, Huang Q. Characteristics of Molten Salt Gasification of Waste PVC. Processes. 2024; 12(2):306. https://doi.org/10.3390/pr12020306
Chicago/Turabian StyleLin, Chengqian, Tianfeng Yang, Siyu Chen, Minjie Li, Shoukang Wang, and Qunxing Huang. 2024. "Characteristics of Molten Salt Gasification of Waste PVC" Processes 12, no. 2: 306. https://doi.org/10.3390/pr12020306
APA StyleLin, C., Yang, T., Chen, S., Li, M., Wang, S., & Huang, Q. (2024). Characteristics of Molten Salt Gasification of Waste PVC. Processes, 12(2), 306. https://doi.org/10.3390/pr12020306






