Performance Evaluation of Modified Biochar as a Polycyclic Aromatic Hydrocarbon Adsorbent and Microbial-Immobilized Carrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation and Chemical Reagents
2.2. Bacterial Strain and Biochar-Immobilized Microorganism Preparation
2.3. Batch Adsorption Experiments of Biochar in Aqueous Solution
2.3.1. Kinetic Adsorption Experiments
2.3.2. Isotherm Adsorption Experiments
2.4. Incubation Experiment
3. Results and Discussion
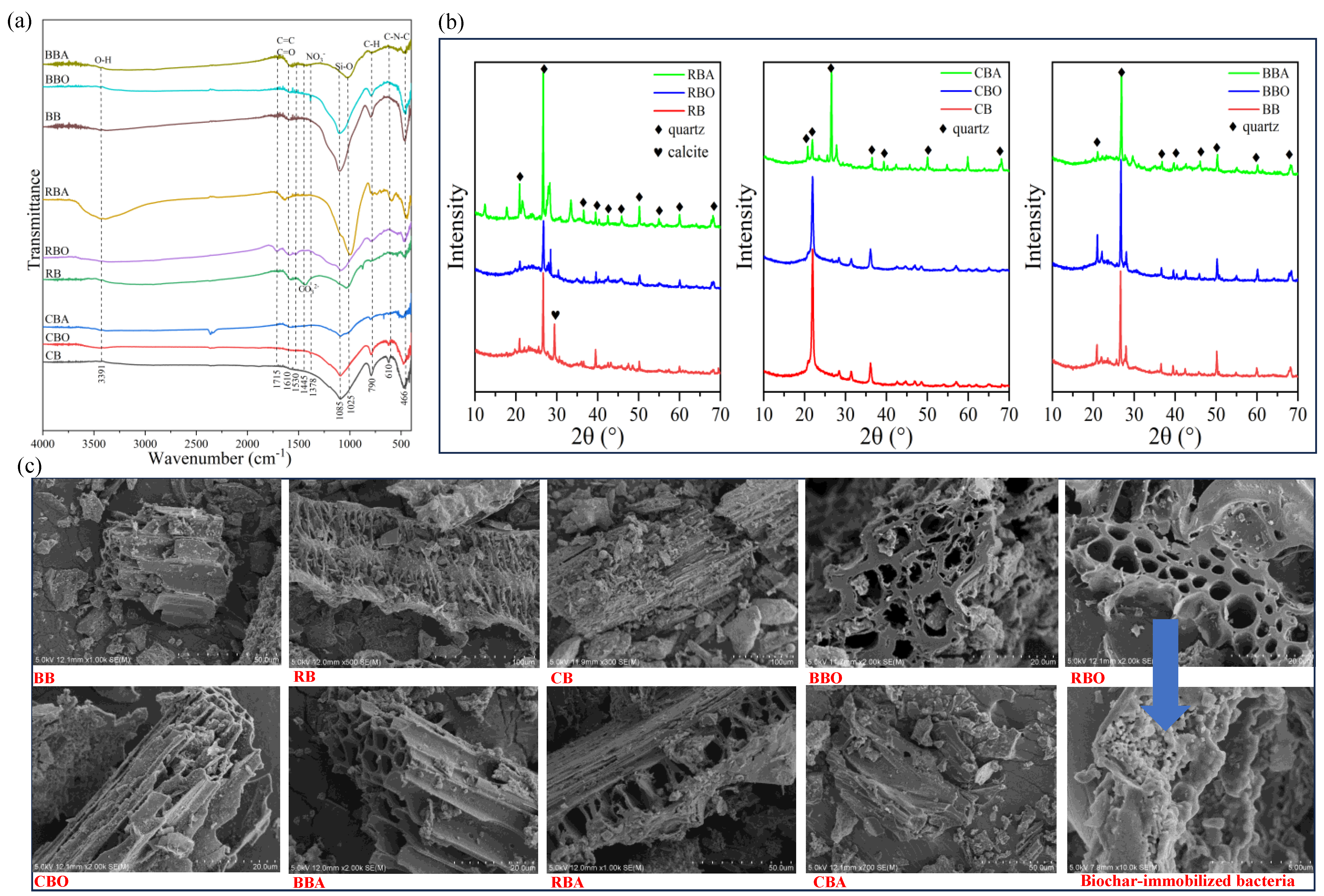
3.1. Characterization of Biochar
3.1.1. Basic Characteristics
3.1.2. Structural Characteristics
3.1.3. Morphological Characteristics
3.2. Adsorption Performance of Biochar
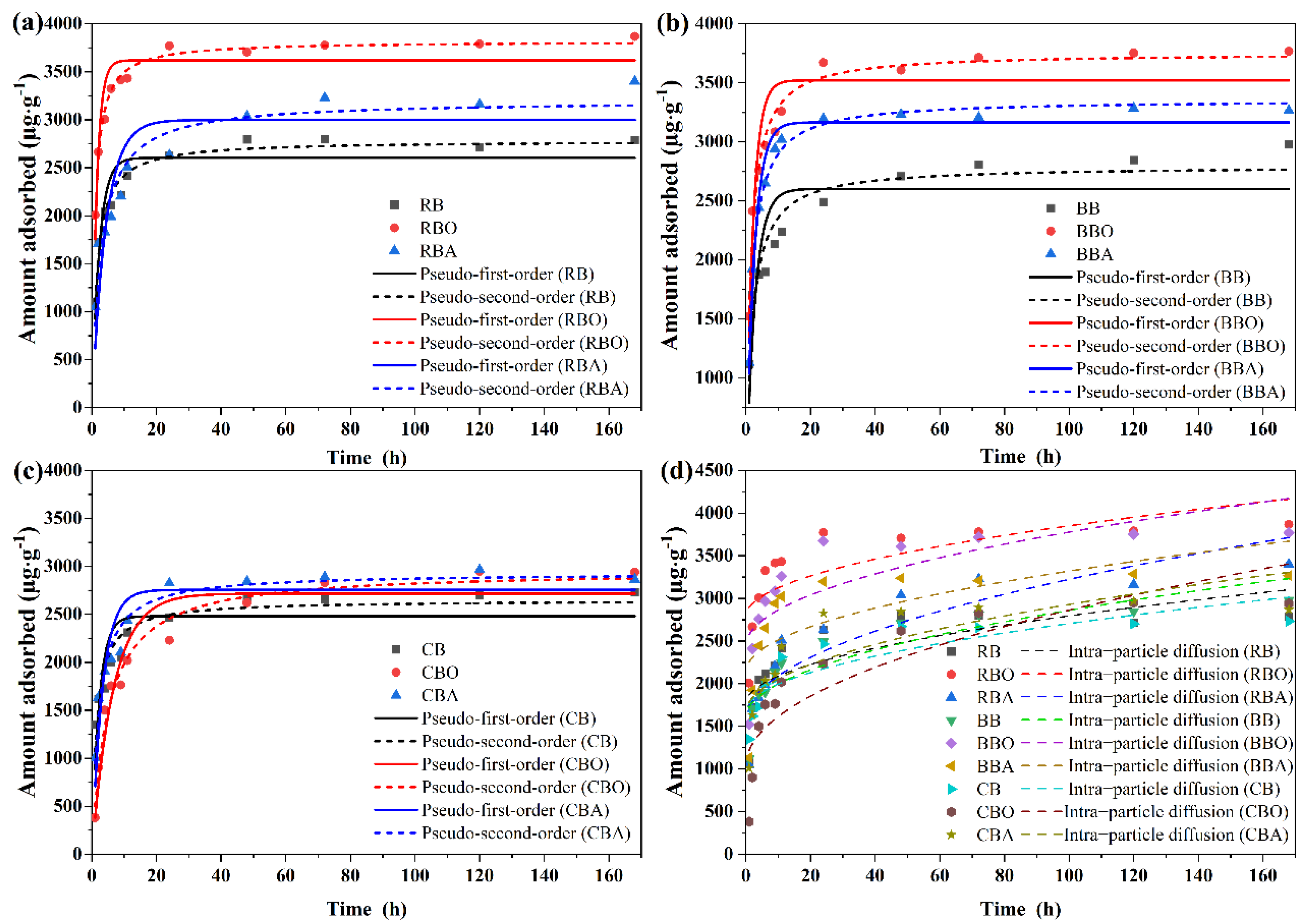
3.2.1. Adsorption Kinetics
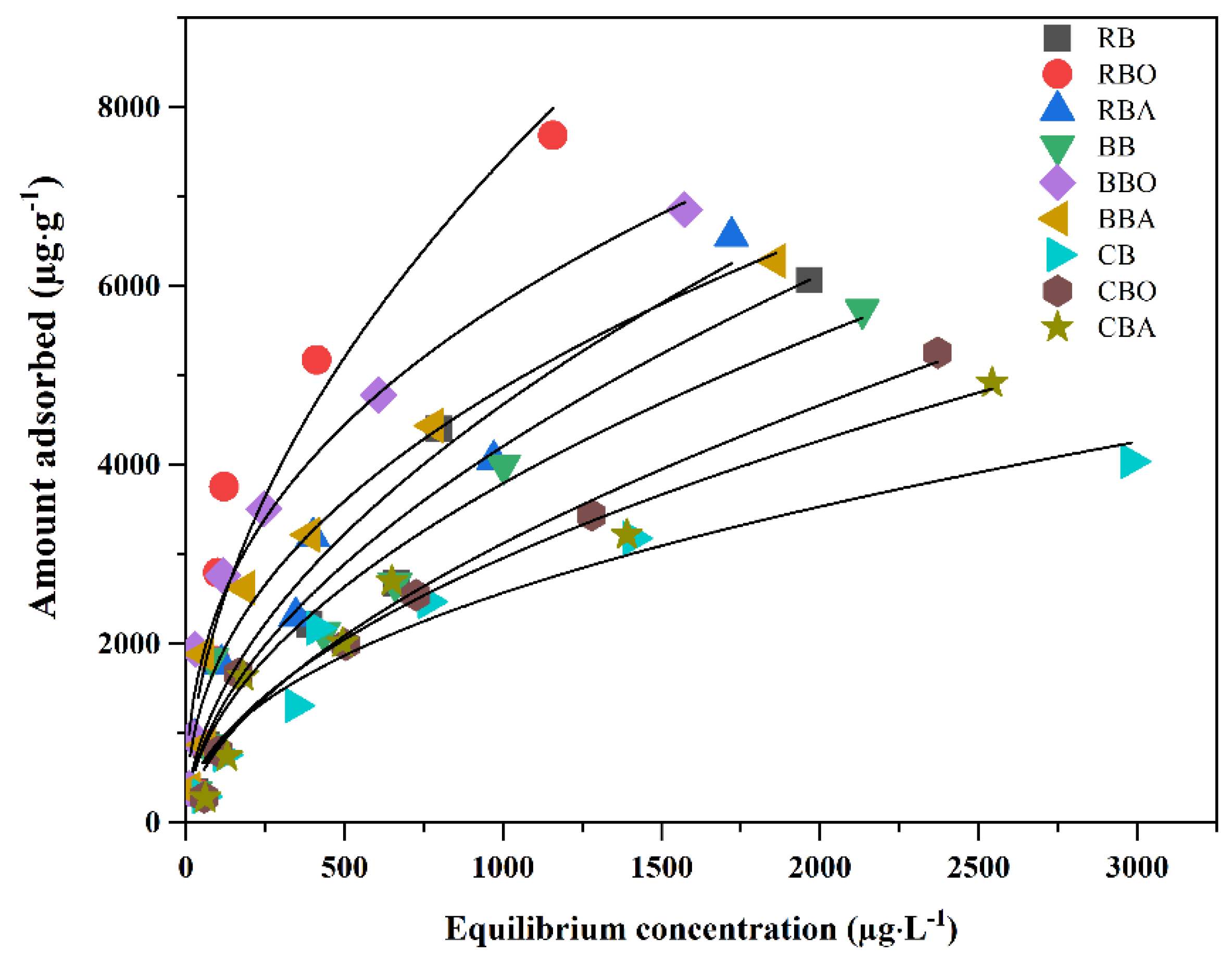
3.2.2. Adsorption Isotherm
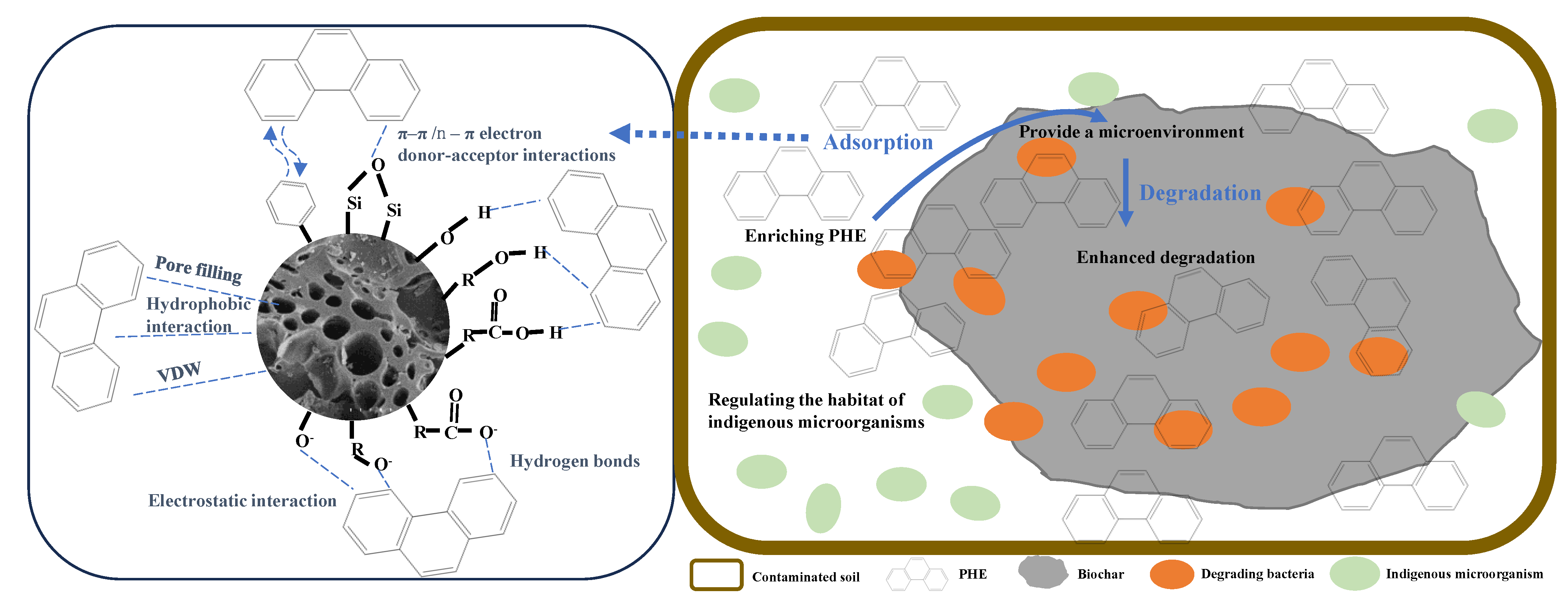
3.2.3. Adsorption Mechanism
3.3. Degradation Performance of Biochar-Immobilized Microorganisms
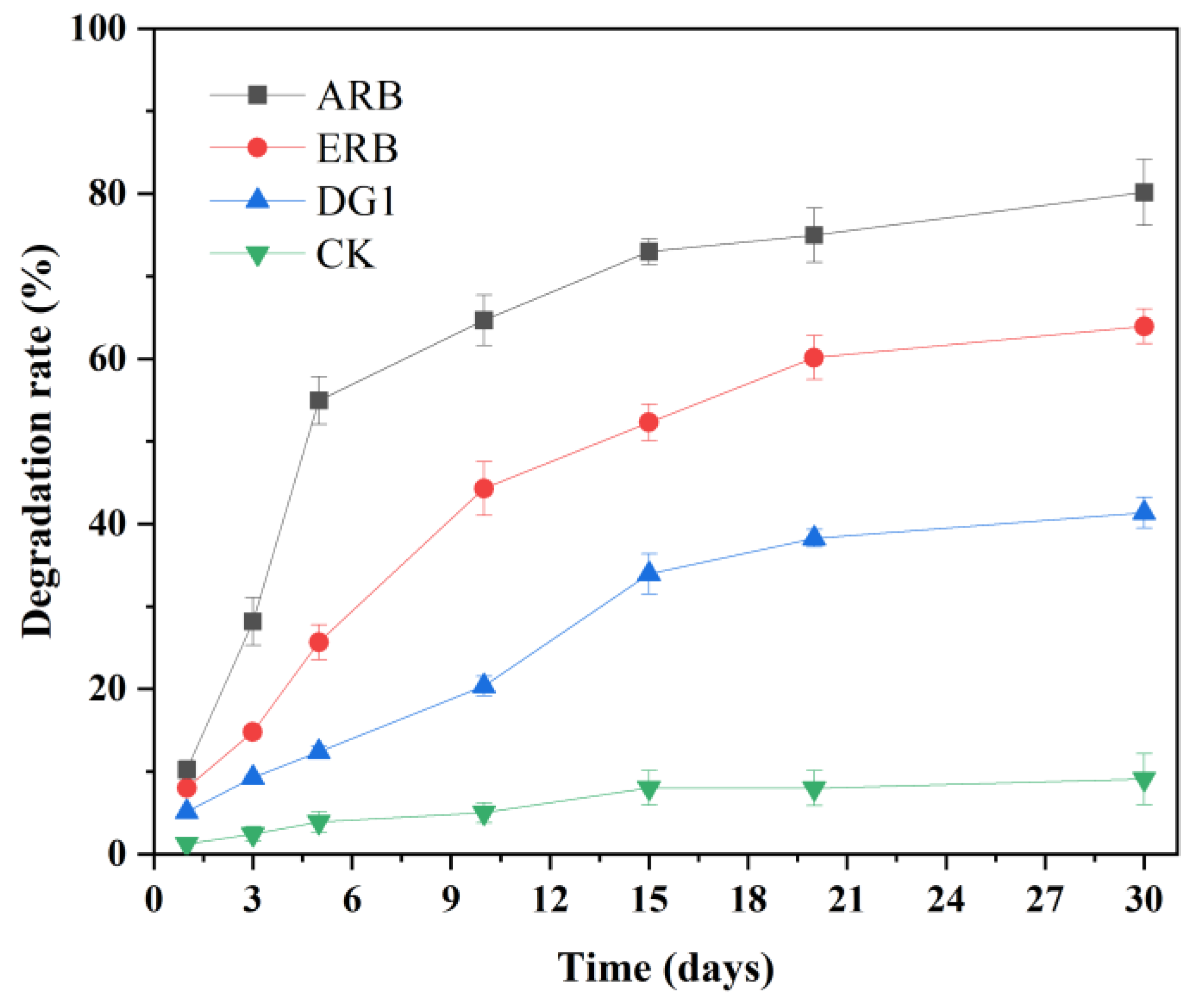
3.3.1. Degradation Performance
3.3.2. Degradation Mechanism
3.4. Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Han, Y.; Duan, Y.; Lai, X.; Fu, R.; Liu, S.; Leong, K.H.; Tu, U.; Zhou, L. Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environ. Res. 2022, 205, 112423. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef]
- Geng, S.; Qin, W.; Cao, W.; Wang, Y.; Ding, A.; Zhu, Y.; Fan, F.; Dou, J. Pilot-scale bioaugmentation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil using an indigenous bacterial consortium in soil-slurry bioreactors. Chemosphere 2022, 287, 132183. [Google Scholar] [CrossRef]
- Wang, H.; Lv, Y.; Bao, J.; Chen, Y.; Zhu, L. Petroleum-contaminated soil bioremediation and microbial community succession induced by application of co-pyrolysis biochar amendment: An investigation of performances and mechanisms. J. Hazard. Mater. 2024, 466, 133600. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tan, X.; Ali, I.; Duan, Z.; Naz, I.; Cao, J.; Ruan, Y.; Wang, Y. More effective application of biochar-based immobilization technology in the environment: Understanding the role of biochar. Sci. Total Environ. 2023, 872, 162021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, Y.H.; Gao, Y.Z.; Zhou, X.; Sun, Y.; Wang, T.; Tang, L.; Ling, W.; Mosa, A.; Wang, J.; et al. Remediation potential of an immobilized microbial consortium with corn straw as a carrier in polycyclic aromatic hydrocarbons contaminated soil. J. Hazard. Mater. 2024, 469, 134091. [Google Scholar] [CrossRef] [PubMed]
- Dike, C.C.; Hakeem, I.G.; Rani, A.; Surapaneni, A.; Khudur, L.; Shah, K.; Ball, A.S. The co-application of biochar with bioremediation for the removal of petroleum hydrocarbons from contaminated soil. Sci. Total Environ. 2022, 849, 157753. [Google Scholar] [CrossRef]
- Valizadeh, S.; Lee, S.S.; Choi, Y.J.; Baek, K.; Jeon, B.; Andrew Lin, K.; Park, Y. Biochar application strategies for polycyclic aromatic hydrocarbons removal from soils. Environ. Res. 2022, 213, 113599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, S.; Zhong, W.; Wei, W. Enhanced methylene blue adsorption onto activated reed-derived biochar by tannic acid. J. Mol. Liq. 2018, 268, 658–666. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.; Wang, X. Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherpa, K.; Bui, X.; Nguyen, V.; Vo, T.; Ho, H.; Chen, C.; Dong, C. Biochar for soil remediation: A comprehensive review of current research on pollutant removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Zhu, L. Role of Pyrogenic Carbon in Parallel Microbial Reduction of Nitrobenzene in the Liquid and Sorbed Phases. Environ. Sci. Technol. 2020, 54, 8760–8769. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, L.; Moghaddam, T.B.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kwak, J.; Lee, Y.; Kim, S.; Choi, M.; Bae, S.; Lee, S.; Park, Y.; Chon, K. Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: Contribution of hydrophobic and π-π interactions. Environ. Pollut. 2021, 270, 116244. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhu, L. Sorption of phenanthrene to biochar modified by base. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Jin, J.; Li, S.; Peng, X.; Liu, W.; Zhang, C.; Yang, Y.; Han, L.; Du, Z.; Sun, K.; Wang, X. HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour. Technol. 2018, 256, 247–253. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Qiao, K.; Tian, W.; Bai, J.; Dong, J.; Zhao, J.; Gong, X.; Liu, S. Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol. Environ. Saf. 2018, 149, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Zhang, M.; Shen, X.; Zhang, X.; Wu, F.; Hu, J.; Wang, B.; Wang, X. Removal of PAHs at high concentrations in a soil washing solution containing TX-100 via simultaneous sorption and biodegradation processes by immobilized degrading bacteria in PVA-SA hydrogel beads. J. Hazard. Mater. 2020, 410, 124533. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Bao, J.; Liu, D.; Gao, X.; Yu, Y.; Zhu, L. Synergistic effects of rice husk biochar and aerobic composting for heavy oil-contaminated soil remediation and microbial community succession evaluation. J. Hazard. Mater. 2023, 448, 130929. [Google Scholar] [CrossRef]
- Xia, M.; Gao, R.; Xu, G.; You, Y.; Li, X.; Dou, J.; Fan, F. Fabrication and investigation of novel monochloroacetic acid fortified, tripolyphosphate-crosslinked chitosan for highly efficient adsorption of uranyl ions from radioactive effluents. J. Hazard. Mater. 2022, 431, 128461. [Google Scholar] [CrossRef]
- Dou, J.; Ding, A.; Liu, X.; Du, Y.; Deng, D.; Wang, J. Anaerobic benzene biodegradation by a pure bacterial culture of Bacillus cereus under nitrate reducing conditions. J. Environ. Sci. 2010, 22, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. J. Hazard. Mater. 2020, 398, 122941. [Google Scholar] [CrossRef] [PubMed]
- Tulcan, R.; Liu, L.H.; Lu, X.X.; Ge, Z.M.; Rojas, D.Y.F.; Silva, D.M. PAHs contamination in ports: Status, sources and risks. J. Hazard. Mater. 2024, 475, 134937. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z.; Hu, Z.; Ngo, H.; Liang, S.; Zhang, J. Adsorption of phenanthrene from aqueous solutions by biochar derived from an ammoniation-hydrothermal method. Sci. Total Environ. 2020, 733, 139267. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Yu, B.; Wu, X.; Xu, J.; Dong, F.; Zheng, Y. Characterization of peanut-shell biochar and the mechanisms underlying its sorption for atrazine and nicosulfuron in aqueous solution. Sci. Total Environ. 2020, 702, 134767. [Google Scholar] [CrossRef]
- Mosley, L.M.; Willson, P.; Hamilton, B.; Butler, G.; Seaman, R. The capacity of biochar made from common reeds to neutralise pH and remove dissolved metals in acid drainage. Environ. Sci. Pollut. Res. 2015, 22, 15113–15122. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Du, C.; Wang, Y.; Wang, L.; Li, X. A novel role of various hydrogen bonds in adsorption, desorption and co-adsorption of PPCPs on corn straw-derived biochars. Sci. Total Environ. 2023, 861, 160623. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Guan, D.; Wang, B.; Zhou, S.; Chen, T.; Wang, J.; Li, Y.; Gao, B. Qualitative and quantitative analysis for Cd2+ removal mechanisms by biochar composites from co-pyrolysis of corn straw and fly ash. Chemosphere 2023, 330, 138701. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Anichini, B.; Bakari, Z.; Bruzzoniti, M.C.; Camisa, R.; Caprini, C.; Checchini, L.; Fibbi, D.; El Ghadraoui, A.; Liguori, F.; et al. Physicochemical properties and sorption capacities of sawdust-based biochars and commercial activated carbons towards ethoxylated alkylphenols and their phenolic metabolites in effluent wastewater from a textile district. Sci. Total Environ. 2020, 708, 135217. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, S.; Wang, Y.; Lu, S.; Gao, Y. Sorptive removal of phenanthrene from aqueous solutions using magnetic and non-magnetic rice husk-derived biochars. R. Soc. Open Sci. 2018, 5, 1723825. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Hu, X.; He, F.; Bean, E.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Applications of carbonaceous adsorbents in the remediation of polycyclic aromatic hydrocarbon-contaminated sediments: A review. J. Clean. Prod. 2020, 255, 120263. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, Y.; Hou, Y.; Arp, H.P.H.; Reid, B.J.; Cai, C. Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere 2017, 182, 316–324. [Google Scholar] [CrossRef]
- Wahla, A.Q.; Anwar, S.; Mueller, J.A.; Arslan, M.; Iqbal, S. Immobilization of metribuzin degrading bacterial consortium MB3R on biochar enhances bioremediation of potato vegetated soil and restores bacterial community structure. J. Hazard. Mater. 2020, 390, 121493. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, T.; Liu, Y.; Liu, J.; Wang, Y.; Wang, P.; Zhou, J.; Chen, M.; Yang, B.; Li, L. Combination of biochar and immobilized bacteria accelerates polyacrylamide biodegradation in soil by both bio-augmentation and bio-stimulation strategies. J. Hazard. Mater. 2020, 405, 124086. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Su, P.J.; Zhang, G.H.; Li, D.; Su, P.; Tang, M.; Zhang, G. Biochar alters the persistence of PAHs in soils by affecting soil physicochemical properties and microbial diversity: A meta-analysis. Ecotoxicol. Environ. Saf. 2023, 266, 115589. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Huang, J.; Chi, J. Contribution of phenanthrene in different binding sites to its biodegradation in biochar-amended soils. Environ. Pollut. 2021, 273, 116481. [Google Scholar] [CrossRef]
- Li, J.; Ou, Y.; Zhang, Y.; Guo, S.; Li, S.; Guo, C.; Dang, Z.; Cao, Z.; Feng, J.; Sun, J. Viability and distribution of bacteria immobilized on Sawdust@silica: The removal mechanism of phenanthrene in soil. Ecotoxicol. Environ. Saf. 2020, 198, 110649. [Google Scholar] [CrossRef]
- Li, X.; Yao, S.; Bian, Y.; Jiang, X.; Song, Y. The combination of biochar and plant roots improves soil bacterial adaptation to PAH stress: Insights from soil enzymes, microbiome, and metabolome. J. Hazard. Mater. 2020, 400, 123227. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Liao, C.; Zhang, M.; Liang, X.; Lu, G.; Yang, C.; Dang, Z. A bio-hybrid material for adsorption and degradation of phenanthrene: Bacteria immobilized on sawdust coated with a silica layer. Rsc. Adv. 2016, 6, 107189–107199. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Xie, H.; Hu, Z.; Liang, S.; Ngo, H.H.; Guo, W.; Chen, X.; Fan, J.; Zhao, C. Intensive removal of PAHs in constructed wetland filled with copper biochar. Ecotoxicol. Environ. Saf. 2020, 205, 111028. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Marcinczyk, M.; Race, M.; Papirio, S.; Esposito, G.; Oleszczuk, P. Low temperature-produced and VFA-coated biochar enhances phenanthrene adsorption and mitigates toxicity in marine sediments. Sep. Purif. Technol. 2022, 296, 121414. [Google Scholar] [CrossRef]

| Biochar | pH | Specific Surface Area (m2·g−1) | Pore Volume (m3·g−1) | Elements | ||||
|---|---|---|---|---|---|---|---|---|
| Carbon (C, %) | Hydrogen (H, %) | Nitrogen (N, %) | H/C | C/N | ||||
| RB 1 | 8.11 | 70.69 | 0.052 | 46.23 | 0.99 | 1.35 | 0.02 | 34.24 |
| RBO 2 | 6.34 | 182.6 | 0.141 | 39.51 | 1.34 | 1.54 | 0.03 | 25.66 |
| RBA 3 | 8.52 | 81.18 | 0.061 | 41.36 | 2.26 | 1.42 | 0.05 | 29.13 |
| BB 4 | 8.25 | 43.42 | 0.045 | 57.29 | 2.08 | 1.35 | 0.04 | 42.44 |
| BBO 5 | 4.56 | 161.0 | 0.168 | 52.96 | 1.97 | 1.88 | 0.04 | 28.17 |
| BBA 6 | 9.24 | 60.58 | 0.049 | 54.08 | 2.11 | 1.72 | 0.04 | 31.44 |
| CB 7 | 9.16 | 22.46 | 0.037 | 65.86 | 1.82 | 1.97 | 0.03 | 33.43 |
| CBO 8 | 6.12 | 69.88 | 0.053 | 62.59 | 2.45 | 2.03 | 0.04 | 30.83 |
| CBA 9 | 10.03 | 50.11 | 0.050 | 60.55 | 1.53 | 2.54 | 0.03 | 23.84 |
| Biochar | PFO | PSO | IPD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (µg·g−1) | k1 (h−1) | R2 | Qe (µg·g−1) | k2 (g·µg−1·h−1) | R2 | b (µg·g−1) | k3 (µg·g−1·h−0.5) | R2 | |
| RB | 2604 | 0.438 | 0.848 | 2782 | 2.375 × 10−4 | 0.973 | 1763 | 103.2 | 0.607 |
| RBO | 3621 | 0.664 | 0.862 | 3819 | 2.819 × 10−4 | 0.988 | 2788 | 106.2 | 0.556 |
| RBA | 2998 | 0.232 | 0.780 | 3201 | 1.137 × 10−4 | 0.924 | 1567 | 165.6 | 0.814 |
| BB | 2602 | 0.363 | 0.723 | 2796 | 1.929 × 10−4 | 0.919 | 1597 | 126.3 | 0.795 |
| BBO | 3521 | 0.473 | 0.864 | 3754 | 1.915 × 10−4 | 0.979 | 2442 | 133.4 | 0.596 |
| BBA | 3166 | 0.397 | 0.966 | 3358 | 1.878 × 10−4 | 0.987 | 2139 | 118.1 | 0.490 |
| CB | 2481 | 0.442 | 0.671 | 2648 | 2.582 × 10−4 | 0.905 | 1665 | 103.9 | 0.747 |
| CBO | 2716 | 0.150 | 0.927 | 2960 | 6.862 × 10−5 | 0.978 | 1037 | 182.6 | 0.776 |
| CBA | 2755 | 0.298 | 0.837 | 2932 | 1.625 × 10−4 | 0.959 | 1654 | 127.4 | 0.659 |
| RB | RBO | RBA | BB | BBO | BBA | CB | CBO | CBA | |
|---|---|---|---|---|---|---|---|---|---|
| k4 | 99.79 | 216.4 | 115.4 | 101.3 | 399.8 | 241.0 | 107.4 | 56.89 | 76.08 |
| n | 0.542 | 0.512 | 0.536 | 0.524 | 0.388 | 0.435 | 0.460 | 0.580 | 0.530 |
| R2 | 0.937 | 0.894 | 0.968 | 0.951 | 0.975 | 0.968 | 0.946 | 0.972 | 0.957 |
| Regression Equation | Degradation Constant (k, d−1) | Half-Life Time (T1/2, d) | Regression Coefficient | |
|---|---|---|---|---|
| ARB | C = 49.21e−0.0671t | 0.0671 | 10.33 | 0.9236 |
| ERB | C = 49.15e−0.0409t | 0.0409 | 16.95 | 0.9613 |
| DG1 | C = 49.12e−0.0212t | 0.0212 | 32.70 | 0.9660 |
| Adsorbent | System | Removal Efficiency | References |
|---|---|---|---|
| Arundo donax-derived biochar-loading copper ions | Constructed wetlands with modified biochar as substrates, the influent phenanthrene was 12.8 ± 1.7 mg·L−1, hydraulic retention time lasted 3 days | 94.09% | [44] |
| Magnetically modified rice husk biochar by a hydrothermal method | Phenanthrene concentration 5–70 mg·L−1, shaken at 150 r·min−1, under 25 °C for 24 h | 97.6 mg·g−1/greater than 85% | [32] |
| Rice straw/wood/bamboo-derived biochar modified by NaOH and washed by HCl | Phenanthrene concentration 0.1–1 mg·L−1, shaken in dark at 130 r·min−1 overnight | 42.9 mg·g−1 | [17] |
| Willow-derived biochar based on volatile fatty acids | Phenanthrene concentration 0.21 mg·L−1, shaken at 120 r·min−1 under ambient temperature in dark | 93% | [45] |
| Phragmites australis-derived biochar produced by ammoniation-hydrothermal method | Phenanthrene concentration 2 mg·L−1, shaken in dark at 25 °C under 125 ± 5 r·min−1 | 1.97 mg·g−1 | [26] |
| Rice husk-derived biochar with nitric acid oxidation | Phenanthrene concentration 2 mg·L−1, shaken in dark at 200 r·min−1 under 25 °C | 38.2 mg·g−1/95.47% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, S.; Mao, S.; Xu, G.; Ding, A.; Chen, F.; Dou, J.; Fan, F. Performance Evaluation of Modified Biochar as a Polycyclic Aromatic Hydrocarbon Adsorbent and Microbial-Immobilized Carrier. Processes 2024, 12, 2939. https://doi.org/10.3390/pr12122939
Geng S, Mao S, Xu G, Ding A, Chen F, Dou J, Fan F. Performance Evaluation of Modified Biochar as a Polycyclic Aromatic Hydrocarbon Adsorbent and Microbial-Immobilized Carrier. Processes. 2024; 12(12):2939. https://doi.org/10.3390/pr12122939
Chicago/Turabian StyleGeng, Shuying, Shushuai Mao, Guangming Xu, Aizhong Ding, Feiyong Chen, Junfeng Dou, and Fuqiang Fan. 2024. "Performance Evaluation of Modified Biochar as a Polycyclic Aromatic Hydrocarbon Adsorbent and Microbial-Immobilized Carrier" Processes 12, no. 12: 2939. https://doi.org/10.3390/pr12122939
APA StyleGeng, S., Mao, S., Xu, G., Ding, A., Chen, F., Dou, J., & Fan, F. (2024). Performance Evaluation of Modified Biochar as a Polycyclic Aromatic Hydrocarbon Adsorbent and Microbial-Immobilized Carrier. Processes, 12(12), 2939. https://doi.org/10.3390/pr12122939







