Plant-Derived Extracellular Vesicles: Natural Nanocarriers for Biotechnological Drugs
Abstract
1. Introduction
2. Plant-Derived Extracellular Vesicles (PDEVs)
3. Biogenesis and Characterization of Plant-Derived Extracellular Vesicles
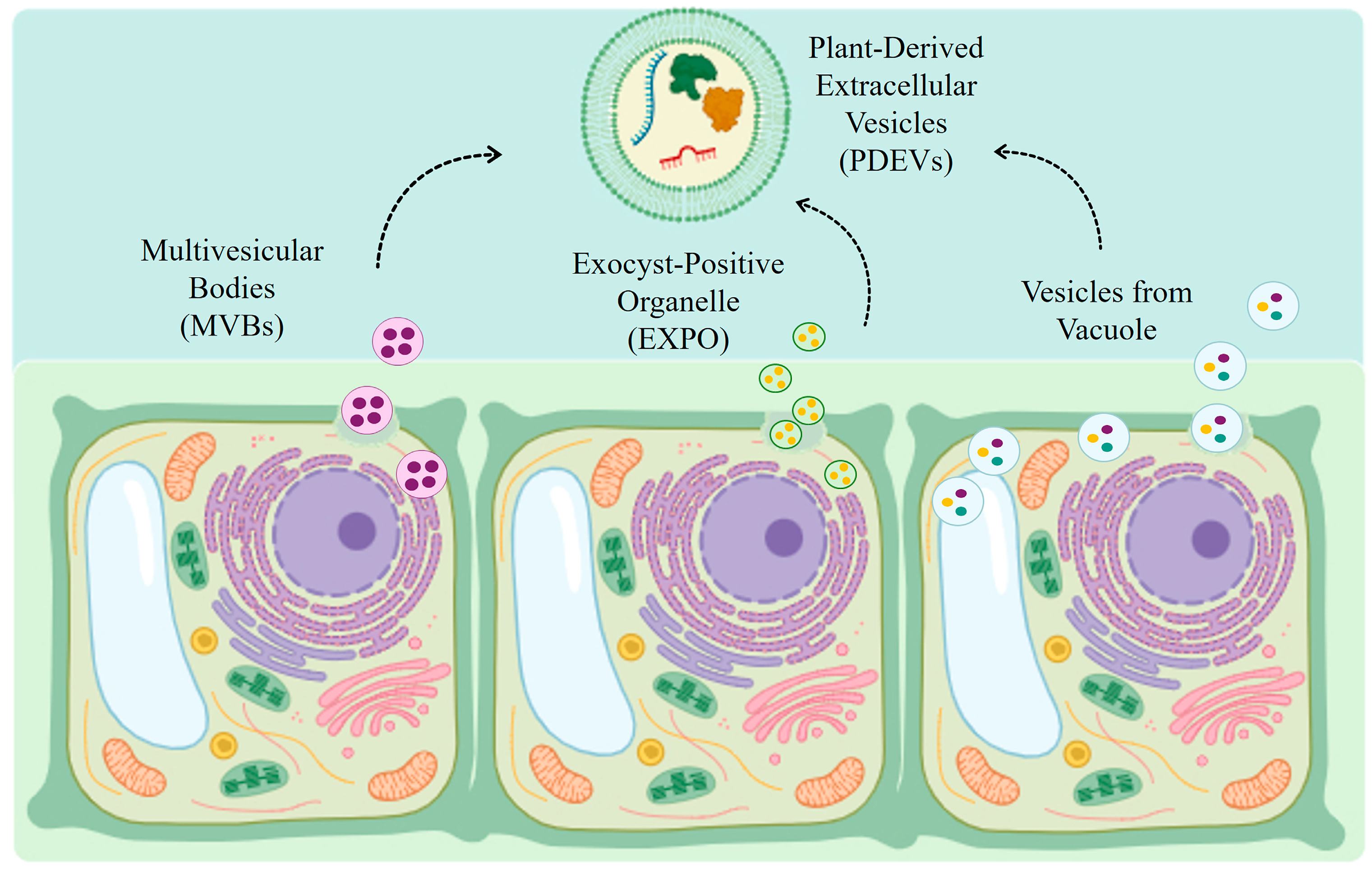
3.1. Biogenesis of Plant-Derived Extracellular Vesicles
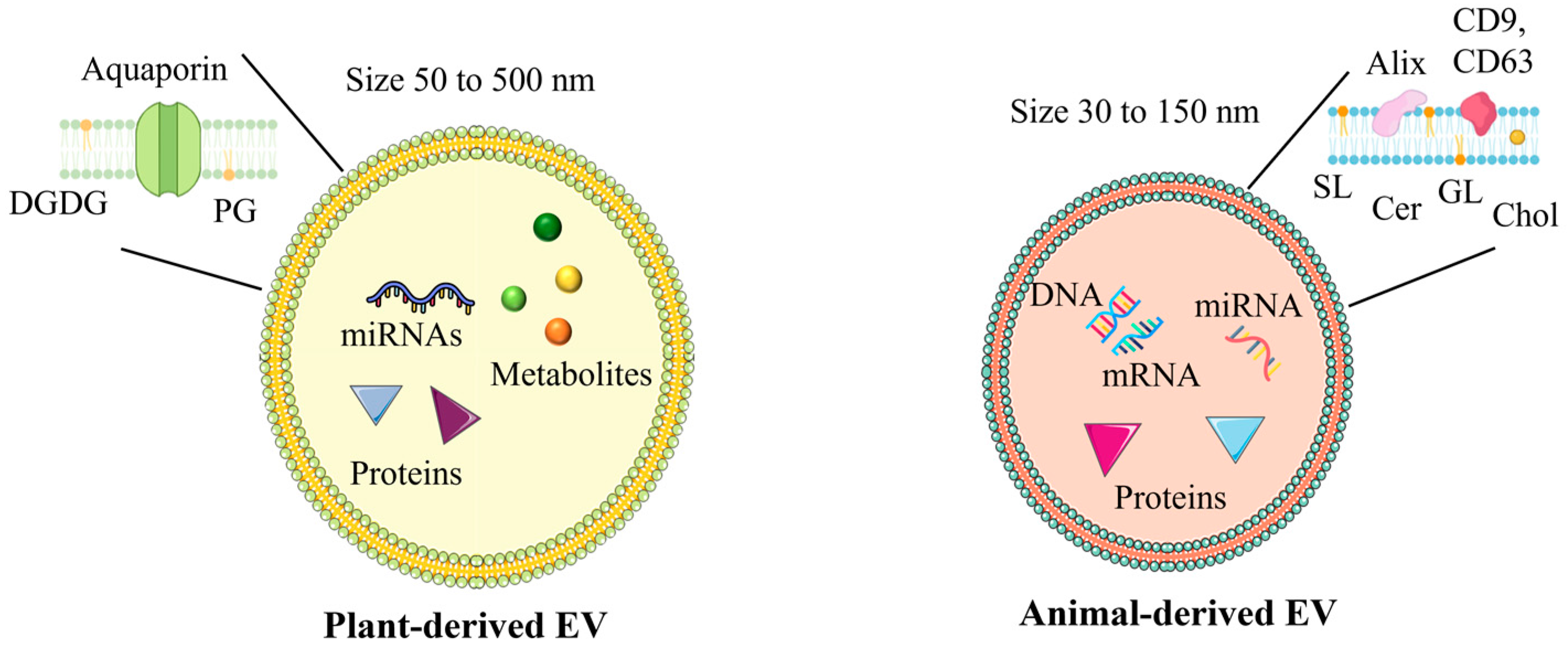
3.2. Plant-Derived Extracellular Vesicles Molecular Cargo
3.3. Differences Between Plant-Derived Extracellular Vesicles and Animal-Derived Extracellular Vesicles
3.4. Comparison Advantages of PDEVs over Synthetic Nanoparticles
4. Isolation and Characterization Techniques for PDEVs
4.1. Isolation Methods
4.1.1. Ultracentrifugation
4.1.2. Density Gradient Ultracentrifugation
4.1.3. Size Exclusion Chromatography (SEC)
4.2. Characterization Techniques
4.2.1. Electron Microscopy
4.2.2. Nanoparticle Tracking Analysis (NTA)
4.2.3. Flow Cytometry
5. Biological Functions of PDEVs
5.1. Role in Plant-Plant Communication and in Plant-Microbe Interactions
5.2. Role in Human Health and Therapeutic Applications
5.2.1. Anti-Inflammatory Effects of PDEVs
5.2.2. Antioxidant Properties of PDEVs
5.2.3. Anticancer Properties of PDEVs
6. Enhancing PDEVs for Drug Delivery Applications
6.1. Engineered PDEVs: Nucleic Acid Delivery
6.2. Surface Functionalization of PDEVs
6.3. Oral Administration of PDEVs for Drug Delivery
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López de las Hazas, M.-C.; Tomé-Carneiro, J.; del Pozo-Acebo, L.; del Saz-Lara, A.; Chapado, L.A.; Balaguer, L.; Rojo, E.; Espín, J.C.; Crespo, C.; Moreno, D.A.; et al. Therapeutic Potential of Plant-Derived Extracellular Vesicles as Nanocarriers for Exogenous miRNAs. Pharmacol. Res. 2023, 198, 106999. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-Derived Extracellular Vesicles: Recent Advancements and Current Challenges on Their Use for Biomedical Applications. J. Extracell. Vesicles 2022, 11, 12283. [Google Scholar] [CrossRef]
- Wang, X.; Xin, C.; Zhou, Y.; Sun, T. Plant-Derived Vesicle-like Nanoparticles: The Next-Generation Drug Delivery Nanoplatforms. Pharmaceutics 2024, 16, 588. [Google Scholar] [CrossRef]
- Kameli, N.; Dragojlovic-Kerkache, A.; Savelkoul, P.; Stassen, F.R. Plant-Derived Extracellular Vesicles: Current Findings, Challenges, and Future Applications. Membranes 2021, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Subha, D.; Harshnii, K.; Madhikiruba, K.G.; Nandhini, M.; Tamilselvi, K.S. Plant Derived Exosome- like Nanovesicles: An Updated Overview. Plant Nano Biol. 2023, 3, 100022. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wang, Z.; Deng, L.; Wang, Y.; Tang, Y.; Luo, L.; Leung, E.L.-H. Extracellular Vesicles as a Novel Mediator of Interkingdom Communication. Cytokine Growth Factor Rev. 2023, 73, 173–184. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe Vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Li, T.; Qi, W.; Miao, Z.; Zhu, L.; Zhang, C.; Jin, H.; Pan, H.; Wang, D. Advances in the Study of Plant-Derived Extracellular Vesicles in the Skeletal Muscle System. Pharmacol. Res. 2024, 204, 107202. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Li, J.; Ruan, R.; Li, Q.; Zhang, X.; Yan, A.; Zhu, H. Plant-Derived Extracellular Vesicles as a Promising Anti-Tumor Approach: A Comprehensive Assessment of Effectiveness, Safety, and Mechanisms. Phytomedicine 2024, 130, 155750. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, Y.; Zhang, K.; Liu, Y.; Liang, Q.; Thakur, A.; Liu, W.; Yan, Y. Plant-Derived Extracellular Vesicles (PDEVs) in Nanomedicine for Human Disease and Therapeutic Modalities. J. Nanobiotechnol. 2023, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Lee, J.H.; Rhee, W.J. Engineered Plant-Derived Extracellular Vesicles for Targeted Regulation and Treatment of Colitis-Associated Inflammation. Theranostics 2024, 14, 5643. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, J.; Huang, K.; Tian, X.; Guo, Y.; Skirtach, A.G.; You, M.; Tan, M.; Su, W. Advances in Preparation and Engineering of Plant-Derived Extracellular Vesicles for Nutrition Intervention. Food Chem. 2024, 457, 140199. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, A.; Barbulova, A.; Cappetta, E.; Cillo, F.; De Palma, M.; Ruocco, M.; Pocsfalvi, G. Plant Extracellular Vesicles: Current Landscape and Future Directions. Plants 2023, 12, 4141. [Google Scholar] [CrossRef] [PubMed]
- Rome, S. Biological Properties of Plant-Derived Extracellular Vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes 2022, 12, 352. [Google Scholar] [CrossRef]
- Zeng, Y.-B.; Deng, X.; Shen, L.-S.; Yang, Y.; Zhou, X.; Ye, L.; Chen, S.; Yang, D.-J.; Chen, G.-Q. Advances in Plant-Derived Extracellular Vesicles: Isolation, Composition, and Biological Functions. Food Funct. 2024, 15, 11319–11341. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, K. Plant-Derived Extracellular Vesicles as Oral Drug Delivery Carriers. J. Control. Release 2022, 350, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kürtösi, B.; Kazsoki, A.; Zelkó, R. A Systematic Review on Plant-Derived Extracellular Vesicles as Drug Delivery Systems. Int. J. Mol. Sci. 2024, 25, 7559. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chang, C.-J.; Liang, M.-L.; Dong, K.-M.; Li, F.-R. Plant-Derived Extracellular Vesicles as Potential Emerging Tools for Cancer Therapeutics. Adv. Ther. 2024, 7, 2400256. [Google Scholar] [CrossRef]
- Cui, L.; Perini, G.; Palmieri, V.; De Spirito, M.; Papi, M. Plant-Derived Extracellular Vesicles as a Novel Frontier in Cancer Therapeutics. Nanomaterials 2024, 14, 1331. [Google Scholar] [CrossRef]
- Kathait, P.; Patel, P.K.; Sahu, A.N. Harnessing Exosomes and Plant-Derived Exosomes as Nanocarriers for the Efficient Delivery of Plant Bioactives. Nanomedicine 2024, 19, 2679–2697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lin, H.; Jiang, X.; Li, W.; Gao, Y.; Li, M.; Yu, Y.; Chen, N.; Gao, J. Exosome-like Nanoparticles Derived from Fruits, Vegetables, and Herbs: Innovative Strategies of Therapeutic and Drug Delivery. Theranostics 2024, 14, 4598. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant Extracellular Vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- Sall, I.M.; Flaviu, T.A. Plant and Mammalian-Derived Extracellular Vesicles: A New Therapeutic Approach for the Future. Front. Bioeng. Biotechnol. 2023, 11, 1215650. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Gu, D.; Nan, J.; Chen, S.; Li, H. Overexpression of S-Adenosyl-l-Methionine Synthetase 2 from Sugar Beet M14 Increased Arabidopsis Tolerance to Salt and Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 847. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Bai, M.; Luo, Q.; Zheng, Q.; Xu, Y.; Li, X.; Jiang, C.; Cho, W.C.; Fan, Z. Edible Plant-Derived Extracellular Vesicles Serve as Promising Therapeutic Systems. Nano TransMed 2023, 2, 100004. [Google Scholar] [CrossRef]
- Woith, E.; Guerriero, G.; Hausman, J.-F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant Extracellular Vesicles and Nanovesicles: Focus on Secondary Metabolites, Proteins and Lipids with Perspectives on Their Potential and Sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef] [PubMed]
- Yugay, Y.; Tsydeneshieva, Z.; Rusapetova, T.; Grischenko, O.; Mironova, A.; Bulgakov, D.; Silant’ev, V.; Tchernoded, G.; Bulgakov, V.; Shkryl, Y. Isolation and Characterization of Extracellular Vesicles from Arabidopsis Thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis. Plants 2023, 12, 3604. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.-G.; Qiao, H. Technology Insight: Plant-Derived Vesicles—How Far from the Clinical Biotherapeutics and Therapeutic Drug Carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef] [PubMed]
- de la Canal, L.; Pinedo, M. Extracellular Vesicles: A Missing Component in Plant Cell Wall Remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, S.; Carata, E.; Mariano, S.; Panzarini, E. Plant Extracellular Vesicles: Investigating Their Utilization as Beneficial Nutrients in Diet. Appl. Sci. 2023, 13, 6656. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous Plant MIR168a Specifically Targets Mammalian LDLRAP1: Evidence of Cross-Kingdom Regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Hou, D.; He, F.; Ma, L.; Cao, M.; Zhou, Z.; Wei, Z.; Xue, Y.; Sang, X.; Chong, H.; Tian, C.; et al. The Potential Atheroprotective Role of Plant MIR156a as a Repressor of Monocyte Recruitment on Inflamed Human Endothelial Cells. J. Nutr. Biochem. 2018, 57, 197–205. [Google Scholar] [CrossRef]
- Ray, R.M.; Lazar, A.D.; Balahura (Stamat), L.R.; Mocanu-Dobranici, A.E.; Costache, M.; Dinescu, S. Chapter 9—Therapeutic Targeting Non-Coding RNAs. In Navigating Non-Coding RNA; Sztuba-Solinska, J., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 349–417. ISBN 978-0-323-90406-3. [Google Scholar]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. AAPS PharmSciTech 2021, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Calzoni, E.; Bertoldi, A.; Cesaretti, A.; Alabed, H.B.R.; Cerrotti, G.; Pellegrino, R.M.; Buratta, S.; Urbanelli, L.; Emiliani, C. Aloe Extracellular Vesicles as Carriers of Photoinducible Metabolites Exhibiting Cellular Phototoxicity. Cells 2024, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Kazemipour-Khabbazi, S.; Raimondo, S.; Dalirfardouei, R. Molecular Mechanisms and Therapeutic Application of Extracellular Vesicles from Plants. Mol. Biol. Rep. 2024, 51, 425. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.; Tsydeneshieva, Z.; Menchinskaya, E.; Rusapetova, T.; Grishchenko, O.; Mironova, A.; Bulgakov, D.; Gorpenchenko, T.; Kazarin, V.; Tchernoded, G.; et al. Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties. Biomedicines 2024, 12, 2142. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Yu, H.; Shi, X.; Zhou, Y.; Alvarez, S.; Naldrett, M.J.; Kachman, S.D.; Ro, S.-H.; Sun, X.; et al. Therapeutic Potential of Garlic Chive-Derived Vesicle-like Nanoparticles in NLRP3 Inflammasome-Mediated Inflammatory Diseases. Theranostics 2021, 11, 9311. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Wu, S.-C.; Chien, H.-Y.; Shen, T.-L.; Hsu, W.-H. Tomato-Fruit-Derived Extracellular Vesicles Inhibit Fusobacterium Nucleatum via Lipid-Mediated Mechanism. Food Funct. 2023, 14, 8942–8950. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, H.; Shi, W.; Chen, L.; Chen, T.; Chen, G.; Wang, W.; Lan, J.; Huang, Z.; Zhang, J.; et al. Aloe Derived Nanovesicle as a Functional Carrier for Indocyanine Green Encapsulation and Phototherapy. J. Nanobiotechnol. 2021, 19, 439. [Google Scholar] [CrossRef]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural Exosome-like Nanovesicles from Edible Tea Flowers Suppress Metastatic Breast Cancer via ROS Generation and Microbiota Modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Hur, J.; Kim, K.P.; Lee, K.; Kang, J.Y. Surface-Functionalizable Plant-Derived Extracellular Vesicles for Targeted Drug Delivery Carrier Using Grapefruit. Adv. Mater. Interfaces 2023, 10, 2300220. [Google Scholar] [CrossRef]
- Latella, R.; Calzoni, E.; Urbanelli, L.; Cerrotti, G.; Porcellati, S.; Emiliani, C.; Buratta, S.; Tancini, B. Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products. Foods 2024, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cai, X.; Wu, Q.; Liao, H.; Liang, S.; Fu, H.; Xiang, Q.; Zhang, S. Extraction, Isolation, and Component Analysis of Turmeric-Derived Exosome-like Nanoparticles. Bioengineering 2023, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.d.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma Membrane Aquaporins Mediates Vesicle Stability in Broccoli. PLoS ONE 2018, 13, e0192422. [Google Scholar] [CrossRef]
- Anusree, K.P.; Ashin, M.; Anusha, R.; Sijisha, K.S.; Priya, S. Optimization of the Filtration and Centrifugation Steps for the Isolation of Exosome-like Nanoparticles (ELNs) from Grapes. Sep. Sci. Technol. 2024, 59, 365–371. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein Biocargo of Citrus Fruit-Derived Vesicles Reveals Heterogeneous Transport and Extracellular Vesicle Populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like Nanoparticles from Mulberry Bark Prevent DSS-induced Colitis via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Özkan, İ.; Koçak, P.; Yıldırım, M.; Ünsal, N.; Yılmaz, H.; Telci, D.; Şahin, F. Garlic (Allium Sativum)-Derived SEVs Inhibit Cancer Cell Proliferation and Induce Caspase Mediated Apoptosis. Sci. Rep. 2021, 11, 14773. [Google Scholar] [CrossRef]
- Chaya, T.; Banerjee, A.; Rutter, B.D.; Adekanye, D.; Ross, J.; Hu, G.; Innes, R.W.; Caplan, J.L. The Extracellular Vesicle Proteomes of Sorghum Bicolor and Arabidopsis Thaliana Are Partially Conserved. Plant Physiol. 2024, 194, 1481–1497. [Google Scholar] [CrossRef]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter Melon Derived Extracellular Vesicles Enhance the Therapeutic Effects and Reduce the Drug Resistance of 5-Fluorouracil on Oral Squamous Cell Carcinoma. J. Nanobiotechnol. 2021, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant Extracellular Vesicles Are Incorporated by a Fungal Pathogen and Inhibit Its Growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant Roots Release Small Extracellular Vesicles with Antifungal Activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Yang, L.; Pan, S.; Zhan, L.; Yuan, F. Characterization of Blueberry Exosome-like Nanoparticles and miRNAs with Potential Cross-Kingdom Human Gene Targets. Food Sci. Hum. Wellness 2024, 13, 869–878. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; López de las Hazas, M.-C.; Tomé-Carneiro, J.; del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic Potential of Broccoli-Derived Extracellular Vesicles as Nanocarriers of Exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived microRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Anti-Oxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Dolma, L.; Damodaran, A.; Panonnummal, R.; Nair, S.C. Exosomes Isolated from Citrus Lemon: A Promising Candidate for the Treatment of Alzheimer’s Disease. Ther. Deliv. 2024, 15, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Tajik, T.; Baghaei, K.; Moghadam, V.E.; Farrokhi, N.; Salami, S.A. Extracellular Vesicles of Cannabis with High CBD Content Induce Anticancer Signaling in Human Hepatocellular Carcinoma. Biomed. Pharmacother. 2022, 152, 113209. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Jian, T.; Gai, Y.; Chen, J. Microbiota and Plant-Derived Vesicles That Serve as Therapeutic Agents and Delivery Carriers to Regulate Metabolic Syndrome. Adv. Drug Deliv. Rev. 2023, 196, 114774. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-Derived Edible Nanoparticle-Based Lipid Nano-Drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef]
- Soprano, E.; Polo, E.; Pelaz, B.; del Pino, P. Biomimetic Cell-Derived Nanocarriers in Cancer Research. J. Nanobiotechnol. 2022, 20, 538. [Google Scholar] [CrossRef]
- Agrahari, V.; Burnouf, P.-A.; Burnouf, T.; Agrahari, V. Nanoformulation Properties, Characterization, and Behavior in Complex Biological Matrices: Challenges and Opportunities for Brain-Targeted Drug Delivery Applications and Enhanced Translational Potential. Adv. Drug Deliv. Rev. 2019, 148, 146–180. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Stremersch, S.; Vandenbroucke, R.E.; Van Wonterghem, E.; Hendrix, A.; De Smedt, S.C.; Raemdonck, K. Comparing Exosome-like Vesicles with Liposomes for the Functional Cellular Delivery of Small RNAs. J. Control. Release 2016, 232, 51–61. [Google Scholar] [CrossRef]
- Feng, J.; Xiu, Q.; Huang, Y.; Troyer, Z.; Li, B.; Zheng, L. Plant-Derived Vesicle-Like Nanoparticles as Promising Biotherapeutic Tools: Present and Future. Adv. Mater. 2023, 35, 2207826. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plant-Derived Edible Nanoparticles and miRNAs: Emerging Frontier for Therapeutics and Targeted Drug-Delivery. ACS Sustain. Chem. Eng. 2019, 7, 8055–8069. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical Challenges of Working with Extracellular Vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; LeClaire, M.; Wohlschlegel, J.; Gimzewski, J. Impact of Isolation Methods on the Biophysical Heterogeneity of Single Extracellular Vesicles. Sci. Rep. 2020, 10, 13327. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-J.; Wang, M.-H.; Ho, C.-T.; Pan, M.-H. Plant-Derived Extracellular Vesicles: A New Revolutionization of Modern Healthy Diets and Biomedical Applications. J. Agric. Food Chem. 2024, 72, 2853–2878. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular Fractions of Sunflower Apoplastic Fluids Are Associated with Potential Exosome Marker Proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Growing Pains: Addressing the Pitfalls of Plant Extracellular Vesicle Research. New Phytol. 2020, 228, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral Administration of Turmeric-Derived Exosome-like Nanovesicles with Anti-Inflammatory and pro-Resolving Bioactions for Murine Colitis Therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of Cabbage Exosome-like Nanovesicles and Investigation of Their Biological Activities in Human Cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Abraham, A.M.; Wiemann, S.; Ambreen, G.; Zhou, J.; Engelhardt, K.; Brüßler, J.; Bakowsky, U.; Li, S.-M.; Mandic, R.; Pocsfalvi, G.; et al. Cucumber-Derived Exosome-like Vesicles and PlantCrystals for Improved Dermal Drug Delivery. Pharmaceutics 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and Characterization of Extracellular Vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and Characterization of Extracellular Vesicles and Future Directions in Diagnosis and Therapy. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2022, 23, 191. [Google Scholar] [CrossRef]
- Gai, C.; Pomatto, M.A.C.; Deregibus, M.C.; Dieci, M.; Piga, A.; Camussi, G. Edible Plant-Derived Extracellular Vesicles for Oral mRNA Vaccine Delivery. Vaccines 2024, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Crescitelli, R.; Lässer, C.; Jang, S.C.; Cvjetkovic, A.; Malmhäll, C.; Karimi, N.; Höög, J.L.; Johansson, I.; Fuchs, J.; Thorsell, A.; et al. Subpopulations of Extracellular Vesicles from Human Metastatic Melanoma Tissue Identified by Quantitative Proteomics after Optimized Isolation. J. Extracell. Vesicles 2020, 9, 1722433. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, D.; Mladenović, D.; Criscuoli, M.; Loria, F.; Veiman, K.-L.; Zocco, D.; Koort, K.; Zarovni, N. Opportunities and Pitfalls of Fluorescent Labeling Methodologies for Extracellular Vesicle Profiling on High-Resolution Single-Particle Platforms. Int. J. Mol. Sci. 2021, 22, 10510. [Google Scholar] [CrossRef] [PubMed]
- Desgeorges, A.; Hollerweger, J.; Lassacher, T.; Rohde, E.; Helmbrecht, C.; Gimona, M. Differential Fluorescence Nanoparticle Tracking Analysis for Enumeration of the Extracellular Vesicle Content in Mixed Particulate Solutions. Methods 2020, 177, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.; Deighan, C.; Bandeira, E.; Kwak, K.J.; Rahman, M.; Nana-Sinkam, P.; Lee, L.J.; Paulaitis, M.E. Analyzing the miRNA Content of Extracellular Vesicles by Fluorescence Nanoparticle Tracking. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 765–770. [Google Scholar] [CrossRef]
- Gul, B.; Syed, F.; Khan, S.; Iqbal, A.; Ahmad, I. Characterization of Extracellular Vesicles by Flow Cytometry: Challenges and Promises. Micron 2022, 161, 103341. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, D.; Battaglia, A.; Ricciardi-Tenore, C.; Colella, F.; Perelli, L.; De Maria, R.; Scambia, G.; Sgambato, A.; Fattorossi, A. Measuring Extracellular Vesicles by Conventional Flow Cytometry: Dream or Reality? Int. J. Mol. Sci. 2020, 21, 6257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, G.; Liu, F.; Xie, M.; Zou, Y.; Wang, S.; Guo, Z.; Dong, J.; Ye, J.; Cao, Y.; et al. An Enzyme-Based System for Extraction of Small Extracellular Vesicles from Plants. Sci. Rep. 2023, 13, 13931. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Yang, H.; Hu, J.; Luo, L.; Yuan, Y.; Liu, L. Bioactive Compounds and Biological Functions of Medicinal Plant-Derived Extracellular Vesicles. Pharmacol. Res. 2024, 200, 107062. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Molecular Responses during Plant Grafting and Its Regulation by Auxins, Cytokinins, and Gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef]
- Abubakar, Y.S.; Sadiq, I.Z.; Aarti, A.; Wang, Z.; Zheng, W. Interplay of Transport Vesicles during Plant-Fungal Pathogen Interaction. Stress Biol. 2023, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-P.; Kwon, S.; Adeshara, T.; Göhre, V.; Feldbrügge, M.; Weiberg, A. Extracellular RNAs Released by Plant-Associated Fungi: From Fundamental Mechanisms to Biotechnological Applications. Appl. Microbiol. Biotechnol. 2023, 107, 5935–5945. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Fan, H.; Wu, J. Emerging Proteins as Precursors of Bioactive Peptides/Hydrolysates with Health Benefits. Curr. Opin. Food Sci. 2022, 48, 100914. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.P.; Paolini, A.; D’Oria, V.; Sarra, A.; Sennato, S.; Bordi, F.; Masotti, A. Extracellular Vesicles Derived From Citrus Sinensis Modulate Inflammatory Genes and Tight Junctions in a Human Model of Intestinal Epithelium. Front. Nutr. 2021, 8, 778998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Bian, Y.; Wang, Q.; Yin, F.; Yin, L.; Zhang, Y.; Liu, J. Blueberry-Derived Exosomes-like Nanoparticles Ameliorate Nonalcoholic Fatty Liver Disease by Attenuating Mitochondrial Oxidative Stress. Acta Pharmacol. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; De Leo, V.; Tamborra, R.; Laselva, O.; Ingrosso, C.; Daniello, V.; Catucci, L.; Losito, I.; Sollitto, F.; Loizzi, D.; et al. Characterization of Anti-Proliferative and Anti-Oxidant Effects of Nano-Sized Vesicles from Brassica oleracea L. (Broccoli). Sci. Rep. 2022, 12, 14362. [Google Scholar] [CrossRef]
- Ramírez-Pavez, T.; García-Peñaranda, A.; Garcia-Ibañez, P.; Yepes-Molina, L.; Carvajal, M.; Ruiz-Alcaraz, A.J.; Moreno, D.A.; García-Peñarrubia, P.; Martínez-Esparza, M. Potential of Sulforaphane and Broccoli Membrane Vesicles as Regulators of M1/M2 Human Macrophage Activity. Int. J. Mol. Sci. 2022, 23, 11141. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Trentini, M.; Zanolla, I.; Zanotti, F.; Tiengo, E.; Licastro, D.; Dal Monego, S.; Lovatti, L.; Zavan, B. Apple Derived Exosomes Improve Collagen Type I Production and Decrease MMPs during Aging of the Skin through Downregulation of the NF-κB Pathway as Mode of Action. Cells 2022, 11, 3950. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-Derived Nanoparticles Alter Macrophage Polarization to Inhibit Melanoma Growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef]
- Potestà, M.; Roglia, V.; Fanelli, M.; Pietrobono, E.; Gismondi, A.; Vumbaca, S.; Nguedia Tsangueu, R.G.; Canini, A.; Colizzi, V.; Grelli, S.; et al. Effect of Microvesicles from Moringa Oleifera Containing miRNA on Proliferation and Apoptosis in Tumor Cell Lines. Cell Death Discov. 2020, 6, 43. [Google Scholar] [CrossRef]
- Feng, T.; Wan, Y.; Dai, B.; Liu, Y. Anticancer Activity of Bitter Melon-Derived Vesicles Extract against Breast Cancer. Cells 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiang, J.; Jin, C.; Ye, L.; Wang, L.; Gao, Y.; Lv, N.; Zhang, J.; You, F.; Qiao, H.; et al. Medicinal Plant-Derived mtDNA via Nanovesicles Induces the cGAS-STING Pathway to Remold Tumor-Associated Macrophages for Tumor Regression. J. Nanobiotechnol. 2023, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Mu, J.; Hu, X.; Samykutty, A.; Zhuang, X.; Deng, Z.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Grapefruit-Derived Nanovectors Deliver miR-18a for Treatment of Liver Metastasis of Colon Cancer by Induction of M1 Macrophages. Oncotarget 2016, 7, 25683. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Canup, B.S.B.; Ngo, V.L.; Denning, T.L.; Garg, P.; Laroui, H. Internalization of Garlic-Derived Nanovesicles on Liver Cells Is Triggered by Interaction with CD98. ACS Omega 2020, 5, 23118–23128. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in AD Mice by Inhibiting Hyperphosphorylation of the Tau Protein through the AKT/GSK-3β Pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Zhuang, X.; Teng, Y.; Samykutty, A.; Mu, J.; Deng, Z.; Zhang, L.; Cao, P.; Rong, Y.; Yan, J.; Miller, D.; et al. Grapefruit-Derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola Exosome-like Nanovesicles to Systemically Deliver Nucleic Acid Medicine via Oral Administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral Administration of Ginger-Derived Nanolipids Loaded With siRNA As a Novel Approach for Efficient siRNA Drug Delivery to Treat Ulcerative Colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef]
- Mao, Y.; Han, M.; Chen, C.; Wang, X.; Han, J.; Gao, Y.; Wang, S. A Biomimetic Nanocomposite Made of a Ginger-Derived Exosome and an Inorganic Framework for High-Performance Delivery of Oral Antibodies. Nanoscale 2021, 13, 20157–20169. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-Y.; Li, C.-Q.; Zhang, Y.-L.; Ma, M.-W.; Cheng, W.; Zhang, G.-J. Emerging Drug Delivery Vectors: Engineering of Plant-Derived Nanovesicles and Their Applications in Biomedicine. Int. J. Nanomed. 2024, 19, 2591–2610. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, K.; Liu, J.; Cheng, D.; Yu, B.; Zhao, N.; Xu, F.-J. Biomimetic Electrodynamic Nanoparticles Comprising Ginger-Derived Extracellular Vesicles for Synergistic Anti-Infective Therapy. Nat. Commun. 2022, 13, 7164. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Boura, E.; Ivanov, V.; Carlson, L.-A.; Mizuuchi, K.; Hurley, J.H. Endosomal Sorting Complex Required for Transport (ESCRT) Complexes Induce Phase-Separated Microdomains in Supported Lipid Bilayers. J. Biol. Chem. 2012, 287, 28144–28151. [Google Scholar] [CrossRef] [PubMed]

| PDEV-Associated Component | Type | Source | References |
|---|---|---|---|
| Lipids | Phosphatidic acid (PA) | Ginger, Citrus, Grapefruit, Grape, Garlic, Tomato, Aloe, Orange, Tea flowers | [33,42,43,44,45,46,47,48,49,50] |
| Phosphatidylethanolamine (PE) | Citrus, Grapefruit, Ginger, Olive Vegetation Water, Garlic, Tomato, Orange, Tea flowers | [42,43,46,48,49,51,52] | |
| Phosphatidylcholine (PC) | Grapefruit, Ginger, Garlic, Tomato, Aloe, Curcuma, Orange, Tea flowers | [33,43,45,46,47,48,49,53] | |
| Digalactosyldiacylglycerol (DGDG) | Garlic, Aloe, Orange | [45,47,48] | |
| Cholesterol esters (CE) | Tomato | [46] | |
| Diacylglycerols (DAG) | Tomato | [46] | |
| Proteins | Aquaporins | Broccoli, Grape, Citrus | [54,55,56] |
| Heat shock proteins | Grape, Citrus, Tomato, Ginger, Mulberry, Garlic | [44,56,57,58,59,60] | |
| Annexins | Grape, Citrus, Ginger, Sorghum bicolor, Arabidopsis thaliana, Bitter melon, Tea flowers | [44,49,56,58,61,62] | |
| Peroxidases | Citrus, Sunflower, Grapefruit, Tomato | [43,56,63,64] | |
| Glyceraldehyde 3 phosphate dehydrogenase | Bitter melon, Citrus, Sunflower, Aloe | [47,56,62,63] | |
| Nucleic acids | miR-156 | Grape, Ginger, Bitter melon, Coconut | [33,58,62,65] |
| miR-159 | Coconut, Bitter melon, Ginger, Grape, Strawberry, Broccoli | [33,39,54,58,62] | |
| miR-166 | Coconut, Ginger, Bitter melon, Strawberry, Blueberry, Broccoli | [39,58,62,65,66,67] | |
| miR-167 | Grape, Broccoli, Bitter melon | [33,62,67] | |
| miR-169 | Grape, Hami melon | [33,68] | |
| miR-172 | Kiwi, Grape, Broccoli, Ginger, Bitter melon, Cabbage | [33,68] | |
| miR-394 | Grape, Broccoli, Bitter melon, | [33,62,67] | |
| miR-396 | Strawberry, Ginger, Broccoli, Blueberry, Bitter melon, | [39,58,62,66,67] | |
| miR-398 | Orange, Broccoli | [48,67] | |
| miT-408 | Grape, Broccoli | [33,67] | |
| Metabolites | Vitamin C | Citrus, Strawberry, Orange, | [39,48,69] |
| Naringin | Grapefruit, Citrus | [43,70] | |
| Gingerol | Ginger | [50] | |
| Cannabidiol | Cannabis | [71] | |
| Trans-δ-Viniferin | Grape | [44] | |
| Anthraquinones | Aloe sp. | [41] | |
| Curcuminoids | Curcuma | [29] | |
| Sulforaphane | Broccoli | [4] |
| PDEVs Source | Biological Activity | In Vitro/In Vivo Effects | References |
|---|---|---|---|
| Ginger | Anti-inflammatory | Suppression of NLRP3 inflammasome activation, a key protein involved in inflammatory diseases such as inflammatory bowel disease (IBD) and reduction in pro-inflammatory cytokines levels. | [113] |
| Mulberry bark | Anti-inflammatory | Stimulation of heat shock protein family A (Hsp70) member 8 (HSPA8) that mediated the activation of the AhR signaling pathway. | [59] |
| Turmeric | Anti-inflammatory | Regulation of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β and promotion of the expression of the antioxidant gene HO-1 in murine colitis models. | [53] |
| Orange | Anti-inflammatory | Reduction in intestinal inflammation and restoration of intestinal barrier integrity by modulating the expression of key genes such as HMOX-1, ICAM1, OCLN, CLDN1, and MLCK. | [114] |
| Garlic | Anti-inflammatory | Reduction in the expression of pro-inflammatory factors IFN-γ and IL-6 in LPS-stimulated HepG2 cells. | [126] |
| Carrot | Anti-inflammatory | Increased expression of the anti-inflammatory cytokine IL-10 in Raw 264.7 macrophages. | [72] |
| Cabbage | Anti-inflammatory | Decreased levels of pro-inflammatory cytokines IL-6 and IL-1β, along with COX-2, in LPS-stimulated Raw 264.7 macrophages, and suppression of NLRP3 inflammasome assembly and activation in primary macrophages from C57BL/6J mice, mediated by ginger’s anti-inflammatory properties. | [91] |
| Broccoli | Anti-inflammatory | Reduced expression of pro-inflammatory cytokines TNF-α, IL-17A, and IFN-γ, coupled with increased expression of the anti-inflammatory cytokine IL-10, in a DSS-induced colitis model using C57BL/6 (B6) mice. | [40] |
| Aloe vera | Antioxidant | Increase expression of key genes such as Nrf2, CAT, HO-1, and SOD. | [47] |
| Carrot | Antioxidant | Prevention of downregulation of Nrf2 expression, protecting H9C2 cardiomyoblastic cells and SH-SY5Y human neuroblastoma cells from oxidative stress. | [90] |
| Strawberry | Antioxidant | Protection against oxidative damage | [39] |
| Blueberry | Antioxidant | Reduction in oxidative stress by affecting the functionality of the mitochondrial protein Bcl-2 and preventing apoptosis in HepG2 cells. | [115] |
| Ginseng | Anticancer | Induction of melanoma cell apoptosis by macrophage polarization toward the M1 phenotype via Toll-like receptor (TLR)-4 and myeloid differentiation antigen 88 (MyD88) signaling pathways. | [120] |
| Moringa oleifera | Anticancer | Upregulation of the antiapoptotic protein Bcl-2 and impairment of mitochondrial membrane potential. | [121] |
| Bitter melon | Anticancer | Inhibition of breast cancer cells proliferation by induction of ROS production. | [122] |
| Tea flower | Anticancer | Cytotoxic activity against breast cancer cells via ROS generation. | [49] |
| Lemon | Anticancer | Growth inhibition of chronic myeloid leukemia tumor cells by activation of the TRAIL-mediated apoptotic process. | [123] |
| Ginger | Anticancer | Inhibition of the proliferation of colon cancer cells by reducing the levels of cell-cycle protein D1 RNA. | [58] |
| Aloe arborescens, Aloe barbadensis, Aloe chinensis | Anticancer | Inhibition of melanoma cell proliferation through anthraquinones photoactivation leading to ROS production. | [41] |
| Grapefruit | Anticancer | Inhibition of liver metastasis dependent on the induction of M1 macrophages (F4/80+IFNγ+IL-12+). | [125] |
| Grape | Anticancer | Apoptosis induction in colon-26 tumor and HT-29 adenocarcinoma cells. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzoni, E.; Bertoldi, A.; Cusumano, G.; Buratta, S.; Urbanelli, L.; Emiliani, C. Plant-Derived Extracellular Vesicles: Natural Nanocarriers for Biotechnological Drugs. Processes 2024, 12, 2938. https://doi.org/10.3390/pr12122938
Calzoni E, Bertoldi A, Cusumano G, Buratta S, Urbanelli L, Emiliani C. Plant-Derived Extracellular Vesicles: Natural Nanocarriers for Biotechnological Drugs. Processes. 2024; 12(12):2938. https://doi.org/10.3390/pr12122938
Chicago/Turabian StyleCalzoni, Eleonora, Agnese Bertoldi, Gaia Cusumano, Sandra Buratta, Lorena Urbanelli, and Carla Emiliani. 2024. "Plant-Derived Extracellular Vesicles: Natural Nanocarriers for Biotechnological Drugs" Processes 12, no. 12: 2938. https://doi.org/10.3390/pr12122938
APA StyleCalzoni, E., Bertoldi, A., Cusumano, G., Buratta, S., Urbanelli, L., & Emiliani, C. (2024). Plant-Derived Extracellular Vesicles: Natural Nanocarriers for Biotechnological Drugs. Processes, 12(12), 2938. https://doi.org/10.3390/pr12122938











