Assessment of the Productivity of Hydrogen and Nano-Carbon Through Liquid-Plasma Cracking of Waste Organic Solvent Using PrxNiyFeO3 Perovskite Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ferrite-Based Perovskites
2.2. Cracking of Waste Organic Solvent Using Liquid Plasma
2.3. Physicochemical Properties of the Perovskites
3. Results and Discussion
3.1. Refinement of the WOS
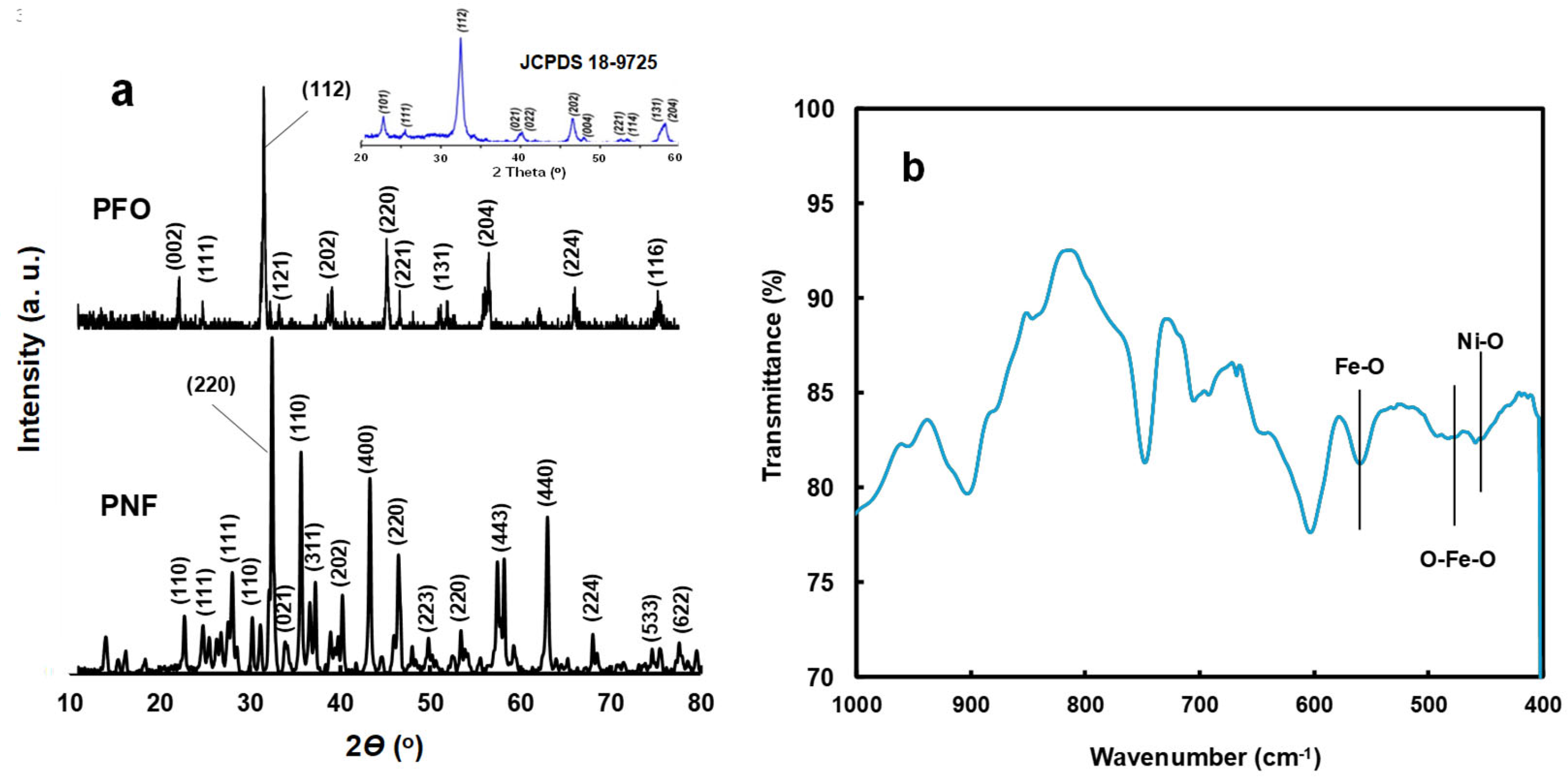
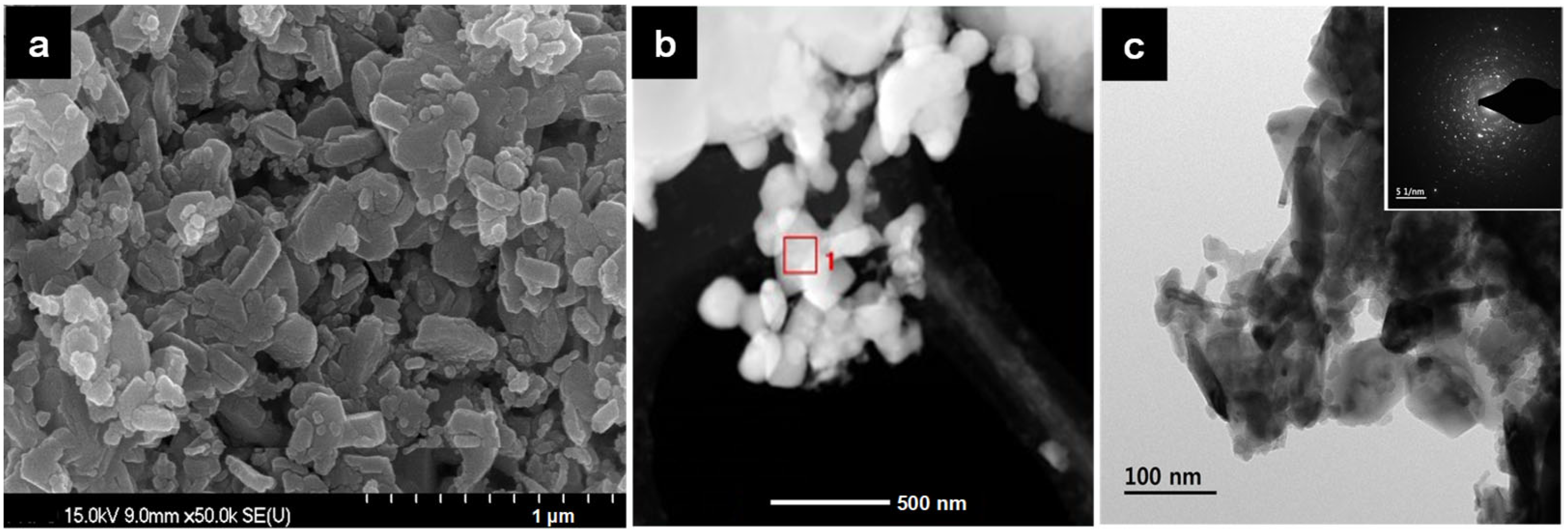
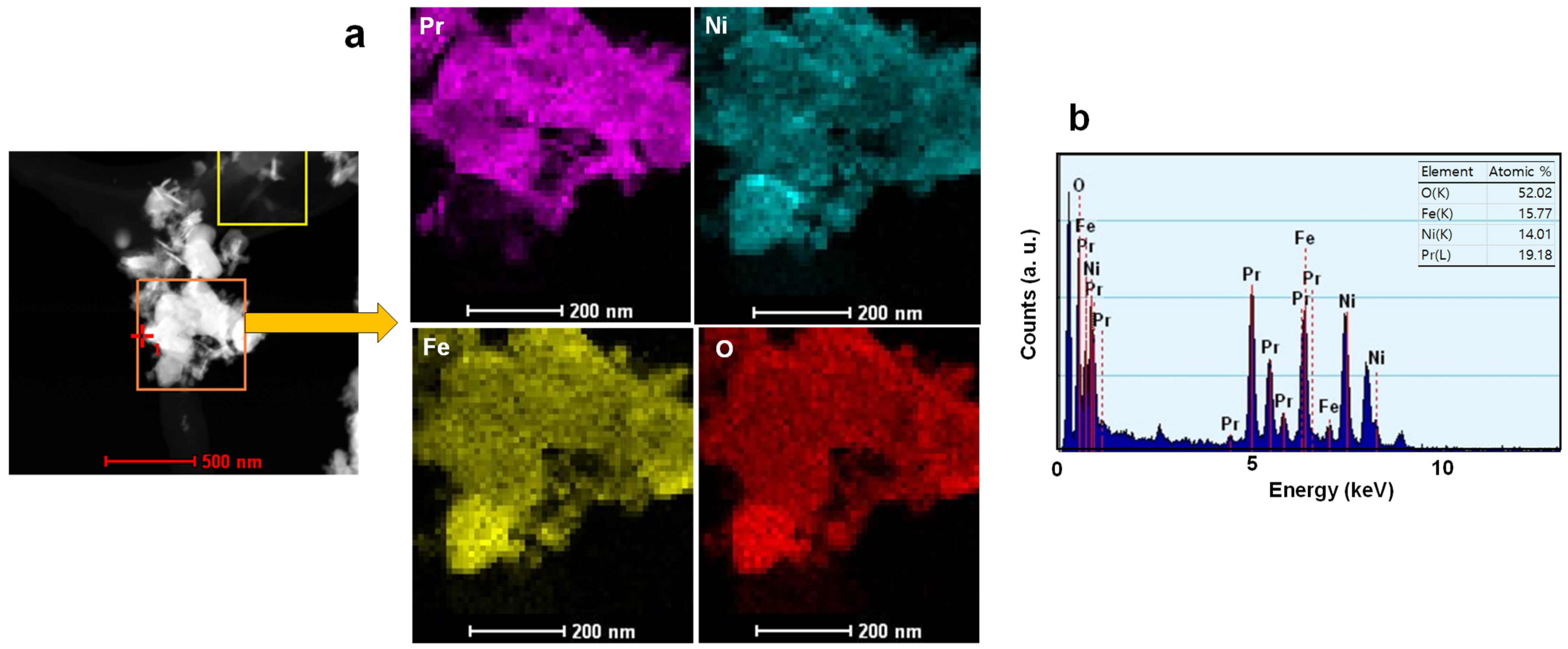
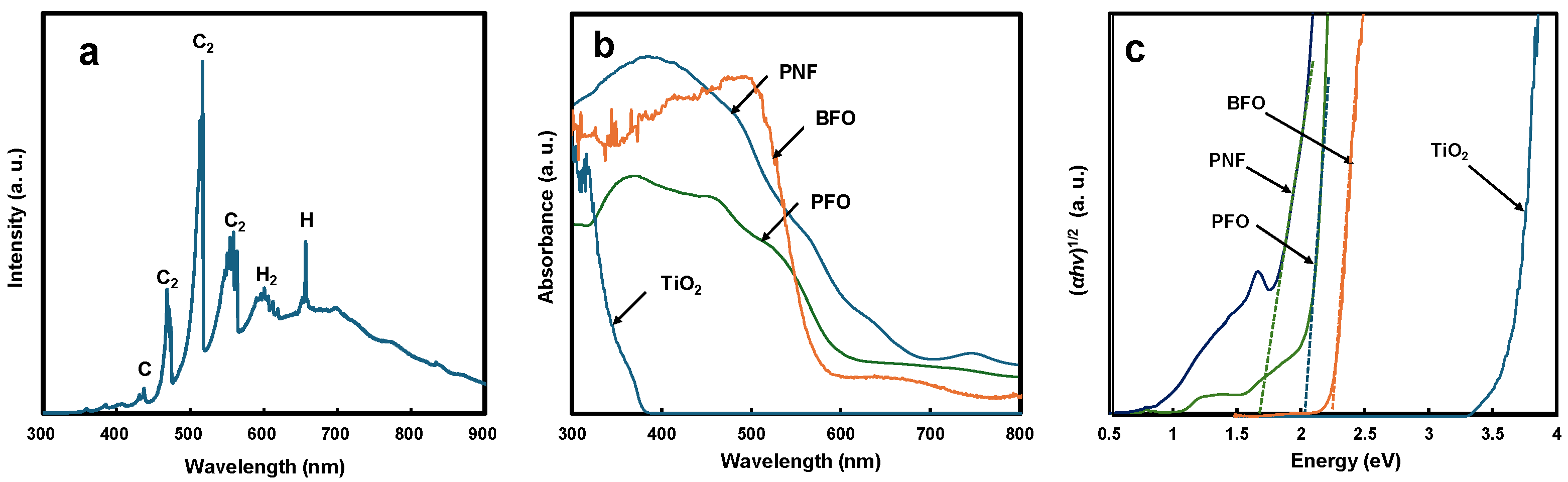
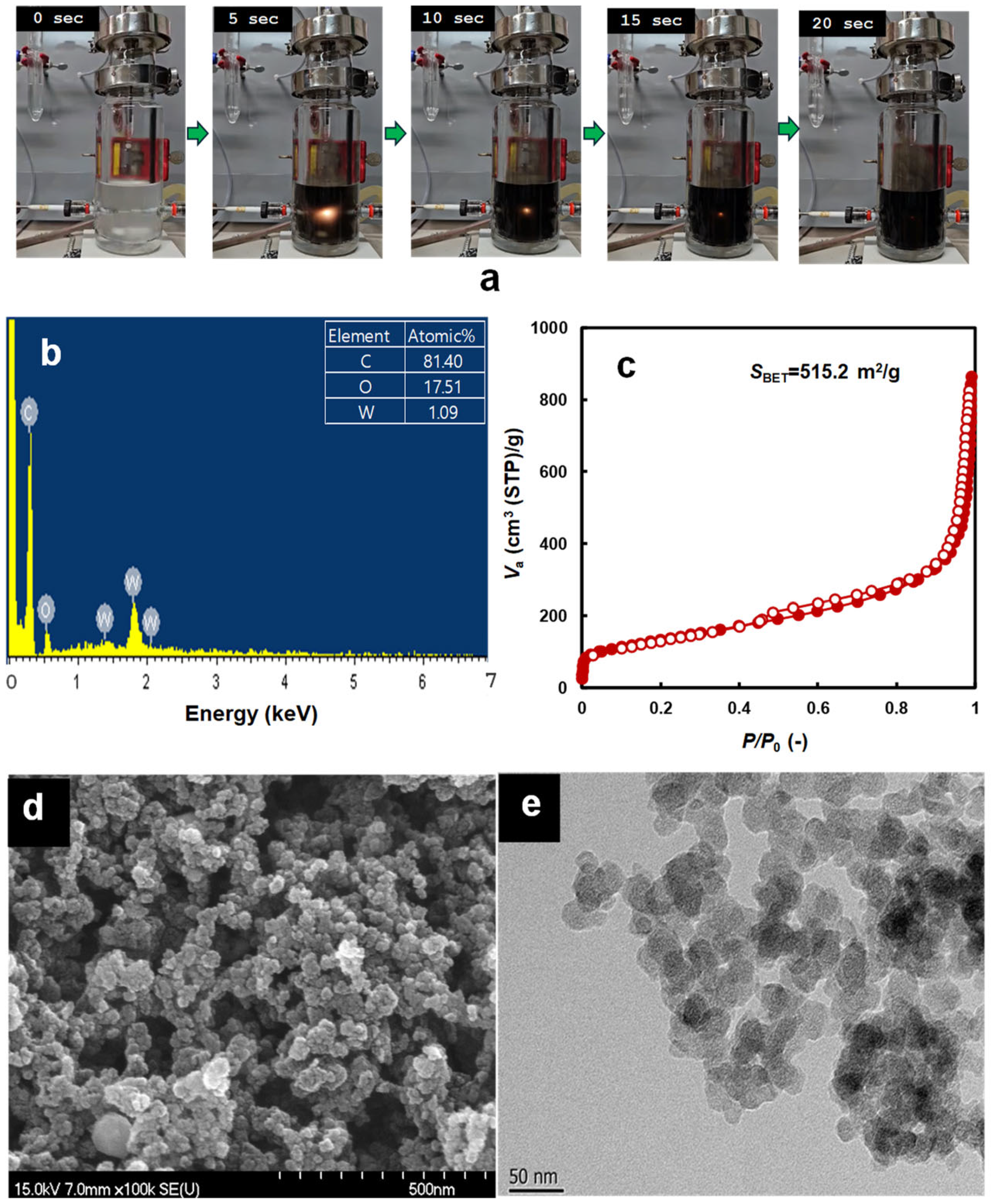
3.2. Physicochemical and Optical Properties of Ferrite-Based Perovskites
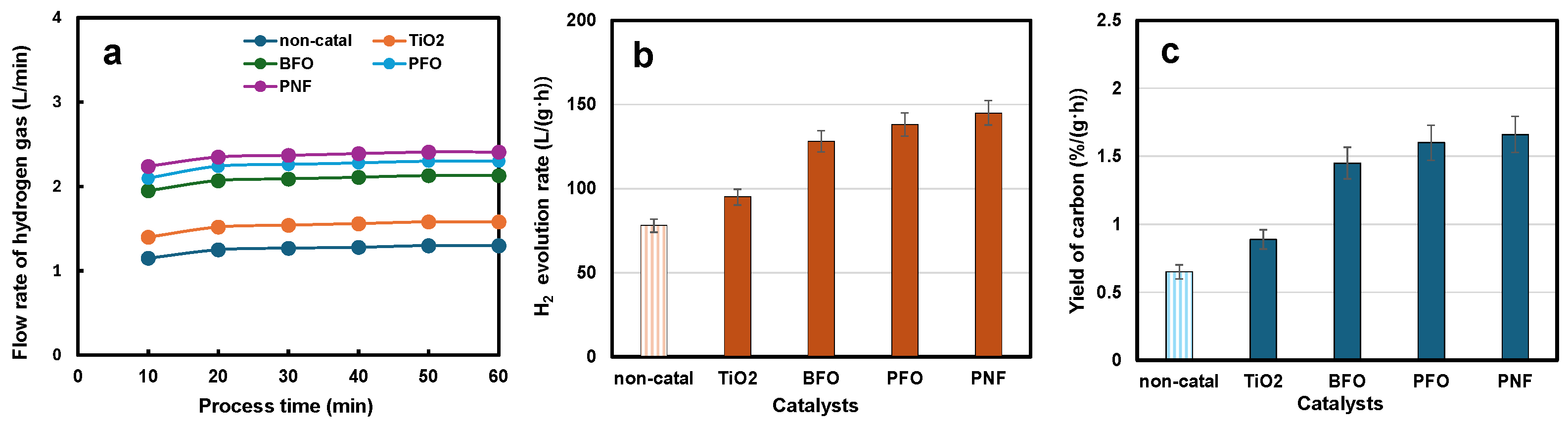
3.3. Production of Hydrogen and Carbon from the Decomposition of ROS by Liquid Plasma
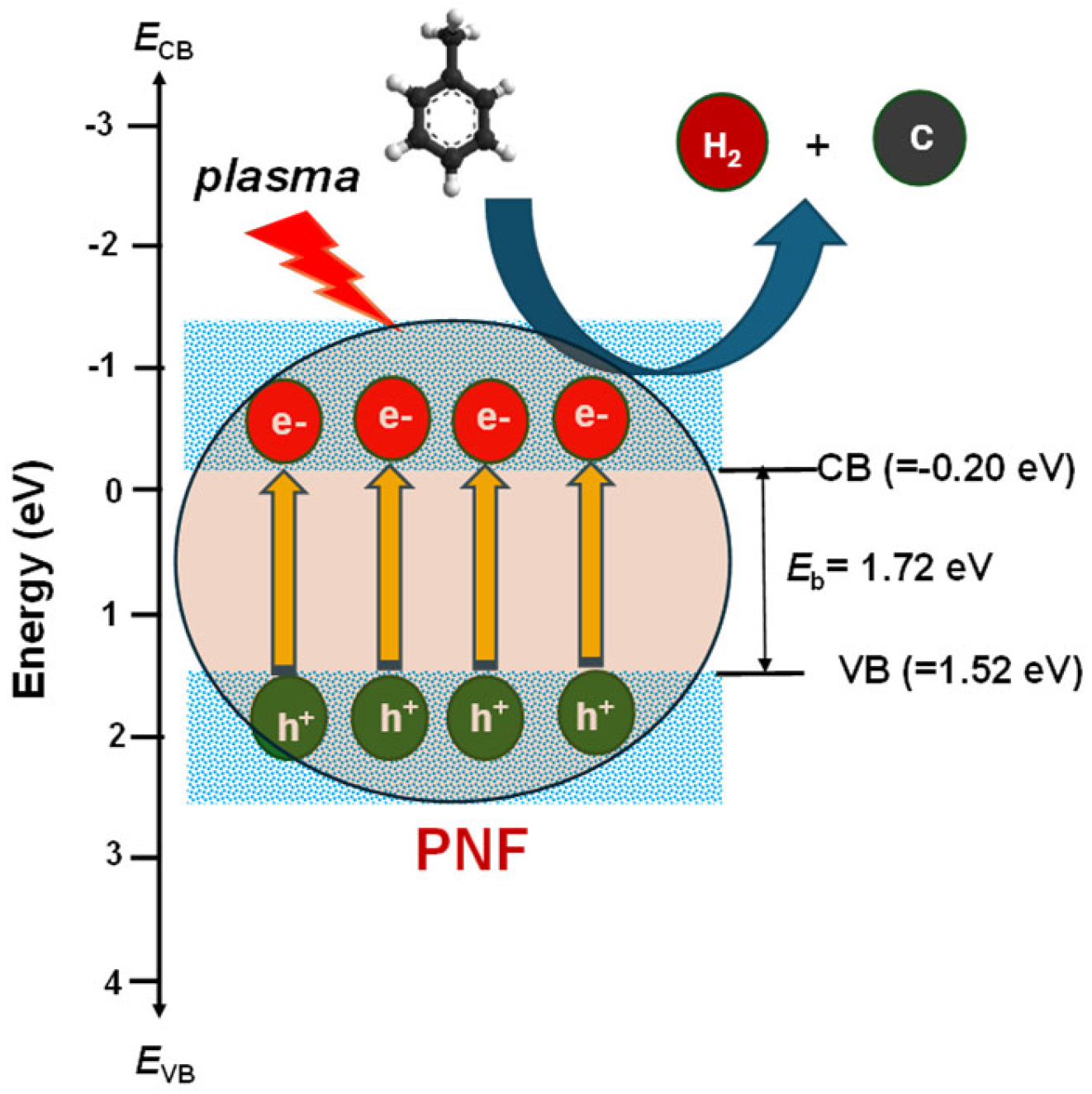
3.4. Hydrogen and Carbon Production Mechanism by Plasma Cracking of WOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Lai, C.; Wei, Z.; Zhou, X.; Liu, S.; Qin, L.; Yi, H.; Fu, Y.; Li, L.; Zhang, M.; et al. Strategies for improving the stability of perovskite for photocatalysis: A review of recent progress. Chemosphere 2023, 344, 140395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chu, Y.; Wang, C.; Zhao, Y.; Chu, W.; Zhao, J. Enhancing photocatalytic hydrogen production efficiency in all-inorganic lead-free double perovskites via silver doping-induced efficient separation of photogenerated carriers. Sep. Purif. Technol. 2025, 357, 130111. [Google Scholar] [CrossRef]
- Sun, K.; Li, Z.; Cao, Y.; Wang, F.; Qyyum, M.A.; Han, N. Recent advancements in perovskite electrocatalysts for clean energy-related applications: Hydrogen production, oxygen electrocatalysis, and nitrogen reduction. Int. J. Hydrogen Energy 2024, 52, 1104–1126. [Google Scholar] [CrossRef]
- Subha, N.; Sankar, A.R.; Navaneethakrishnan, S.; Lavanya, J.; Aakash, M. Perovskite-based Z-scheme photocatalytic system for hydrogen production. Catal. Commun. 2024, 187, 106903. [Google Scholar] [CrossRef]
- Tabish, A.; Varghese, A.M.; Wahab, M.A.; Karanikolos, G.N. Perovskites in the energy grid and CO2 conversion: Current context and future directions. Catalysts 2020, 10, 95. [Google Scholar] [CrossRef]
- Bienkowski, K.; Solarska, R.; Trinh, L.; Widera-Kalinowska, J.; Al-Anesi, B.; Liu, M.; Grandhi, G.K.; Vivo, P.; Oral, B.; Yılmaz, B.; et al. Halide perovskites for photoelectrochemical water splitting and CO2 reduction: Challenges and opportunities. ACS Catal. 2024, 14, 9. [Google Scholar] [CrossRef]
- Al-Gamal, A.G.; Yehia, F.; Elmasry, M.R.; El-Khair, M.A.A.; Kandeel, H.S.; Elseman, A.M.; Kim, D.-H.; Kabel, K.I. Perovskite materials for hydrogen evolution: Processes, challenges and future prospectives. Int. J. Hydrogen Energy 2024, 79, 1113–1138. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Sun, H.T. Metal-doped lead halide perovskites: Synthesis, properties, and optoelectronic applications. Chem. Mater. 2018, 30, 6589–6613. [Google Scholar] [CrossRef]
- Sundar, D.; Karuppasamy, L.; Gurusamy, L.; Liu, C.-H.; Wu, J.J. Perovskites-like composites for CO2 photoreduction into hydrocarbon fuels. Curr. Opin. Green Sustain. Chem. 2022, 33, 100563. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Wang, J.; Shen, Q.; Zhang, Y.; Ding, C.; Bai, Y.; Jiang, G.; Li, Z.; Gaponik, N. Boosting photocatalytic CO2 Reduction on CsPbBr3 perovskite nanocrystals by immobilizing metal complexes. Chem. Mater. 2020, 32, 1517–1525. [Google Scholar] [CrossRef]
- Bach, A.-U.-R.; Nabi, I.; Chen, Y.; Li, Z.; Iqbal, A.; Liu, W.; Afridi, M.N.; Arifeen, A.; Jin, W.; Yang, L. Environmental application of perovskite material for organic pollutant-enriched wastewater treatment. Coord. Chem. Rev. 2023, 495, 215378. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Y.; Dai, J.; Li, T.; Liu, Y.; Dai, X.; Xiao, X.; Deng, Y.; Gruverman, A.; Zeng, X.C.; et al. Metallic surface doping metal halide perovskites. Nat. Commun. 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, G.; Fu, H.; Zheng, W.; Ma, L.; Chen, X. Doping engineering of lead halide perovskite nanocrystals for advanced optoelectronic applications. Coord. Chem. Rev. 2024, 519, 216119. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Current trends in strategies to improve photocatalytic performance of perovskites materials for solar to hydrogen production. Renew. Sustain. Energy Rev. 2020, 132, 110073. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Attia, A.; Hussain, S.; Khan, M.I.; Sadaf, A.; Seliem, A.F.; Mohammed, A.Y.A.; Ibrahim, M.M. Tuning the band gap edges of perovskite material by Cd doping for achieving high current density in perovskite solar cells. Ceram. Int. 2023, 49, 20465–20469. [Google Scholar] [CrossRef]
- Mantry, S.P.; Bharti, N.K.; Modak, B. Enhancement of visible light-driven water splitting and CO2 conversion activities of SrTiO3 by tuning the dopant charge state. J. Phys. Chem. C 2024, 128, 4383–4394. [Google Scholar] [CrossRef]
- Wang, X.-D.; Huang, Y.-H.; Liao, J.-F.; Wei, J.-F.; Li, W.-G.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B. Surface passivated halide perovskite single-crystal for efficient photoelectrochemical synthesis of dimethoxydihydrofuran. Nat. Commun. 2021, 12, 1202. [Google Scholar] [CrossRef]
- Kumar, V.; Kathiravan, A.; Jhonsi, M.A. Beyond lead halide perovskites: Crystal structure, bandgaps, photovoltaic properties and future stance of lead-free halide double perovskites. Nano Energy 2024, 125, 109523. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Aldsmasy, M.H.; Frasca, C.; Abate, A.; Zhao, K.; Hu, Y. Advances in the synthesis of halide perovskite single crystals for optoelectronic applications. Chem. Matt. 2023, 35, 2683–2712. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Kharat, A.N.; Taghavinia, N. Moisture stability in nanostructured perovskite solar cells. Mater. Lett. 2019, 237, 356–360. [Google Scholar] [CrossRef]
- Qin, K.; Dong, B.; Wang, S. Improving the stability of metal halide perovskite solar cells from material to structure. J. Energy Chem. 2019, 33, 90–99. [Google Scholar] [CrossRef]
- Liao, D.; Li, P.; Shai, X.; Huang, W.; Liu, S.; Li, H.; Shen, Y.; Wang, M. Recent progress on stability issues of organic–inorganic hybrid lead perovskite-based solar cells. RSC Adv. 2016, 6, 89356–89366. [Google Scholar] [CrossRef]
- Miah, M.H.; Khandaker, M.U.; Rahman, M.B.; Nur-E-Alam, M.; Islam, M.A. Band gap tuning of perovskite solar cells for enhancing the efficiency and stability: Issues and prospects. RSC Adv. 2024, 14, 15876–15906. [Google Scholar] [CrossRef]
- Raza, M.A.; Li, F.; Que, M.; Zhu, L.; Chen, X. Photocatalytic reduction of CO2 by halide perovskites: Recent advances and future perspectives. Mater. Adv. 2021, 2, 7187–7209. [Google Scholar] [CrossRef]
- Chang, Y.K.; Chen, N.-W.; Chen, T.Y.; Shuang, Y.M.; Yen, Y.-S. Enhancing efficiency and stability in perovskite solar cells through blended hole transporting materials incorporating benzo[g]quinoxaline-conjugated small molecules. ACS Appl. Energy Mater. 2024, 7, 1287–1297. [Google Scholar] [CrossRef]
- Al-Shujaa, S.; Zhao, P.; He, D.; Al-Anesi, B.; Feng, Y.; Xia, J.; Zhang, B.; Zhang, Y. Improving the efficiency and stability of perovskite solar cells by refining the perovskite-electron transport layer interface and shielding the absorber from UV effects. ACS Appl. Mater. Interfaces 2024, 16, 28493–28504. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Jimenez, M.; Yubero, C.; Calzada, M.D. Study on the reforming of alcohols in a surface wave discharge (SWD) at atmospheric pressure. J. Phys. D 2008, 41, 175201. [Google Scholar] [CrossRef]
- Sarmiento, B.; Brey, J.J.; Viera, I.G.; Gonzalez-Elipe, A.R.; Cotrino, J.; Rico, V.J. Hydrogen production by reforming of hydrocarbons and alcohols in a dielectric barrier discharge. J. Power Sources 2007, 169, 140–143. [Google Scholar] [CrossRef]
- Deminsky, M.; Jivotov, V.; Potapkin, B.; Rusanov, V. Plasma assisted production of hydrogen from hydrocarbons. Pure Appl. Chem. 2002, 74, 413–418. [Google Scholar] [CrossRef]
- Hrycak, B.; Czylkowski, D.; Miotk, R.; Dors, M.; Jasinski, M.; Mizeraczyk, J. Application of atmospheric pressure microwave plasma source for hydrogen production from ethanol. Int. J. Hydrogen Energy 2014, 39, 14184–14190. [Google Scholar] [CrossRef]
- Sun, D.P.; Yang, X.H.; Liu, Y.Y.; Chen, Y.Q. Study on decomposition products of methanol in AC discharge by spectroscopy. Spectrosc. Spectr. Anal. 2008, 28, 1983–1986. [Google Scholar] [CrossRef]
- Aubry, O.; Met, C.; Khacef, A.; Cormier, J.M. On the use of a non-thermal plasma reactor for ethanol steam reforming. Chem. Eng. J. 2005, 106, 241–247. [Google Scholar] [CrossRef]
- Yan, Z.C.; Li, C.; Lin, W.H. Hydrogen generation by glow discharge plasma electrolysis of methanol solutions. Int. J. Hydrogen Energy 2009, 34, 48–55. [Google Scholar] [CrossRef]
- Kabashima, H.; Einaga, H.; Futamura, S. Hydrogen generation from water, methane, and methanol with nonthermal plasma. IEEE Trans. Ind. Appl. 2003, 39, 340–345. [Google Scholar] [CrossRef]
- Kabashima, H.; Einaga, H.; Futamura, S. Hydrogen generation from water with nonthermal plasma. Chem. Lett. 2001, 30, 1314–1315. [Google Scholar] [CrossRef]
- Li, H.Q.; Zou, J.J.; Zhang, Y.P.; Liu, C.J. Novel plasma methanol decomposition to hydrogen using corona discharges. Chem. Lett. 2004, 33, 744–745. [Google Scholar] [CrossRef]
- Chernyak, V.Y.; Olszewski, S.V.; Yukhymenko, V.; Solomenko, E.V.; Prysiazhnevych, I.V.; Naumov, V.V.; Levko, D.S.; Shchedrin, A.I.; Ryabtsev, A.V.; Demchina, V.P.; et al. Plasma-assisted reforming of ethanol in dynamic plasma-liquid system: Experiments and modeling. IEEE Trans. Plasma Sci. 2008, 36, 2933–2939. [Google Scholar] [CrossRef]
- Bromberg, L.; Cohn, D.R.; Rabinovich, A. Plasma reformer fuel cell system for decentralized power applications. Int. J. Hydrogen Energy 1997, 22, 83–94. [Google Scholar] [CrossRef]
- Chung, K.-H.; Lam, S.S.; Park, Y.-K.; Kim, S.-J.; Jung, S.-C. Application of liquid-phase plasma for the production of hydrogen and carbon from the plasma-induced cracking of liquid hydrocarbons. Fuel 2022, 328, 125297. [Google Scholar] [CrossRef]
- Brown, O.F. A comparative study of fuels for on-board hydrogen production for fuel-cell-powered automobiles. Int. J. Hydrogen Energy 2001, 26, 381–397. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, L.; Mlotek, M.; Krawczyk, K. Efficient plasma technology for the production of green hydrogen from ethanol and water. Energies 2022, 15, 2777. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen production technologies overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Seroglazova, A.S.; Chebanenko, M.I.; Popkov, V.I. Synthesis, structure, and photo-Fenton activity of PrFeO3-TiO2 mesoporous nanocomposites. Condens. Matter. Interphase 2021, 23, 543–547. [Google Scholar] [CrossRef]
- Tijare, S.N.; Bakardjieva, S.; Subrt, J.; Joshi, M.V.; Rayalu, S.S.; Hishita, S.; Labhsetwar, N. Synthesis and visible light photocatalytic activity of nanocrystalline PrFeO3 perovskite for hydrogen generation in ethanol-water system. J. Chem. Sci. 2014, 126, 517–525. [Google Scholar] [CrossRef]
- Kimura, S.; Arai, F.; Ikezawa, M. Mixed valence of praseodymium oxides. In Proceedings of the 11th International Conference on Vacuum Ultraviolet Radiation Physics, Toshima, Japan, 27 August–1 September 1995; Volume 78, p. 135. [Google Scholar] [CrossRef]
- Pradhan, G.; Parida, K. Fabrication of iron-cerium mixed oxide: An efficient photocatalyst for dye degradation. Int. J. Eng. Sci. Tech. 2010, 2, 53. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Deng, Y.; Qian, Y.; Jia, X.; Ma, M.; Yang, C.; Liu, K.; Wang, Z.; Qu, S.; et al. The application of perovskite materials in solar water splitting. J. Semicond. 2020, 41, 011701. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrogen Energy 2010, 35, 12161. [Google Scholar] [CrossRef]
- Pandey, S.K.; Bindu, R.; Bhatt, P.; Chaudhari, S.M.; Piple, A.V. Synthesis and investigation of structural and electronic properties of Pr1−xCaxFeO3 (0≤x≤0.2) compounds. Phys. B 2005, 365, 47–54. [Google Scholar] [CrossRef]
- Ito, A.; Masumoto, H.; Goto, T. Microstructure and electrical conductivity of SrRuO3 thin films prepared by laser ablation. Mater. Trans. 2006, 11, 2808. [Google Scholar] [CrossRef][Green Version]
- Dupin, J.; Gonbeau, D.; Vinatire, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319. [Google Scholar] [CrossRef]
- Ponce, S.; Pena, M.A.; Fierro, J.L.G. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl. Catal. B Environ. 2000, 24, 193. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]

| Component | WOS (Conc., %) | ROS (Conc., %) |
|---|---|---|
| Benzene | 1.23 | 0.07 |
| 1,2-dichloroethane | 2.71 | 0.02 |
| Trichloroethylene | 0.06 | 0 |
| Toluene | 46.79 | 98.67 |
| Tetrachloroethylene | 0.03 | 0.01 |
| Ethylbenzene | 0.22 | 0.02 |
| p,m-xylene | 3.54 | 0.08 |
| o-xylene | 2.46 | 1.13 |
| Water | 42.96 | 0 |
| total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-C.; You, C.-S.; Chung, K.-H. Assessment of the Productivity of Hydrogen and Nano-Carbon Through Liquid-Plasma Cracking of Waste Organic Solvent Using PrxNiyFeO3 Perovskite Catalysts. Processes 2024, 12, 2932. https://doi.org/10.3390/pr12122932
Jung S-C, You C-S, Chung K-H. Assessment of the Productivity of Hydrogen and Nano-Carbon Through Liquid-Plasma Cracking of Waste Organic Solvent Using PrxNiyFeO3 Perovskite Catalysts. Processes. 2024; 12(12):2932. https://doi.org/10.3390/pr12122932
Chicago/Turabian StyleJung, Sang-Chul, Chan-Seo You, and Kyong-Hwan Chung. 2024. "Assessment of the Productivity of Hydrogen and Nano-Carbon Through Liquid-Plasma Cracking of Waste Organic Solvent Using PrxNiyFeO3 Perovskite Catalysts" Processes 12, no. 12: 2932. https://doi.org/10.3390/pr12122932
APA StyleJung, S.-C., You, C.-S., & Chung, K.-H. (2024). Assessment of the Productivity of Hydrogen and Nano-Carbon Through Liquid-Plasma Cracking of Waste Organic Solvent Using PrxNiyFeO3 Perovskite Catalysts. Processes, 12(12), 2932. https://doi.org/10.3390/pr12122932







