Synthesis, Rheology, Morphology, and Mechanical Properties of Biodegradable PVA-Based Composite Films: A Review on Recent Progress
Abstract
:1. Introduction
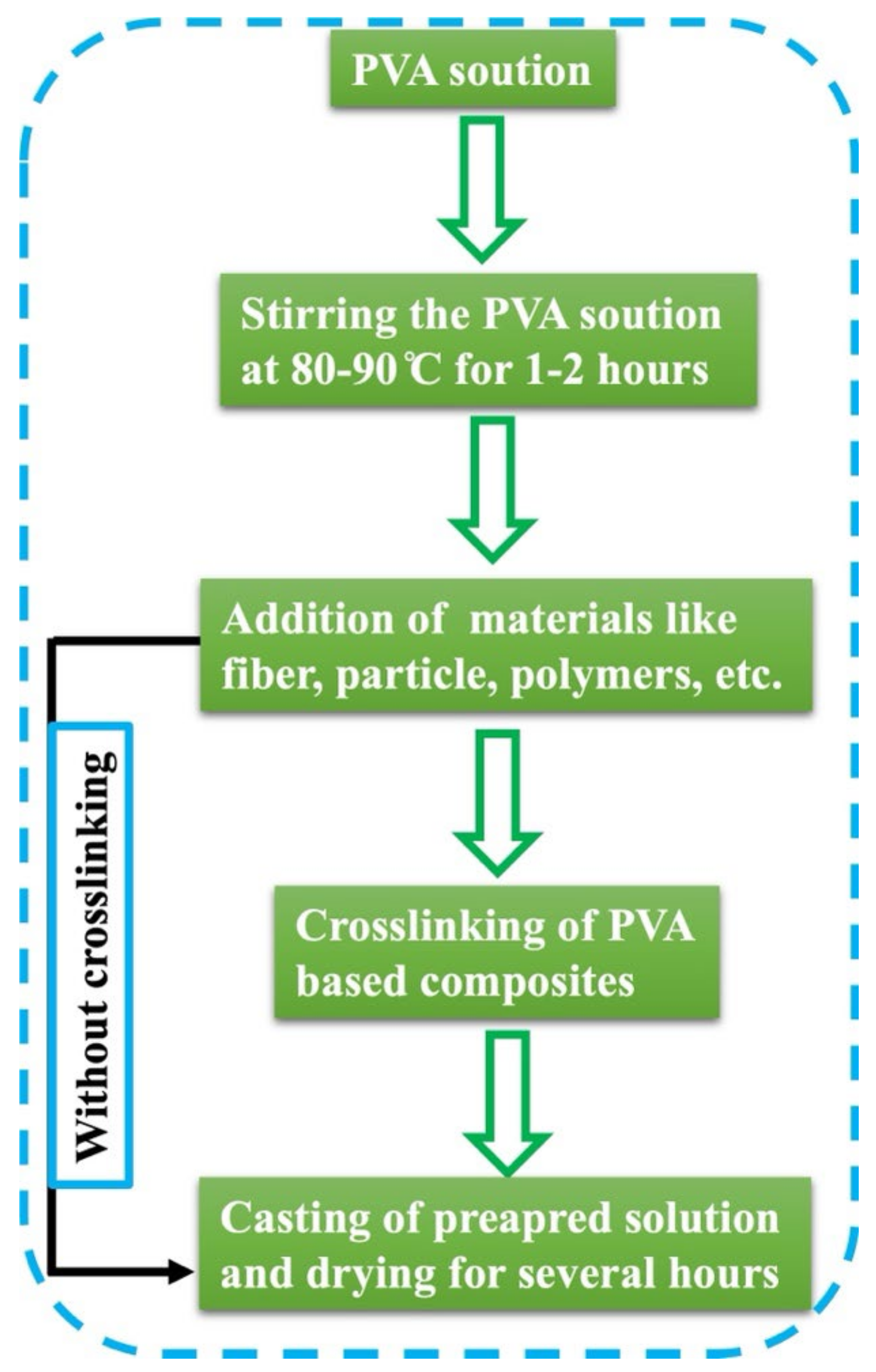
2. Synthesis
2.1. Synthesis of PVA-Based Composite Films
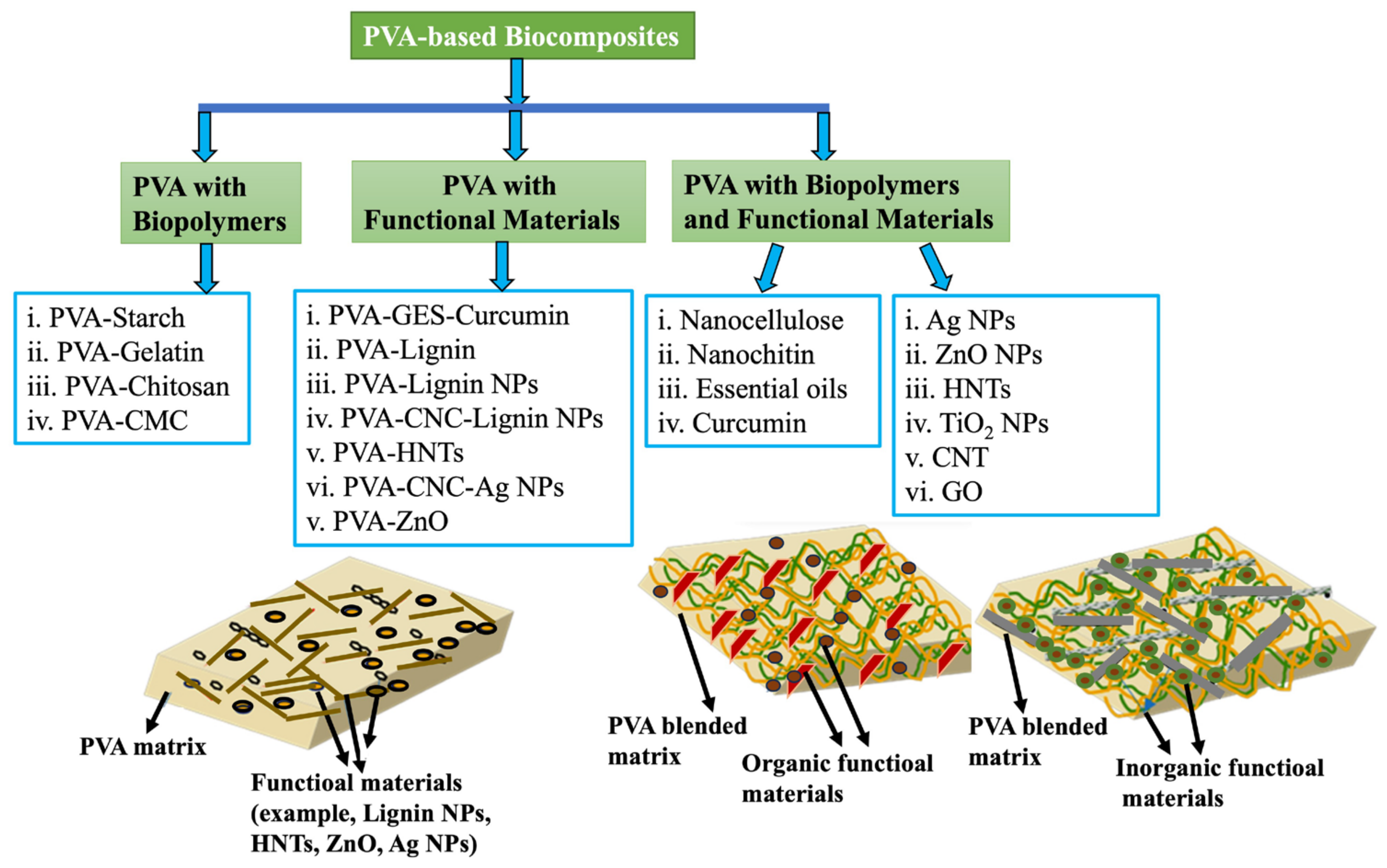
2.2. PVA-Based Biopolymer/Inorganic Nanoparticles/Functional Composite Films
3. Morphology of PVA-Based Composite Films
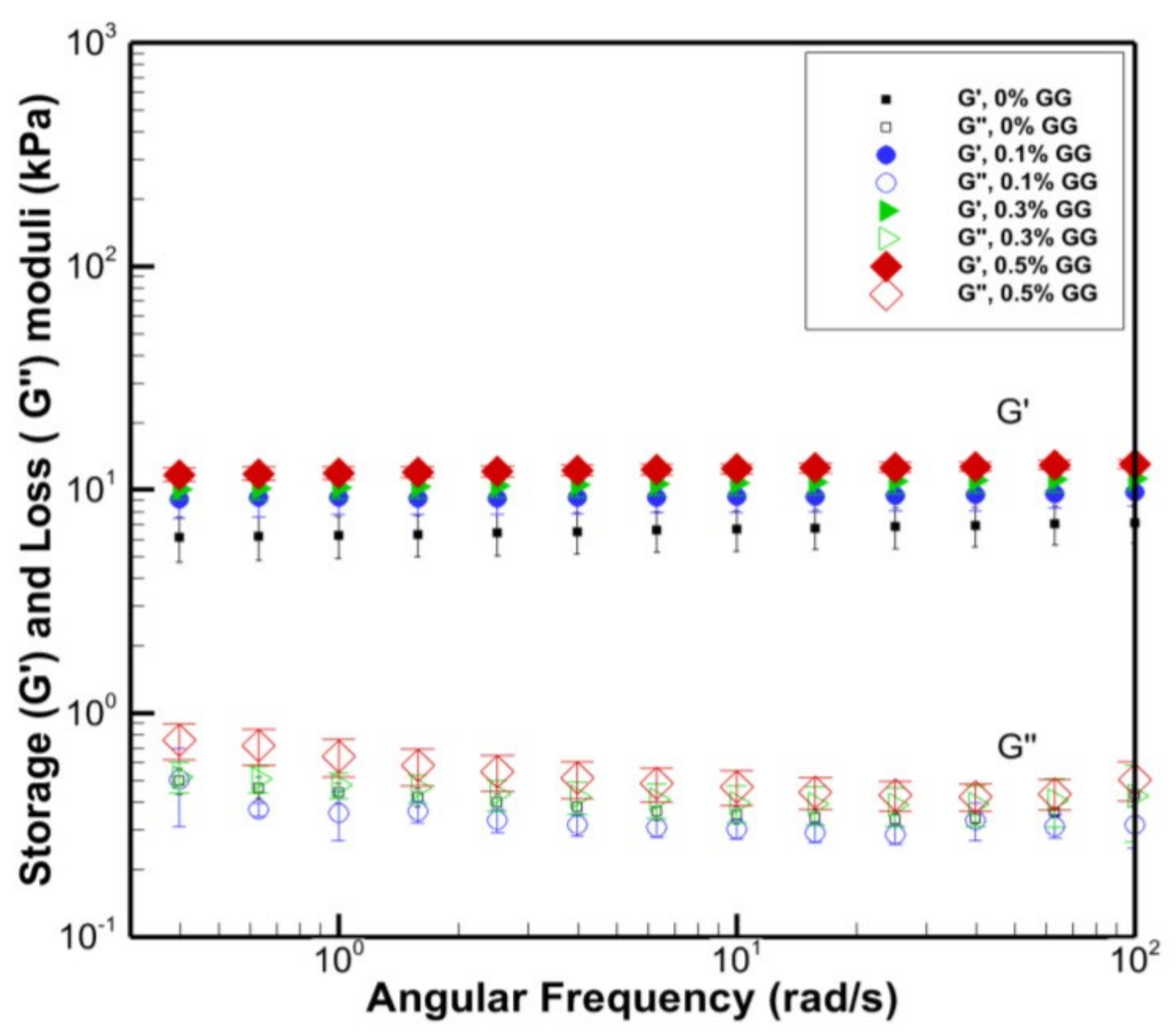
4. Rheological Properties of PVA-Based Composite Films
5. Mechanical Properties of PVA-Based Composite Films
6. Summary and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | Poly vinyl alcohol |
| PBC | PVA-based composites |
| GO | Graphene oxide |
| CMC | Carboxymethyl cellulose |
| CNT | Carbon nanotube |
| HNTs | Halloysite nanotubes |
| PANI | Polyaniline |
| PL | Photoluminescence |
| rGO | Reduced graphene oxide |
| CS | Chitosan |
| MFC | Micro fibrillated cellulose |
| TMC | Trimesoyl chloride |
| CNF | Cellulose nanofibers |
| HA | Hydroxyapatite |
| GG | Gellan gum |
| CNC | Cellulose nanocrystal |
| PNIPAm | Poly(N-isopropylacrylamide) |
| EMImAc | 1-ethyl-3-methylimidazolium acetate |
| LCST | Lower critical solution temperature |
| DMA | Dynamic mechanical analysis |
| GO | Graphene oxide |
References
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag. Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Imam, S.H.; Mao, L. Composite Films Based on Biorelated Agro-Industrial Waste and Poly(vinyl alcohol). Preparation and Mechanical Properties Characterization. Biomacromolecules 2001, 2, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- O’Looney, J. Marking Progress Toward Service Integration. Adm. Soc. Work 1997, 21, 31–65. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Vettumperumal, R.; Kokila, R. A study of linear optical properties of ternary blends PVA/CMC/aloe vera biofilm for UV shielding. Appl. Nanosci. 2021, 11, 669–678. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym.-Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Chakraborty, N.; Jeong, J.-H. Easy fabrication of PVA-CaO-CuO composite films for efficient photocatalyst: Towards distinct luminescence property, morphology and thermal stability. Inorg. Chem. Commun. 2024, 170, 113287. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Pal, S.; Hoque, M.M.; Alam, M.R.; Younus, M.; Kobayashi, H. Simple Fabrication of PVA–ZnS Composite Films with Superior Photocatalytic Performance: Enhanced Luminescence Property, Morphology, and Thermal Stability. ACS Omega 2019, 4, 6144–6153. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Akter, M.; Amin, M.K.; Younus, M.; Chakraborty, N. Synthesis, Luminescence and Thermal Properties of PVA–ZnO–Al2O3 Composite Films: Towards Fabrication of Sunlight-Induced Catalyst for Organic Dye Removal. J. Polym. Environ. 2018, 26, 3371–3381. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, antioxidant and swelling behaviour of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wu, Y.; Li, G.; Han, Y.; Chu, F. Transparent Nanocomposite Films of Lignin Nanospheres and Poly(vinyl alcohol) for UV-Absorbing. Ind. Eng. Chem. Res. 2018, 57, 1207–1212. [Google Scholar] [CrossRef]
- E, S.; Ning, D.; Wang, Y.; Huang, J.; Jin, Z.; Ma, Q.; Yang, K.; Lu, Z. Ternary Synergistic Strengthening and Toughening of Bio-Inspired TEMPO-Oxidized Cellulose Nanofibers/Borax/Polyvinyl Alcohol Composite Film with High Transparency. ACS Sustain. Chem. Eng. 2020, 8, 15661–15669. [Google Scholar] [CrossRef]
- Luis, L.; Alexander, G.; Lilian, A.; Cristian, T. Manufacture of β-chitin nano- and microparticles from jumbo squid pen (Dosidicus gigas) and evaluation of their effect on mechanical properties and water vapour permeability of polyvinyl alcohol/chitosan films. J. Food Eng. 2021, 290, 110230. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, G.; Chen, S.-C.; Song, F.; Wang, Y.-Z. Green Fabrication of High-Performance Chitin Nanowhiskers/PVA Composite Films with a “Brick-and-Mortar” Structure. ACS Sustain. Chem. Eng. 2020, 8, 17807–17815. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.; Liu, Y. Developing poly(vinyl alcohol)/chitosan films incorporate with d-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw. Appl. Surf. Sci. 2018, 449, 591–602. [Google Scholar] [CrossRef]
- Shojaee Kang Sofla, M.; Mortazavi, S.; Seyfi, J. Preparation and characterization of polyvinyl alcohol/chitosan blends plasticized and coMPatibilized by glycerol/polyethylene glycol. Carbohydr. Polym. 2020, 232, 115784. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Radhakrishnan, E.K. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- Kahvand, F.; Fasihi, M. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. Int. J. Biol. Macromol. 2019, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Nair, I.C.; Siengchin, S.; Parameswaranpillai, J.; Radhakrishnan, E.K. Lipopeptide and zinc oxide nanoparticles blended polyvinyl alcohol-based nanocomposite films as antimicrobial coating for biomedical applications. Process Biochem. 2021, 102, 220–228. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Rathinavel, S.; Saravanakumar, S.S. Development and Analysis of Silver Nano Particle Influenced PVA/Natural Particulate Hybrid Composites with Thermo-Mechanical Properties. J. Polym. Environ. 2021, 29, 1894–1907. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Chen, W.; Tao, X.; Xue, P.; Cheng, X. Enhanced mechanical properties and morphological characterizations of poly(vinyl alcohol)–carbon nanotube composite films. Appl. Surf. Sci. 2005, 252, 1404–1409. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Antioxidant and antimicrobial poly(vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Lamarra, J.; Rivero, S.; Pinotti, A. Nanocomposite bilayers based on poly(vinyl alcohol) and chitosan functionalized with gallic acid. Int. J. Biol. Macromol. 2020, 146, 811–820. [Google Scholar] [CrossRef]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and antimicrobial properties of carbohydrate-based films enriched with cinnamon essential oil by Pickering emulsion method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Patil, D.S.; Shaikh, J.S.; Dalavi, D.S.; Kalagi, S.S.; Patil, P.S. Chemical synthesis of highly stable PVA/PANI films for supercapacitor application. Mater. Chem. Phys. 2011, 128, 449–455. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Ding, C.; Qiao, Z. A review of the application of polyvinyl alcohol membranes for fuel cells. Ionics 2022, 28, 1–13. [Google Scholar] [CrossRef]
- Sonker, A.K.; Wagner, H.D.; Bajpai, R.; Tenne, R.; Sui, X. Effects of tungsten disulphide nanotubes and glutaric acid on the thermal and mechanical properties of polyvinyl alcohol. Compos. Sci. Technol. 2016, 127, 47–53. [Google Scholar] [CrossRef]
- Kuljanin, J.; Čomor, M.I.; Djoković, V.; Nedeljković, J.M. Synthesis and characterization of nanocomposite of polyvinyl alcohol and lead sulfide nanoparticles. Mater. Chem. Phys. 2006, 95, 67–71. [Google Scholar] [CrossRef]
- Mehto, A.; Mehto, V.R.; Chauhan, J. Preparation and Characterization of Polyvinyl Alcohol (PVA)/ZrO2 Composite Membranes. Phys. Status Solidi (B) 2023, 260, 2300164. [Google Scholar] [CrossRef]
- Sirotkin, N.; Khlyustova, A.; Costerin, D.; Naumova, I.; Kalazhokov, Z.; Kalazhokov, K.; Titov, V.; Agafonov, A. Synthesis of chitosan/PVA/metal oxide nanocomposite using underwater discharge plasma: Characterization and antibacterial activities. Polym. Bull. 2023, 80, 5655–5674. [Google Scholar] [CrossRef]
- Algelal, H.; Kareem, S.; Mohammed, K.; Khamees, E.; Abed, A.; Alkhayatt, A.; Al-Okbi, R. Synthesis of PVA-Fe2O3-TiO2 hybrid structure for biomedical application. J. Optoelectron. Biomed. Mater. 2022, 14, 43–51. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Zerefa, E.; Adimasu, Y. PVA assisted ZnO based mesoporous ternary metal oxides nanomaterials: Synthesis, optimization, and evaluation of antibacterial activity. Mater. Res. Express 2020, 7, 045011. [Google Scholar] [CrossRef]
- Ali, A.I.; Salim, S.A.; Kamoun, E.A. Novel glass materials-based (PVA/PVP/Al2O3/SiO2) hybrid composite hydrogel membranes for industrial applications: Synthesis, characterization, and physical properties. J. Mater. Sci. Mater. Electron. 2022, 33, 10572–10584. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2020, 81, 106186. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Hamdi, M.; Fortunati, E.; Kenny, J.M.; Buonocore, G.G.; Lavorgna, M. Synergistic Effect of Halloysite and Cellulose Nanocrystals on the Functional Properties of PVA Based Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 794–800. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef]
- Morales, A.; Andrés, M.Á.; Labidi, J.; Gullón, P. UV–vis protective poly(vinyl alcohol)/bio-oil innovative films. Ind. Crops Prod. 2019, 131, 281–292. [Google Scholar] [CrossRef]
- Mahmoud, K.H. Synthesis, characterization, optical and antimicrobial studies of polyvinyl alcohol–silver nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 434–440. [Google Scholar] [CrossRef]
- Oun, A.A.; Shin, G.H.; Kim, J.T. Antimicrobial, antioxidant, and pH-sensitive polyvinyl alcohol/chitosan-based composite films with aronia extract, cellulose nanocrystals, and grapefruit seed extract. Int. J. Biol. Macromol. 2022, 213, 381–393. [Google Scholar] [CrossRef]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.N. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef]
- Adami, R.; Lamberti, P.; Casa, M.; D’Avanzo, N.; Ponticorvo, E.; Cirillo, C.; Sarno, M.; Bychanok, D.; Kuzhir, P.; Yu, C.; et al. Synthesis and Electrical Percolation of Highly Amorphous Polyvinyl Alcohol/Reduced Graphene Oxide Nanocomposite. Materials 2023, 16, 4060. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Netravali, A.N. Fabrication and characterization of biodegradable composites based on microfibrillated cellulose and polyvinyl alcohol. Compos. Sci. Technol. 2012, 72, 1588–1594. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Li, C.; Liang, M.; Lu, C.; Deng, Y. Mechanochemical activation of cellulose and its thermoplastic polyvinyl alcohol ecocomposites with enhanced physicochemical properties. Carbohydr. Polym. 2011, 83, 257–263. [Google Scholar] [CrossRef]
- Hyder, M.N.; Chen, P. Pervaporation dehydration of ethylene glycol with chitosan–poly(vinyl alcohol) blend membranes: Effect of CS–PVA blending ratios. J. Membr. Sci. 2009, 340, 171–180. [Google Scholar] [CrossRef]
- Ueda, T.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Kobayashi, Y.; Ito, H. Structural, rheological, and mechanical properties of polyvinyl alcohol composites reinforced with cellulose nanofiber treated by ultrahigh-pressure homogenizer. Mater. Today Commun. 2022, 33, 104316. [Google Scholar] [CrossRef]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G.; McCall, R.D. Preparation and characterization of poly(vinyl alcohol) nanocomposites made from cellulose nanofibers. J. Appl. Polym. Sci. 2009, 113, 2238–2247. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and BiocoMPatibility Properties. Materials 2020, 13, 2077. [Google Scholar] [CrossRef]
- Rumon, M.M.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Sarkar, S.D.; Alam, M.M.; Roy, C.K. Nanomaterials for Self-Healing Hydrogels. Emerg. Appl. Nanomater 2023, 141, 270–293. [Google Scholar]
- Rumon, M.M.H.; Sarkar, S.D.; Uddin, M.M.; Alam, M.M.; Karobi, S.N.; Ayfar, A.; Azam, M.S.; Roy, C.K. Graphene oxide based crosslinker for simultaneous enhancement of mechanical toughness and self-healing capability of conventional hydrogels. RSC Adv. 2022, 12, 7453–7463. [Google Scholar] [CrossRef] [PubMed]
- Shakil, A.R.; Begum, M.L.; Shaikh, M.A.A.; Sultana, S.; Rahman, M.S.; Rumon, M.M.H.; Roy, C.K.; Haque, M.A. Jute Fiber Reinforced Hydrogel Composite for Removal of Methylene Blue Dye from Water. Dhaka Univ. J. Sci. 2022, 70, 59–64. [Google Scholar] [CrossRef]
- Vasconcelos, H.C.; Carrêlo, H.; Eleutério, T.; Meirelles, M.G.; Özmenteş, R.; Amorim, R. Rheology of Cellulosic Microfiber Suspensions Under Oscillatory and Rotational Shear for Biocomposite Applications. Compounds 2024, 4, 688–707. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Huang, X.; Zhang, J.; Zhang, W. A novel high-molecule inorganic composite material with excellent elasticity and toughness used in tunnel isolation—Material design and basic mechanical properties. Constr. Build. Mater. 2024, 444, 137810. [Google Scholar] [CrossRef]
- Shi, X.; Wu, B.; Dong, X.; Zhang, Q.; Lu, W.; Chen, X. Preparation and Rheological Properties of the PVA/CMC/Gel Composite for Mining. ACS Omega 2024, 9, 28253–28267. [Google Scholar] [CrossRef]
- Tavassoli, M.; Bahramian, B.; Abedi-Firoozjah, R.; Jafari, N.; Javdani, H.; Sadeghi, S.M.; Hadavifar, S.; Majnouni, S.; Ehsani, A.; Roy, S. Comprehensive Review on Polyvinyl Alcohol-Based Electrospun Nanofibers for Food Packaging: Applications, Developments, and Future Horizon. Food Bioprocess Technol. 2024. [Google Scholar] [CrossRef]
- Dananjaya, V.; Marimuthu, S.; Yang, R.; Grace, A.N.; Abeykoon, C. Synthesis, properties, applications, 3D printing and machine learning of graphene quantum dots in polymer nanocomposites. Prog. Mater. Sci. 2024, 144, 101282. [Google Scholar] [CrossRef]
- Gonçalves, J.; Caliceti, P. Optimizing Pharmacological and Immunological Properties of Therapeutic Proteins Through PEGylation: Investigating Key Parameters and Their IMPact. Drug Des. Dev. Ther. 2024, 18, 5041–5062. [Google Scholar] [CrossRef]
- Quennouz, N.; Hashmi, S.M.; Choi, H.S.; Kim, J.W.; Osuji, C.O. Rheology of cellulose nanofibrils in the presence of surfactants. Soft Matter 2016, 12, 157–164. [Google Scholar] [CrossRef]
- Sarvestani, A.S.; Picu, C.R. Network model for the viscoelastic behavior of polymer nanocomposites. Polymer 2004, 45, 7779–7790. [Google Scholar] [CrossRef]
- Burrell, G.L.; Dunlop, N.F.; Separovic, F. Non-Newtonian viscous shear thinning in ionic liquids. Soft Matter 2010, 6, 2080–2086. [Google Scholar] [CrossRef]
- Wei, M.; Lin, K.; Sun, L. Shear thickening fluids and their applications. Mater. Des. 2022, 216, 110570. [Google Scholar] [CrossRef]
- Dardas, D. Survey of Applicable Methods for Determining Viscoelastic Effects in Ferroelectric and Antiferroelectric Chiral Liquid Crystals. Materials 2024, 17, 3993. [Google Scholar] [CrossRef]
- Etienne, D.; Nicolas, H.; Alain, F.; François, G. Looking over liquid silicone rubbers: (2) mechanical properties vs. network topology. ACS Appl. Mater. Interfaces 2012, 4, 3353–3363. [Google Scholar] [CrossRef]
- Hong, G.-W.; Wan, J.; Park, Y.; Chang, K.; Chan, L.K.; Lee, K.W.; Yi, K.-H. Rheological Characteristics of Hyaluronic Acid Fillers as Viscoelastic Substances. Polymers 2024, 16, 2386. [Google Scholar] [CrossRef]
- Li, Z.; Bhardwaj, A.; He, J.; Zhang, W.; Tran, T.T.; Li, Y.; McClung, A.; Nuguri, S.; Watkins, J.J.; Lee, S.-W. Nanoporous amorphous carbon nanopillars with lightweight, ultrahigh strength, large fracture strain, and high damping capability. Nat. Commun. 2024, 15, 8151. [Google Scholar] [CrossRef]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Madhusudanan, M.; Chowdhury, M. Advancements in Novel Mechano-Rheological Probes for Studying Glassy Dynamics in Nanoconfined Thin Polymer Films. ACS Polym. Au 2024, 4, 342–391. [Google Scholar] [CrossRef]
- Gurt, A.; Khonsari, M. A Review of the Rheological Consistency of Materials. Lubricants 2024, 12, 236. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of the Ratio of Protein to Water on the Weak Gel Nonlinear Viscoelastic Behavior of Fish Myofibrillar Protein Paste from Alaska Pollock. Gels 2024, 10, 737. [Google Scholar] [CrossRef]
- Fakhari, A.; Fernandes, C. Single-Bubble Rising in Shear-Thinning and Elastoviscoplastic Fluids Using a Geometric Volume of Fluid Algorithm. Polymers 2023, 15, 3437. [Google Scholar] [CrossRef] [PubMed]
- Marnot, A.; Koube, K.; Jang, S.; Thadhani, N.; Kacher, J.; Brettmann, B. Material extrusion additive manufacturing of high particle loaded suspensions: A review of materials, processes and challenges. Virtual Phys. Prototyp. 2023, 18, e2279149. [Google Scholar] [CrossRef]
- Pawlak, A. Crystallization of Polymers with a Reduced Density of Entanglements. Crystals 2024, 14, 385. [Google Scholar] [CrossRef]
- Sangroniz, L.; Fernández, M.; Santamaria, A. Polymers and rheology: A tale of give and take. Polymer 2023, 271, 125811. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Moseson, D.E.; Lee, H.-G.; Esteghamatian, A.; Thipsay, P. Role of rheology in formulation and process design of hot melt extruded amorphous solid dispersions. Int. J. Pharm. 2024, 664, 124651. [Google Scholar] [CrossRef]
- Cao, C.; Killips, A.; Li, X. Advances in the Science and Engineering of Metal Matrix Nanocomposites: A Review. Adv. Eng. Mater. 2024, 26, 2400217. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef]
- Hor, J.L.; Wang, H.; Fakhraai, Z.; Lee, D. Effects of polymer–nanoparticle interactions on the viscosity of unentangled polymers under extreme nanoconfinement during capillary rise infiltration. Soft Matter 2018, 14, 2438–2446. [Google Scholar] [CrossRef]
- Schmid, F. Understanding and Modeling Polymers: The Challenge of Multiple Scales. ACS Polym. Au 2023, 3, 28–58. [Google Scholar] [CrossRef]
- Teodorescu, M.; Morariu, S.; Bercea, M.; Săcărescu, L. Viscoelastic and structural properties of poly(vinyl alcohol)/poly(vinylpyrrolidone) hydrogels. RSC Adv. 2016, 6, 39718–39727. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, S.-C.; Lim, K.; Ramaswamy, Y.; Jabbarzadeh, A. Tribological and Rheological Properties of Poly(vinyl alcohol)-Gellan Gum Composite Hydrogels. Polymers 2022, 14, 3830. [Google Scholar] [CrossRef] [PubMed]
- Meree, C.E.; Schueneman, G.T.; Meredith, J.C.; Shofner, M.L. Rheological behavior of highly loaded cellulose nanocrystal/poly(vinyl alcohol) composite suspensions. Cellulose 2016, 23, 3001–3012. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic properties of poly (vinyl alcohol) hydrogels with cellulose nanocrystals fabricated through sodium chloride addition: Rheological evidence of double network formation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125577. [Google Scholar] [CrossRef]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- Yilmazer, S.; Schwaller, D.; Mésini, P.J. Beyond Sol-Gel: Molecular Gels with Different Transitions. Gels 2023, 9, 273. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Sharma, B. Viscoelastic investigation of graphene oxide grafted PVA biohybrid using ostwald modeling for packaging applications. Polym. Test. 2020, 91, 106791. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J.; Liu, T.; Chen, H.; Jiang, N. Compositional, Structural, and Biomechanical Properties of Three Different Soft Tissue–Hard Tissue Insertions: A CoMParative Review. ACS Biomater. Sci. Eng. 2024, 10, 2659–2679. [Google Scholar] [CrossRef]
- Roy, C.K.; Guo, H.L.; Sun, T.L.; Bin Ihsan, A.; Kurokawa, T.; Takahata, M.; Nonoyama, T.; Nakajima, T.; Gong, J.P. Self-adjustable adhesion of polyampholyte hydrogels. Adv. Mater. 2015, 27, 7344–7348. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Uddin, M.M.; Roy, C.K.; Hossen, M.J.; Sujan, M.I.; Azam, M.S. Mechanically tough and highly stretchable poly(acrylic acid) hydrogel cross-linked by 2D graphene oxide. RSC Adv. 2020, 10, 10949–10958. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Akib, A.A.; Sarkar, S.D.; Khan, M.A.R.; Uddin, M.M.; Nasrin, D.; Roy, C.K. Polysaccharide-Based Hydrogels for Advanced Biomedical Engineering Applications. ACS Polym. Au 2024, 4, 463–486. [Google Scholar] [CrossRef]
- Akib, A.A.; Rumon, M.M.H.; Moniruzzaman, M.; Kumar Roy, C.; Chowdury, A.-N. PLA-PEG Diblock Copolymer Micelle as Nanocarrier for Anti-Obesity Drug Delivery System. ECS Trans. 2022, 107, 19031. [Google Scholar] [CrossRef]
- Rajankunte Mahadeshwara, M.; Al-Jawad, M.; Hall, R.M.; Pandit, H.; El-Gendy, R.; Bryant, M. How Do Cartilage Lubrication Mechanisms Fail in Osteoarthritis? A Comprehensive Review. Bioengineering 2024, 11, 541. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Zhang, X. Promoting Articular Cartilage Regeneration through Microenvironmental Regulation. J. Immunol. Res. 2024, 2024, 4751168. [Google Scholar] [CrossRef]
- Benson, J.M.; Moore, A.C.; Schrader, J.; Burris, D.L. Adhesion–Lubrication Paradox of Articular Cartilage. Langmuir 2024, 40, 13810–13818. [Google Scholar] [CrossRef]
- Choudhari, A.; Gupta, A.K.; Kumar, A.; Kumar, A.; Gupta, A.; Chowdhury, N.; Kumar, A. Wear and Friction Mechanism Study in Knee and Hip Rehabilitation: A Comprehensive Review. In Applications of Biotribology in Biomedical Systems; Kumar, A., Kumar, A., Kumar, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 345–432. [Google Scholar]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Berni, M.; Marchiori, G.; Baleani, M.; Giavaresi, G.; Lopomo, N.F. Biomechanics of the Human Osteochondral Unit: A Systematic Review. Materials 2024, 17, 1698. [Google Scholar] [CrossRef]
- Hiranaka, T.; Furumatsu, T.; Yokoyama, Y.; Higashihara, N.; Tamura, M.; Kawada, K.; Ozaki, T. Weight loss enhances meniscal healing following transtibial pullout repair for medial meniscus posterior root tears. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 143–150. [Google Scholar] [CrossRef]
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022, 52, 201–235. [Google Scholar] [CrossRef]
- Ondrésik, M.; Oliveira, J.M.; Reis, R.L. Knee Articular Cartilage. In Regenerative Strategies for the Treatment of Knee Joint Disabilities; Oliveira, J.M., Reis, R.L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 3–20. [Google Scholar]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- Marchiori, G.; Berni, M.; Boi, M.; Filardo, G. Cartilage mechanical tests: Evolution of current standards for cartilage repair and tissue engineering. A literature review. Clin. Biomech. 2019, 68, 58–72. [Google Scholar] [CrossRef]
- Cárdenas-Aguazaco, W.; Lara-Bertrand, A.L.; Prieto-Abello, L.; Barreto-López, N.; Camacho, B.; Silva-Cote, I. Exploring calcium-free alternatives in endochondral bone repair tested on In vivo trials—A review. Regen. Ther. 2024, 26, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Ang, K.; Guan, P.; Li, J.; Meng, H.; Yang, J.; Fan, L.; Sun, Y. Application of adhesives in the treatment of cartilage repair. Interdiscip. Med. 2024, 2, e20240015. [Google Scholar] [CrossRef]
- Nordberg, R.C.; Bielajew, B.J.; Takahashi, T.; Dai, S.; Hu, J.C.; Athanasiou, K.A. Recent advancements in cartilage tissue engineering innovation and translation. Nat. Rev. Rheumatol. 2024, 20, 323–346. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Rosenwasser, M.P.; Buckwalter, J.A.; Malinin, T.I.; Mow, V.C. Interspecies coMParisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef]
- Sokoloff, L. The Joints and Synovial Fluid: Volume II; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Almarza, A.J.; Athanasiou, K.A. Design Characteristics for the Tissue Engineering of Cartilaginous Tissues. Ann. Biomed. Eng. 2004, 32, 2–17. [Google Scholar] [CrossRef]
- Chen, Y.; Song, J.; Wang, S.; Liu, W. PVA-Based Hydrogels: Promising Candidates for Articular Cartilage Repair. Macromol. Biosci. 2021, 21, 2100147. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef]
- Baniasadi, M.; Baniasadi, H.; Azimi, R.; Khosravi Dehaghi, N. Fabrication and characterization of a wound dressing composed of polyvinyl alcohol/nanochitosan/Artemisia ciniformis extract: An RSM study. Polym. Eng. Sci. 2020, 60, 1459–1473. [Google Scholar] [CrossRef]
- Huesca-Urióstegui, K.; García-Valderrama, E.J.; Gutierrez-Uribe, J.A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Nanofiber Systems as Herbal Bioactive Compounds Carriers: Current Applications in Healthcare. Pharmaceutics 2022, 14, 191. [Google Scholar] [CrossRef]
- Temel-Soylu, T.M.; Keçeciler-Emir, C.; Rababah, T.; Özel, C.; Yücel, S.; Basaran-Elalmis, Y.; Altan, D.; Kirgiz, Ö.; Seçinti, İ.E.; Kaya, U.; et al. Green Electrospun Poly(vinyl alcohol)/Gelatin-Based Nanofibrous Membrane by Incorporating 45S5 Bioglass Nanoparticles and Urea for Wound Dressing Applications: Characterization and In Vitro and In Vivo Evaluations. ACS Omega 2024, 9, 21187–21203. [Google Scholar] [CrossRef]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Graça, M.F.P.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef]
- Shastri, S.S.; Varma, P.; Kandasubramanian, B. Enhancing Drug Delivery with Electrospun Biopolymer Nanofibers. Biomed. Mater. Devices 2024. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, W.; Wang, Q.; Zang, J.; Zhang, Y.; Han, G. Preparation of electrospun chitosan/poly(ethylene oxide) composite nanofibers reinforced with cellulose nanocrystals: Structure, morphology, and mechanical behavior. Compos. Sci. Technol. 2019, 182, 107774. [Google Scholar] [CrossRef]
- Wang, K. Calibration scheduling with time slot cost. Theor. Comput. Sci. 2020, 821, 1–14. [Google Scholar] [CrossRef]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Kharaghani, D.; Khan, M.Q.; Tamada, Y.; Ogasawara, H.; Inoue, Y.; Saito, Y.; Hashmi, M.; Kim, I.S. Fabrication of electrospun antibacterial PVA/Cs nanofibers loaded with CuNPs and AgNPs by an in-situ method. Polym. Test. 2018, 72, 315–321. [Google Scholar] [CrossRef]
- Kiro, A.; Bajpai, J.; Bajpai, A.K. Designing of silk and ZnO based antibacterial and noncytotoxic bionanocomposite films and study of their mechanical and UV absorption behavior. J. Mech. Behav. Biomed. Mater. 2017, 65, 281–294. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Ceseracciu, L.; Heredia-Guerrero, J.A.; Anyfantis, G.C.; Cingolani, R.; Athanassiou, A.; Bayer, I.S. Effect of trifluoroacetic acid on the properties of polyvinyl alcohol and polyvinyl alcohol–cellulose composites. Chem. Eng. J. 2015, 277, 242–251. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Gu, K.; Yao, J.; Shao, Z.; Chen, X. Poly(vinyl alcohol) Hydrogels with Integrated Toughness, Conductivity, and Freezing Tolerance Based on Ionic Liquid/Water Binary Solvent Systems. ACS Appl. Mater. Interfaces 2021, 13, 29008–29020. [Google Scholar] [CrossRef]
- Li, Y.; Umer, R.; Samad, Y.A.; Zheng, L.; Liao, K. The effect of the ultrasonication pre-treatment of graphene oxide (GO) on the mechanical properties of GO/polyvinyl alcohol composites. Carbon 2013, 55, 321–327. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Nassar, M.A. Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohydr. Polym. 2010, 79, 694–699. [Google Scholar] [CrossRef]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. BioResources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Sharma, P.; Singh, V.K.; Chauhan, S. A Comprehensive Review on the Engineering of BiocoMPatible Polyvinyl Alcohol Composites with Enhanced Properties Using Carbonaceous Fillers. J. Mater. Environ. Sci. 2023, 14, 560–581. [Google Scholar]
- Kamboj, G.; Gaff, M.; Smardzewski, J.; Haviarová, E.; Hui, D.; Rezaei, F.; Sethy, A.K. Effect of cellulose nanofiber and cellulose nanocrystals reinforcement on the strength and stiffness of PVAc bonded joints. Compos. Struct. 2022, 295, 115821. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Alcalà, M.; Espinach, F.X.; Mutjé, P.; Delgado-Aguilar, M. Research on the Strengthening Advantages on Using Cellulose Nanofibers as Polyvinyl Alcohol Reinforcement. Polymers 2020, 12, 974. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Yan, C.-F. Mechanical Properties of Cellulose Nanofibril (CNF)- and Cellulose Nanocrystal (CNC)-Based Nanocomposites. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 393–443. [Google Scholar]
- Yue, Y.; Han, J.; Han, G.; French, A.D.; Qi, Y.; Wu, Q. Cellulose nanofibers reinforced sodium alginate-polyvinyl alcohol hydrogels: Core-shell structure formation and property characterization. Carbohydr. Polym. 2016, 147, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Si, Y.; Wang, X.; Jia, X.; Guo, X.; Xu, Y. Poly(vinyl alcohol) Nanocrystal-Assisted Hydrogels with High Toughness and Elastic Modulus for Three-Dimensional Printing. ACS Appl. Nano Mater. 2019, 2, 707–715. [Google Scholar] [CrossRef]






| Nanocomposites | Synthesis Method | Remarks | Applications | Ref. |
|---|---|---|---|---|
| PVA–ZnS | Solvent casting | Continuously stirred for 5 h at 60 °C | MB removal | [9] |
| PVA–ZnO–Al2O3 | Solvent casting | Sonicated for 2 h; temperature 80–90 °C | MB removal | [10] |
| PVA–CaO–CuO | Solvent casting | Acid-catalyzed polymerization, ultrasonication | MB removal | [8] |
| PVA–ZrO2 | Solution casting | Synthesized at room temperature | Membrane for filtration | [39] |
| Chitosan/PVA/MeOx (Cu2O, ZnO) | Plasma | DC power supply maintaining voltage up to 5 kV and resistor 0.5 k Ohm | Antimicrobial activity | [40] |
| PVA–Fe2O3–TiO2 | Chemical blending process | Continuous stirring | Biomedical | [41] |
| PVA–ZnO | Sol–gel | Stirring at ~115 °C for about 15 min | Antimicrobial activity | [42] |
| PVA/PVP/Al2O3/SiO2 | Dip-coating | Ultrasonication, pH ~7 at 70 °C for 3 h | Probable applications in optoelectronic devices | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman Khan, M.M.; Rumon, M.M.H.; Islam, M. Synthesis, Rheology, Morphology, and Mechanical Properties of Biodegradable PVA-Based Composite Films: A Review on Recent Progress. Processes 2024, 12, 2880. https://doi.org/10.3390/pr12122880
Rahman Khan MM, Rumon MMH, Islam M. Synthesis, Rheology, Morphology, and Mechanical Properties of Biodegradable PVA-Based Composite Films: A Review on Recent Progress. Processes. 2024; 12(12):2880. https://doi.org/10.3390/pr12122880
Chicago/Turabian StyleRahman Khan, Mohammad Mizanur, Md. Mahamudul Hasan Rumon, and Mobinul Islam. 2024. "Synthesis, Rheology, Morphology, and Mechanical Properties of Biodegradable PVA-Based Composite Films: A Review on Recent Progress" Processes 12, no. 12: 2880. https://doi.org/10.3390/pr12122880
APA StyleRahman Khan, M. M., Rumon, M. M. H., & Islam, M. (2024). Synthesis, Rheology, Morphology, and Mechanical Properties of Biodegradable PVA-Based Composite Films: A Review on Recent Progress. Processes, 12(12), 2880. https://doi.org/10.3390/pr12122880








