1. Introduction
Low-grade waste heat resources refer to the heat generated during industrial production or the geothermal energy that has not yet been effectively utilized. Recovering and utilizing this low-grade energy through waste heat recovery technology has been critical in promoting energy utilization efficiency and reducing carbon emissions [
1]. Low-grade waste heat, typically found as low-temperature hot water, presents considerable potential for recovery [
2]. To alleviate the current energy shortage and environmental pollution problems, it is essential not only to focus on developing new energy sources but also to explore new technologies to improve the efficiency of low-grade energy utilization usage.
Currently, the mainstream methods for low-grade energy conversion are the Kalina cycle and the organic Rankine cycle (ORC) [
3,
4]. The research on the ORC mainly focuses on the cycle configurations, the refrigerant selections [
5], and the expansion device developments [
6]. Various ORC cycle configurations include transcritical cycles, multi-pressure evaporation cycles, regenerative cycles, and cycles with injection boosters [
7]. Bamorovat Abadi et al. [
8] investigated the performance of refrigerant mixtures compared to pure refrigerants. The results demonstrated that refrigerant mixtures could enhance the exergy efficiency of both the evaporator and condenser. Shahrooz et al. [
9] analyzed 25 pure refrigerants and 104 refrigerant mixtures, comparing the performance of refrigerant mixtures with their pure refrigerants. The findings showed that refrigerant mixtures offered performance improvements and higher exergy efficiency. Zhao et al. [
10] reviewed expansion devices for ORC systems, including scroll, screw, piston, vane, turbine, and injector types, guiding the selection of expansion devices in ORC systems. Cao et al. [
4] discussed the working fluid selection, cycle improvement methods, integrated systems, and applications of ORC. ORC is used in a variety of cycle forms, such as transcritical cycles [
11], trilateral cycles, multiple evaporation pressure cycles [
12], and organic flash cycles [
13]. In order to achieve better thermodynamic performance, the original cycle form is usually improved by the addition of the regenerator [
14], the reheater, or the vapor ejector [
15].
The Kalina cycle utilizes ammonia water as the working fluid, with the commonly used KCS-11 configuration being suitable for waste heat recovery in the range of 100–200 °C [
16,
17]. Compared to the ORC, the Kalina cycle incorporates a gas-liquid separator for component regulation [
18]. Although the Kalina cycle offers higher thermodynamic efficiency, its heat source utilization is lower due to the incomplete evaporation process. Kumar Singh et al. [
19] integrated the Kalina cycle with a parabolic trough collector, achieving a maximum thermal efficiency of 48.17%. Anish Modi et al. [
20] proposed four configurations of the Kalina cycle based on different regenerators and compared their cycle efficiency and cooling water requirements, identifying the optimal layout. Parvathy S. et al. [
21] improved the performance of the Kalina cycle by employing a two-stage expansion, resulting in a 4.04% increase in cycle thermal efficiency.
Ammonia water is a common zeotropic fluid known for its “temperature glide” behavior [
22], allowing it to match the temperature variations of the waste heat during the evaporation process, thereby reducing irreversibilities in the heat exchange within the evaporator. However, ammonia is flammable and toxic, which poses risks for large-scale power generation systems. In recent years, binary zeotropic refrigerant mixtures composed of different boiling point substances have gained widespread application [
23,
24,
25]. Zeotropic mixture refrigerants can reduce irreversible losses during the two-phase heat transfer of ORC compared to pure refrigerants due to the better matching of the temperature distribution to the heat source/sink, enabling an increase in the mean temperature of the heat supply and/or a decrease in the mean temperature of the heat rejection, which leads to better thermodynamic performance [
26,
27]. However, zeotropic mixture refrigerants have lower phase change heat transfer coefficients and require larger heat transfer areas, making them incur higher capital investment costs [
28]. Drawing inspiration from both the auto-cascade cycle and the Kalina cycle, Zhao et al. [
26] proposed an auto-cascade Rankine cycle (ARC) using zeotropic mixtures to minimize thermodynamic irreversibilities. Compared to the ORC, the ARC achieved a 13.1% increase in thermal efficiency and a 13.7% improvement in energy utilization.
The dual-pressure evaporation cycle significantly reduces irreversibilities in the evaporator by employing a dual-pressure evaporation strategy [
27], thereby greatly enhancing heat source utilization and power generation. Li et al. [
28] introduced the system layout, process characteristics, and component arrangement of dual-pressure evaporation ORCs, along with an overview of cycle structure enhancements and potential application fields. Zheng et al. [
29] investigated the effects of zeotropic mixtures (R245fa and R152a) on two types of dual-pressure series and parallel ORCs. The results indicated that using zeotropic refrigerant mixtures improved the net power output of both systems. Zheng et al. [
30] also proposed a parallel dual-pressure Kalina cycle system for harnessing geothermal energy. The findings revealed that the maximum net power output is increased by 19.9% and the exergy efficiency is increased by 33.3% compared to the simple Kalina cycle. Shokati et al. [
31] compared the simple ORC, dual-pressure ORC, and Kalina cycle, and the results revealed that the dual-pressure ORC had the highest net power output, surpassing the simple ORC by 15.22%. Additionally, the Kalina cycle exhibited the lowest cost, with its optimal value being 52.09% lower than that of the dual-pressure ORC.
As previously discussed, the organic Rankine cycle (ORC) is known for its high heat source utilization efficiency, while the auto-cascade Rankine cycle (ARC) has excellent thermal efficiency. Considering a dual-pressure evaporation strategy, combining the ARC with the ORC can enhance the overall performance of the cycle system. This study proposes dual-pressure ORC configurations in both series and parallel forms and provides a comparative study and parametric analysis. The structure of the paper is summarized as follows: The second section describes the proposed systems; the third section details the system thermodynamic models; the fourth section compares the two proposed cycles and performs exergy analysis, and then examines the effects of crucial parameters on the thermodynamic performance of the system.
2. System Description
The schematic of the series auto-cascade organic Rankine cycle (SAORC) is shown in
Figure 1. The working fluid absorbs heat from industrial wastewater in evaporator 1, turning into a high-pressure vapor–liquid mixture with a quality of about 0.5. This mixture is separated into saturated vapor and liquid in a vapor–liquid separator. The vapor contains more low-boiling fluid, while the liquid contains more high-boiling fluid. The high-pressure liquid is then throttled to an intermediate pressure and enters evaporator 2, where it absorbs more heat and becomes superheated vapor. Meanwhile, the high-pressure vapor expands in turbine 2 to produce power, while the intermediate-pressure vapor powers turbine 1. The exhaust from turbine 1 is condensed, pressurized, and combined with the low-pressure vapor from turbine 2 before entering condenser 2 for further condensation. The condensed fluid is then pressurized and sent back to evaporator 1 to repeat the cycle.
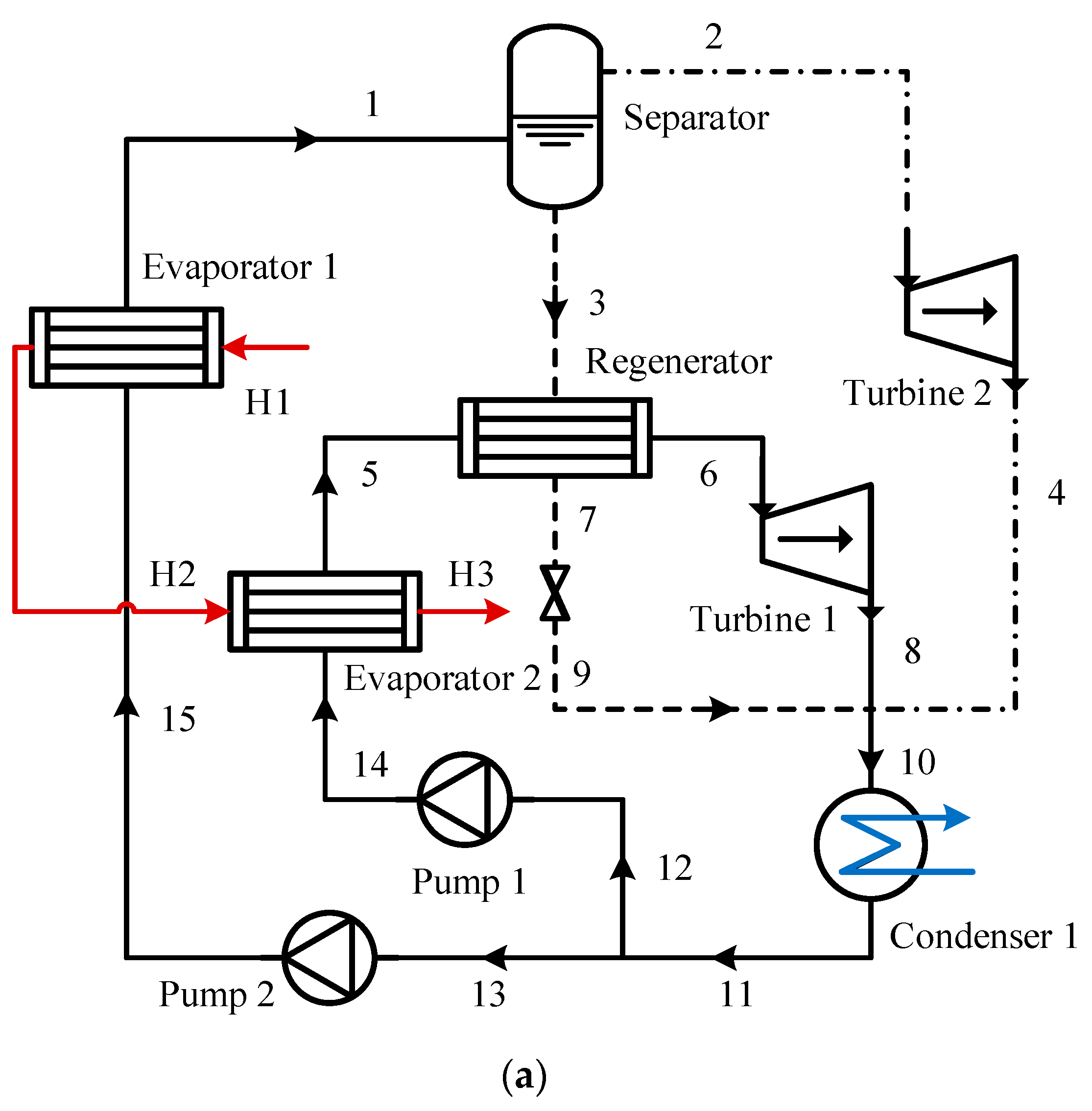
The PAORC diagram (
Figure 2) shows the system where subcooled liquid at point 11 is split into two streams. One is pressurized by pump 2 and enters evaporator 1 (point 15), while the other is pressurized by pump 1 to intermediate pressure and enters evaporator 2 (point 14). Waste heat passes through both evaporators, heating the zeotropic working fluid. The solution at point 1 is separated into saturated vapor (point 2) and liquid (point 3) in a separator. The vapor expands in turbine 2, generating power, while the liquid heats the vapor through a regenerator and becomes superheated (point 5). The superheated vapor at point 6 expands in turbine 1. Low-pressure vapors from points 4 and 8, along with the working fluid, combine at point 10 and enter the condenser. The condensed fluid then returns through pumps 1 and 2 to complete the cycle.
In the SAORC, the intermediate pressure is reduced by a throttling valve, which acts as a conventional ORC. The valve forms a series configuration with the high-pressure portion of the auto-cascade organic Rankine cycle. The PAORC combines the auto-cascade organic Rankine cycle with the conventional ORC, where the high-pressure section uses the auto-cascade configuration and the intermediate-pressure section uses the conventional configuration, achieving a parallel combination of the two cycles.