Experimental Study on Biodiesel Production in a Continuous Tubular Reactor with a Static Mixer
Abstract
1. Introduction
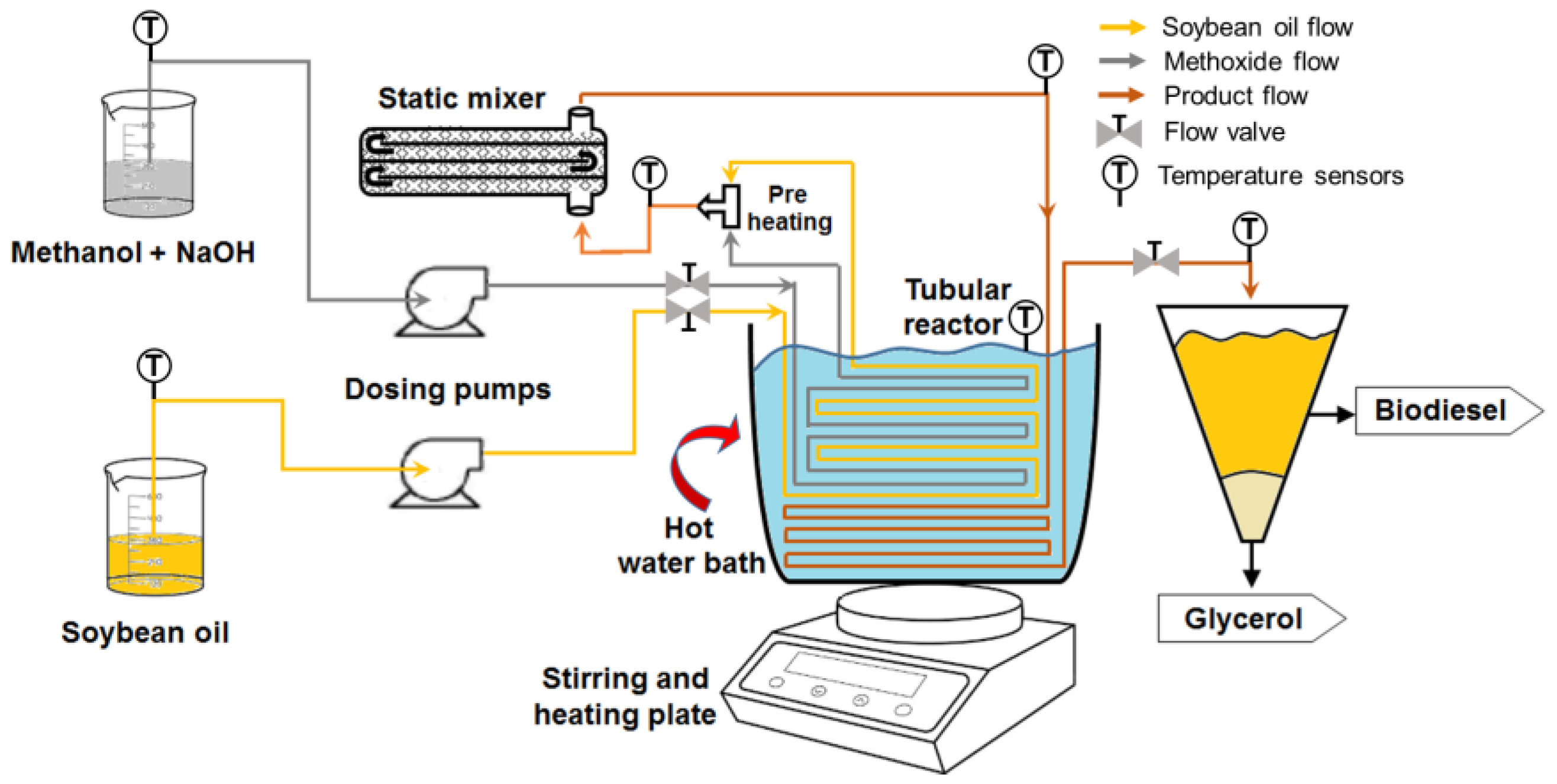
2. Experimental Setup and Procedure
2.1. Materials and Methods
2.2. Production of Biodiesel
2.3. Statistical Analysis
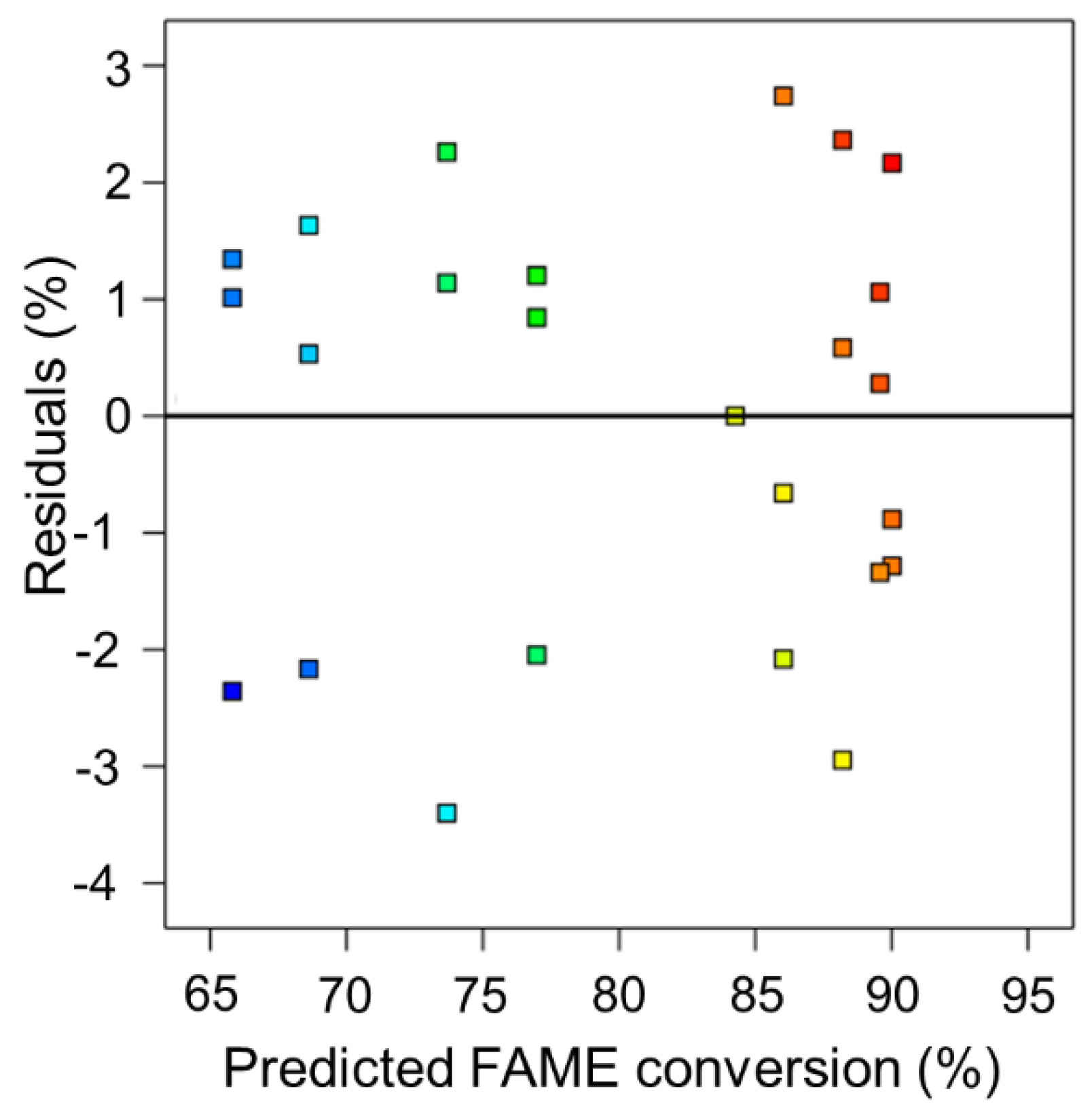
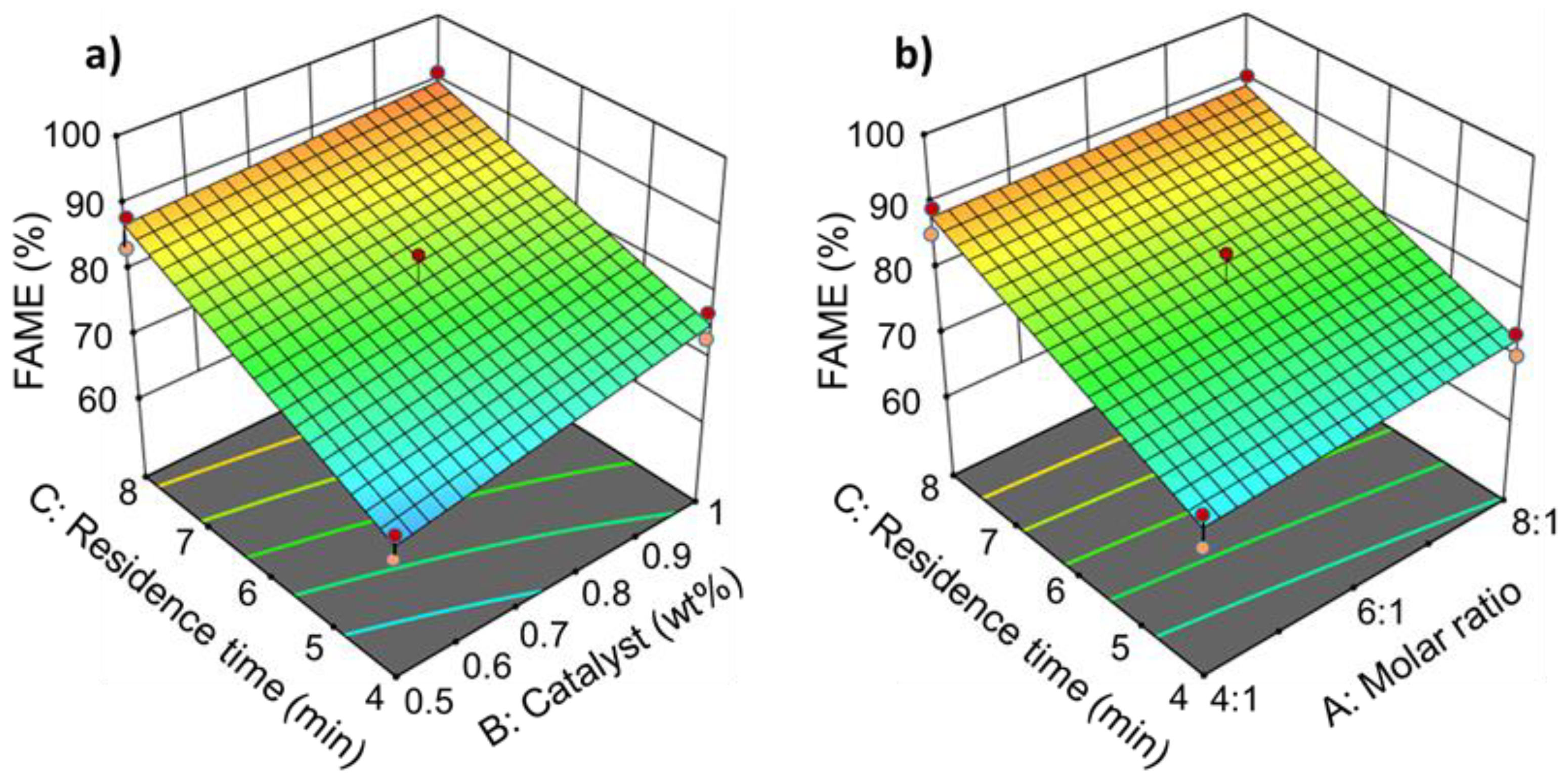
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razm, S.; Brahimi, N.; Hammami, R.; Dolgui, A. A production planning model for biorefineries with biomass perishability and biofuel transformation. Int. J. Prod. Econ. 2023, 258, 108773. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O.; et al. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Kumer-Roy, D.; Zoynal-Abedin, M. Potentiality of biodiesel and bioethanol production from feedstock in Bangladesh: A review. Heliyon 2022, 8, e11213. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza, P.L.; Nishidaa, V.S.; Zevallos, L.A.; Zandoná, A.; Ricardo-Soccol, R. Integrated sugarcane biorefinery for first- and second-generation bioethanol production using imidazole pretreatment. J. Clean. Prod. 2022, 381, 135179. [Google Scholar] [CrossRef]
- Zabaruddin, N.H.; Abdullah, L.C.; Mohamed, N.H.; Choong, T.S.Y. Optimization Using Response Surface Methodology (RSM) for Biodiesel Synthesis Catalyzed by Radiation-Induced Kenaf Catalyst in Packed-Bed Reactor. Processes 2020, 8, 1289. [Google Scholar] [CrossRef]
- Brásio, A.S.R.; Romanenko, A.; Leal, J.; Santos, L.O.; Fernandes, N.C.P. Nonlinear model predictive control of biodiesel production via transesterification of used vegetable oils. J. Process. Control 2013, 23, 1471–1479. [Google Scholar] [CrossRef]
- Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M.B.; Akhtar, M.N.; Iqbal, H.M.N. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials—A review. Fuel 2022, 328, 125254. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Da Silva, C.; de Castilhos, F.; Oliveira, J.V.; Filho, L.C. Continuous production of soybean biodiesel with compressed ethanol in a microtube reactor. Fuel Process. Technol. 2010, 91, 1274–1281. [Google Scholar] [CrossRef]
- Gopi, R.; Thangarasu, V.; Vinayakaselvi, A.; Ramanathan, A. A critical review of recent advancements in continuous flow reactors and prominent integrated microreactors for biodiesel production. Renew. Sustain. Energy Rev. 2022, 154, 111869. [Google Scholar] [CrossRef]
- Sun, X.; Opulencia, M.J.C.; Alexandrovich, T.P.; Khan, A.; Algarni, M.; Abdelrahman, A. Modeling and optimization of vegetable oil biodiesel production with heterogeneous nano catalytic process: Multi-layer perceptron, decision regression tree, and K-Nearest Neighbor methods. Environ. Technol. Innov. 2022, 27, 102794. [Google Scholar] [CrossRef]
- Meshalkin, V.; Sapunov, V.; Kozlovskiy, R.; Kozlovskiy, I.; Staroverov, D.; Luganskiy, A.; Voronov, M. Experimentally Calculated Study of the Effectiveness on the Process of Non-Catalytic Synthesis of Biodiesel in Reactors of Various Type. Processes 2021, 9, 1488. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V.V. Application of Tubular Reactor Technologies for the Acceleration of Biodiesel Production. Bioengineering 2022, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Somnuk, K.; Prasit, T.; Prateepchaikul, G. Effects of mixing technologies on continuous methyl ester production: Comparison of using plug flow, static mixer, and ultrasound clamp. Energ. Convers. Manag. 2017, 140, 91–97. [Google Scholar] [CrossRef]
- Sungwornpatansakul, P.; Hiroi, J.; Nigahara, Y.; Jayasinghe, T.K.; Yoshikawa, K. Enhancement of biodiesel production reaction employing the static mixing. Fuel Process. Technol. 2013, 116, 1–8. [Google Scholar] [CrossRef]
- Somnuk, K.; Soysuwan, N.; Prateepchaikul, G. Continuous process for biodiesel production from palm fatty acid distillate (PFAD) using helical static mixers as reactors. Renew. Energy 2019, 131, 100–110. [Google Scholar] [CrossRef]
- Huang, S.; Cui, Z.; Zhu, R.; Chen, C.; Song, S.; Song, J.; Wang, M.; Tan, T. Design and development of a new static mixing bioreactor for enzymatic bioprocess: Application in biodiesel production. Renew. Energy 2022, 197, 922–931. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Abbasi, O.A.; Alalqam, W.M.; Alwadi, A.A.; Alnajim, M.M. Experimental and CFD simulation studies of biodiesel production in an in-house Tesla-shaped microreactor. Clean. Energy Systems 2024, 7, 100098. [Google Scholar] [CrossRef]
- Baydir, E.; Aras, O. Increasing biodiesel production yield in narrow channel tubular reactors. Chem. Eng. Process. Process. Intensif. 2022, 170, 108719. [Google Scholar] [CrossRef]
- Bizualem, Y.D.; Nurie, A.G.; Nadew, T.T. A review on biodiesel micromixers: Types of micromixers, configurations, and flow patterns. Heliyon 2024, 10, e34790. [Google Scholar] [CrossRef] [PubMed]
- Oo, Y.M.; Somnuk, K. Investigation of free fatty acid reduction from mixed crude palm oil using 3D-printed rotor–stator hydrodynamic cavitation: An experimental study of geometric characteristics of the inner hole. Ultrason. Sonochemistry 2023, 98, 106472. [Google Scholar] [CrossRef]
- Shaah, M.A.; Hossain, M.S.; Allafi, F.; Kadirb, M.O.A.; Ahmad, M.I. Biodiesel production from candlenut oil using a non-catalytic supercritical methanol transesterification process: Optimization, kinetics, and thermodynamic studies. RSC Adv. 2022, 12, 9845–9861. [Google Scholar] [CrossRef]
- Barrachini, A.L.; Vernier, A.J.; Albarello, M.; de Castilhos, F. Non-catalytic production of fatty acid methyl esters from degummed soybean oil and supercritical dimethyl carbonate in a tubular reactor. J. Supercrit. Fluids 2023, 198, 105921. [Google Scholar] [CrossRef]
- Veljković, V.B.; Veličković, A.V.; Avramović, J.M.; Stamenković, O.S. Modeling of biodiesel production: Performance comparison of box–Behnken, face central composite or full factorial design. Chin. J. Chem. Eng. 2018, 27, 1690–1698. [Google Scholar] [CrossRef]
- Santana, H.S.; Tortola, D.S.; Reis, E.M.; Silva, J.L.; Taranto, O.P. Transesterification reaction of sunflower oil and ethanol for biodiesel synthesis in microchannel reactor: Experimental and simulation studies. Chem. Eng. J. 2016, 302, 752–762. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Bangjang, T.; Kaewchada, A.; Jaree, A. Biodiesel production from rice bran oil fatty acid distillate via supercritical hydrolysis–esterification–transesterification in a microreactor. Energy Rep. 2023, 9, 5299–5305. [Google Scholar] [CrossRef]
- Okolie, J.A.; Escobar, J.I.; Umenweke, G.; Khanday, W.; Okoye, P.U. Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Fuel 2022, 307, 121821. [Google Scholar] [CrossRef]
- Martínez Arias, E.L.; Fazzio Martins, P.; Jardini Munhoz, A.L.; Gutierrez-Rivera, L.; MacIel Filho, R. Continuous synthesis and in situ monitoring of biodiesel production in different microfluidic devices. Ind. Eng. Chem. Res. 2012, 51, 10755–10767. [Google Scholar] [CrossRef]
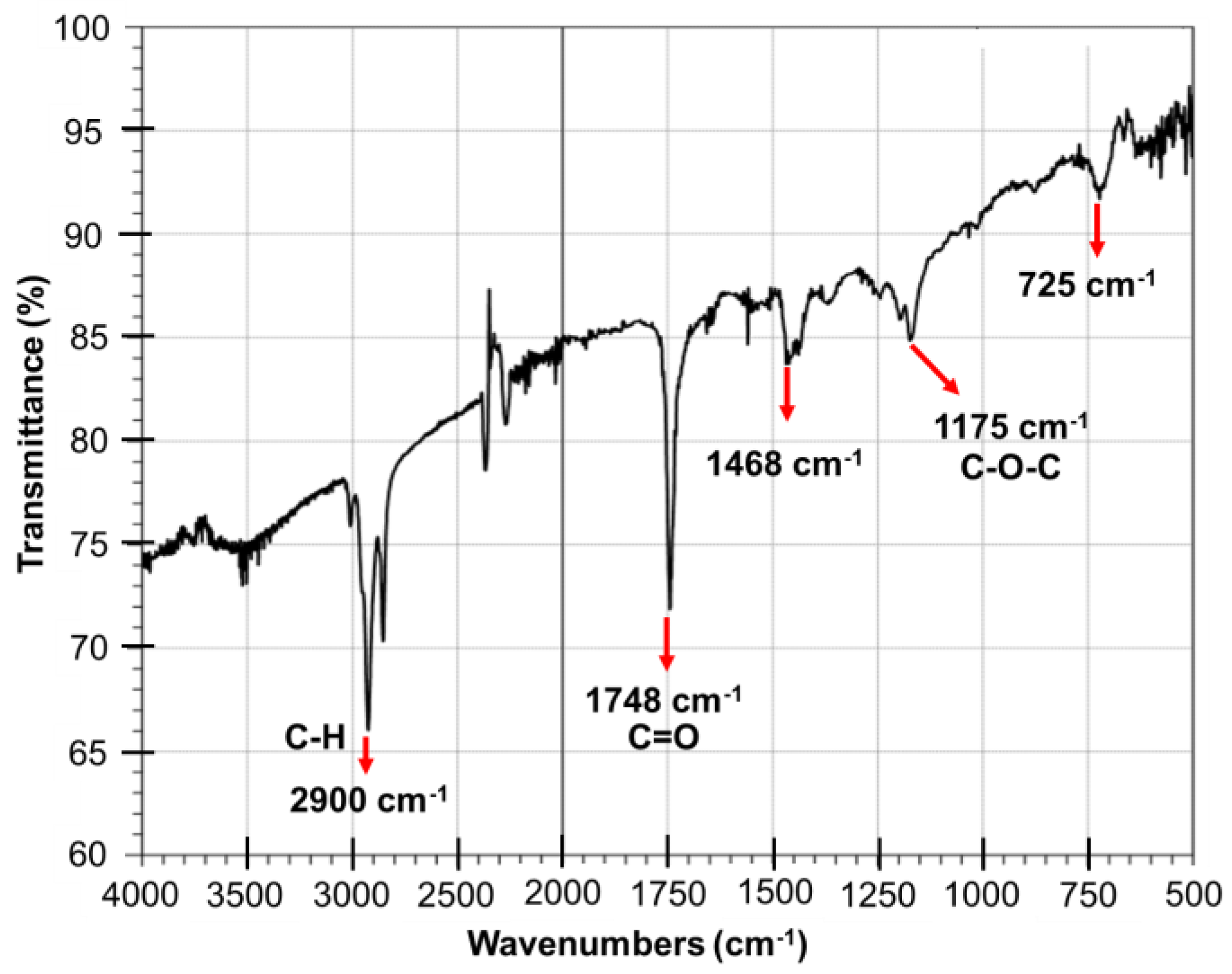
- Rosset, M.; Perez-Lopez, O.W. FTIR spectroscopy analysis for monitoring biodiesel production by heterogeneous catalyst. Vib. Spectrosc. 2019, 105, 102990. [Google Scholar] [CrossRef]
- Pourhoseini, S.H.; Karimian, A.; Ficarella, A. A new environmental friend and commercial biodiesel from Salicornia persica Akhani: Studies of synthesis, physico chemical analysis and flame analysis. Int. J. Eng. Sci. Technol. 2024, 54, 101716. [Google Scholar] [CrossRef]
- Berrios, M.; Gutiérrez, M.C.; Martín, M.A.; Martín, A. Application of the factorial design of experiments to biodiesel production from lard. Fuel Process. Technol. 2009, 90, 1447–1451. [Google Scholar] [CrossRef]
- de Jesus, A.A.; de Santana Souza, D.F.; de Oliveira, J.A.; de Deus, M.S.; da Silva, M.G.; Franceschi, E.; da Silva Egues, S.M.; Dariva, C. Mathematical modeling and experimental esterification at supercritical conditions for biodiesel production in a tubular reactor. Energy Convers. Manag. 2018, 171, 1697–1703. [Google Scholar] [CrossRef]
- Gholami, A.; Pourfayaz, F.; Hajinezhad, A.; Mohadesi, M. Biodiesel production from Norouzak (Salvia leriifolia) oil using choline hydroxide catalyst in a microchannel reactor. Renew. Energy 2019, 136, 993–1001. [Google Scholar] [CrossRef]
- Sakthivel, S.; Halder, S.; Gupta, P.D. Optimisation of process variables for production of biodiesel in packed bed reactor using response surface methodology. Int. J. Ambient. Energy 2013, 34, 83–91. [Google Scholar] [CrossRef]
- Pongraktham, K.; Somnuk, K. Circulation process of methyl ester production from pretreated sludge palm oil using CaO/ABS catalytic static mixer coupled with an ultrasonic clamp. Ultrason. Sonochemistry 2024, 111, 107138. [Google Scholar] [CrossRef]
- Juera-Ong, P.; Pongraktham, K.; Oo, Y.M.; Somnuk, K. Reduction in free fatty acid concentration in sludge palm oil using heterogeneous and homogeneous catalysis: Process optimization, and reusable heterogeneous catalysts. Catalysts 2022, 12, 1007. [Google Scholar] [CrossRef]
- Rashid, W.N.W.A.; Uemura, Y.; Kusakabe, K.; Osman, N.B.; Abdullah, B. Synthesis of Biodiesel from Palm Oil in Capillary Millichannel Reactor: Effect of Temperature, Methanol to Oil Molar Ratio, and KOH Concentration on FAME Yield. Procedia Chem. 2014, 9, 165–171. [Google Scholar] [CrossRef]
- Abdullahi, K.; Ojonugwa, S.S.; Yusuff, A.S.; Umaru, M.; Mohammed, I.A.; Olutoye, M.A.; Aberuagba, F. Optimization of biodiesel production from Allamanda Seed Oil using design of experiment. Fuel Commun. 2023, 14, 100081. [Google Scholar] [CrossRef]
- Awogbemi, O.; Inambao, F.; Onuh, E.I. Modelling and Optimization of Transesterification of Waste Sunflower Oil to Fatty Acid Methyl Ester: A case of Response Surface Methodology vs. Taguchi Orthogonal Approach. Int. J. Eng. Res. Technol. 2019, 12, 2346–2361. Available online: https://www.irphouse.com (accessed on 9 March 2024).
- Miladinović, M.R.; Stamenković, O.S.; Banković, P.T.; Milutinović-Nikolić, A.D.; Jovanović, D.M.; Veljković, V.B. Modeling and optimization of sunflower oil methanolysis over quicklime bits in a packed bed tubular reactor using the response surface methodology. Energy Convers. Manag. 2016, 130, 25–33. [Google Scholar] [CrossRef]



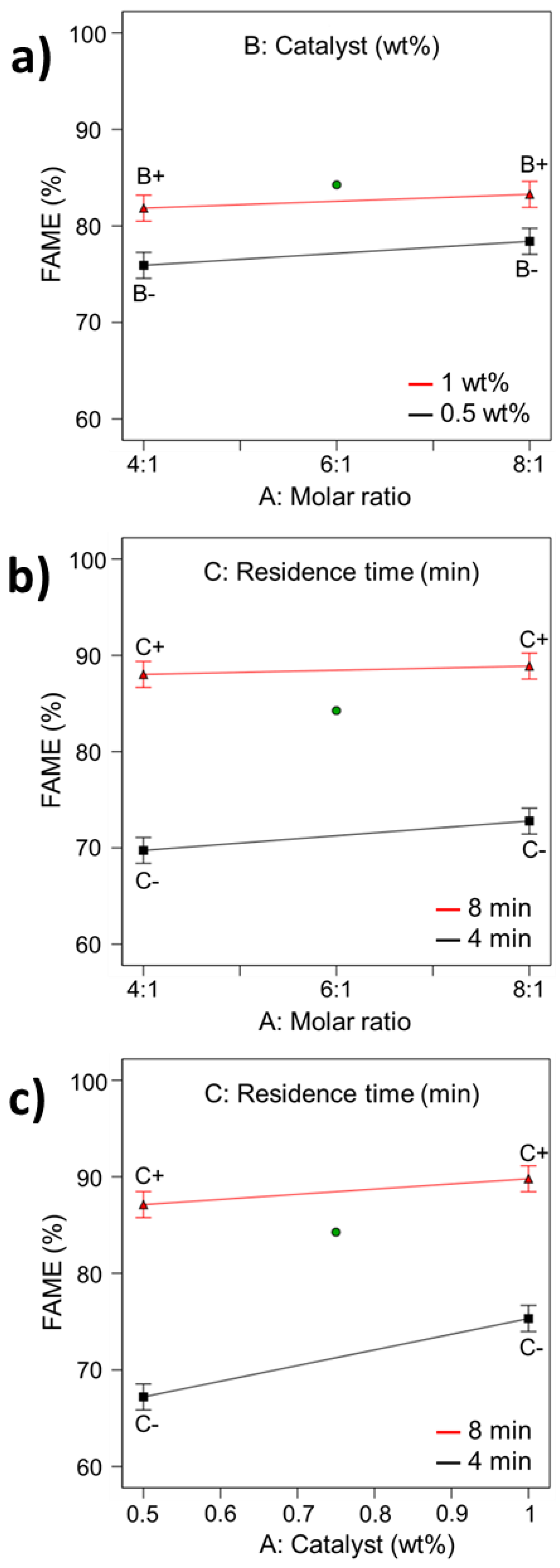
| Run | Molar Ratio | Catalyst (wt%) | Residence Time (min) | FAME a Conversion (%) | FAME b Conversion (%) |
|---|---|---|---|---|---|
| 1 | 4:1 | 0.5 | 4 | 65.53 ± 2.04 | 65.81 |
| 2 | 8:1 | 0.5 | 4 | 68.21 ± 1.95 | 68.62 |
| 3 | 4:1 | 1 | 4 | 73.68 ± 2.99 | 73.68 |
| 4 | 8:1 | 1 | 4 | 76.57 ± 1.77 | 76.98 |
| 5 | 6:1 | 0.75 | 6 | 84.26 ± 2.29 | 84.26 |
| 6 | 4:1 | 0.5 | 8 | 85.83 ± 2.47 | 86.03 |
| 7 | 8:1 | 0.5 | 8 | 88.42 ± 2.70 | 88.20 |
| 8 | 4:1 | 1 | 8 | 90.01 ± 1.88 | 91.17 |
| 9 | 8:1 | 1 | 8 | 89.12 ± 1.22 | 89.57 |
| Parameter | Value |
|---|---|
| Temperature (°C) | 60 (±0.5) |
| Residence time (min) | 2, 4, 6, 8, 10 |
| [NaOH] (wt%) | 0.3, 0.6, 0.9 |
| Molar ratio | 6:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Quiroz, A.; Carrera-Avendaño, E.d.J.; Acevedo-Quiroz, N.; Alvarez-Gutiérrez, P.E.; Borunda, M.; Adam-Medina, M. Experimental Study on Biodiesel Production in a Continuous Tubular Reactor with a Static Mixer. Processes 2024, 12, 2859. https://doi.org/10.3390/pr12122859
Acevedo-Quiroz A, Carrera-Avendaño EdJ, Acevedo-Quiroz N, Alvarez-Gutiérrez PE, Borunda M, Adam-Medina M. Experimental Study on Biodiesel Production in a Continuous Tubular Reactor with a Static Mixer. Processes. 2024; 12(12):2859. https://doi.org/10.3390/pr12122859
Chicago/Turabian StyleAcevedo-Quiroz, Abisai, Edgardo de Jesús Carrera-Avendaño, Noemi Acevedo-Quiroz, Peggy Elizabeth Alvarez-Gutiérrez, Monica Borunda, and Manuel Adam-Medina. 2024. "Experimental Study on Biodiesel Production in a Continuous Tubular Reactor with a Static Mixer" Processes 12, no. 12: 2859. https://doi.org/10.3390/pr12122859
APA StyleAcevedo-Quiroz, A., Carrera-Avendaño, E. d. J., Acevedo-Quiroz, N., Alvarez-Gutiérrez, P. E., Borunda, M., & Adam-Medina, M. (2024). Experimental Study on Biodiesel Production in a Continuous Tubular Reactor with a Static Mixer. Processes, 12(12), 2859. https://doi.org/10.3390/pr12122859







