Methanol Production via Power-to-Liquids: A Comparative Simulation of Two Pathways Using Green Hydrogen and Captured CO2
Abstract
1. Introduction
1.1. CO2 Capture
- Pre-combustion capture
- Post-combustion capture
- Oxyfuel combustion (combustion with pure oxygen)
- Absorption processes use chemical or physical solvents and are commonly used in fossil-fueled power plants [8]. In continuous operation, CO2-rich flue gas enters the absorber, where the solvent absorbs the CO2. The CO2-rich stream is sent to a regenerator for desorption, and the solvent is recycled [10]. Common absorbents include monoethanolamine (MEA), diethanolamine (DEA), N-methyldiethanolamine (MDEA), and di-2-propanolamine (DIPA) [11]. Studies show that MEA is the most efficient, with a CO2 capture rate of over 90% [12].
- In adsorption, solid sorbents are used to capture CO2 on their surface. Key criteria for selecting a sorbent include high specific surface area, high selectivity, and strong regeneration capability. Typical sorbents are molecular sieves, activated carbon, zeolites, calcium oxides, hydrotalcites, and lithium zirconate [4].
- Cryogenic processes separate CO2 by cooling the flue gas to very low temperatures, eliminating the need for chemical solvents, but they face challenges such as pressure drops and ice formation [13].
- Membrane technologies use selective barriers to filter CO2 from flue gases, but their effectiveness is limited to low CO2 concentrations and pressure [14].
1.2. Power-to-X
- Power-to-Hydrogen (P-t-H),
- Power-to-Gas (P-t-G),
- Power-to-Liquids (P-t-L),
- Power-to-Chemicals (P-t-C), and
- Power-to-Power (P-t-P).
1.3. H2 Production via Water Electrolysis
- Anion Exchange Membrane (AEM) electrolysis is an emerging technology for H2 production [25]. In AEM electrolysis, conventional membranes are replaced by anion exchange membranes that allow the migration of OH− [26]. Water is reduced at the cathode, producing H2 and hydroxyl ions, which recombine at the anode to form oxygen [24].
1.4. Methanol Synthesis
2. Methods
2.1. CO2 Capture
2.1.1. Reactions
2.1.2. Process Flow Diagram and Feed Solution in the CO2 Capture Simulation
2.2. Water Electrolysis
2.2.1. Reactions
2.2.2. Process Flow Diagram and Feed Solution in the Water Electrolysis Simulation
2.3. Methanol Synthesis
2.3.1. Kinetic Model for the Methanol Synthesis and RWGS Reactor
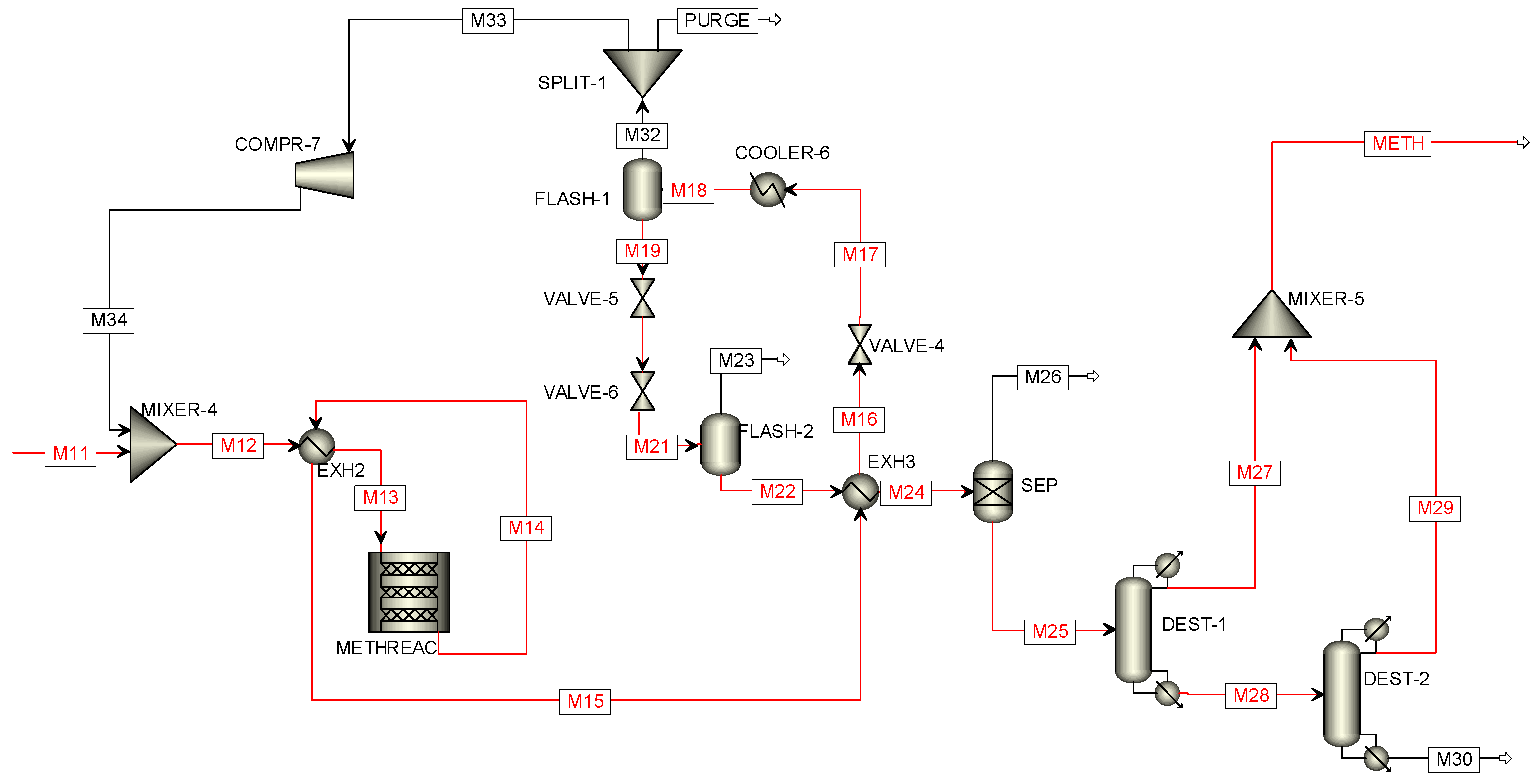
2.3.2. Direct Methanol Synthesis
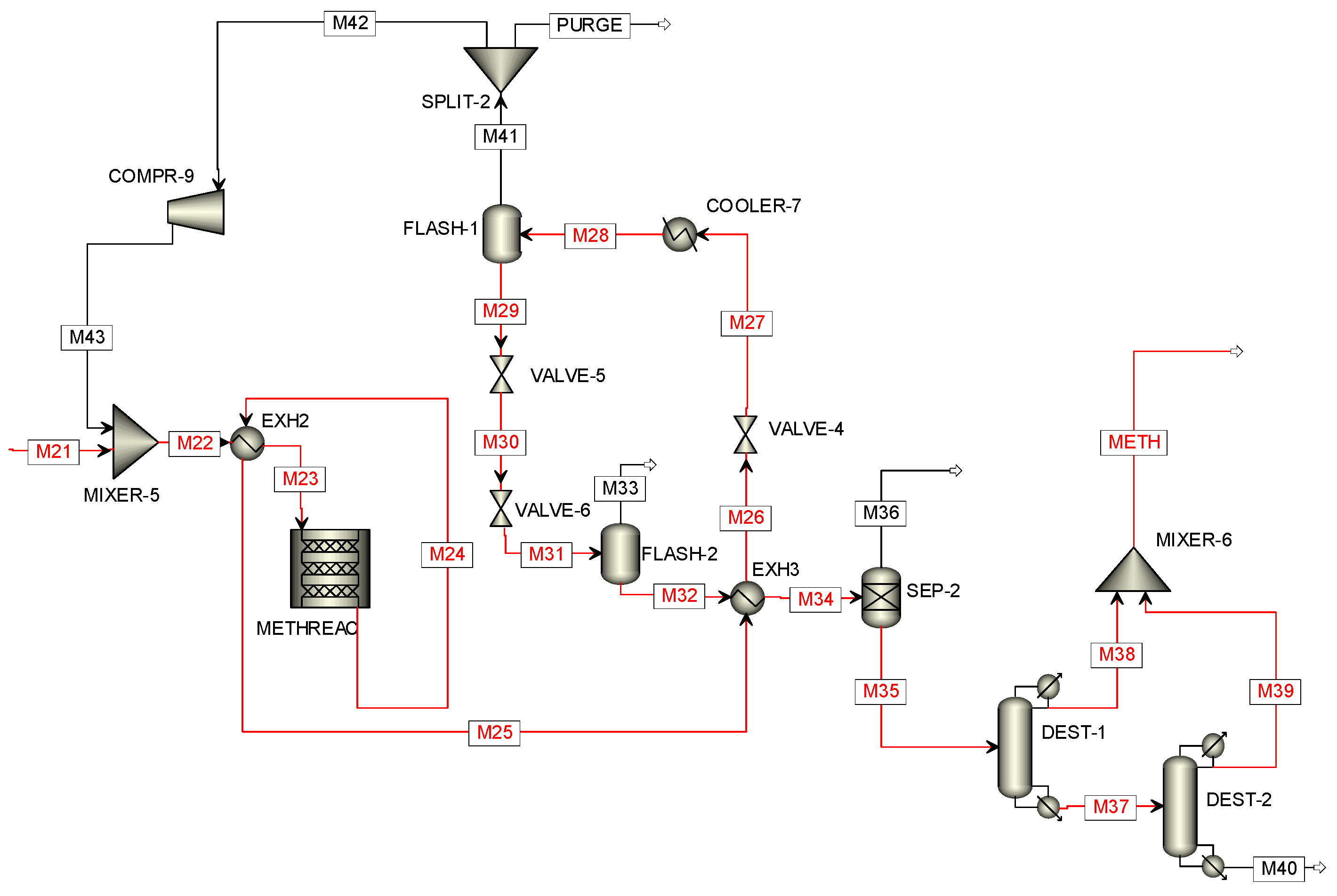
2.3.3. Two-Step Methanol Synthesis
2.4. Sensitivity Analysis
2.5. Evaluation of Carbon Emissions, Electricity and Utility Costs
3. Results and Discussion
3.1. CO2 Capture
3.1.1. Results of the CO2 Capture Simulation in Aspen Plus
3.1.2. Results of Heat Integration, Utility Costs, and Carbon Emissions Analysis of CO2 Capture in the Aspen Energy Analyzer
3.2. Water Electrolysis
3.2.1. Results of the Water Electrolysis Simulation in Aspen Plus
3.2.2. Results of Heat Integration, Utility Costs, and Carbon Emissions Analysis of Water Electrolysis in the Aspen Energy Analyzer
3.3. Direct Methanol Synthesis
3.3.1. Results of the Direct Methanol Synthesis Simulation in Aspen Plus
3.3.2. Results of Heat Integration, Utility Costs, and Carbon Emissions Analysis of Direct Methanol Synthesis in the Aspen Energy Analyzer
3.4. Two-Step Methanol Synthesis
3.4.1. Results of the Two-Step Methanol Synthesis Simulation in Aspen Plus
3.4.2. Results of Heat Integration, Utility Costs, and Carbon Emissions Analysis of Two-Step Methanol Synthesis in the Aspen Energy Analyzer
3.5. Sensitivity Analysis of the Direct and Two-Step Methanol Synthesis
3.6. Comparison of the Direct and Two-Step Methanol Synthesis Paths
3.7. Comparison of Results and Model Validation
3.8. Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Hu, Y.; Chu, J.; Yao, Y.; Lv, H.; Xu, C. Investigation of renewable methanol production from CO2 hydrogeneration based on spectral splitting and heat integration. Fuel 2023, 352, 129041. [Google Scholar] [CrossRef]
- Van-Dal, É.S.; Bouallou, C. Design and simulation of a methanol production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Borhani, T.N.G.; El-Naas, M.H. Carbon Capture. In Exergetic, Energetic and Environmental Dimensions; Elsevier: Amsterdam, The Netherlands, 2018; pp. 997–1016. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Blomen, E.; Hendriks, C.; Neele, F. Capture technologies: Improvements and promising developments. Energy Procedia 2009, 1, 1505–1512. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef]
- Pfaff, I.; Kather, A. Comparative thermodynamic analysis and integration issues of CCS steam power plants based on oxy-combustion with cryogenic or membrane based air separation. Energy Procedia 2009, 1, 495–502. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, Economic, and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]
- Ma’mun, S.; Dindore, V.Y.; Svendsen, H.F. Kinetics of the Reaction of Carbon Dioxide with Aqueous Solutions of 2-((2-Aminoethyl)amino)ethanol. Ind. Eng. Chem. Res. 2007, 46, 385–394. [Google Scholar] [CrossRef]
- Veawab, A.; Aroonwilas, A.; Tontiwachwuthikul, P. CO2 absorption performance of aqueous alkanolamines in packed columns. ACS Div. Fuel Chem. Prepr. 2002, 47, 49–50. [Google Scholar]
- Göttlicher, G.; Pruschek, R. Comparison of CO2 removal systems for fossil-fuelled power plant processes. Energy Convers. Manag. 1997, 38, S173–S178. [Google Scholar] [CrossRef]
- Rackley, S.A. Membrane separation systems. In Carbon Capture and Storage; Elsevier: Amsterdam, The Netherlands, 2017; pp. 187–225. [Google Scholar] [CrossRef]
- Koj, J.C.; Wulf, C.; Zapp, P. Environmental impacts of power-to-X systems—A review of technological and methodological choices in Life Cycle Assessments. Renew. Sustain. Energy Rev. 2019, 112, 865–879. [Google Scholar] [CrossRef]
- Ince, A.C.; Colpan, C.O.; Hagen, A.; Serincan, M.F. Modeling and simulation of Power-to-X systems: A review. Fuel 2021, 304, 121354. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Buffo, G.; Marocco, P.; Ferrero, D.; Lanzini, A.; Santarelli, M. Power-to-X and power-to-power routes. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 529–557. [Google Scholar] [CrossRef]
- Yusuf, M.; Bazli, L.; Alam, M.A.; Masood, F.; Keong, L.K.; Noor, A.; Hellgardt, K.; Abdullah, B. Hydrogen production via natural gas reforming: A comparative study between DRM, SRM and BRM techniques. In Proceedings of the 2021 Third International Sustainability and Resilience Conference: Climate Change IEEE, Sakheer, Bahrain, 15–16 November 2021; pp. 155–158. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Zoulias, E.; Varkaraki, E.; Lymberopoulos, N.; Christodoulou, C.; Karagiorgis, G. A Review on Water Electrolysis. Cyprus J. Sci. Technol. 2004, 4, 41–71. [Google Scholar]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Varone, A.; Ferrari, M. Power to liquid and power to gas: An option for the German Energiewende. Renew. Sustain. Energy Rev. 2015, 45, 207–218. [Google Scholar] [CrossRef]
- Dell’Aversano, S.; Villante, C.; Gallucci, K.; Vanga, G.; Di Giuliano, A. E-Fuels: A Comprehensive Review of the Most Promising Technological Alternatives towards an Energy Transition. Energies 2024, 17, 3995. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, Y. An overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493. [Google Scholar] [CrossRef]
- Wasmus, S.; Küver, A. Methanol oxidation and direct methanol fuel cells: A selective review. J. Electroanal. Chem. 1999, 461, 14–31. [Google Scholar] [CrossRef]
- Malik, M.I.; Abatzoglou, N.; Achouri, I.E. Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst. Catalysts 2021, 11, 893. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, J.; Cui, M.; Han, B. Synthesis of acetic acid via methanol hydrocarboxylation with CO2 and H2. Nat. Commun. 2016, 7, 11481. [Google Scholar] [CrossRef]
- Adeoye, A.A.; Passarini, F.; De Maron, J.; Tabanelli, T.; Cavani, F.; Cespi, D. Methyl Methacrylate Production Processes: A Comparative Analysis of Alternatives Using the Life Cycle Assessment Methodology. ACS Sustain. Chem. Eng. 2023, 11, 17355–17370. [Google Scholar] [CrossRef]
- Modic, R.; Porschen, J.; Schoengen, A.; Wirges, R. Process for the Production of Dimethyl Terephthalate from P-xylene and Methanol. U.S. Patent 4,642,369, 10 February 1987. [Google Scholar]
- Yoshiro Ashina, M.F. Process for Producing Methylamines. U.S. Patent 4,485,261, 27 November 1984. [Google Scholar]
- Yandrapu, V.P.; Kanidarapu, N.R. Conceptual Design of Methyl Chloride Production Processes: A Review. Period. Polytech. Chem. Eng. 2022, 66, 341–353. [Google Scholar] [CrossRef]
- Maleki, A.; Bahadori, F. Simulation and multi-objective optimization of the dimethyl carbonate production process. Sci. Rep. 2023, 13, 16900. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Douglas, P.L. Optimisation of Methyl Tert-Butyl-Ether (MTBE) Synthesis Processes using Aspen-Plus. Dev. Chem. Eng. Miner. Process. 2002, 10, 89–103. [Google Scholar] [CrossRef]
- Liu, G.; Hagelin-Weaver, H.; Welt, B. A Concise Review of Catalytic Synthesis of Methanol from Synthesis Gas. Waste 2023, 1, 228–248. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas Production from Biomass Gasification: Influences of Feedstock Properties, Reactor Type, and Reaction Parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Wernicke, H.-J.; Plass, L.; Schmidt, F. Methanol Generation. In Methanol: The Basic Chemical and Energy Feedstock of the Future; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–301. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E.; Perathoner, S.; Spadaccini, L. Waste-to-methanol: Process and economics assessment. Bioresour. Technol. 2017, 243, 611–619. [Google Scholar] [CrossRef]
- Li, Y.; Nahil, M.A.; Williams, P.T. Hydrogen/Syngas Production from Different Types of Waste Plastics Using a Sacrificial Tire Char Catalyst via Pyrolysis–Catalytic Steam Reforming. Energy Fuels 2023, 37, 6661–6673. [Google Scholar] [CrossRef]
- Poto, S.; Vico van Berkel, D.; Gallucci, F.; Fernanda Neira d’Angelo, M. Kinetic modelling of the methanol synthesis from CO2 and H2 over a CuO/CeO2/ZrO2 catalyst: The role of CO2 and CO hydrogenation. Chem. Eng. J. 2022, 435, 134946. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Methanol. In Applications in Design and Simulation of Sustainable Chemical Processes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–145. [Google Scholar] [CrossRef]
- Anicic, B.; Trop, P.; Goricanec, D. Comparison between two methods of methanol production from carbon dioxide. Energy 2014, 77, 279–289. [Google Scholar] [CrossRef]
- Mbatha, S.; Everson, R.C.; Musyoka, N.M.; Langmi, H.W.; Lanzini, A.; Brilman, W. Power-to-methanol process: A review of electrolysis, methanol catalysts, kinetics, reactor designs and modelling, process integration, optimisation, and techno-economics. Sustain. Energy Fuels 2021, 5, 3490–3569. [Google Scholar] [CrossRef]
- Schwiderowski, P.; Ruland, H.; Muhler, M. Current developments in CO2 hydrogenation towards methanol: A review related to industrial application. Curr. Opin. Green Sustain. Chem. 2022, 38, 100688. [Google Scholar] [CrossRef]
- Portha, J.-F.; Parkhomenko, K.; Kobl, K.; Roger, A.-C.; Arab, S.; Commenge, J.-M.; Falk, L. Kinetics of Methanol Synthesis from Carbon Dioxide Hydrogenation over Copper–Zinc Oxide Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13133–13145. [Google Scholar] [CrossRef]
- Samiee, L.; GhasemiKafrudi, E. Assessment of different kinetic models of carbon dioxide trans-formation to methanol via hydrogenation, over a Cu/ZnO/Al2O3 catalyst. React. Kinet. Mech. Catal. 2021, 133, 801–823. [Google Scholar] [CrossRef]
- Al-Kalbani, H.; Xuan, J.; García, S.; Wang, H. Comparative energetic assessment of methanol production from CO2: Chemical versus electrochemical process. Appl. Energy 2016, 165, 1–13. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Madeddu, C.; Errico, M.; Baratti, R. CO2 Capture by Reactive Absorption-Stripping; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- AspenTech. Rate-Based Model of the CO2 Capture Process by MEA Using Aspen Plus. Available online: http://www.aspentech.com (accessed on 11 December 2023).
- Sánchez, M.; Amores, E.; Abad, D.; Rodríguez, L.; Clemente-Jul, C. Aspen Plus model of an alkaline electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 3916–3929. [Google Scholar] [CrossRef]
- Ulleberg, O. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrogen Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Graaf, G.H.; Scholtens, H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Intra-particle diffusion limitations in low-pressure methanol synthesis. Chem. Eng. Sci. 1990, 45, 773–783. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Arab Aboosadi, Z.; Jahanmiri, A.H. Synthesis gas production in a novel hydrogen and oxygen perm-selective membranes tri-reformer for methanol production. J. Nat. Gas Sci. Eng. 2012, 9, 149–159. [Google Scholar] [CrossRef]
- Santos, M.F.; Bresciani, A.E.; Ferreira, N.L.; Bassani, G.S.; Alves, R.M.B. Carbon dioxide conversion via reverse water-gas shift reaction: Reactor design. J. Environ. Manag. 2023, 345, 118822. [Google Scholar] [CrossRef]
- Esmaili, P.; Dincer, I.; Naterer, G.F. Development and analysis of an integrated photovoltaic system for hydrogen and methanol production. Int. J. Hydrogen Energy 2015, 40, 11140–11153. [Google Scholar] [CrossRef]
- Sollai, S.; Porcu, A.; Tola, V.; Ferrara, F.; Pettinau, A. Renewable methanol production from green hydrogen and captured CO2: A techno-economic assessment. J. CO2 Util. 2023, 68, 102345. [Google Scholar] [CrossRef]








| Acidic Conditions | Alkaline Conditions | |
|---|---|---|
| Anode | H2O → 1/2O2 + 2H+ + 2e− | 2OH− → 1/2O2 + H2O + 2e− |
| Cathode | 2H+ + 2e− → H2 | 2H2O + 2e− → H2 + 2OH− |
| Variable | Value |
|---|---|
| F [kmol/h] | 16,560 |
| T [K] | 313.15 |
| p [bar] | 1 |
| xCO2 | 0.14 |
| xH2O | 0.07 |
| xN2 | 0.79 |
| Parameter | Value | Unit |
|---|---|---|
| Tstack | 70 | °C |
| p | 6.7 | bar |
| P | 0.65 | GW |
| xH2O | 0.0111 | / |
| Parameter | Value | |
|---|---|---|
| Process Flow | STACK-IN | H2ODEFIC |
| F [kmol/h] | 728,354 | 6433 |
| T [°C] | 70 | 25 |
| p [bar] | 7 | 1 |
| xH2O | 0.74 | 1 |
| xKOH | 0.26 | 0 |
| Parameter | Value |
|---|---|
| Reactor type | Reactor with specified temperature |
| T [°C] | 284 |
| p [bar] | 78 |
| l [m] | 2 |
| d [m] | 1 |
| Catalyst | yes |
| mCAT [kg] | 6000 |
| ε | 0.4 |
| Parameter | Value |
|---|---|
| Reactor type | Reactor with specified temperature |
| T [°C] | 750 |
| p [bar] | 20 |
| l [m] | 12 |
| d [m] | 0.1 |
| Catalyst | yes |
| mCAT [kg] | 2.3552 |
| ε | 0.528 |
| Label | Type | Price |
|---|---|---|
| U-1 | Cooling Water | 0.21 $/GJ |
| U-2 | Electricity | 0.0775 $/kWh |
| U-3 | Low-pressure Steam | 1.90 $/GJ |
| U-4 | Refrigerant 1 | 2.74 $/GJ |
| U-5 | Medium-pressure Steam | 2.20 $/GJ |
| U-6 | Fire heater | 4.25 $/GJ |
| Stream | FLUEGAS | Z5 | CO2-OUT | CO2 |
|---|---|---|---|---|
| F [kmol/h] | 16,560 | 4278 | 2144 | 2000 |
| xCO2 | 0.141 | 0.478 | 0.967 | 1 |
| Utility | Required Energy [MW] | Cost of Utilities [M$/a] |
|---|---|---|
| Cold Utility | 69.2 | 0.46 |
| Electricity | 0.6 | 0.41 |
| Σ | 69.8 | 0.87 |
| Streams | STACK- IN | H2-OUT | H2ODEFIC | E2 | H2-PROD | H2 |
|---|---|---|---|---|---|---|
| F [kmol/h] | 728,354 | 367,181 | 6433 | 6280 | 6029 | 6000 |
| xH2O | 0.7431 | 0.7288 | 1 | 0.0446 | 0.0048 | 0 |
| xH2 | 0 | 0.0164 | 0 | 0.9554 | 0.9951 | 1 |
| Utility | Required Energy [MW] | Optimized Energy Required [MW] | Possible Energy Savings [%] |
|---|---|---|---|
| Hot utilities | 650 | 611.8 | 5.87 |
| Cold utilities | 172.4 | 143.2 | 22.15 |
| Electricity | 0.165 | / | / |
| Σ | 822.4 | 746 | 9.28 |
| Utility | Cost of Utilities [M$/a] | Optimized Utility Cost [M$/a] | Possible Monetary Savings [%] |
|---|---|---|---|
| Hot utilities | 38.97 | 36.68 | 5.88 |
| Cold utilities | 1.87 | 0.93 | 50.44 |
| Electricity | 0.11 | / | / |
| Σ | 40.96 | 37.61 | 8.18 |
| Stream | M11 | M13 | M14 | M19 | M22 | METH |
|---|---|---|---|---|---|---|
| F [kmol/h] | 8000 | 8993 | 5015 | 3992 | 3988 | 1983.8 |
| xCO2 | 0.25 | 0.2229 | 0.0013 | 0.0003 | 0 | 0 |
| xCO | 0 | 0.03 | 0.0557 | 0.0004 | 0 | 0 |
| xH2 | 0.75 | 0.7467 | 0.1474 | 0 | 0 | 0 |
| xCH3OH | 0 | 0 | 0.3971 | 0.4982 | 0.4986 | 0.9997 |
| Utility | Required Energy [MW] | Optimized Energy Required [MW] | Possible Energy Savings [%] |
|---|---|---|---|
| Hot utilities | 47.1 | 18 | 29.1 |
| Cold utilities | 75.5 | 46.4 | 29.1 |
| Electricity | 25.3 | / | / |
| Σ | 147.9 | 89.7 | 39.4 |
| Utility | Cost of Utilities [M$/a] | Optimized Utility Cost [M$/a] | Possible Monetary Savings [%] |
|---|---|---|---|
| Hot utilities | 5.8 | 2.8 | 51.5 |
| Cold utilities | 3.9 | 0.42 | 89.1 |
| Electricity | 17.2 | / | / |
| Σ | 26.8 | 20.4 | 23.9 |
| Stream | M9 | M10 | M21 | M22 | M24 | M29 | M32 | METH |
|---|---|---|---|---|---|---|---|---|
| F [kmol/h] | 3600 | 3600 | 7169 | 8038 | 4059 | 3163 | 3157 | 1957 |
| xCO2 | 0.5556 | 0.3248 | 0.1631 | 0.1458 | 0.0010 | 0.0004 | 0.0002 | 0 |
| xCO | 0 | 0.2308 | 0.1159 | 0.1313 | 0.0576 | 0.0006 | 0.0001 | 0 |
| xH2 | 0.4444 | 0.2137 | 0.7210 | 0.7225 | 0.1629 | 0.0009 | 0 | 0 |
| xCH3OH | 0 | 0 | 0 | 0.0004 | 0.4908 | 0.6289 | 0.6289 | 0.9998 |
| Utility | Required Energy [MW] | Optimized Energy Required [MW] | Possible Energy Savings [%] |
|---|---|---|---|
| Hot utilities | 69.5 | 29.4 | 57.7 |
| Cold utilities | 81.4 | 41.3 | 49.3 |
| Electricity | 24.1 | / | / |
| Σ | 175 | 94.8 | 45.8 |
| Utility | Cost of Utilities [M$/a] | Optimized Utility Cost [M$/a] | Possible Monetary Savings [%] |
|---|---|---|---|
| Hot utilities | 9.7 | 4.6 | 52.6 |
| Cold utilities | 3 | 0.4 | 87.6 |
| Electricity | 16.4 | / | / |
| Σ | 29 | 21.3 | 26.5 |
| Parameter | Direct Synthesis | Two-Step Synthesis |
|---|---|---|
| Methanol synthesis [kmol/h] | 1983.8 | 1957 |
| Ratio of methanol produced [kmol of methanol/kmol of H2] | 0.331 | 0.326 |
| Molar ratio [CO2:CO:H2] | 1:0.1:3.4 | 1:0.9:5 |
| Required energy [MW] | 147.9 | 175 |
| Methanol produced per MW [kmol of methanol/MW] | 13.4 | 11.2 |
| Cost of Utilities [M$/a] | 26.8 | 29 |
| Optimized required energy [MW] | 89.7 | 94.8 |
| Optimized Cost of Utilities [M$/a] | 20.4 | 21.3 |
| Optimal H2 feed [kmol/h] | 5950 | 5850 |
| Molar ratio at optimal H2 feed [CO2:CO:H2] | 1:0.30:3.09 | 1:0.90:4.94 |
| Carbon emissions [kg/h] | 22,728 | 33,367 |
| Carbon emissions after optimization [kg/h] | 8.933 | 16.576 |
| Catalyst | Cu/ZnO/Al2O3 | Cu/ZnO/Al2O3 and Ni/MgAl2O4 |
| Type | Process Parameters | Catalyst for Methanol Reactor | Reactor Feed [molar %] | kmol of Methanol Produced per kmol of H2 | Reference |
|---|---|---|---|---|---|
| Direct | 284 °C 78 bar | Cu/ZnO/Al2O3 | CO:2.90 CO2:22.32 H2:74.78 | 0.331 | This study |
| Direct | 220 °C 50 bar | Cu/ZnO/Al2O3 | CO2:30 H2:70 | 0.282 | Esmaili et al. [61] |
| Direct | 250 °C 16 bar | Cu/ZnO/ZrO2 | CO2:25 H2:75 | 0.301 | Anicic et al. [47] |
| Direct | 210 °C 65 bar | Cu/Zn/Al | CO2:24.2 H2:75.8 | 0.302 | Sollai et al. [62] |
| Two-step | 284 °C 78 bar | Cu/ZnO/Al2O3 | CO:13.04 CO2:14.49 H2:72.46 | 0.326 | This study |
| Two-step | 250 °C 16 bar | Cu/ZnO/ZrO2 | CO:16.67 CO2:16.67 H2:66.67 | 0.324 | Anicic et al. [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hren, D.T.; Bogataj, M.; Nemet, A. Methanol Production via Power-to-Liquids: A Comparative Simulation of Two Pathways Using Green Hydrogen and Captured CO2. Processes 2024, 12, 2843. https://doi.org/10.3390/pr12122843
Hren DT, Bogataj M, Nemet A. Methanol Production via Power-to-Liquids: A Comparative Simulation of Two Pathways Using Green Hydrogen and Captured CO2. Processes. 2024; 12(12):2843. https://doi.org/10.3390/pr12122843
Chicago/Turabian StyleHren, David Tian, Miloš Bogataj, and Andreja Nemet. 2024. "Methanol Production via Power-to-Liquids: A Comparative Simulation of Two Pathways Using Green Hydrogen and Captured CO2" Processes 12, no. 12: 2843. https://doi.org/10.3390/pr12122843
APA StyleHren, D. T., Bogataj, M., & Nemet, A. (2024). Methanol Production via Power-to-Liquids: A Comparative Simulation of Two Pathways Using Green Hydrogen and Captured CO2. Processes, 12(12), 2843. https://doi.org/10.3390/pr12122843






