New Insights into Red and White Quinoa Protein Isolates: Nutritional, Functional, Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Isolation
2.2. Amino Acid Analysis
2.3. Phenols and Flavonoids
2.4. Scanning Electron Microscopy (SEM)
2.5. Zeta Potential
2.6. Particle Size Distribution
2.7. Differential Scanning Calorimetry (DSC)
2.8. Rheological Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Amino Acids
3.2. Phenols and Flavonoids in White Quinoa Protein Isolate (WQPI) and Red Quinoa Protein Isolate (RQPI)
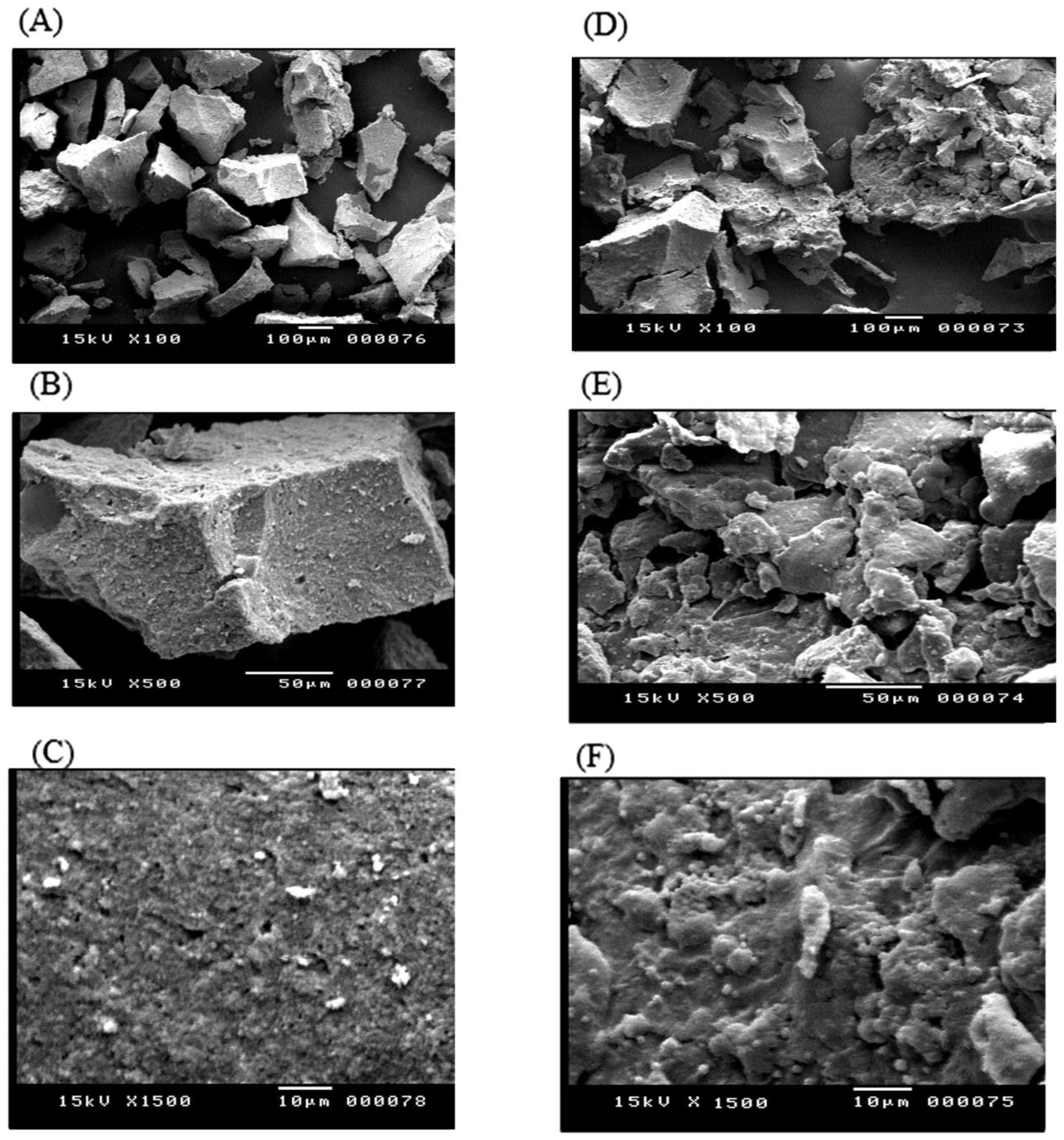
3.3. Scanning Electron Microscopy (SEM)
3.4. Zeta Potential
3.5. Particle Size Distribution
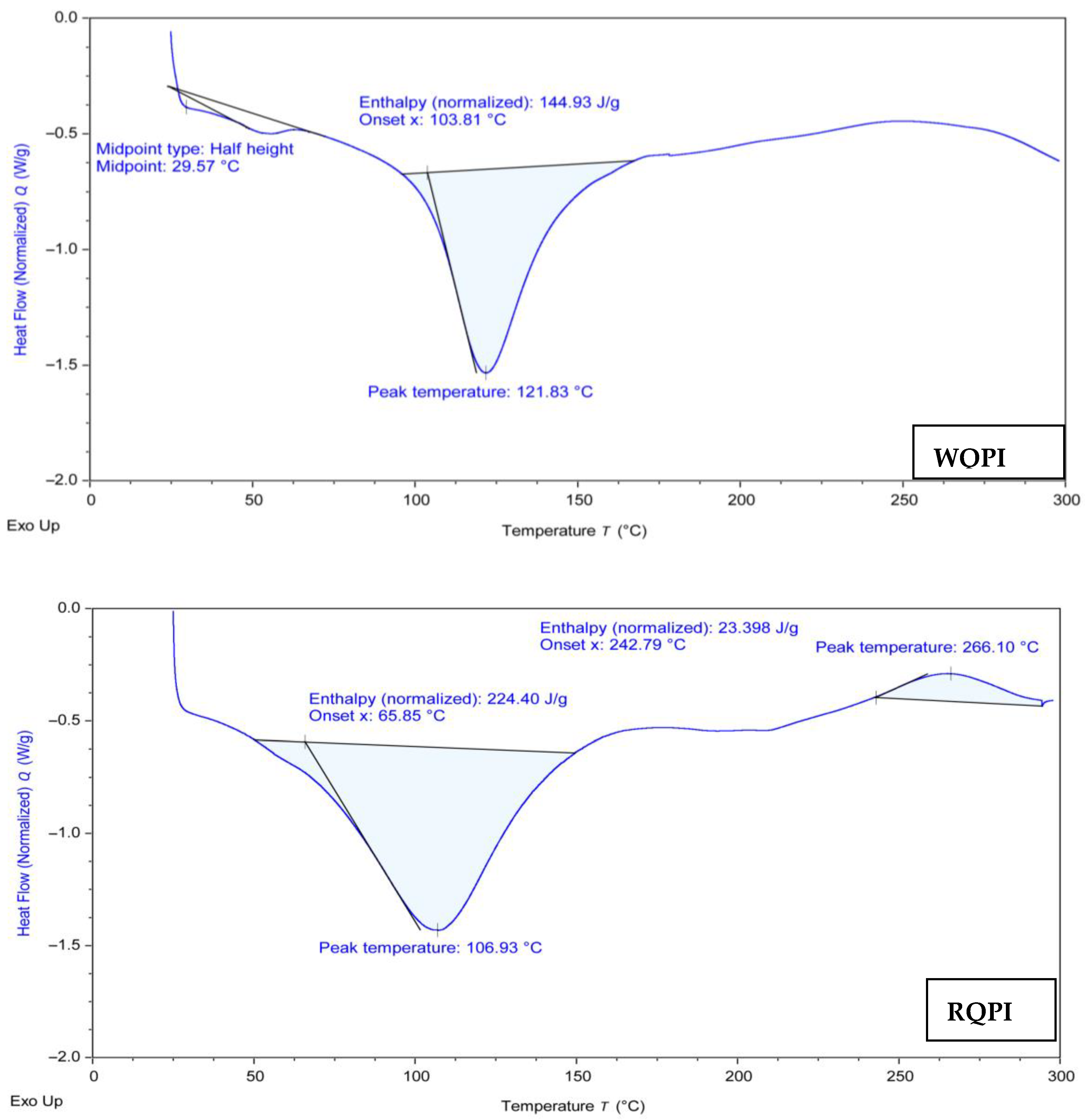
3.6. Differential Scanning Calorimetry (DSC)
3.7. Rheological Properties
3.7.1. Rotational Test
3.7.2. Oscillatory Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valencia-Chamorro, S.A. Quinoa: Overview. Encycl. Food Grains 2016, 1, 341–348. [Google Scholar]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M.; Hamed, H.A.; Kobacy, W.; Mahmoud, E.A.; El-Geddawy, M.M.A.; Kang, S.; et al. Quinoa Proteins (Chenopodium quinoa Willd.) Fractionated by Ultrafiltration Using Ceramic Membranes: The Role of PH on Physicochemical and Conformational Properties. Food Chem. 2011, 10, 104700. [Google Scholar]
- Taylor, J.R.N.; Parker, M.L. Quinoa. In Pseudocereals and Less Common Cereals; Springer: Berlin/Heidelberg, Germany, 2002; pp. 93–122. [Google Scholar]
- Hamed, H.A.; Kobacy, W.; Mahmoud, E.A.; El-Geddawy, M.M.A. Looking for a Novel Vegan Protein Supplement from Faba Bean, Lupine, and Soybean: A Dietary and Industrial Standpoint. Plant Foods Hum. Nutr. 2024, 79, 90–97. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M. Study of Some Physicochemical and Functional Properties of Quinoa (Chenopodium Quinoa Willd) Protein Isolates. J. Agric. Food Chem. 2008, 56, 4745–4750. [Google Scholar] [CrossRef] [PubMed]
- Mondor, M.; Aksay, S.; Drolet, H.; Roufik, S.; Farnworth, E.; Boye, J.I. Influence of Processing on Composition and Antinutritional Factors of Chickpea Protein Concentrates Produced by Isoelectric Precipitation and Ultrafiltration. Innov. Food Sci. Emerg. Technol. 2009, 10, 342–347. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa Protein: Composition, Structure and Functional Properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Acosta, C.; Carpio, C.; Vilcacundo, R.; Carrillo, W. Identification of Proteins Isolate from Amaranth (Amaranthus caudatus) by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis with Water and NaCl 0.1 m Solvents. Asian J. Pharm. Clin. Res. 2016, 9, 331–334. [Google Scholar]
- Cordero-De-Los-Santos, M.Y.; Osuna-Castro, J.A.; Borodanenko, A.; Paredes-López, O. Physicochemical and Functional Characterisation of Amaranth (Amaranthus hypochondriacus) Protein Isolates Obtained by Isoelectric Precipitation and Micellisation. Food Sci. Technol. Int. 2005, 11, 269–280. [Google Scholar] [CrossRef]
- Martínez, E.N.; Añón, M.C. Composition and Structural Characterization of Amaranth Protein Isolates. An Electrophoretic and Calorimetric Study. J. Agric. Food Chem. 1996, 44, 2523–2530. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’ s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant Properties of Domesticated and Wild Rubus Species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Hossain, M.I.; Hossain, M.A. Determinants of Capital Structure and Testing of Theories: A Study on the Listed Manufacturing Companies in Bangladesh. Int. J. Econ. Financ. 2015, 7, 176–190. [Google Scholar] [CrossRef]
- Alsaleem, K.A.; Elfaruk, M.S.; Hammam, A.R.A. Estimation of Antioxidants Activity and Capacity to Capture Free Radical Using 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay. Aust. J. Basic Appl. Sci. 2020, 14, 86–93. [Google Scholar]
- Piñuel, L.; Boeri, P.; Zubillaga, F.; Barrio, D.A.; Torreta, J.; Cruz, A.; Vásquez, G.; Pinto, A.; Carrillo, W. Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation. Plants 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Nemli, E.; Ozkan, G.; Gultekin Subasi, B.; Cavdar, H.; Lorenzo, J.M.; Zhao, C.; Capanoglu, E. Interactions between Proteins and Phenolics: Effects of Food Processing on the Content and Digestibility of Phenolic Compounds. J. Sci. Food Agric. 2024, 104, 2535–2550. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Cao, B.; Wei, X.; Shen, Z.; Su, N. Assessment and Comparison of Nutritional Qualities of Thirty Quinoa (Chenopodium Quinoa Willd.) Seed Varieties. Food Chem. X 2023, 19, 100808. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Manrique, G.D.; Dimitrov, K. Optimization of Antioxidant Phenolic Compounds Extraction from Quinoa (Chenopodium quinoa) Seeds. J. Food Sci. Technol. 2015, 52, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, J.; Guo, X.; Lei, Y.; Yang, M. Effects of Ultrasonic Treatment on the Structure, Functional Properties of Chickpea Protein Isolate and Its Digestibility In Vitro. Foods 2022, 11, 880. [Google Scholar] [CrossRef]
- Tonon, R.V.; Pedro, R.B.; Grosso, C.R.F.; Hubinger, M.D. Microencapsulation of Flaxseed Oil by Spray Drying: Effect of Oil Load and Type of Wall Material. Dry. Technol. 2012, 30, 1491–1501. [Google Scholar] [CrossRef]
- TUNG, M.A. Rheology of Protein Dispersions. J. Texture Stud. 1978, 9, 3–31. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.-E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Ranhotra, G.S.; Gelroth, J.A.; Glaser, B.K.; Lorenz, K.J.; Johnson, D.L. Composition and Protein Nutritional Quality of Quinoa. Cereal Chem. 1993, 70, 303. [Google Scholar]
- Ogungbenle, H.N.; Oshodi, A.A.; Oladimeji, M.O. The Proximate and Effect of Salt Applications on Some Functional Properties of Quinoa (Chenopodium quinoa) Flour. Pak. J. Nutr. 2009, 8, 49–52. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, Anti-Inflammatory, and Antitumor Activities of Phenolic Compounds from White, Red, and Black Chenopodium Quinoa Seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and α-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of Oryzanol and Ferulic Acid on the Glucose Metabolism of Mice Fed with a High-Fat Diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef]
- Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Simultaneous Determination of Phenolic Compounds and Saponins in Quinoa (Chenopodium quinoa Willd) by a Liquid Chromatography–Diode Array Detection–Electrospray Ionization–Time-of-Flight Mass Spectrometry Methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef]
- Kazantseva, V.V.; Goncharuk, E.A.; Fesenko, A.N.; Shirokova, A.V.; Zagoskina, N.V. Features of the phenolics’ formation in seedlings of different varieties of buckwheat (Fagopyrum Esculentum Moench). Agric. Biol. 2015, 50, 611–619. [Google Scholar] [CrossRef]
- Das, D.; Panesar, P.S.; Saini, C.S. PH Shifting Treatment of Ultrasonically Extracted Soybean Meal Protein Isolate: Effect on Functional, Structural, Morphological and Thermal Properties. Process Biochem. 2022, 120, 227–238. [Google Scholar] [CrossRef]
- Li, X.; Da, S.; Li, C.; Xue, F.; Zang, T. Effects of High-intensity Ultrasound Pretreatment with Different Levels of Power Output on the Antioxidant Properties of Alcalase Hydrolyzates from Quinoa (Chenopodium quinoa Willd.) Protein Isolate. Cereal Chem. 2018, 95, 518–526. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, Molecular and Thermal Properties of High-Intensity Ultrasound (HIUS) Treated Protein Isolates from Album (Chenopodium album) Seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S. Effects of Protein Concentration and Oil-Phase Volume Fraction on the Stability and Rheology of Menhaden Oil-in-Water Emulsions Stabilized by Whey Protein Isolate with Xanthan Gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Malik, A.M.; Singh, A. Pseudocereals Proteins-A Comprehensive Review on Its Isolation, Composition and Quality Evaluation Techniques. Food Chem. Adv. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Mapiour, M.; Amira, A. Critical Influences of Plasma PH on Human Protein Properties for Modeling Considerations: Size, Charge, Conformation, Hydrophobicity, and Denaturation. J. Compos. Sci. 2023, 7, 28. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Yuan, X.; Zhao, S.; Kang, Z.; Zhu, M.; He, H.; Ma, H. Physicochemical, Conformational and Functional Changes of Quinoa Protein Affected by High-Pressure Homogenization. LWT 2023, 173, 114343. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Method Development to Increase Protein Enrichment During Dry Fractionation of Starch-Rich Legumes. Food Bioprocess Technol. 2015, 8, 1495–1502. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.; Li, Y. Drying Methods Affect Physicochemical and Functional Properties of Quinoa Protein Isolate. Food Chem. 2021, 339, 127823. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; Van der Goot, A.J.; Boom, R.M. Dry Fractionation for Sustainable Production of Functional Legume Protein Concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Goyal, M.; Chaudhuri, T.K.; Kuwajima, K. Irreversible Denaturation of Maltodextrin Glucosidase Studied by Differential Scanning Calorimetry, Circular Dichroism, and Turbidity Measurements. PLoS ONE 2014, 9, e115877. [Google Scholar] [CrossRef]
- Sánchez, H.A.C.; Jovanović, M.R.; Kumar, S.; Morozov, A.; Shankar, V.; Subramanian, G.; Wilson, H.J. Understanding Viscoelastic Flow Instabilities: Oldroyd-B and Beyond. J. Nonnewton. Fluid Mech. 2022, 302, 104742. [Google Scholar] [CrossRef]
- Schuller, T.; Fanzio, P.; Galindo-Rosales, F.J. Analysis of the Importance of Shear-Induced Elastic Stresses in Material Extrusion. Addit. Manuf. 2022, 57, 102952. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Liu, W.; Zou, L.; McClements, D.J. A Review of the Rheological Properties of Dilute and Concentrated Food Emulsions. J. Texture Stud. 2020, 51, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kierulf, A.; Whaley, J.; Liu, W.; Enayati, M.; Tan, C.; Perez-Herrera, M.; You, Z.; Abbaspourrad, A. Protein Content of Amaranth and Quinoa Starch Plays a Key Role in Their Ability as Pickering Emulsifiers. Food Chem. 2020, 315, 126246. [Google Scholar] [CrossRef]
- Kimbell, G.; Azad, M.A. 3D Printing: Bioinspired Materials for Drug Delivery. In Bioinspired and Biomimetic Materials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–318. [Google Scholar]
- Kinsella, J.E.; Morr, C.V. Milk Proteins: Physicochemical and Functional Properties. Crit. Rev. Food Sci. Nutr. 1984, 21, 197–262. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.H.; Kavanagh, G.M.; Ross-Murphy, S.B. Globular Protein Gelation—Theory and Experiment. Food Hydrocoll. 2001, 15, 383–400. [Google Scholar] [CrossRef]
- Damodaran, S. Amino Acids, Peptides and Proteins. Fennema’s Food Chem. 2008, 4, 425–439. [Google Scholar]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]


| Amino Acids | WQPI (g/100 g) | RQPI (g/100 g) | p-Value | |
|---|---|---|---|---|
| Essential amino acids | Aspartic | 13.244 ± 3.73 | 12.685 ± 3.29 | 0.6 |
| Phenylalanine | 2.825 ± 1.38 | 3.815 ± 1.15 | 0.47 | |
| Isoleucine | 6.871 ± 3.20 | 9.898 ± 3.08 | 0.03 * | |
| Leucine | 2.750 ± 1.59 | 3.627 ± 1.23 | 0.52 | |
| Histidine | 10.520 ± 3.14 | 4.747 ± 1.63 | 0.00 ** | |
| Methionine | 4.813 ± 1.07 | 9.253 ± 3.18 | 0.00 ** | |
| Threonine | 5.903 ± 2.26 | 3.956 ± 1.24 | 0.16 | |
| Valine | 4.311 ± 1.41 | 7.957 ± 2.19 | 0.01 * | |
| Lysine | 3.767 ± 1,56 | 3.380 ± 1.33 | 0.77 | |
| Non-essential amino acids | Proline | 6.616 ± 1.35 | 3.160 ± 1.12 | 0.01 * |
| Tyrosine | 5.417 ± 2.61 | 8.777 ± 2.06 | 0.02 * | |
| Cysteine | 5.59 ± 2.73 | 1.273 ± 0.22 | 0.00 ** | |
| Alanine | 3.811 ± 1.27 | 4.576 ± 1.57 | 0.57 | |
| Glutamic | 6.937 ± 1.11 | 6.210 ± 1.35 | 0.59 | |
| Arginine | 9.027 ± 2.71 | 5.917 ± 2.06 | 0.03 * | |
| Glycine | 3.299 ± 1.08 | 2.126 ± 0.52 | 0.39 | |
| Serine | 6.849 ± 1.41 | 3.797 ± 1.31 | 0.03 * | |
| Tryptophan | Not determined | |||
| Compounds | WQPI Conc. (µg/g) | RQPI Conc. (µg/g) | p-Value | |
|---|---|---|---|---|
| Phenols | Gallic acid | 155.50 ± 12.97 | 243.40 ± 12.65 | 0.00 ** |
| Protocatechuic acid | ND | 17.83 ± 3.89 | ||
| Cinnamic acid | 0.49 ± 0.12 | 1.28 ± 0.25 | 0.38 | |
| p-Hydroxybenzoic acid | 28.47 ± 4.74 | 35.34 ± 5.29 | 0.000 ** | |
| Syringic acid | 5.27 ± 2.32 | 23.99 ± 8.47 | 0.00 ** | |
| Ellagic acid | 0.00 | 0.00 | 0.00 | |
| Coumaric acid | 28.93 ± 5.21 | 54.70 ± 9.35 | 0.00 ** | |
| Vanillin | 12.15 ± 3.17 | 17.07 ± 3.11 | 0.00 ** | |
| Ferulic acid | 89.12 + 10.83 | 116.54 ± 10.92 | 0.00 ** | |
| Total | 319.93 ± 10.86 | 510.15 ± 10.48 | 0.00 ** | |
| Flavonoids | Naringenin | 6.27 ± 82 | 10.19 ± 2.56 | 0.00 ** |
| Daidzein | 2.09 ± 1.62 | 2.49 ± 1.67 | 0.38 | |
| Catechin | 11.40 ± 5.47 | 6.59 ± 2.13 | 0.00 ** | |
| Rutin | (30.1 + 7.52) | 39.67 ± 7.72 | 0.00 ** | |
| Quercetin | 8.86 ± 5.48 | 20.08 ± 5.44 | 0.00 ** | |
| Total | 58.72 ± 8.22 | 70.02 ± 7.38 | 0.00 ** |
| Sample | η [m·Pa·s] | p | p2 |
|---|---|---|---|
| WQPI | 47.7 | −0.638 | 0.407044 |
| RQPI | 99.8 | −0.401 | 0.160801 |
| WQPI | RQPI | ||
|---|---|---|---|
| G′ point (storage modulus) Pa | Maximum | 54.863 | 171.07 |
| Minimum | 1.2199 | 1.1868 | |
| G″ point (loss modulus) Pa | Maximum | 8.9093 | 31.166 |
| Minimum | 1.7479 | 2.0035 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaleem, K.A.; Moftah, R.F.; El-Geddawy, M.M.A. New Insights into Red and White Quinoa Protein Isolates: Nutritional, Functional, Thermal Properties. Processes 2024, 12, 2822. https://doi.org/10.3390/pr12122822
Alsaleem KA, Moftah RF, El-Geddawy MMA. New Insights into Red and White Quinoa Protein Isolates: Nutritional, Functional, Thermal Properties. Processes. 2024; 12(12):2822. https://doi.org/10.3390/pr12122822
Chicago/Turabian StyleAlsaleem, Khalid A., Rofida F. Moftah, and Mennatallah M. A. El-Geddawy. 2024. "New Insights into Red and White Quinoa Protein Isolates: Nutritional, Functional, Thermal Properties" Processes 12, no. 12: 2822. https://doi.org/10.3390/pr12122822
APA StyleAlsaleem, K. A., Moftah, R. F., & El-Geddawy, M. M. A. (2024). New Insights into Red and White Quinoa Protein Isolates: Nutritional, Functional, Thermal Properties. Processes, 12(12), 2822. https://doi.org/10.3390/pr12122822








