Comparative Analysis of Volatile Components and Sensory Profiles of Four Basil Varieties Based on HS-SPME and SD Coupled with GC-MS
Abstract
Highlights
- Forty-seven and sixty-six volatiles were found in fresh basils and EOs, respectively.
- Drying and extraction process of basils reduced ethers and altered aroma quality.
- PCA and OPLS-DA revealed differences between fresh basil and EO volatile profiles.
- Sensory analysis also showed processing affecting aromas intensity and quality.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Volatile Extraction Using the HS-SPME Method and Analyses
2.3. EO Extraction Using the SD Method and Analyses
2.4. GC-MS Analysis
2.5. Sensory Evaluation
2.6. Data Processing and Statistical Analyses
3. Results and Discussion
3.1. Volatile Components of Four Fresh Basil Varieties Analyzed by HS-SPME/GC-MS
3.2. Extraction of EOs from Four Basil Varieties Using the SD Method
3.3. Volatile Components of EOs from Four Basil Varieties Analyzed by GC-MS
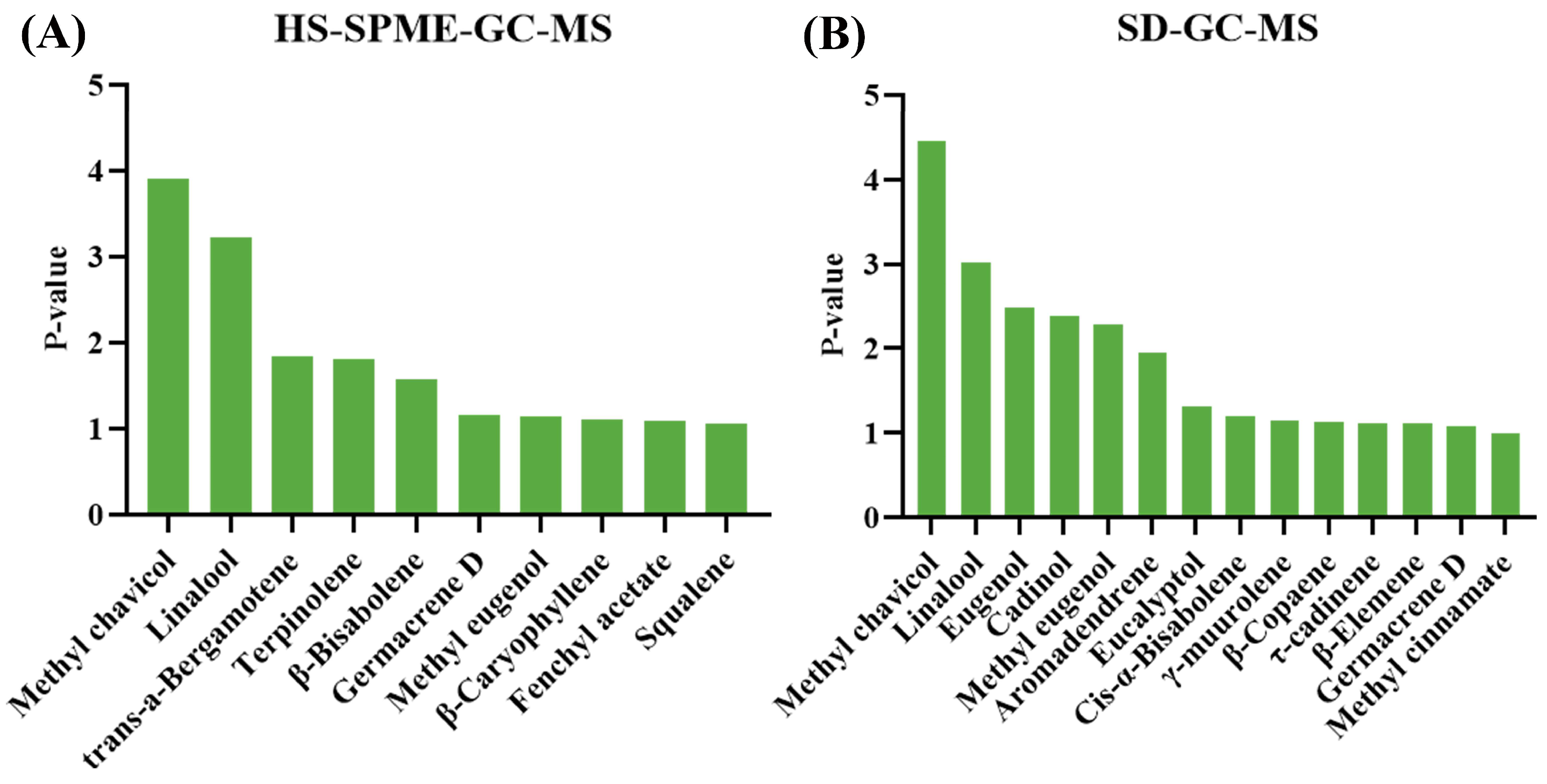
3.4. Comparative Analysis of Volatile Fractions Across Different Basil Varieties
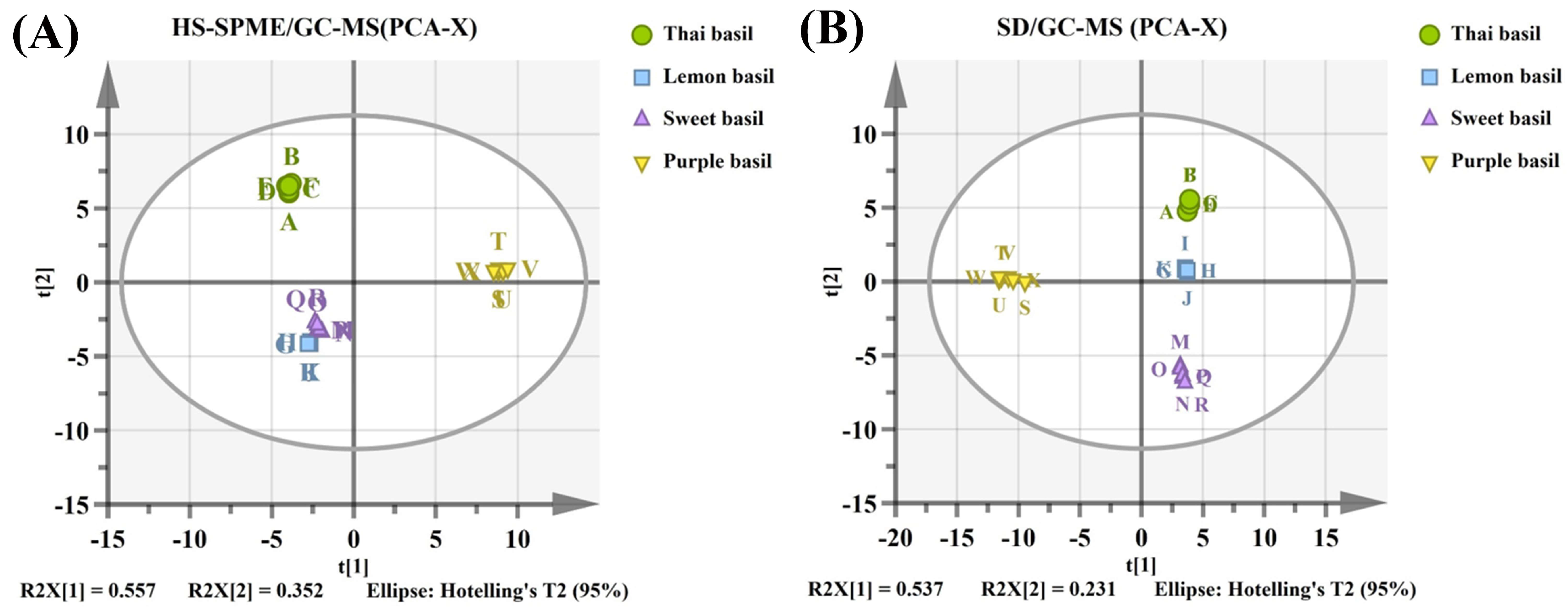
3.5. PCA-OPLS-DA to Determine Differential Components of Different Basil Varieties
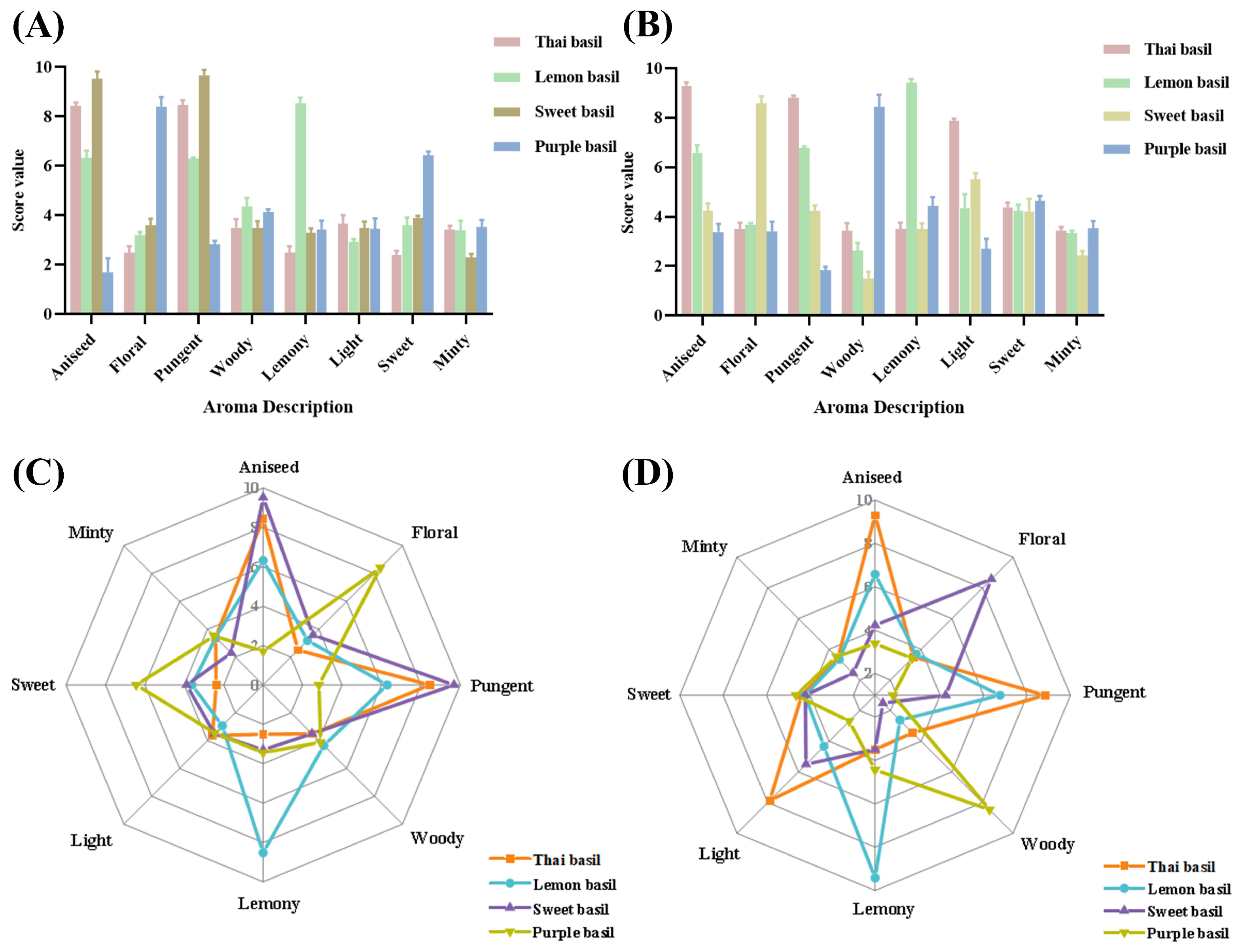
3.6. Sensory Evaluation of Aroma in Different Basil Varieties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, W.L.; Shahrajabian, M.H.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Backiam, A.D.S.; Duraisamy, S.; Karuppaiya, P.; Balakrishnan, S.; Sathyan, A.; Kumarasamy, A.; Raju, A. Analysis of the main bioactive compounds from Ocimum basilicum for their antimicrobial and antioxidant activity. Biotechnol. Appl. Biochem. 2023, 70, 2038–2051. [Google Scholar] [CrossRef] [PubMed]
- Elhindi, K.; El Din, A.S.; Salam, E.A.; Elgorban, A. Amelioration of salinity stress in different basil (Ocimum basilicum L.) varieties by vesicular-arbuscular mycorrhizal fungi. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2016, 66, 583–592. [Google Scholar] [CrossRef]
- Alves, V.C.; Kalbina, I.; Nilsen, A.; Aronsson, M.; Rosenqvist, E.; Jansen, M.A.K.; Qian, M.; Öström, Å.; Hyötyläinen, T.; Strid, Å. Integration of non-target metabolomics and sensory analysis unravels vegetable plant metabolite signatures associated with sensory quality: A case study using dill (Anethum graveolens). Food Chem. 2021, 344, 128714. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Lee, R.; Merchant, E.V.; Juliani, H.R.; Simon, J.E.; Tepper, B.J. Descriptive aroma profiles of fresh sweet basil cultivars (Ocimum spp.): Relationship to volatile chemical composition. J. Food Sci. 2021, 86, 3228–3239. [Google Scholar] [CrossRef]
- da Silva, W.M.F.; Kringel, D.H.; de Souza, E.J.D.; da Rosa Zavareze, E.; Dias, A.R.G. Basil essential oil: Methods of extraction, chemical composition, biological activities, and food applications. Food Bioprocess Technol. 2021, 15, 1–27. [Google Scholar] [CrossRef]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Comparison of chemical composition and biological properties of essential oils obtained by hydrodistillation and steam distillation of Laurus nobilis L. Plant Food Hum. Nutr. 2020, 75, 495–504. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yang, J.J.; Wu, G.J.; Jiang, J.G. Comparative antioxidant, anticancer and antimicrobial activities of essential oils from Semen Platycladi by different extraction methods. Ind. Crop. Prod. 2020, 146, 112206. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Graziani, G.; Romano, R.; De Pascale, S.; Rouphael, Y.; Corrado, G. Comparative analysis of aromatic and nutraceutical traits of six basils from Ocimum genus grown in floating raft culture. Sci. Hortic. 2023, 322, 112382. [Google Scholar] [CrossRef]
- Ma, X.J.; Han, J.; Wang, T.; Jiang, R.Y.; Wang, H.; Wang, X.; Yao, R.S. Extraction process optimization, composition analysis and sensory evaluation of volatile oil from Thai Basil (Ocimum basilicum var. thyrsiflora). Xiandai Shipin Keji 2022, 38, 309–317. [Google Scholar] [CrossRef]
- Kuang, C.L.; Lv, D.; Shen, G.H.; Li, S.S.; Luo, Q.Y.; Zhang, Z.Q. Chemical composition and antimicrobial activities of volatile oil extracted from Chrysanthemum morifolium Ramat. J. Food Sci. Technol. 2018, 55, 2786–2794. [Google Scholar] [CrossRef]
- Farag, M.A.; El Kersh, D.M.; Rasheed, D.M.; Heiss, A.G. Volatiles distribution in Nigella species (black cumin seeds) and in response to roasting as analyzed via solid-phase microextraction (SPME) coupled to chemometrics. Ind. Crop. Prod. 2017, 108, 564–571. [Google Scholar] [CrossRef]
- Donato, F.D.; Biancolillo, A.; Mazzulli, D.; Rossi, L.; D’Archivio, A.A. HS-SPME/GC-MS volatile fraction determination and chemometrics for the discrimination of typical Italian Pecorino cheeses. Microchem. J. 2021, 165, 106133. [Google Scholar] [CrossRef]
- Xie, J.Y.; Li, X.J.; Li, W.; Ding, H.; Yin, J.X.; Bie, S.T.; Li, F.Y.; Tian, C.W.; Han, L.F.; Yang, W.Z.; et al. Characterization of the key volatile organic components of different parts of fresh and dried perilla frutescens based on headspace-gas chromatography-ion mobility spectrometry and headspace solid phase microextraction-gas chromatography-mass spectrometry. Arab. J. Chem. 2023, 16, 104867. [Google Scholar] [CrossRef]
- Tarchoune, I.; Baâtour, O.; Harrathi, J.; Cioni, P.L.; Lachaâl, M.; Flamini, G.; Ouerghi, Z. Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J. Plant Nutr. Soil Sci. 2013, 176, 748–755. [Google Scholar] [CrossRef]
- Mahmoud, E.; Starowicz, M.; Ciska, E.; Topolska, J.; Farouk, A. Determination of volatiles, antioxidant activity, and polyphenol content in the postharvest waste of Ocimum basilicum L. Food Chem. 2021, 375, 131692. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Redeo, A.J.D.; Mitcham, E.J. Chilling temperatures and controlled atmospheres alter key volatile compounds implicated in basil aroma and flavor. Front. Plant Sci. 2023, 14, 1218734. [Google Scholar] [CrossRef] [PubMed]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- Tangpao, T.; Chung, H.H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai Basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Lech, K.; Barrachina, Á.A.C.; Szumny, A. Chemical determinants of dried Thai basil (O. basilicum var. thyrsiflora) aroma quality. Ind. Crop. Prod. 2020, 155, 112769. [Google Scholar] [CrossRef]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant activities of a new chemotype of Piper cubeba L. fruit essential oil (Methyleugenol/Eugenol): In silico molecular docking and ADMET studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Nakib, R.; Flores, M.S.R.; Escuredo, O.; Ouelhadj, A.; Coello, M.C.S. Retama sphaerocarpa, Atractylis serratuloides and Eruca sativa honeys from Algeria: Pollen dominance and volatile profiling (HS-SPME/GC-MS). Microchem J. 2022, 174, 107088. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Yu, H.T.; Yu, X.Y.; Zhu, L.J.; Yu, Z.F. Comparison of volatile compounds in Chrysanthemum nankingense during storage based on HS-SPME-GC-MS and E-nose. J. Food Meas. Charact. 2023, 17, 3134–3148. [Google Scholar] [CrossRef]
- Jordán, M.J.; Quílez, M.; Luna, M.C.; Bekhradi, F.; Sotomayor, J.A.; Sánchez-Gómez, P.; Gil, M.I. Influence of water stress and storage time on preservation of the fresh volatile profile of three basil genotypes. Food Chem. 2017, 221, 169–177. [Google Scholar] [CrossRef]
- Ali, J.S.; Azeem, M.; Mannan, A.; Zia, M. Chemical composition, antibacterial and antioxidative activities of Monotheca buxifolia (Falc.) A. DC leaves essential oil. Nat. Prod. Res. 2022, 36, 5848–5851. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and caryophyllene oxide: A variety of chemical transformations and biological activities. Chem. Pap. 2022, 76, 1–39. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Li, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer properties of eugenol: A review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Bellik, F.Z.; Ali, F.B.; Alsafra, Z.; Eppe, G. Effect of different parameters on volatile composition of the different parts of Cymbopogon schoenanthus L. spreng (Poaceae) extracted by Headspace Solid-phase Microextraction and Hydrodistillation. J. Essent. Oil Bear. Plants 2021, 24, 841–862. [Google Scholar] [CrossRef]
- Silva, A.C.R.; Bizzo, H.R.; Vieira, R.F.; Bringel, J.B.A.; Azevedo, D.A.; Uekane, T.M.; Rezende, C.M. Characterization of volatile and odor-active compounds of the essential oil from Bidens graveolens Mart. (Asteraceae). Flavour Frag. J. 2019, 35, 79–87. [Google Scholar] [CrossRef]
- Mahendran, G.; Vimolmangkang, S. Chemical compositions, antioxidant, antimicrobial, and mosquito larvicidal activity of Ocimum americanum L. and Ocimum basilicum L. leaf essential oils. BMC Complement. Altern. Med. 2023, 23, 390. [Google Scholar] [CrossRef] [PubMed]
- Guclu, G.; Keser, D.; Kelebek, H.; Keskin, M.; Sekerli, Y.E.; Soysal, Y.; Selli, S. Impact of production and drying methods on the volatile and phenolic characteristics of fresh and powdered sweet red peppers. Food Chem. 2020, 338, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Y.; Wen, Y.H.; Peng, Y.; Chen, T.J.; Chen, J.J.; Yang, J.L.; Gong, T.; Zhu, P. Advances in biosynthesis of cadinane sesquiterpenes. Chin. J. Biotechnol. 2021, 37, 1952–1967. [Google Scholar] [CrossRef]
- Zhang, H.X.; Huang, T.; Liao, X.N.; Zhou, Y.H.; Chen, S.X.; Chen, J.; Xiong, W.M. Extraction of camphor tree essential oil by steam distillation and supercritical CO2 extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Yang, R.W.; Zhang, H.; Wang, S.L.; Chen, D.; Lin, S.Y. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, Y.L.; Wang, Y.G.; Zhao, Y.Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem. 2014, 171, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.G.; Shi, G.Y.; Yang, H.; Wang, X.M.; Zhao, H.Y.; Zhao, S.H. Effects of drying methods on the nutritional aspects, flavor, and processing properties of Chinese chestnuts. J. Food Sci. Technol. 2018, 55, 3391–3398. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Wang, Y.; Liu, Y.M. Geographical origins and varieties identification of hops (Humulus lupulus L.) by multi-metal elements fingerprinting and the relationships with functional ingredients. Food Chem. 2019, 289, 522–530. [Google Scholar] [CrossRef]
- Oliveira, L.F.C.; Tega, D.U.; Duarte, G.H.B.; Barbosa, L.D.; Ribeiro, H.C.; Castello, A.C.D.; Sawaya, A.C.H.F.; Sussulini, A. Foodomics for agroecology: Differentiation of volatile profile in mint (Mentha × gracilis Sole) from permaculture, organic and conventional agricultural systems using HS-SPME/GC-MS. Food Res. Int. 2022, 155, 111107. [Google Scholar] [CrossRef]
- Miyazato, H. Volatile composition and the key aroma compounds of the Citrus tachibana (Makino) Tanaka peel essential oil. J. Essent. Oil Bear. Plants 2018, 21, 924–937. [Google Scholar] [CrossRef]
- Yu, Z.; Dong, W.H.; Wang, Y.L.; Li, W.; Guo, Z.Y.; Mei, W.L.; Dai, H.F. Identification of aroma-active components from cultivated agarwood ‘Qi-Nan’ based on GC-O-MS combined with aroma extract dilution analysis. Flavour Frag. J. 2023, 38, 392–403. [Google Scholar] [CrossRef]



| Categories | ID | Compounds | Linear Retention Index | CAS Number | Relative Mass fraction (%) | |||
|---|---|---|---|---|---|---|---|---|
| Thai Basil | Lemon Basil | Sweet Basil | Purple Basil | |||||
| Ketone | 1 | Fechone | 1385 | 4695-62-9 | ND | ND | ND | 0.12 ± 0.01 a |
| 2 | Camphor | 1515 | 76-22-2 | 1.08 ± 0.07 a | ND | 0.11 ± 0.01 c | 0.19 ± 0.02 b | |
| Subtotal | 1.08 | - | 0.11 | 0.31 | ||||
| Ester | 3 | Fenchyl acetate | 1559 | 13851-11-1 | 1.72 ± 0.03 a | ND | ND | 0.38 ± 0.01 b |
| 4 | Bornyl acetate | 1591 | 76-49-3 | 0.23 ± 0.02 a | ND | ND | 0.26 ± 0.04 a | |
| 5 | Methyl cinnamate | 2069 | 103-26-4 | 0.59 ± 0.02 a | ND | ND | ND | |
| Subtotal | 2.54 | - | - | 0.64 | ||||
| Ether | 6 | 2-Methyl-5-(prop-1-en-2-yl)cyclohexanol | 1377 | 619-01-2 | ND | ND | ND | 0.08 ± 0.02 a |
| 7 | Methyl chavicol | 1671 | 140-67-0 | 66.53 ± 1.65 b | 90.18 ± 0.33 a | 89.19 ± 0.15 a | 0.74 ± 0.02 c | |
| 8 | Isoestragole | 1988 | 104-46-1 | ND | ND | 0.11 ± 0.01 b | 0.55 ± 0.03 a | |
| 9 | Methyl eugenol | 2010 | 93-15-2 | 0.76 ± 0.03 d | 1.36 ± 0.05 c | 1.85 ± 0.04 b | 2.14 ± 0.05 a | |
| 10 | Eugenol | 2138 | 97-53-0 | Tr | ND | Tr | 1.45 ± 0.04 a | |
| Subtotal | 67.29 | 91.54 | 91.15 | 5.74 | ||||
| 11 | Sesquisabinen | 1668 | 58319-04-3 | 0.14 ± 0.04 a | ND | ND | ND | |
| 12 | Germacrene D | 1721 | 23986-74-5 | 0.98 ± 0.02 d | 1.05 ± 0.04 c | 1.74 ± 0.06 b | 2.75 ± 0.05 a | |
| Terpene | 13 | β-Myrcene | 1153 | 123-35-3 | ND | Tr | ND | 0.27 ± 0.05 a |
| 14 | γ- Terpinene | 1228 | 99-85-4 | ND | ND | ND | 0.09 ± 0.01 a | |
| 15 | (Z)-13,7-Dimethyl-3,6-octatriene | 1244 | 3338-55-4 | ND | ND | ND | 0.08 ± 0.02 a | |
| 16 | Terpinolene | 1267 | 586-62-9 | Tr | Tr | ND | 0.16 ± 0.03 a | |
| 17 | α-Copaene | 1488 | 3856-25-5 | 0.08 ± 0.01 c | 0.23 ± 0.01 b | 0.24 ± 0.01 b | 0.27 ± 0.03 a | |
| 18 | β-Cubebene | 1544 | 13744-15-5 | 0.15 ± 0.03 a | 0.15 ± 0.03 a | 0.14 ± 0.01 a | 0.16 ± 0.01 a | |
| 19 | α- Bergamotene | 1593 | 17699-05-7 | 0.10 ± 0.04 b | Tr | 0.07 ± 0.03 b | 0.24 ± 0.04 a | |
| 20 | β-Caryophyllene | 1601 | 87-44-5 | 0.30 ± 0.03 c | 2.62 ± 0.04 b | 2.80 ± 0.06 a | 0.20 ± 0.03 d | |
| 21 | (-)-β-Elemene | 1603 | 515-13-9 | 0.33 ± 0.07 b | Tr | ND | 0.75 ± 0.07 a | |
| 22 | α-Cedrene | 1604 | 469-61-4 | ND | ND | ND | 0.08 ± 0.02 a | |
| 23 | α-Caryophyllene | 1607 | 6753-98-6 | 1.00 ± 0.11 a | 0.48 ± 0.02 c | 0.56 ± 0.06 c | 0.68 ± 0.03 b | |
| 24 | trans-a-Bergamotene | 1608 | 13474-59-4 | 7.23 ± 0.66 b | 0.78 ± 0.03 c | 0.82 ± 0.03 c | 19.77 ± 1.01 a | |
| 25 | α-Guaiene | 1614 | 3691-12-1 | 0.32 ± 0.03 b | ND | ND | 1.12 ± 0.13 a | |
| 26 | β-Longipinene | 1622 | 41432-70-6 | ND | ND | ND | 0.46 ± 0.08 a | |
| 27 | cis-β-Farnesene | 1666 | 28973-97-9 | 1.12 ± 0.09 a | 0.20 ± 0.03 b | 0.24 ± 0.02 b | ND | |
| 28 | trans -β-Famesene | 1695 | 18794-84-8 | 0.33 ± 0.04 a | 0.09 ± 0.01 b | 0.10 ± 0.02 b | 0.09 ± 0.01 b | |
| 29 | β-Selinene | 1717 | 17066-67-0 | 0.66 ± 0.04 a | 0.08 ± 0.02 b | ND | ND | |
| 30 | α-Bulnesene | 1727 | 3691-11-0 | 0.32 ± 0.04 b | ND | ND | 1.15 ± 0.22 a | |
| 31 | (+)-Bicyclogermacrene | 1749 | 24703-35-3 | ND | ND | Tr | 1.61 ± 0.25 a | |
| 32 | β-Bisabolene | 1751 | 495-61-4 | ND | 0.94 ± 0.07 a | 0.12 ± 0.01 b | ND | |
| 33 | Naphthalene,1,2,3,5,6,8α-hexahydro-4,7-dimethyl-1-(1-methylethyl) | 1765 | 16729-01-4 | ND | 0.27 ± 0.03 a | ND | ND | |
| 34 | β-Sesquiphellandrene | 1779 | 20307-83-9 | 0.09 ± 0.01 b | ND | 0.05 ± 0.03 b | 0.30 ± 0.05 a | |
| 35 | α-Bisabolene | 1785 | 25532-79-0 | ND | 0.98 ± 0.21 a | ND | ND | |
| 36 | cis-Calamenene | 1838 | 22339-23-7 | 1.54 ± 0.05 a | ND | ND | ND | |
| Subtotal | 14.69 | 7.87 | 6.88 | 30.23 | ||||
| Alcohol (includes a phenolic compound) | 37 | 2-Methoxy-3-(2-propenyl) phenol | 2065 | 1941-12-4 | ND | ND | ND | 0.78 ± 0.02 a |
| 38 | 2,7-dimethyl-2,6-Octadien-1-ol | 1175 | 22410-74-8 | ND | ND | ND | 0.15 ± 0.01 a | |
| 39 | Eucalyptol | 1210 | 470-82-6 | 1.09 ± 0.02 a | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.43 ± 0.07 c | |
| 40 | Bicyclo[3.1.0]hexan-2-ol,2-methyl-5-(1-methylethyl)- | 1374 | 546-79-2 | 0.19 ± 0.03 b | ND | ND | 0.36 ± 0.02 a | |
| 41 | trans-β-Terpineol | 1438 | 7299-41-4 | 0.36 ± 0.04 a | Tr | ND | ND | |
| 42 | Linalool | 1545 | 78-70-6 | 9.19 ± 1.11 b | ND | ND | 58.56 ± 1.66 a | |
| 43 | δ-Terpineol | 1586 | 7299-42-5 | 0.07 ± 0.01 b | 0.08 ± 0.01 b | Tr | 0.24 ± 0.05 a | |
| 44 | Terpinene-1-ol-4 | 1606 | 20126-76-5 | 0.75 ± 0.01 a | ND | ND | ND | |
| 45 | (+)-α-Terpineol | 1704 | 7785-53-7 | ND | ND | ND | 1.19 ± 0.20 a | |
| 46 | Borneol | 1712 | 507-70-0 | 0.06 ± 0.01 a | ND | Tr | ND | |
| 47 | τ-Juniper alcohol | 1736 | 5937-11-1 | ND | 0.09 ± 0.03 b | ND | 1.20 ± 0.19 a | |
| Subtotal | 11.71 | 0.25 | 0.08 | 62.13 | ||||
| Total | 97.31 | 99.66 | 98.22 | 99.05 | ||||
| Categories | ID | Compounds | Linear Retention Index | CAS Number | Relative Mass Fraction (%) | |||
|---|---|---|---|---|---|---|---|---|
| Thai Basil | Lemon Basil | Sweet Basil | Purple Basil | |||||
| Ketone | 1 | Fenchone | 1384 | 7787-20-4 | 0.36 ± 0.02 a | 0.08 ± 0.01 b | 0.09 ± 0.02 b | 0.25 ± 0.04 a |
| 2 | Camphor | 1513 | 76-22-2 | 0.15 ± 0.04 b | ND | 0.39 ± 0.08 a | ND | |
| 48 | Menthone | 1482 | 10458-14-7 | ND | ND | ND | 0.14 ± 0.05 a | |
| 49 | D-(+)-Carvone | 1717 | 2244-16-8 | Tr | ND | 0.19 ± 0.04a | ND | |
| Subtotal | 0.51 | 0.08 | 0.67 | 0.39 | ||||
| Ester | 50 | β-Terpinyl acetate | 1366 | 10198-23-9 | ND | ND | ND | 0.14 ± 0.04 a |
| 3 | Fenchyl acetate | 1558 | 13851-11-1 | 0.14 ± 0.04 a | ND | ND | ND | |
| 4 | Bornyl acetate | 1592 | 76-49-3 | 0.54 ± 0.07 b | 0.17 ± 0.05 c | 1.79 ± 0.44 a | ND | |
| 5 | Methyl cinnamate | 2070 | 103-26-4 | ND | ND | 0.1 ± 0.03 b | 3.22 ± 0.40 a | |
| 51 | Glyceryl linolenate | 2165 | 18465-99-1 | ND | ND | ND | 0.40 ± 0.06 a | |
| Subtotal | 0.68 | 0.17 | 1.89 | 3.76 | ||||
| Ether | 7 | Methyl chavicol | 1671 | 140-67-0 | 65.27 ± 0.94 b | 81.03 ± 1.7 a | 29.34 ± 0.91 c | 3.71 ± 0.43 d |
| 9 | Methyl eugenol | 2010 | 93-15-2 | 12.35 ± 0.80 a | 7.22 ± 0.82 c | 8.94 ± 0.48 b | 0.49 ± 0.05 d | |
| 10 | Eugenol | 2138 | 97-53-0 | Tr | Tr | 13.26 ± 0.82 a | 0.83 ± 0.03 b | |
| Subtotal | 77.62 | 88.25 | 51.54 | 5.03 | ||||
| Terpene | 12 | Germacrene D | 1721 | 23986-74-5 | ND | Tr | ND | 3.64 ± 0.43 a |
| 52 | α -Pinene | 1015 | 80-56-8 | 0.22 ± 0.04 b | ND | ND | 0.29 ± 0.03 a | |
| 53 | β-Phellandrene | 1142 | 555-10-2 | 0.22 ± 0.04 a | ND | ND | ND | |
| 13 | β-Myrcene | 1153 | 123-35-3 | 0.61 ± 0.03 a | ND | 0.09 ± 0.02 c | 0.21 ± 0.04 b | |
| 54 | Ocimene | 1227 | 13877-91-3 | 0.94 ± 0.05 a | 0.30 ± 0.04 b | 0.30 ± 0.03 b | ND | |
| 55 | 4-Thujanol | 1355 | 546-79-2 | ND | ND | ND | 0.18 ± 0.06 a | |
| 56 | (-)-α-Cubebene | 1455 | 17699-14-8 | ND | Tr | ND | 0.09 ± 0.02 a | |
| 17 | α-Copaene | 1490 | 3856-25-5 | ND | 0.19 ± 0.02 a | ND | ND | |
| 18 | β-Cubebene | 1544 | 13744-15-5 | 0.95 ± 0.05 a | 0.28 ± 0.05 b | ND | ND | |
| 57 | Cedrene | 1568 | 11028-42-5 | ND | 0.17 ± 0.03 a | ND | ND | |
| 58 | β-Copaene | 1592 | 18252-44-3 | ND | 1.58 ± 0.02 a | Tr | ND | |
| 19 | α-Bergamotene | 1593 | 17699-05-7 | ND | Tr | ND | 0.27 ± 0.04 a | |
| 23 | α-Caryophyllene | 1594 | 6753-98-6 | ND | 0.42 ± 0.10 a | 0.40 ± 0.08 a | ND | |
| 59 | 5-Germacratriene | 1598 | 37839-63-7 | ND | ND | 1.52 ± 0.11 a | ND | |
| 21 | (-)-β-Elemene | 1600 | 515-13-9 | 0.90 ± 0.07 b | Tr | 0.8 ± 0.08 b | 4.34 ± 0.47 a | |
| 60 | β-Guaiene; | 1602 | 88-84-6 | 0.38 ± 0.06 b | ND | 0.21 ± 0.05 c | 1.12 ± 0.13 a | |
| 61 | Aromadendrene | 1608 | 489-39-4 | 3.92 ± 0.10 b | 0.71 ± 0.06 d | 2.77 ± 0.43 c | 13.19 ± 1.32 a | |
| 20 | β-Caryophyllene | 1609 | 87-44-5 | 0.86 ± 0.08 b | 1.73 ± 0.11 a | 0.13 ± 0.03 d | 0.48 ± 0.06 c | |
| 26 | β-Longipinene | 1622 | 41432-70-6 | 0.32 ± 0.04 b | 0.11 ± 0.02 c | 0.17 ± 0.05 c | 1.31 ± 0.09 a | |
| 28 | trans-β-Farnesene | 1693 | 18794-84-8 | ND | 0.19 ± 0.03 a | 0.12 ± 0.05 b | ND | |
| 62 | γ-Muurolene | 1698 | 10208-80-7 | 1.40 ± 0.13 a | ND | ND | ND | |
| 29 | β-Selinene | 1716 | 17066-67-0 | ND | ND | ND | 0.16 ± 0.07 a | |
| 63 | β-Bulnesene | 1730 | 3772-93-8 | 0.74 ± 0.05 a | ND | 0.63 ± 0.03 b | ND | |
| 64 | α-Selinene | 1734 | 473-13-2 | ND | Tr | Tr | 0.40 ± 0.03 a | |
| 65 | δ-Guaiene | 1745 | 3691-11-0 | ND | ND | ND | 2.58 ± 0.10 a | |
| 31 | (+)Bicyclogermacrene | 1747 | 24703-35-3 | 0.75 ± 0.08 c | ND | 0.83 ± 0.03 b | 2.17 ± 0.03 a | |
| 66 | (+)-δ-Cadinene | 1768 | 483-76-1 | 0.23 ± 0.05 c | 0.11 ± 0.02 c | 1.46 ± 0.04 b | 4.52 ± 0.47 a | |
| 67 | cis-α-Bisabolene | 1785 | 17627-44-0 | ND | 1.79 ± 0.03 a | ND | ND | |
| 36 | cis-Calamenene | 1838 | 22339-23-7 | ND | ND | 0.40 ± 0.08 a | ND | |
| 68 | Zingiberene | 2039 | 495-60-3 | ND | 0.22 ± 0.04 a | ND | ND | |
| Subtotal | 12.44 | 7.80 | 9.83 | 34.95 | ||||
| Alcohol | 69 | Pogostol | 1822 | 21698-41-9 | ND | ND | ND | 0.33 ± 0.04 a |
| 39 | Eucalyptol | 1212 | 470-82-6 | 3.26 ± 0.09 b | 1.20 ± 0.09 d | 4.25 ± 0.05 a | 2.30 ± 0.52 c | |
| 70 | α-Acorenol | 1534 | 28400-11-5 | ND | 0.09 ± 0.02 a | ND | ND | |
| 42 | Linalool | 1559 | 78-70-6 | 3.33 ± 0.02 c | 0.52 ± 0.07 d | 26.95 ± 0.37 a | 20.08 ± 1.14 b | |
| 71 | Spathulenol | 1580 | 6750-60-3 | ND | ND | 0.09 ± 0.02 a | 0.76 ± 0.08 a | |
| 72 | 4-Thujanol | 1610 | 546-79-2 | ND | ND | 0.60 ± 0.01 a | ND | |
| 73 | α-Terpineol | 1701 | 98-55-5 | ND | Tr | ND | 0.58 ± 0.01 a | |
| 74 | (-)-α-Terpineol | 1705 | 10482-56-1 | 0.21 ± 0.04 c | 0.12 ± 0.16 c | 0.96 ± 0.03 b | 1.12 ± 0.13 a | |
| 46 | Borneol | 1712 | 507-70-0 | 0.10 ± 0.02 a | ND | ND | ND | |
| 47 | τ-Juniper alcohol | 1735 | 5937-11-1 | 1.17 ± 0.03 b | 0.26 ± 0.03 c | 0.37 ± 0.03 c | 18.18 ± 0.80 a | |
| 75 | 13-Heptadecyn-1-ol | 1742 | 56554-77-9 | ND | 0.14 ± 0.03 a | ND | ND | |
| 76 | Terpinen-4-ol | 1755 | 562-74-3 | ND | ND | 0.11 ± 0.02 a | 0.09 ± 0.02 a | |
| 77 | α-Bisabolol | 1786 | 515-69-5 | Tr | ND | ND | 1.58 ± 0.07 a | |
| 78 | Nerol | 1809 | 106-25-2 | ND | Tr | ND | 0.82 ± 0.03 a | |
| 79 | Costol | 1936 | 515-20-8 | ND | ND | ND | 0.46 ± 0.06 a | |
| 80 | (-)-Epiglobulol | 2012 | 88728-58-9 | ND | ND | ND | 0.23 ± 0.04 a | |
| 81 | (-)-Cubenol | 2059 | 21284-22-0 | 0.20 ± 0.03 a | ND | 0.73 ± 0.04 a | ND | |
| 82 | Piperonyl alcohol | 2065 | 495-76-1 | ND | Tr | ND | 0.21 ± 0.06 a | |
| 83 | Cubebol | 2070 | 23445-02-5 | ND | ND | Tr | 0.33 ± 0.02 a | |
| 84 | 9-Decen-1-ol | 2086 | 13019-22-2 | ND | ND | ND | 2.63 ± 0.05 a | |
| 85 | 13-Heptadecyn-1-ol | 2116 | 56554-77-9 | ND | ND | ND | 0.13 ± 0.03 a | |
| 86 | α-Cadinol | 2184 | 481-34-5 | Tr | Tr | 0.32 ± 0.04 b | 4.59 ± 0.06 a | |
| 87 | Ergosterol | 2218 | 57-87-4 | ND | ND | ND | 0.23 ± 0.04 a | |
| 88 | Androstenediol | 2232 | 521-17-5 | ND | ND | ND | 0.22 ± 0.08 a | |
| Subtotal | 8.27 | 2.33 | 34.38 | 54.87 | ||||
| Total | 99.52 | 98.43 | 97.41 | 99.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Liu, J.; Liu, Q.; Jin, Z.; Zhu, H.; Han, H.; Ma, X. Comparative Analysis of Volatile Components and Sensory Profiles of Four Basil Varieties Based on HS-SPME and SD Coupled with GC-MS. Processes 2024, 12, 2789. https://doi.org/10.3390/pr12122789
Jiang R, Liu J, Liu Q, Jin Z, Zhu H, Han H, Ma X. Comparative Analysis of Volatile Components and Sensory Profiles of Four Basil Varieties Based on HS-SPME and SD Coupled with GC-MS. Processes. 2024; 12(12):2789. https://doi.org/10.3390/pr12122789
Chicago/Turabian StyleJiang, Rongyue, Jinzhen Liu, Qingchuan Liu, Zhigang Jin, Huixia Zhu, Huipei Han, and Xiaojing Ma. 2024. "Comparative Analysis of Volatile Components and Sensory Profiles of Four Basil Varieties Based on HS-SPME and SD Coupled with GC-MS" Processes 12, no. 12: 2789. https://doi.org/10.3390/pr12122789
APA StyleJiang, R., Liu, J., Liu, Q., Jin, Z., Zhu, H., Han, H., & Ma, X. (2024). Comparative Analysis of Volatile Components and Sensory Profiles of Four Basil Varieties Based on HS-SPME and SD Coupled with GC-MS. Processes, 12(12), 2789. https://doi.org/10.3390/pr12122789





