Linking Cultivation Conditions to the Fatty Acid Profile and Nutritional Value of Chlorella sorokiniana Lipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Samples of Chlorella sorokiniana
2.2. Analysis of Fatty Acids
2.2.1. Reagents and Solutions
2.2.2. Sample Treatment and Derivatization
2.2.3. Analysis by Gas Chromatography
2.3. Calculation of Nutritional Indices of Chlorella sorokiniana Biomass Samples
2.3.1. Atherogenic Index (AI) and Thrombogenic Index (TI)
2.3.2. Thrombogenic Index (TI)
2.3.3. Hypocholesterolemic/Hypercholesterolemic (H/H) Ratio
2.3.4. Omega-3/Omega-6 Fatty Acid (ω3/ω6) Ratio
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition of C. sorokiniana Biomass
3.2. Effect of Cultivation Factors on FA Composition
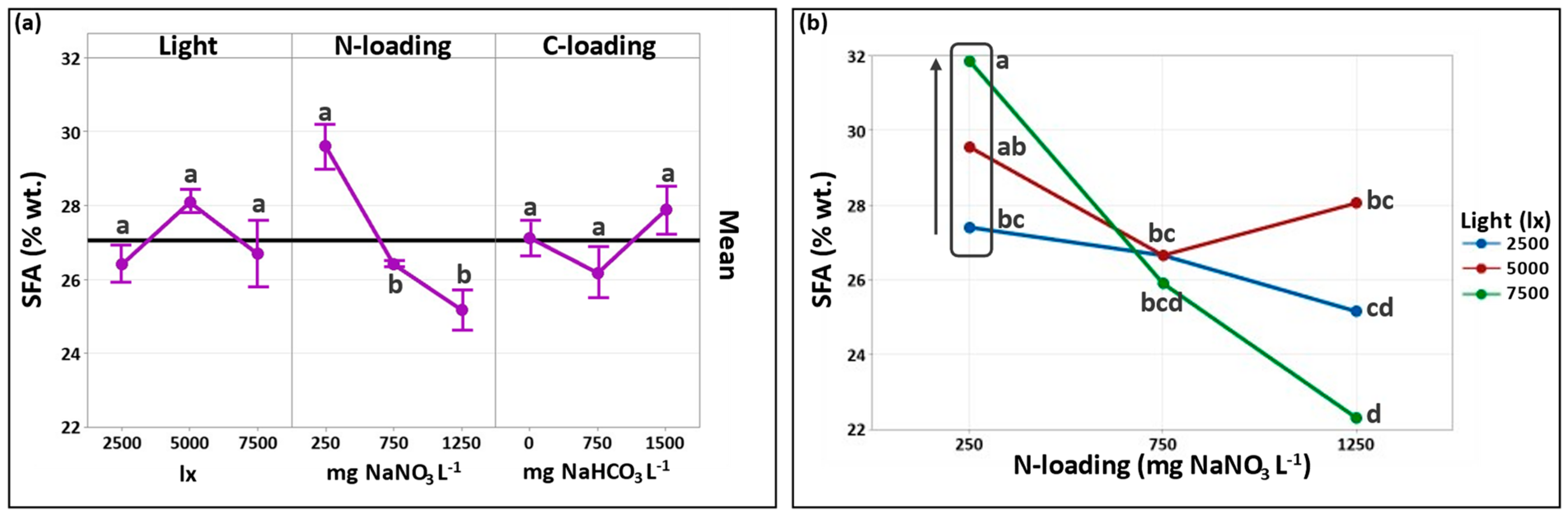
3.2.1. Effect of Cultivation Conditions on SFA Formation
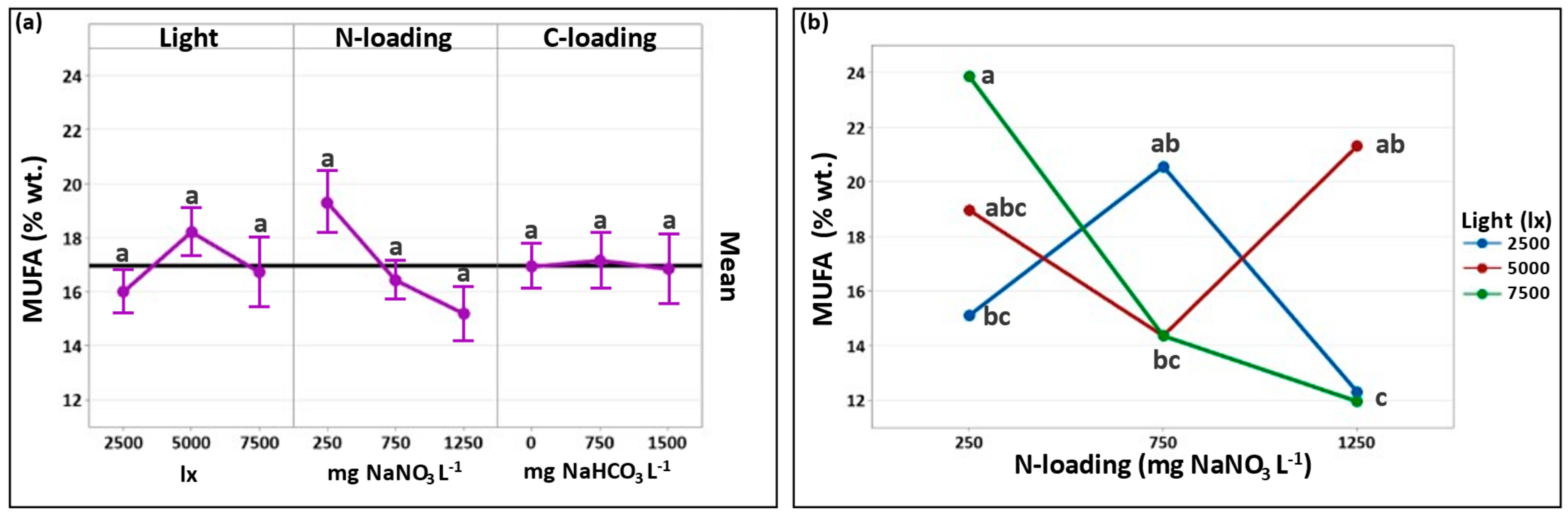
3.2.2. Effect of Cultivation Conditions on MUFA Formation
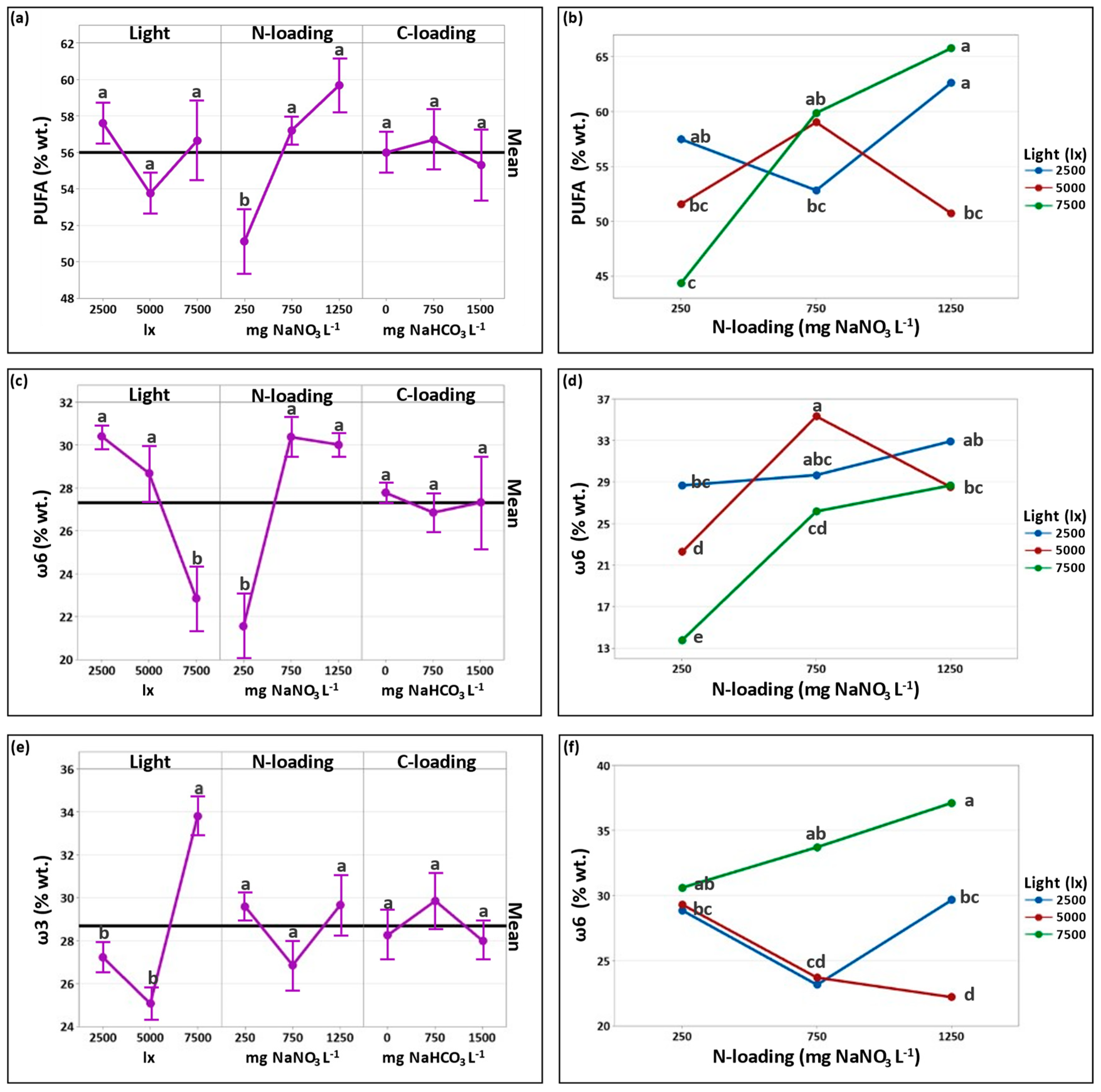
3.2.3. Effect of Cultivation Conditions on PUFA Formation
3.3. Effect of Cultivation Factors on the Nutritional Indices of C. sorokiniana Lipids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perdana, B.A.; Chaidir, Z.; Kusnanda, A.J.; Dharma, A.; Zakaria, I.J.; Syafrizayanti; Bayu, A.; Putra, M.Y. Omega-3 Fatty Acids of Microalgae as a Food Supplement: A Review of Exogenous Factors for Production Enhancement. Algal Res. 2021, 60, 102542. [Google Scholar] [CrossRef]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative Sources of N-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef] [PubMed]
- Guesnet, P.; Alessandri, J.-M. Docosahexaenoic Acid (DHA) and the Developing Central Nervous System (CNS)—Implications for Dietary Recommendations. Biochimie 2011, 93, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. Alpha-Linolenic Acid: An Omega-3 Fatty Acid with Neuroprotective Properties—Ready for Use in the Stroke Clinic? BioMed Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Ferrara, P.; Corsello, G.; Quattrocchi, E.; Dell’Aquila, L.; Ehrich, J.; Giardino, I.; Pettoello-Mantovani, M. Caring for Infants and Children Following Alternative Dietary Patterns. J. Pediatr. 2017, 187, 339–340.e1. [Google Scholar] [CrossRef]
- Amjad Khan, W.; Chun-Mei, H.; Khan, N.; Iqbal, A.; Lyu, S.-W.; Shah, F. Bioengineered Plants Can Be a Useful Source of Omega-3 Fatty Acids. BioMed Res. Int. 2017, 2017, e7348919. [Google Scholar] [CrossRef]
- Sehl, A.; Caderby, E.; Bouhouda, S.; Rébeillé, F.; Griffiths, H.; Gomes, S.D.R. How Do Algae Oils Change the Omega-3 Polyunsaturated Fatty Acids Market? OCL 2022, 29, 20. [Google Scholar] [CrossRef]
- Papapanagiotou, G.; Gkelis, S. Taxonomic Revision of Commercially Used Arthrospira (Cyanobacteria) Strains: A Polyphasic Approach. Eur. J. Phycol. 2019, 54, 595–608. [Google Scholar] [CrossRef]
- Petkov, G.; Garcia, G. Which Are Fatty Acids of the Green Alga Chlorella? Biochem. Syst. Ecol. 2007, 35, 281–285. [Google Scholar] [CrossRef]
- Freitas, H.R. Chlorella vulgaris as a Source of Essential Fatty Acids and Micronutrients: A Brief Commentary. Open Plant Sci. J. 2017, 10, 92–99. [Google Scholar] [CrossRef][Green Version]
- Takic, M.; Pokimica, B.; Petrovic-Oggiano, G.; Popovic, T. Effects of Dietary α-Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases. Molecules 2022, 27, 4471. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of Microalgae with High Lipid Content and Their Potential as Sources of Nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty Acids of Microalgae: Diversity and Applications. Rev. Environ. Sci. Biotechnol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the Physiology of Chlorella vulgaris Cultivated in Sweet Sorghum Bagasse Hydrolysate for Sustainable Algal Biomass and Lipid Production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty Acid Profile of Microalgal Oils as a Criterion for Selection of the Best Feedstock for Biodiesel Production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Mastropetros, S.G.; Tsigkou, K.; Cladas, Y.; Priya, A.K.; Kornaros, M. Effect of Nitrogen, Salinity, and Light Intensity on the Biomass Composition of Nephroselmis sp.: Optimization of Lipids Accumulation (Including EPA). Mar. Drugs 2023, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Hu, C. Effects of Temperature and Its Combination with High Light Intensity on Lipid Production of Monoraphidium dybowskii Y2 from Semi-Arid Desert Areas. Bioresour. Technol. 2018, 265, 407–414. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Pagarette, A.; Rooks, P.; Ali, S.T. The Effect of Sodium Bicarbonate Supplementation on Growth and Biochemical Composition of Marine Microalgae Cultures. J. Appl. Phycol. 2013, 25, 153–165. [Google Scholar] [CrossRef]
- Ghosh, A.; Samadhiya, K.; Kashyap, M.; Anand, V.; Sangwan, P.; Bala, K. The Use of Response Surface Methodology for Improving Fatty Acid Methyl Ester Profile of Scenedesmus vacuolatus. Environ. Sci. Pollut. Res. 2020, 27, 27457–27469. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- González-Félix, M.L.; Maldonado-Othón, C.A.; Perez-Velazquez, M. Effect of Dietary Lipid Level and Replacement of Fish Oil by Soybean Oil in Compound Feeds for the Shortfin Corvina (Cynoscion parvipinnis). Aquaculture 2016, 454, 217–228. [Google Scholar] [CrossRef]
- Monteiro, M.; Matos, E.; Ramos, R.; Campos, I.; Valente, L.M.P. A Blend of Land Animal Fats Can Replace up to 75% Fish Oil without Affecting Growth and Nutrient Utilization of European Seabass. Aquaculture 2018, 487, 22–31. [Google Scholar] [CrossRef]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of Dietary Incorporation of Linseed Alone or Together with Tomato-Red Pepper Mix on Laying Hens’ Egg Yolk Fatty Acids Profile and Health Lipid Indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty Acid Profile of Milk from Saanen and Swedish Landrace Goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-Promoting Ingredients from Four Selected Azorean Macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef]
- Duarte, B.; Goessling, J.W.; Fonseca, V.F.; Jacobsen, S.-E.; Matos, A.R. Quinoa Variety Identification Based on Fatty Acid Composition and Multivariate Chemometrics Approaches. J. Food Compos. Anal. 2022, 114, 104798. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [CrossRef]
- Papapanagiotou, G.; Samara, C.; Chatzidoukas, C. Managing the Interactions of Illumination, Nitrogen, and Sodium Bicarbonate for Autotrophic Biomass Production and Macromolecules Accumulation in the Microalga Chlorella sorokiniana. Algal Res. 2024, 80, 103550. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; García, C. Development in Lipid Analysis: Some New Extraction Techniques and in Situ Transesterification. Lipids 2000, 35, 1167–1177. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Carlsson, N.-G.; Undeland, I. Quantification of Total Fatty Acids in Microalgae: Comparison of Extraction and Transesterification Methods. Anal. Bioanal. Chem. 2014, 406, 7313–7322. [Google Scholar] [CrossRef]
- Georgiou, D.; Exarhopoulos, S.; Charisis, A.; Simitsis, S.; Papapanagiotou, G.; Samara, C.; Katsiapi, M.; Kountrias, G.; Bouras, S.; Katsoulas, N.; et al. Valorization of Monoraphidium sp. Microalgal Biomass for Human Nutrition Applications. J. Appl. Phycol. 2024, 36, 1293–1309. [Google Scholar] [CrossRef]
- Agilent Technologies Inc. Comprehensive Analysis of FAMEs, Fatty Acids, and Triglycerides. Available online: https://www.agilent.com/cs/library/brochures/5991-8763EN_gc_fame_brochure.pdf (accessed on 30 October 2024).
- Ulbricht, T.L.; Southgate, D.A. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Mierliță, D. Effects of Diets Containing Hemp Seeds or Hemp Cake on Fatty Acid Composition and Oxidative Stability of Sheep Milk. S. Afr. J. Anim. Sci. 2018, 48, 504–515. [Google Scholar] [CrossRef]
- Mukhametov, A.; Yerbulekova, M.; Aitkhozhayeva, G.; Tuyakova, G.; Dautkanova, D. Effects of ω-3 Fatty Acids and Ratio of ω-3/ω-6 for Health Promotion and Disease Prevention. Food Sci. Technol 2022, 42, 58321. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, O.H.; Jo, S.-W.; Kim, H.; Jeong, M.R.; Park, K.M.; Lee, K.I.; Yoon, H.-S. Biochemical Composition of a Korean Domestic Microalga Chlorella vulgaris KNUA027. Microbiol. Biotechnol. Lett. 2016, 44, 400–407. [Google Scholar] [CrossRef]
- Kumaran, M.; Palanisamy, K.M.; Bhuyar, P.; Maniam, G.P.; Rahim, M.H.A.; Govindan, N. Agriculture of Microalgae Chlorella vulgaris for Polyunsaturated Fatty Acids (PUFAs) Production Employing Palm Oil Mill Effluents (POME) for Future Food, Wastewater, and Energy Nexus. Energy Nexus 2023, 9, 100169. [Google Scholar] [CrossRef]
- Moradi-Kheibari, N.; Ahmadzadeh, H.; Lyon, S.R. Correlation of Total Lipid Content of Chlorella vulgaris With the Dynamics of Individual Fatty Acid Growth Rates. Front. Mar. Sci. 2022, 9, 837067. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on Cell Growth, Lipid Production and CO2 Addition of Microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, Y.-S.; Yoon, H.-S. Characterization of Chlorella sorokiniana and Chlorella vulgaris Fatty Acid Components under a Wide Range of Light Intensity and Growth Temperature for Their Use as Biological Resources. Heliyon 2020, 6, e04447. [Google Scholar] [CrossRef]
- Yusof, Y.A.M.; Basari, J.M.H.; Mukti, N.A.; Sabuddin, R.; Muda, A.R.; Sulaiman, S.; Makpol, S.; Ngah, W.Z.W. Fatty Acids Composition of Microalgae Chlorella vulgaris Can Be Modulated by Varying Carbon Dioxide Concentration in Outdoor Culture. Afr. J. Biotechnol. 2011, 10, 13536–13542. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, B.; She, X.; Zhao, F.; Cao, Y.; Ren, D.; Lu, J. Lipid Production and Composition of Fatty Acids in Chlorella vulgaris Cultured Using Different Methods: Photoautotrophic, Heterotrophic, and Pure and Mixed Conditions. Ann. Microbiol. 2014, 64, 1239–1246. [Google Scholar] [CrossRef]
- Bertoldi, F.C.; Sant’Anna, E.; Braga, M.V.D.C.; Oliveira, J.L.B. Lipids, Fatty Acids Composition and Carotenoids of Chlorella vulgaris Cultivated in Hydroponic Wastewater. Grasas Aceites 2006, 57, 270–274. [Google Scholar] [CrossRef]
- Hajjar Rakhmadumila, D.; Setiani Muntalif, B. Artificial Produced Water as a Medium to Grow Chlorella sp. for Biodiesel Production. E3S Web Conf. 2020, 148, 02005. [Google Scholar] [CrossRef]
- Jay, M.I.; Kawaroe, M.; Effendi, H. Lipid and Fatty Acid Composition Microalgae Chlorella vulgaris Using Photobioreactor and Open Pond. IOP Conf. Ser. Earth Environ. Sci. 2018, 141, 012015. [Google Scholar] [CrossRef]
- Liu, C.-P.; Lin, L. Ultrastructural Study and Lipid Formation of Isochrysis sp. CCMP1324. Bot. Bull. Acad. Sin. 2001, 42, 207–214. [Google Scholar]
- Wacker, A.; Becher, P.; von Elert, E. Food Quality Effects of Unsaturated Fatty Acids on Larvae of the Zebra Mussel Dreissena polymorpha. Limnol. Oceanogr. 2002, 47, 1242–1248. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of Light Intensity and Photoperiod on Biomass and Fatty Acid Composition of the Microalgae, Chlorella vulgaris. Aquacult. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Jane Chizie, O.; Nkechinyere Onyekwere, N.; Christiana Nwakego, O. Effects of Light on Cell Growth, Chlorophyll, and Carotenoid Contents of Chlorella sorokiniana and Ankistrodesmus falcatus in Poultry Dropping Medium. J. Appl. Biol. Biotech. 2021, 9, 157–163. [Google Scholar] [CrossRef]
- Rosa, A.; Deidda, D.; Serra, A.; Deiana, M.; Dessi, M.A.; Pompei, R. Omega-3 Fatty Acid Composition and Biological Activity of Three Microalgae Species. Int. J. Food Agric. Environ. 2005, 3, 120. [Google Scholar]
- Stirk, W. Lipid Productivity and Fatty Acid Composition in Chlorella and Scenedesmus Strains Grown in Nitrogen-Stressed Conditions. J. Appl. Phycol. 2013, 25, 233–243. [Google Scholar]
- Negi, S.; Barry, A.N.; Friedland, N.; Sudasinghe, N.; Subramanian, S.; Pieris, S.; Holguin, F.O.; Dungan, B.; Schaub, T.; Sayre, R. Impact of Nitrogen Limitation on Biomass, Photosynthesis, and Lipid Accumulation in Chlorella sorokiniana. J. Appl. Phycol. 2016, 28, 803–812. [Google Scholar] [CrossRef]
- Viso, A.-C.; Marty, J.-C. Fatty Acids from 28 Marine Microalgae. Phytochemistry 1993, 34, 1521–1533. [Google Scholar] [CrossRef]
- Wright, D.C.; Berg, L.R.; Patterson, G.W. Effect of Cultural Conditions on the Sterols and Fatty Acids of Green Algae. Phytochemistry 1980, 19, 783–785. [Google Scholar] [CrossRef]
- Chia, M.A.; Lombardi, A.T.; Melão, M.D.G.G.; Parrish, C.C. Effects of Cadmium and Nitrogen on Lipid Composition of Chlorella vulgaris (Trebouxiophyceae, Chlorophyta). Eur. J. Phycol. 2013, 48, 1–11. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and Morphological Changes Triggered by Nitrogen Stress in the Oleaginous Microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Y.; Shang, C.; Wang, Z.; Xu, J.; Yuan, Z. Characterization of Lipid and Fatty Acids Composition of Chlorella zofingiensis in Response to Nitrogen Starvation. J. Biosci. Bioeng. 2015, 120, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Miao, X. Lipid Turnover and SQUAMOSA Promoter-Binding Proteins Mediate Variation in Fatty Acid Desaturation under Early Nitrogen Deprivation Revealed by Lipidomic and Transcriptomic Analyses in Chlorella pyrenoidosa. Front. Plant Sci. 2022, 13, 987354. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.D.F.R.; Giraldi, L.A.; Winck, F.V. From Feasting to Fasting: The Arginine Pathway as a Metabolic Switch in Nitrogen-Deprived Chlamydomonas Reinhardtii. Cells 2023, 12, 1379. [Google Scholar] [CrossRef]
- Wei, L.; You, W.; Xu, Z.; Zhang, W. Transcriptomic Survey Reveals Multiple Adaptation Mechanisms in Response to Nitrogen Deprivation in Marine Porphyridium cruentum. PLoS ONE 2021, 16, e0259833. [Google Scholar] [CrossRef]
- Cohen, Z.; Khozin-Goldberg, I. 10—Searching for Polyunsaturated Fatty Acid-Rich Photosynthetic Microalgae. In Single Cell Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2010; pp. 201–224. ISBN 978-1-893997-73-8. [Google Scholar]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of Light Intensity on Growth and Lipid Production in Microalgae Grown in Wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of Light Intensity on Physiological Changes, Carbon Allocation and Neutral Lipid Accumulation in Oleaginous Microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Frumento, D.; Casazza, A.A.; Al Arni, S.; Converti, A. Cultivation of Chlorella vulgaris in Tubular Photobioreactors: A Lipid Source for Biodiesel Production. Biochem. Eng. J. 2013, 81, 120–125. [Google Scholar] [CrossRef]
- Najafabadi, A.H.; Malekzadeh, M.; Jalilian, F.; Vossoughi, M.; Pazuki, G. Effect of Various Carbon Sources on Biomass and Lipid Production of Chlorella vulgaris during Nutrient Sufficient and Nitrogen Starvation Conditions. Bioresour. Technol. 2015, 180, 311–317. [Google Scholar] [CrossRef]
- Nayak, M.; Suh, W.I.; Lee, B.; Chang, Y.K. Enhanced Carbon Utilization Efficiency and FAME Production of Chlorella sp. HS2 through Combined Supplementation of Bicarbonate and Carbon Dioxide. Energy Convers. Manag. 2018, 156, 45–52. [Google Scholar] [CrossRef]
- Wu, T.; Yu, L.; Zhang, Y.; Liu, J. Characterization of Fatty Acid Desaturases Reveals Stress-Induced Synthesis of C18 Unsaturated Fatty Acids Enriched in Triacylglycerol in the Oleaginous Alga Chromochloris zofingiensis. Biotechnol. Biofuels 2021, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, Fatty Acid, and Pigment Content of Chlorella vulgaris under Different Light Regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Krzemińska, I.; Piasecka, A.; Nosalewicz, A.; Simionato, D.; Wawrzykowski, J. Alterations of the Lipid Content and Fatty Acid Profile of Chlorella protothecoides under Different Light Intensities. Bioresour. Technol. 2015, 196, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Belkoura, M.; Benider, A.; El Antari, A.; Dauta, A. Effect of Environmental Conditions on the Fatty Acid Composition of the Green Alga Chlorella sorokiniana SHIHIRA et KRAUSS. Algol. Stud./Arch. Hydrobiol. Suppl. 2000; 97, 93–101. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of Temperature and Nitrogen Concentration on the Growth and Lipid Content of Nannochloropsis oculata and Chlorella vulgaris for Biodiesel Production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S. Nitrogen Starvation Strategies and Photobioreactor Design for Enhancing Lipid Content and Lipid Production of a Newly Isolated Microalga Chlorella vulgaris ESP-31: Implications for Biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef]
- Breuer, G.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. The Impact of Nitrogen Starvation on the Dynamics of Triacylglycerol Accumulation in Nine Microalgae Strains. Bioresour. Technol. 2012, 124, 217–226. [Google Scholar] [CrossRef]
- Klyachko-Gurvich, G.L.; Tsoglin, L.N.; Doucha, J.; Kopetskii, J.; Shebalina, I.B.; Semenenko, V.E. Desaturation of Fatty Acids as an Adaptive Response to Shifts in Light Intensity 1. Physiol. Plant. 1999, 107, 240–249. [Google Scholar] [CrossRef]
- Rueda, A.; Seiquer, I.; Olalla, M.; Giménez, R.; Lara, L.; Cabrera-Vique, C. Characterization of Fatty Acid Profile of Argan Oil and Other Edible Vegetable Oils by Gas Chromatography and Discriminant Analysis. J. Chem. 2014, 2014, 843908. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Yu-Poth, S.; Sabaté, J.; Ratcliffe, H.E.; Zhao, G.; Etherton, T.D. Nuts and Their Bioactive Constituents: Effects on Serum Lipids and Other Factors That Affect Disease Risk. Am. J. Clin. Nutr. 1999, 70, 504S–511S. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Da Silva, R.C.; Gioielli, L.A.; de Almeida Gonçalves, M.I.; Grimaldi, R.; Gonçalves, L.A.G.; Kieckbusch, T.G. Physico-Chemical Properties of Brazilian Cocoa Butter and Industrial Blends. Part I Chemical Composition, Solid Fat Content and Consistency. Grasas Aceites 2012, 63, 79–88. [Google Scholar] [CrossRef]


| Cultivation Factors | C. sorokiniana Biomass Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | |
| Light intensity (lx) | 2500 | 2500 | 2500 | 5000 | 5000 | 5000 | 7500 | 7500 | 7500 |
| (L) | (L) | (L) | (M) | (M) | (M) | (H) | (H) | (H) | |
| N-loading (mg NaNO3 L−1) | 250 | 750 | 1250 | 250 | 750 | 1250 | 250 | 750 | 1250 |
| (L) | (M) | (H) | (L) | (M) | (H) | (L) | (M) | (H) | |
| C-loading (mg NaHCO3 L−1) | 0 | 750 | 1500 | 750 | 1500 | 0 | 1500 | 0 | 750 |
| (L) | (M) | (H) | (M) | (H) | (L) | (H) | (L) | (M) | |
| Fatty Acid | C. sorokiniana Biomass Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | |
| Palmitic acid—C16:0 | 26.0 ± 2.4 | 26.7 ± 0.3 | 24.8 ± 0.4 | 29.6 ± 0.8 | 26.1 ± 0.4 | 27.9 ± 0.0 | 30.5 ± 0.3 | 25.9 ± 0.2 | 22.3 ± 0.3 |
| Palmitoleic acid—C16:1 cis 9 | 1.2 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.4 | 1.3 ± 0.2 | 1.0 ± 0.2 |
| Stearic acid—C18:0 | 1.5 ± 1.0 | n.d. | 0.4 ± 0.2 | n.d. | 0.6 ± 0.0 | 0.2 ± 0.2 | 1.4 ± 0.1 | n.d. | n.d. |
| Oleic acid—C18:1 cis 9 | 12.2 ± 1.1 | 19.3 ± 0.9 | 10.0 ± 0.5 | 17.3 ± 4.2 | 12 ± 0.8 | 19.7 ± 4.0 | 21.6 ± 5.9 | 13.1 ± 2.1 | 10.0 ± 0.4 |
| Elaidic acid— C18:1 trans 9 | 1.7 ± 0.3 | 0.5 ± 0.5 | 1.5 ± 0.1 | 0.9 ± 0.3 | 1.6 ± 0.0 | 0.7 ± 0.7 | 1.5 ± 0.1 | n.d. | 1.0 ± 0.1 |
| Linoleic acid—C18:2 cis 9,12 | 28.7 ± 3.0 | 29.7 ± 0.0 | 32.9 ± 0.3 | 22.3 ± 3.9 | 35.3 ± 1.8 | 28.5 ± 0.8 | 13.8 ± 0.9 | 26.2 ± 0.8 | 28.7 ± 2.0 |
| α- Linolenic acid—C18:3 cis 9,12,15 | 28.9 ± 1.8 | 23.2 ± 0.0 | 29.7 ± 0.1 | 29.3 ± 1.5 | 23.7 ± 1.4 | 22.2 ± 2.0 | 30.6 ± 6.1 | 33.7 ± 2.9 | 37.1 ± 1.7 |
| Total SFAs | 27.4 ± 3.3 | 26.7 ± 0.3 | 25.2 ± 0.5 | 29.6 ± 0.8 | 26.7 ± 0.5 | 28.1 ± 0.2 | 31.9 ± 0.5 | 25.9 ± 0.2 | 22.3 ± 0.3 |
| Total MUFAs | 15.1 ± 1.3 | 20.6 + 0.4 | 12.3 ± 0.6 | 19.0 ± 4.5 | 14.4 ± 0.9 | 21.3 ± 3.0 | 23.9 ± 6.4 | 14.4 ± 2.0 | 12.0 ± 0.6 |
| Total PUFAs | 57.5 ± 4.7 | 52.8 ± 0.1 | 62.6 ± 0.2 | 51.6 ± 5.4 | 59.0 ± 0.4 | 50.7 ± 2.8 | 44.4 ± 7.0 | 59.9 ± 2.2 | 65.8 ± 0.3 |
| ω-3 | 28.9 ± 1.8 | 23.2 ± 0.0 | 29.7 ± 0.1 | 29.3 ± 1.5 | 23.7 ± 1.4 | 22.2 ± 2.0 | 30.6 ± 6.1 | 33.7 ± 2.9 | 37.1 ± 1.7 |
| ω-6 | 28.7 ± 3.0 | 29.7 ± 0.0 | 32.9 ± 0.3 | 22.3 ± 3.9 | 35.3 ± 1.8 | 28.5 ± 0.8 | 13.8 ± 0.9 | 26.2 ± 0.8 | 28.7 ± 2.0 |
| Factor | DF | Adjusted Sum of Squares and p-Values | |||||
|---|---|---|---|---|---|---|---|
| SFA | UFA | MUFA | PUFA | ω-3 | ω-6 | ||
| Light intensity | 2 | 9.748 | 9.568 | 15.303 | 48.591 | 248.47 | 187.954 |
| p-value for light intensity | (0.436) | (0.443) | (0.779) | (0.650) | (0.009) | (0.029) | |
| Factor contribution | 6.89% | 6.76% | 3.84% | 5.50% | 52.30% | 26.94% | |
| N-source loading | 2 | 62.831 | 63.341 | 53.563 | 233.364 | 30.630 | 299.081 |
| p-value for N-source loading | (0.019) | (0.019) | (0.436) | (0.163) | (0.428) | (0.007) | |
| Factor contribution | 44.47% | 44.76% | 13.44% | 26.39% | 6.45% | 42.92% | |
| C-source loading | 2 | 8.874 | 8.698 | 0.323 | 5.741 | 12.230 | 2.521 |
| p-value for C-source loading | (0.467) | (0.474) | (0.995) | (0.949) | (0.702) | (0.936) | |
| Factor Contribution | 6.28% | 6.15% | 0.01% | 0.65% | 2.57% | 0.36% | |
| Error | 11 | 59.831 | 59.909 | 329.150 | 596.393 | 183.78 | 207.241 |
| Total | 17 | 141.284 | 141.516 | 398.340 | 884.089 | 475.11 | 696.798 |
| R-sq | 57.65% | 57.67% | 17.37% | 32.54% | 61.32% | 70.26% | |
| Factor | Adjusted Sum of Squares and p-Values | ||||||
|---|---|---|---|---|---|---|---|
| DF | SFA | UFA | MUFA | PUFA | ω-3 | ω-6 | |
| Light intensity | 2 | 9.748 | 9.568 | 15.303 | 48.591 | 248.47 | 187.95 |
| p-value for light intensity | (0.225) | (0.242) | (0.540) | (0.317) | (0.002) | (0.002) | |
| Factor contribution | 6.89% | 6.76% | 3.84% | 5.50% | 52.30% | 26.94% | |
| N-source loading | 2 | 62.831 | 63.341 | 53.56 | 233.36 | 30.63 | 299.08 |
| p-value for N-source loading | (0.003) | (0.004) | (0.155) | (0.020) | (0.226) | (0.000) | |
| Factor contribution | 44.47% | 44.76 | 13.44% | 26.39% | 6.45% | 42.92% | |
| First order interaction | 4 | 43.876 | 42.832 | 225.24 | 434.85 | 117.92 | 145.75 |
| p-value for first order interaction | (0.040) | (0.047) | (0.023) | (0.013) | (0.059) | (0.020) | |
| Factor contribution | 31.06% | 30.27% | 56.54% | 49.19% | 24.82% | 20.92% | |
| Error | 9 | 24.830 | 25.775 | 104.23 | 167.28 | 78.09 | 64.01 |
| Total | 17 | 141.284 | 141.516 | 398.34 | 884.09 | 475.11 | 696.80 |
| R-sq | 82.43% | 81.79% | 73.83% | 81.08% | 83.56% | 90.81% | |
| C. sorokiniana Biomass Sample | Nutritional Index | |||
|---|---|---|---|---|
| AI | TI | H/H Ratio | ω3/ω6 Ratio | |
| A | 0.36 ± 0.05 | 0.25 ± 0.04 | 2.71 ± 0.39 | 1.01 ± 0.04 |
| B | 0.36 ± 0.01 | 0.28 ± 0.0 | 2.70 ± 0.07 | 0.78 ± 0.00 |
| C | 0.33 ± 0.01 | 0.22 ± 0.01 | 2.93 ± 0.06 | 0.90 ± 0.01 |
| D | 0.42 ± 0.02 | 0.27 ± 0.02 | 2.33 ± 0.11 | 1.35 ± 0.17 |
| E | 0.36 ± 0.01 | 0.28 ± 0.02 | 2.73 ± 0.06 | 0.68 ± 0.07 |
| F | 0.39 ± 0.00 | 0.30 ± 0.01 | 2.52 ± 0.03 | 0.78 ± 0.03 |
| G | 0.45 ± 0.01 | 0.29 ± 0.03 | 2.17 ± 0.04 | 2.22 ± 0.21 |
| H | 0.35 ± 0.00 | 0.21 ± 0.01 | 2.82 ± 0.02 | 1.29 ± 0.15 |
| I | 0.29 ± 0.01 | 0.17 ± 0.01 | 3.4 ± 0.05 | 1.31 ± 0.15 |
| Factor | DF | Adjusted Sum of Squares and p-Values | |||
|---|---|---|---|---|---|
| AΙ | ΤΙ | H/H | ω3/ω6 | ||
| Light intensity | 2 | 0.004150 | 0.011402 | 0.2727 | 1.9033 |
| p-value for light intensity | (0.095) | (0.018) | (0.078) | (0.000) | |
| Factor contribution | 10.00% | 30.10% | 11.51% | 49.51% | |
| N-source loading | 2 | 0.017393 | 0.004350 | 0.9161 | 1.3117 |
| p-value for N-source loading | (0.002) | (0.138) | (0.003) | (0.000) | |
| Factor contribution | 41.93% | 11.49% | 38.67% | 34.12% | |
| First order interaction | 4 | 0.013914 | 0.014234 | 0.8230 | 0.3831 |
| p-value for first order interaction | (0.019) | (0.038) | (0.019) | (0.055) | |
| Factor contribution | 33.55% | 37.59% | 34.74% | 9.97% | |
| Error | 9 | 0.006022 | 104.23 | 167.28 | 78.09 |
| Total | 17 | 0.041479 | 0.037872 | 2.3689 | 3.8442 |
| R-sq | 85.48% | 79.18% | 84.92% | 93.60% | |
| Lipid Source | AI | TI | H/H | ω3/ω6 | Reference |
|---|---|---|---|---|---|
| C. sorokiniana | 0.36 | 0.25 | 2.71 | 1.14 | Present study |
| Sunflower oil | 0.06 | 0.18 | 18.63 | 0.01 | [6,81] |
| Soybean oil | 0.11 | 0.21 | 9.28 | 0.15 | [6,81] |
| Sesame oil | 0.11 | 0.34 | 8.77 | 0.01 | [6,81] |
| Olive oil | 0.16 | 0.39 | 6.01 | 0.14 | [6,81] |
| Peanut butter | 0.17 | 0.45 | 6.42 | 0.01 | [6,82] |
| Cocoa butter | 0.67 | 3.09 | 1.50 | 0.02 | [6,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapanagiotou, G.; Charisis, A.; Samara, C.; Kalogianni, E.P.; Chatzidoukas, C. Linking Cultivation Conditions to the Fatty Acid Profile and Nutritional Value of Chlorella sorokiniana Lipids. Processes 2024, 12, 2770. https://doi.org/10.3390/pr12122770
Papapanagiotou G, Charisis A, Samara C, Kalogianni EP, Chatzidoukas C. Linking Cultivation Conditions to the Fatty Acid Profile and Nutritional Value of Chlorella sorokiniana Lipids. Processes. 2024; 12(12):2770. https://doi.org/10.3390/pr12122770
Chicago/Turabian StylePapapanagiotou, Georgia, Aggelos Charisis, Christina Samara, Eleni P. Kalogianni, and Christos Chatzidoukas. 2024. "Linking Cultivation Conditions to the Fatty Acid Profile and Nutritional Value of Chlorella sorokiniana Lipids" Processes 12, no. 12: 2770. https://doi.org/10.3390/pr12122770
APA StylePapapanagiotou, G., Charisis, A., Samara, C., Kalogianni, E. P., & Chatzidoukas, C. (2024). Linking Cultivation Conditions to the Fatty Acid Profile and Nutritional Value of Chlorella sorokiniana Lipids. Processes, 12(12), 2770. https://doi.org/10.3390/pr12122770










