Efficient Photocatalytic Degradation of Methylene Blue and Methyl Orange Using Calcium-Polyoxometalate Under Ultraviolet Irradiation
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of H60N6Na2Ca2W12O60 Cluster
2.3. Material Characterization
2.4. Photocatalytic Degradation
3. Results and Discussions
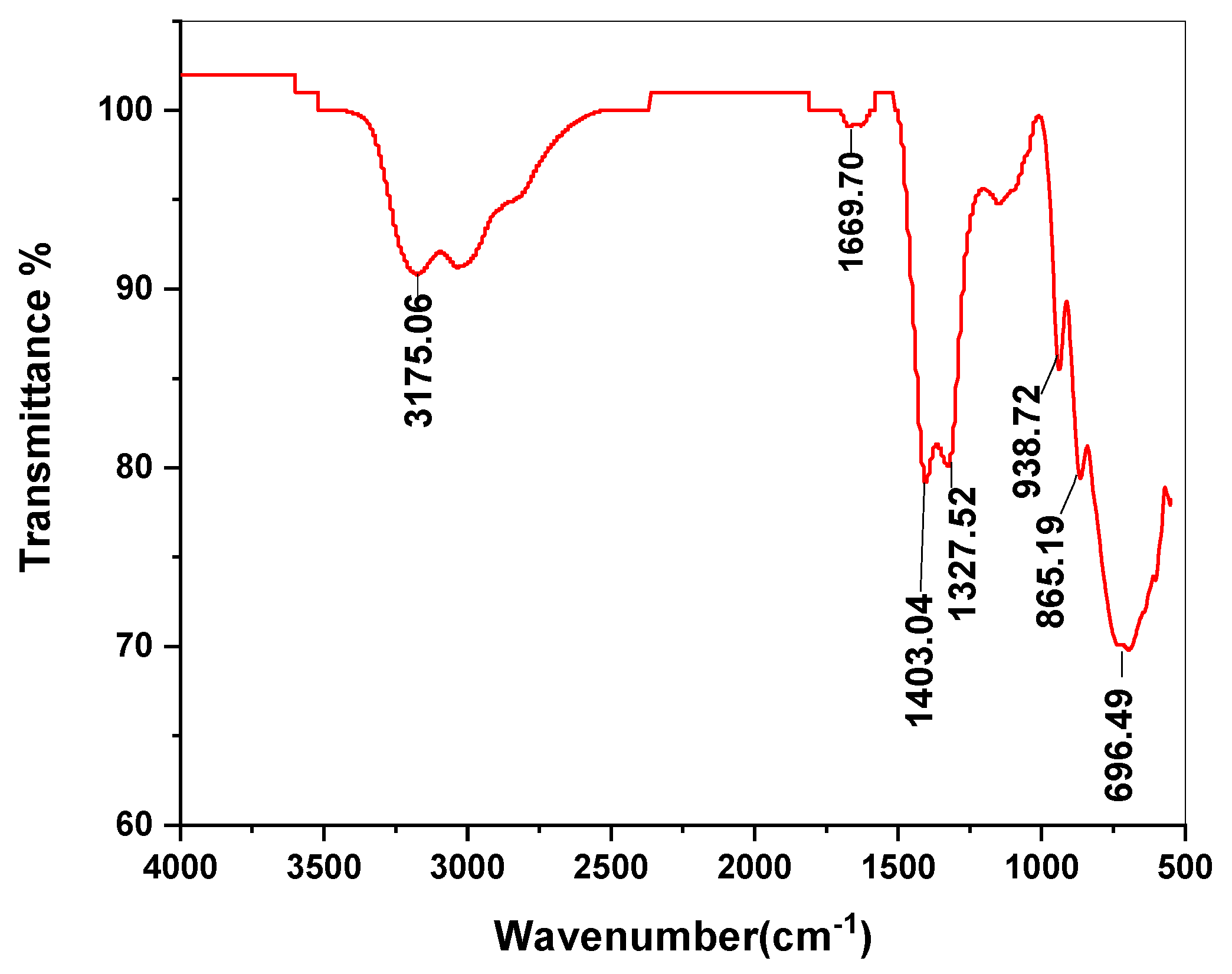
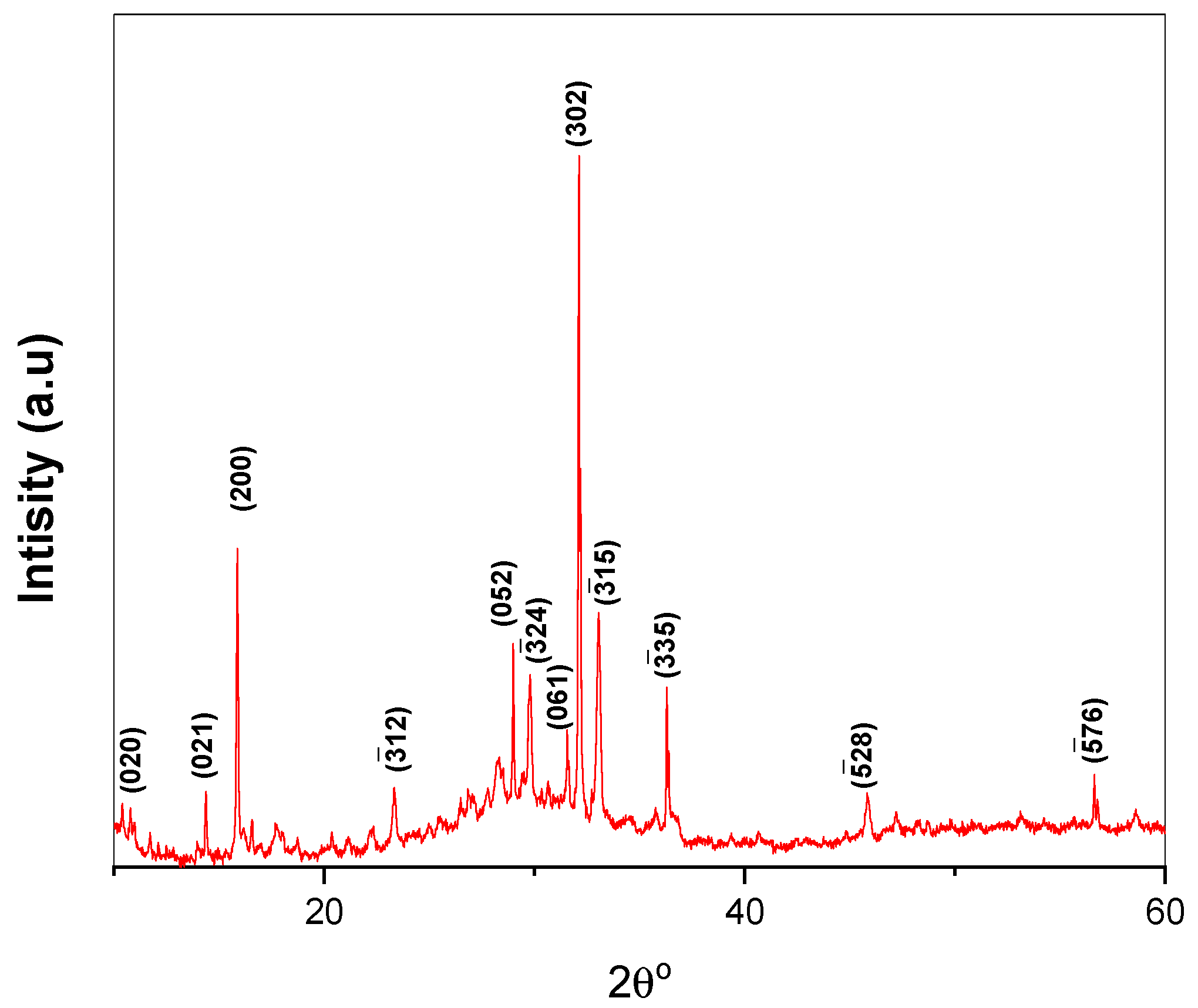
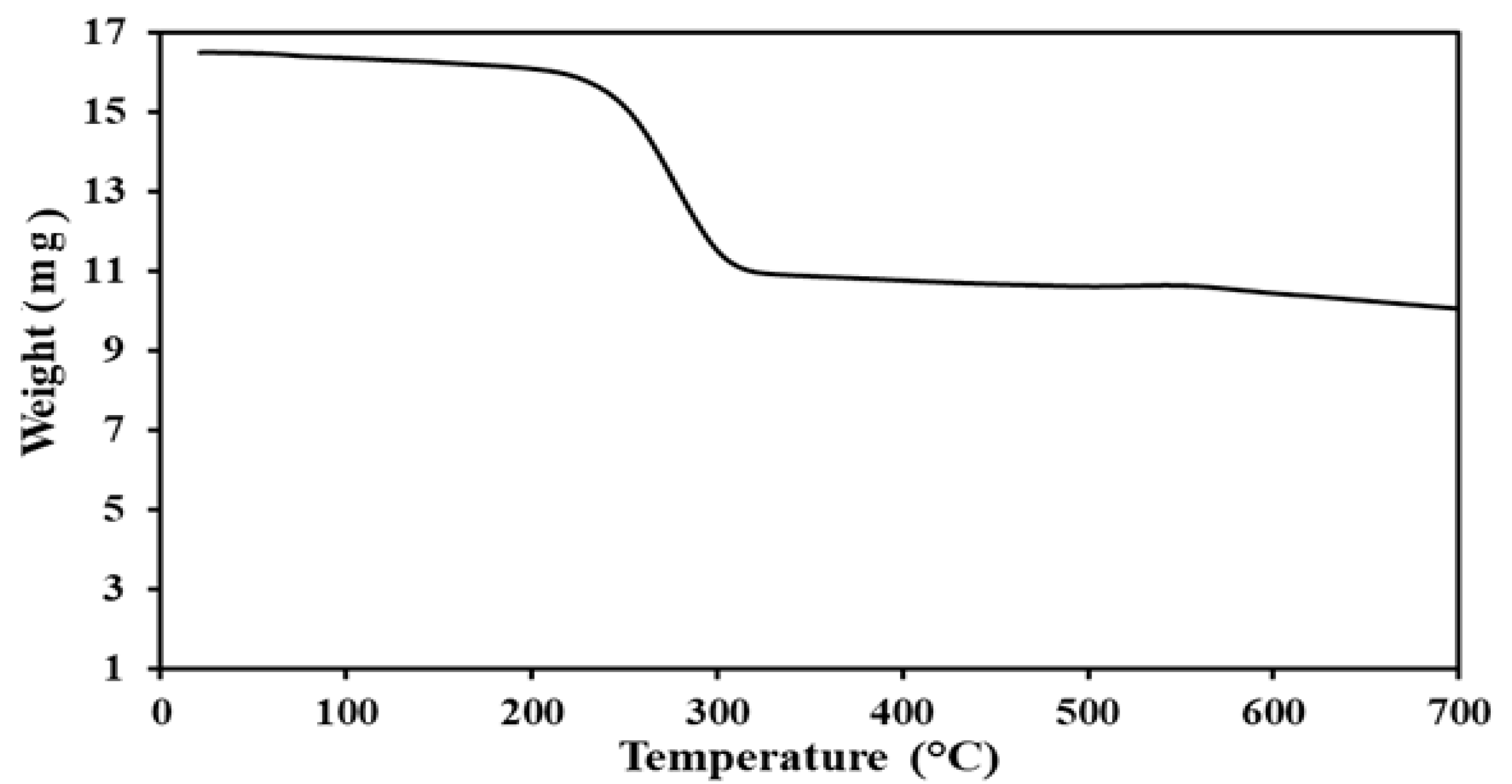
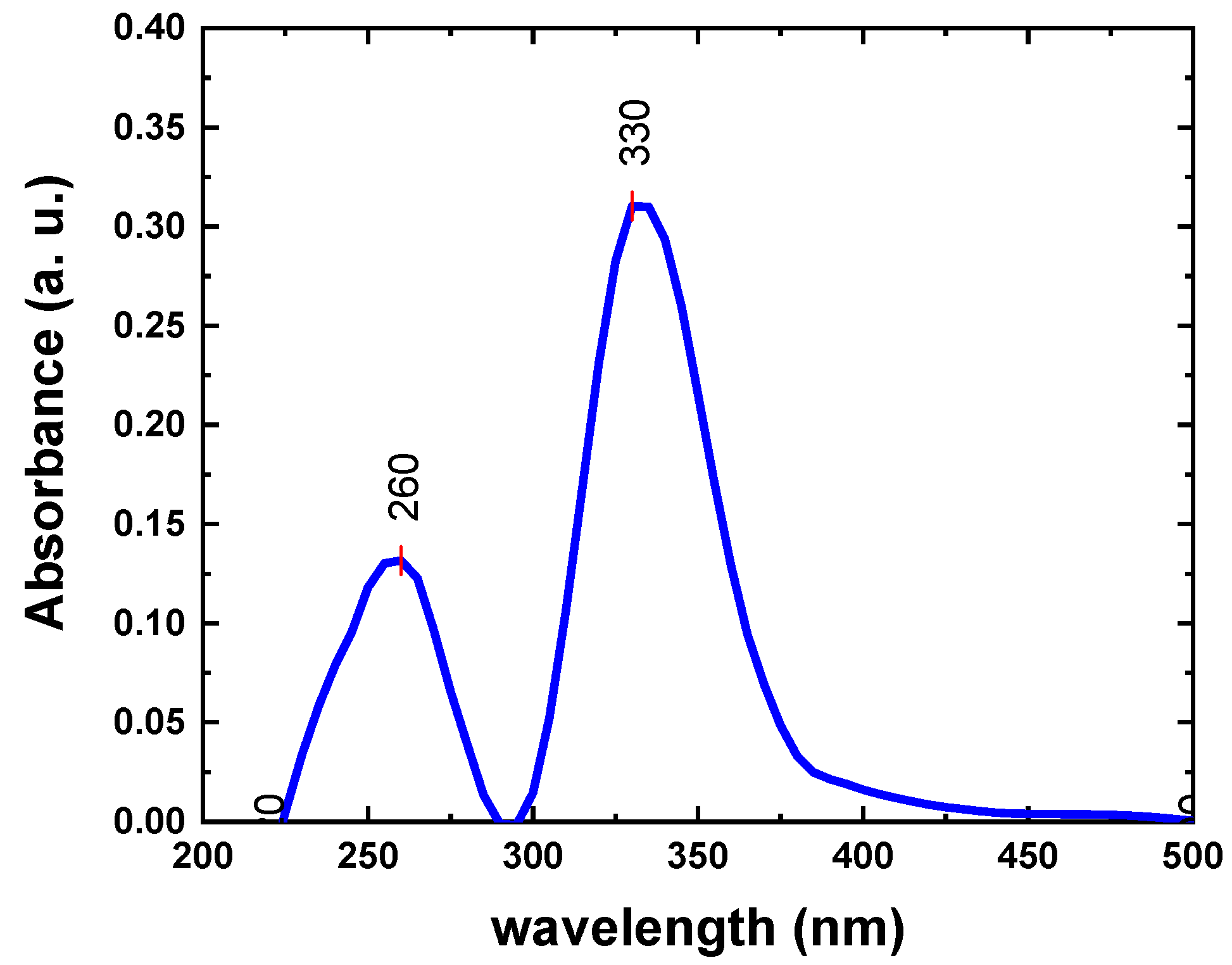
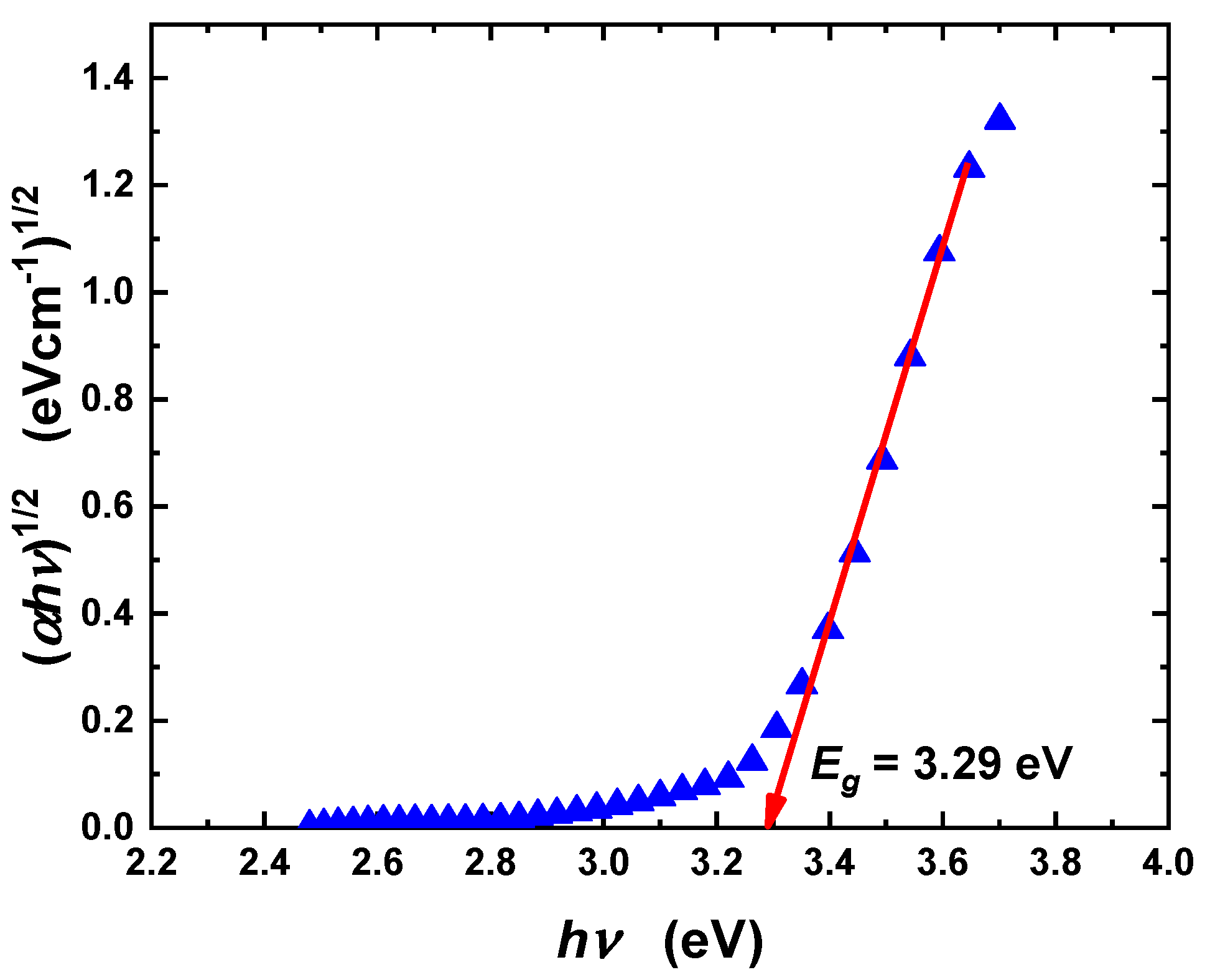
3.1. Characterization of Ca-POM Cluster
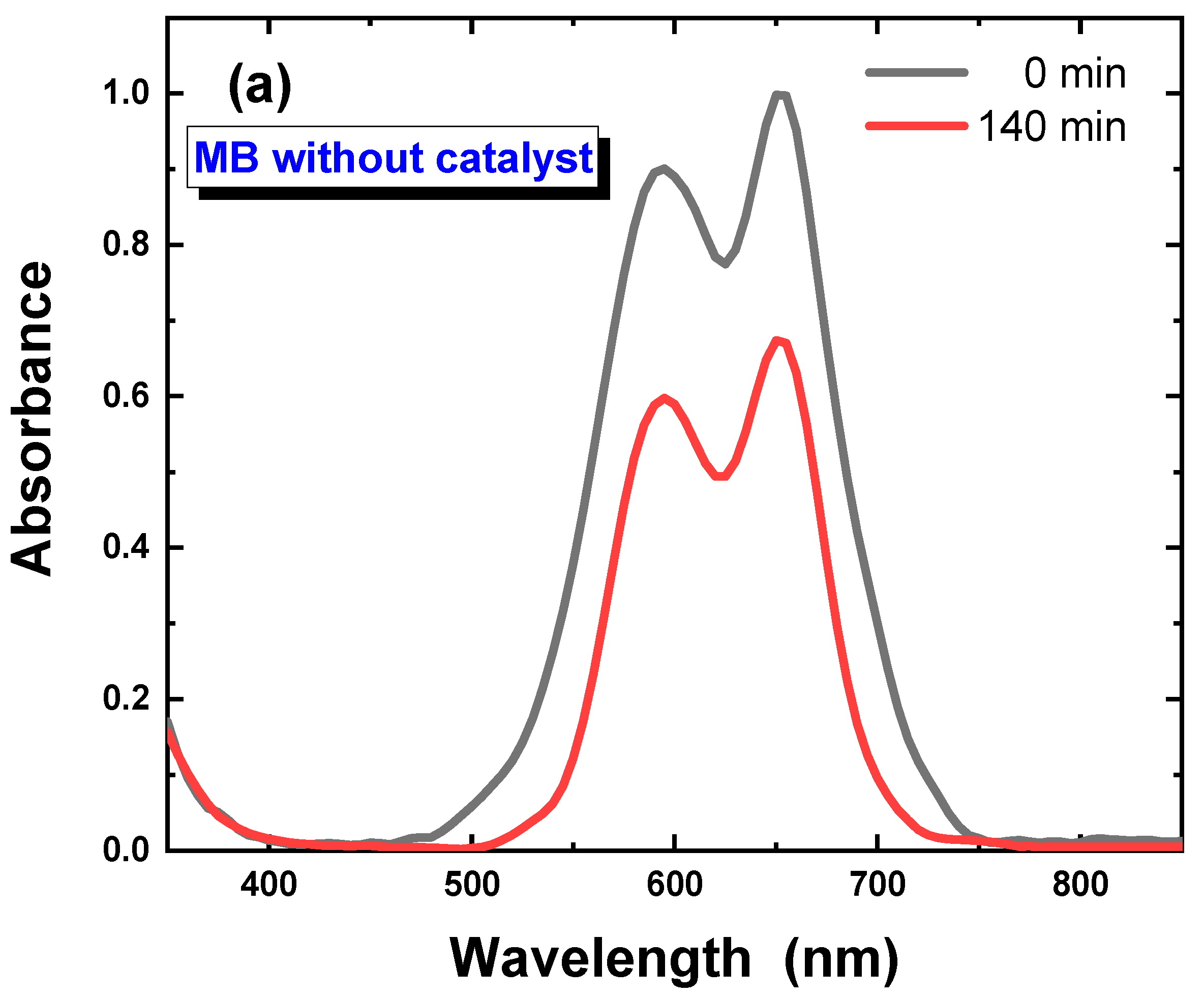
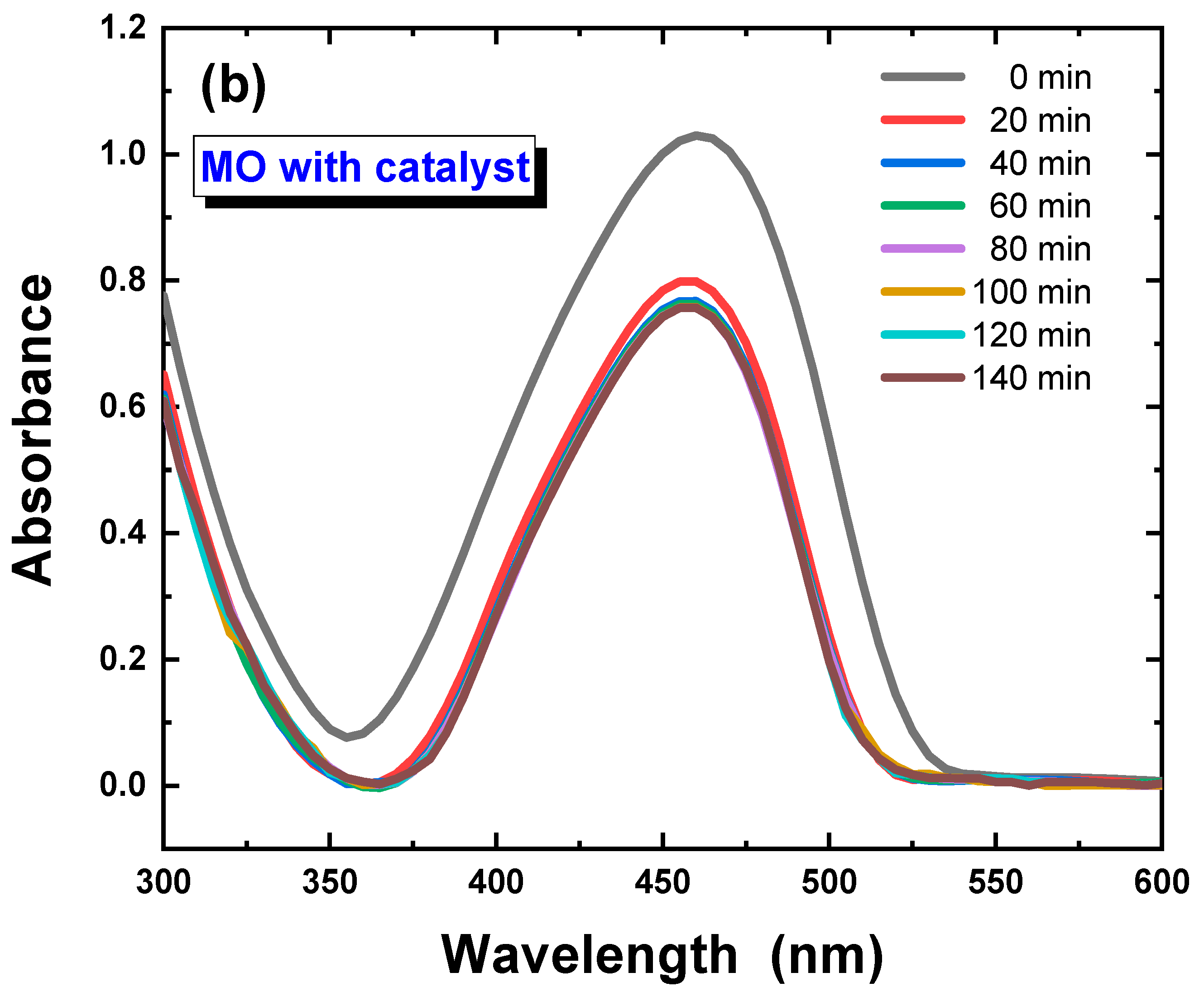
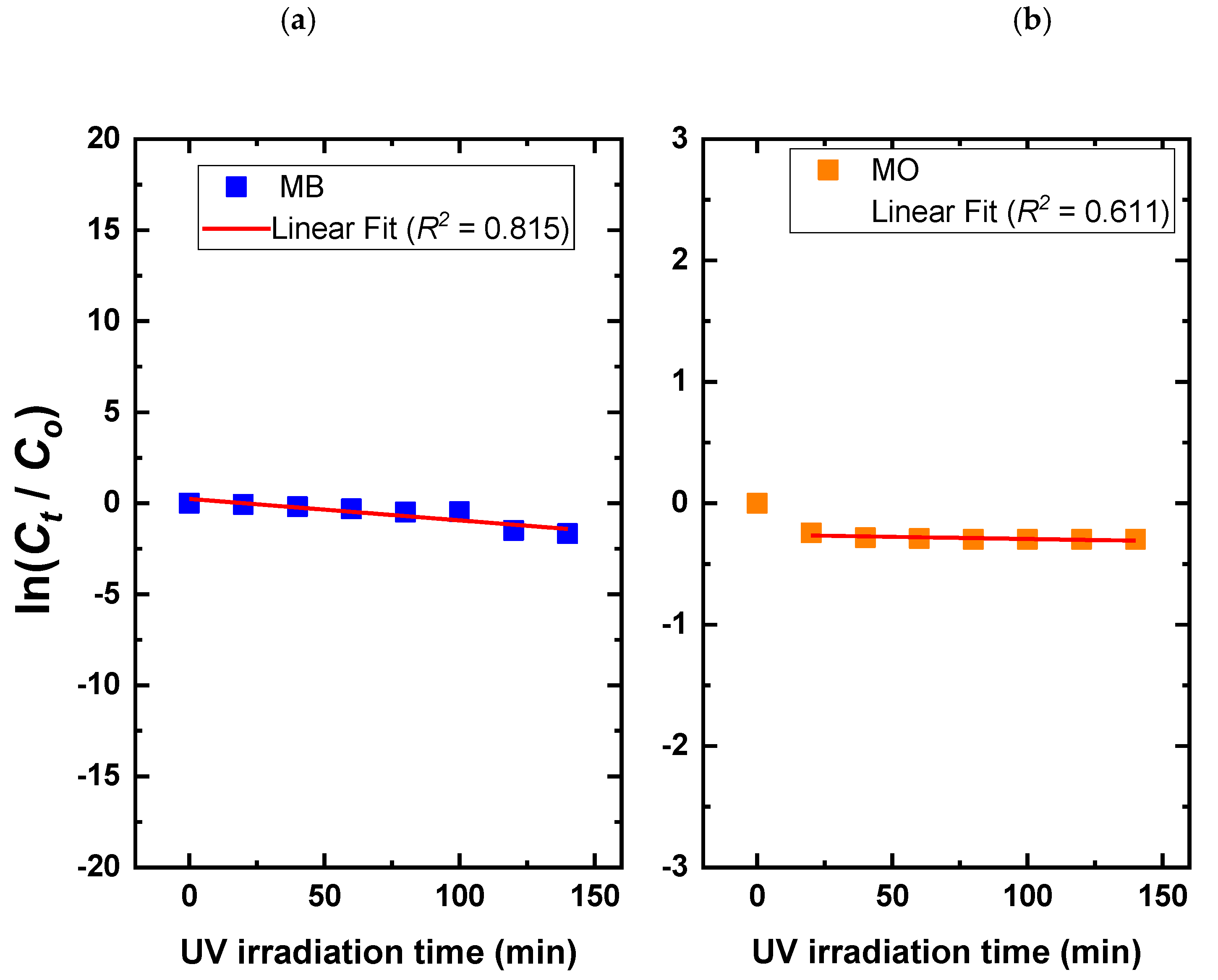
3.2. Photocatalysis Investigations of Ca–POM
3.3. Photocatalytic Mechanism
| Catalytic | Dye | ƛ irra (nm) | Optical Band Gap/eV | PCE% | kapp (min−1) | Time/min | Reference |
|---|---|---|---|---|---|---|---|
| Ca–POM | MB | 254 | 3.29 | 81.21 | 1.17 × 10−2 | 140 | Current work |
| Ca–POM | MO | 254 | 3.29 | 25.80 | 3.58 × 10−4 | 140 | Current work |
| NiO-ZnONCs | MB | 365 | 3.69 | 72 | 1.50 × 10−2 | 80 | [51] |
| Cr2O3-CNT NPs | MB | >420 | - | 68 | 9.80 × 10−4 | 120 | [52] |
| ZnO NPs | MB | 254 | 71.1 | 4.30 × 10−3 | 150 | [53] | |
| TCPP/CuPOM/TiO2 | MB | UV | 3.2 | 49 | - | 80 | [54] |
| TCPP/ZnPOM/TiO2 | MB | UV | 3.2 | 44 | - | 80 | [54] |
| CdS nanocrystals | MB | UV | 2.91 | 35 | 3.42 × 10−2 | 30 | [55] |
| PMo11V | MB | UV | - | 50.8 | - | 120 | [39] |
| Cu-POM-TiO2 | MB | UV | - | 41.58 | 2.9 × 10−2 | 180 | [40] |
| Zn-POM-TiO2 | MB | UV | - | 35.34 | 2.6 × 10−2 | 180 | [40] |
4. Comparative Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thang, N.Q.; Sabbah, A.; Chen, L.C.; Chen, K.H.; Thi, C.M.; Van Viet, P. High-efficient photocatalytic degradation of commercial drugs for pharmaceutical wastewater treatment prospects: A case study of Ag/g-C3N4/ZnO nanocomposite materials. Chemosphere 2021, 282, 130971. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lv, S.; Wang, S.; Bao, M.; Zhang, X.; Gao, Y.; Liu, Y.Y.; Zhang, Z.G.; Zheng, L.B.; Ke, J. Construction of efficient g-C3N4/NH2-UiO-66 (Zr) heterojunction photocatalysts for wastewater purification. Sep. Purif. Technol. 2021, 274, 118973. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive Removal of Dyes from Aqueous Solution onto Carbon Nanotubes: A Review. Adv. Colloid Interface Sci. 2013, 193, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of Methylene Blue on Activated Carbon Produced from Flamboyant Pods (Delonix regia): Study of Adsorption Isotherms and Kinetic Models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Alamrani, N.A.; Al-Aoh, H.A.; Aljohani, M.M.H.; Bani-Atta, S.A.; Sobhi, M.; Khalid, M.S.; Darwish, A.A.A.; Keshk, A.A.; Abdelfattah, M.A.A. Wastewater purification from permanganate ions by sorption on the Ocimum basilicum leaves powder modified by zinc chloride. J. Chem. 2021, 2021, 5561829. [Google Scholar] [CrossRef]
- Mustafa, S.K.; Al-Aoh, H.A.; Bani-Atta, S.A.; Alrawashdeh, L.R.; Aljohani, M.M.; Alsharif, M.A.; Darwish, A.; Al-Shehri, H.; Ahmad, M.A.; Al-Tweher, J.N.; et al. Enhance the adsorption behavior of methylene blue from wastewater by using ZnCl2 modified neem (Azadirachta indica) leaves powder. Desalination Water Treat. 2020, 209, 367–378. [Google Scholar] [CrossRef]
- Bani-Atta, S.A. Zinc chloride modification of sage leaves powder and its application as an adsorbent for KMnO4 removal from aqueous solutions. Mater. Res. Express 2020, 7, 095511. [Google Scholar] [CrossRef]
- Bani-Atta, S.A. Potassium permanganate dye removal from synthetic wastewater using a novel, low-cost adsorbent modified from the powder of Foeniculum vulgare seeds. Sci. Rep. 2022, 12, 4547. [Google Scholar] [CrossRef]
- Bani-Atta, S.A.; Al-Aoh, H.A.; Aljohani, M.M.; Keshka, A.A.; Al-Shehri, H.S.; Mustafa, S.K.; Alamrani, N.A.; Darwish, A.A.; Sobhi, M. Methylene Blue sorption by the chemically modified Ocimum basilicum leaves powder. Desalination Water Treat. 2021, 222, 237–245. [Google Scholar] [CrossRef]
- Bani-Atta, S.A.; Al-Aoh, H.A. Methylene blue dye elimination from synthetic wastewater by modified adsorbent produced from Foeniculum vulgare waste: Thermodynamic, equilibrium, and kinetic studies. Desalination Water Treat. 2024, 320, 100649. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewater: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, L.; Feng, C.; Liu, H.; Zhang, H.; Peng, B.; Chen, Z.; Xie, Q. Intimate coupling of photocatalysis and biodegradation for wastewater treatment: Mechanisms, recent advances and environmental applications. Water Res. 2020, 175, 115673. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Iftekhar, S.; Park, Y.; Joseph, J.; Srivastava, V.; Khan, M.A.; Makvandi, P.; Sillanpaa, M.; Varma, R.S. An overview on non-spherical semiconductors for heterogeneous photocatalytic degradation of organic water contaminants. Chemosphere 2021, 280, 130907. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Jaisai, M.; Dutta, J. Efficient solar photocatalytic degradation of textile wastewater using ZnO/ZTO composites. Appl. Catal. B Environ. 2015, 163, 1–8. [Google Scholar] [CrossRef]
- Hill, C.L. Introduction: Polyoxometalates multicomponent molecular vehicles to probe fundamental issues and practical problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate Clusters, Nanostructures, and Materials: From Self-Assembly to Designer Materials and Devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Pope, M.T. Borrás-Almenar, J.J., Coronado EMüller, A., Pope, M.T., Eds.; Polyoxometalate Molecular Science; Springer: Dordrecht, The Netherlands, 2003; pp. 3–31. [Google Scholar]
- Long, D.L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building Blocks for Functional Nanoscale Systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-Saiz, C.; Gómez-García, C.J. Recent Advances in Polyoxometalate-Containing Molecular Conductors. Coord. Chem. Rev. 2005, 249, 1776–1796. [Google Scholar] [CrossRef]
- Alsulaim, G.M.; Aboraia, A.M.; Hamdalla, T.A.; Darwish, A.A.A. High-Efficient Photocatalytic Degradation of Industrial Dyes (MB, RhD, and MB/RhD) Using Zn100−xSmxO Nanoparticles. Phys. Scr. 2023, 98, 065920. [Google Scholar] [CrossRef]
- Papaconstantinou, E. Photochemistry of Polyoxometallates of Molybdenum and Tungsten and/or Vanadium. Chem. Soc. Rev. 1989, 18, 1–31. [Google Scholar] [CrossRef]
- Streb, C. New Trends in Polyoxometalate Photoredox Chemistry: From Photosensitisation to Water Oxidation Catalysis. Dalton Trans. 2012, 41, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Wales, D.J.; Newton, G.N. Shining a light on the photosensitisation of organic–inorganic hybrid polyoxometalates. Dalton Trans. 2018, 47, 5120–5136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Nomiya, K.; Sugie, Y.; Miyazaki, T.; Miwa, M. Catalysis by heteropolyacids—ix. Photocatalytic oxidation of isopropyl alcohol to acetone under oxygen using tetrabutylammonium decatungstate. Polyhedron 1986, 5, 1267–1271. [Google Scholar] [CrossRef]
- Omwoma, S.; Gore, C.T.; Ji, Y.; Hu, C.; Song, Y.F. Environmentally benign polyoxometalate materials. Coord. Chem. Rev. 2015, 286, 17–29. [Google Scholar] [CrossRef]
- Kim, S.; Yeo, J.; Choi, W. Simultaneous conversion of dye and hexavalent chromium in visible light-illuminated aqueous solution of polyoxometalate as an electron transfer catalyst. Appl. Catal. B Environ. 2008, 84, 148–155. [Google Scholar] [CrossRef]
- Troupis, A.; Hiskia, A.; Papaconstantinou, E. Photocatalytic reduction and recovery of copper by polyoxometalates. Environ. Sci. Technol. 2002, 36, 5355–5362. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Arslan-Alaton, I.; Ferry, J.L. Application of polyoxotungstates as environmental catalysts: Wet air oxidation of acid dye Orange II. Dye. Pigment. 2002, 54, 25–36. [Google Scholar] [CrossRef]
- Chai, F.; Wang, L.; Xu, L.; Wang, X.; Huang, J. Degradation of dye on polyoxotungstate nanotube under molecular oxygen. Dye. Pigment. 2008, 76, 113–117. [Google Scholar] [CrossRef]
- Chen, W.L.; Chen, B.W.; Tan, H.Q.; Li, Y.G.; Wang, Y.H.; Wang, E.B. Ionothermal syntheses of three transition-metal-containing polyoxotungstate hybrids exhibit the photocatalytic and electrocatalytic properties. J. Solid. State Chem. 2010, 183, 310–321. [Google Scholar] [CrossRef]
- Suhaimi, N.A.; Roslan, N.N.; Amirul, N.B.; Lau, H.L.; Hasman, A.A.; Nur, M.; Lim, J.W.; Usman, A. Unraveling the photocatalytic degradation kinetics and efficiency of methylene blue, rhodamine B, and auramine O in their ternary mixture: Diffusion and conformational insights. Reac. Kinet. Mech. Cat. 2024, 137, 3441–3462. [Google Scholar] [CrossRef]
- Li, H.; Gao, S.; Cao, M.; Cao, R. Self-assembly of polyoxometalate–thionine multilayer films on magnetic microspheres as photocatalyst for methyl orange degradation under visible light irradiation. J. Colloid. Interface Sci. 2013, 394, 434–440. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, Q.Q.; Cui, Z.W.; Shi, S.; Long, J.Y.; Wang, X.L.; Fei, B.L. A novel octamolybdate-based organic–inorganic hybrid as photo-Fenton-like catalyst for degradation of methylene blue. Appl. Organomet. Chem. 2023, 37, e6966. [Google Scholar] [CrossRef]
- Taghdiri, M. Selective adsorption and photocatalytic degradation of dyes using polyoxometalate hybrid supported on magnetic activated carbon nanoparticles under sunlight, visible, and UV irradiation. Int. J. Photoenergy 2017, 2017, 8575096. [Google Scholar] [CrossRef]
- Shi, C.; Kang, N.; Wang, C.; Yu, K.; Lv, J.; Wang, C.; Zhou, B. An inorganic–organic hybrid nanomaterial with a core–shell structure constructed by using Mn–BTC and Ag₅[BW₁₂O₄₀] for supercapacitors and photocatalytic dye degradation. Nanoscale Adv. 2022, 4, 4358–4365. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; An, C.; Liu, H.; Wu, Q. Preparation of vanadium-substituted polyoxometalate doped carbon nitride hybrid materials POM/g-C₃N₄ and their photocatalytic oxidation performance. Mater. Lett. 2020, 262, 126954. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Rodríguez, A.; Utria, D.; Vallejo, W.; Puello, E.; Zarate, X.; Schott, E. Photocatalytic degradation of methylene blue by the Anderson-type polyoxomolybdates/TiO₂ thin films. Polyhedron 2018, 149, 163–170. [Google Scholar] [CrossRef]
- Atta, S.; Haddad, S.; AlDamen, M. Different Polyoxometalate Structures Obtained from the Na₁₁H[H₂₋ₓBi₂W₂₀O₇₀(HWO₃)ₓ]·46H₂O (x = 1.4). Jordanian J. Eng. Chem. Ind. 2018, 1, 1–2. [Google Scholar]
- Laugier, J.; Bochu, B. LMGP-Suite of Programs for the Interpretation of X-Ray Experiments; ENSP/Laboratoire des Matériaux et du Génie Physique: Saint Martin d’Hères, France, 2000. [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- El-Zaidia, E.F.M.; Ali, H.A.M.; Hamdalla, T.A.; Darwish, A.A.A.; Hanafy, T.A. Optical linearity and bandgap analysis of Erythrosine B doped in polyvinyl alcohol films. Opt. Mater. 2020, 100, 109661. [Google Scholar] [CrossRef]
- Qashou, S.I.; Rashad, M.; Mahmoud, A.Z.; Darwish, A.A.A. The promotion of Indeno [1, 2-b] flourene-6, 12 dione thin film to be changed into stable aromatic compound under the effect of annealing treatment. J. Mater. 2019, 162, 199–207. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Guo, R.; Shan, D.; Zhao, Y.; Linghu, X.; Shu, Y.; Wang, B. Synthesis of nano-sized Ag₃PW₁₂O₄₀/ZnO heterojunction as a photocatalyst for degradation of organic pollutants under simulated sunlight. Arab. J. Chem. 2022, 15, 103659. [Google Scholar] [CrossRef]
- Patil, K.; Pawar, R.; Talap, P. Self-aggregation of methylene blue in aqueous medium and aqueous solutions of Bu₄NBr and urea. Phys. Chem. Chem. Phys. 2000, 2, 4313–4317. [Google Scholar] [CrossRef]
- Xiong, S.F.; Yin, Z.L.; Yuan, Z.F.; Yan, W.B.; Yang, W.Y.; Liu, J.J.; Zhang, F. Dual-frequency (20/40 kHz) ultrasonic assisted photocatalysis for degradation of methylene blue effluent: Synergistic effect and kinetic study. Ultrason. Sonochem 2012, 19, 756–761. [Google Scholar] [CrossRef]
- Mebed, A.M.; Alshammari, K.; Ezzeldien, M.; Al-Ghamdi, S.A.; Abd-Elnaiem, A.M.; Abd El-Aal, M.; Hamad, D. Enhancement of the photodegradation performance towards methylene blue and rhodamine B using AgₓSn₁₋ₓO₂ nanocomposites. J. Alloys Compd. 2024, 1009, 176977. [Google Scholar] [CrossRef]
- Li, F.R.; Ji, T.; Chen, W.L. A tri-vanadium-capped Keggin phosphomolybdate: Synthesis, characterization, photocatalytic and bifunctional electrocatalytic properties. Tungsten 2022, 4, 99–108. [Google Scholar] [CrossRef]
- Abdul Rahman, I.; Ayob, M.T.M.; Radiman, S. Enhanced photocatalytic performance of NiO-decorated ZnO nanowhiskers for methylene blue degradation. J. Nanotechnol. 2014, 2014, 212694. [Google Scholar] [CrossRef]
- Chen, M.L.; Cho, K.Y.; Oh, W.C. Synthesis and photocatalytic behaviors of Cr₂O₃–CNT/TiO₂ composite materials under visible light. J. Mater. Sci. 2010, 45, 6611–6616. [Google Scholar] [CrossRef]
- Azmina, M.S.; Md Nor, R.; Rafaie, H.A.; Razak, N.S.A.; Sani, S.F.A.; Osman, Z. Enhanced photocatalytic activity of ZnO nanoparticles grown on porous silica microparticles. Appl. Nanoscience 2017, 7, 885–892. [Google Scholar] [CrossRef]
- Sanguino, A.; Diaz-Uribe, C.; Duran, F.; Vallejo, W.; Guzman, L.; Ruiz, D.; Puello, E.; Quiñones, C.; Schott, E.; Zarate, X. Photocatalytic degradation of methylene blue under visible light using TiO₂ thin films impregnated with porphyrin and Anderson-type polyoxometalates (Cu and Zn). Catalysts 2022, 12, 1169. [Google Scholar] [CrossRef]
- Chang, S.K.; Abbasi, Z.; Khushbakht, F.; Ullah, I.; Rehman, F.U.; Hafeez, M. Rapid pH-dependent photocatalytic degradation of methylene blue by CdS nanorods synthesized through hydrothermal process. Arab. J. Chem. 2024, 17, 105422. [Google Scholar] [CrossRef]





| Condition | Range | Optimized Condition |
|---|---|---|
| Amount of POM precursor | 0.5–2 g | 1.49 g |
| pH | 4–7 | 6 |
| Temperature | 60–90 °C | 80 °C |
| Reaction time | 1–2 h | 2 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bani-Atta, S.A.; Darwish, A.A.A.; Shwashreh, L.; Alotaibi, F.A.; Al-Tweher, J.N.; Al-Aoh, H.A.; El-Zaidia, E.F.M. Efficient Photocatalytic Degradation of Methylene Blue and Methyl Orange Using Calcium-Polyoxometalate Under Ultraviolet Irradiation. Processes 2024, 12, 2769. https://doi.org/10.3390/pr12122769
Bani-Atta SA, Darwish AAA, Shwashreh L, Alotaibi FA, Al-Tweher JN, Al-Aoh HA, El-Zaidia EFM. Efficient Photocatalytic Degradation of Methylene Blue and Methyl Orange Using Calcium-Polyoxometalate Under Ultraviolet Irradiation. Processes. 2024; 12(12):2769. https://doi.org/10.3390/pr12122769
Chicago/Turabian StyleBani-Atta, Suhair A., A. A. A. Darwish, Leena Shwashreh, Fatimah A. Alotaibi, Jozaa N. Al-Tweher, Hatem A. Al-Aoh, and E. F. M. El-Zaidia. 2024. "Efficient Photocatalytic Degradation of Methylene Blue and Methyl Orange Using Calcium-Polyoxometalate Under Ultraviolet Irradiation" Processes 12, no. 12: 2769. https://doi.org/10.3390/pr12122769
APA StyleBani-Atta, S. A., Darwish, A. A. A., Shwashreh, L., Alotaibi, F. A., Al-Tweher, J. N., Al-Aoh, H. A., & El-Zaidia, E. F. M. (2024). Efficient Photocatalytic Degradation of Methylene Blue and Methyl Orange Using Calcium-Polyoxometalate Under Ultraviolet Irradiation. Processes, 12(12), 2769. https://doi.org/10.3390/pr12122769









