Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants
Abstract
1. Introduction
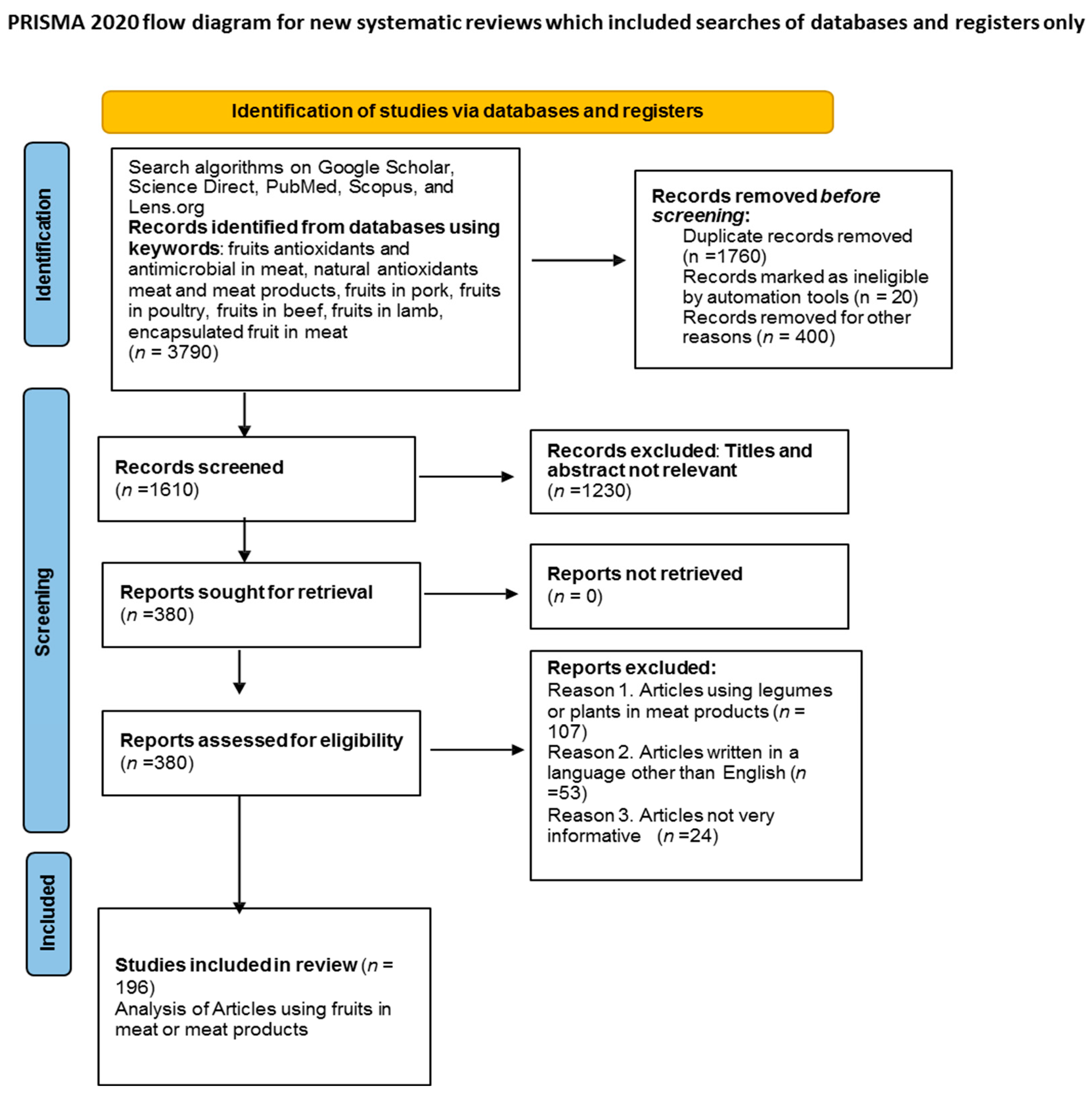
2. Research Methodology
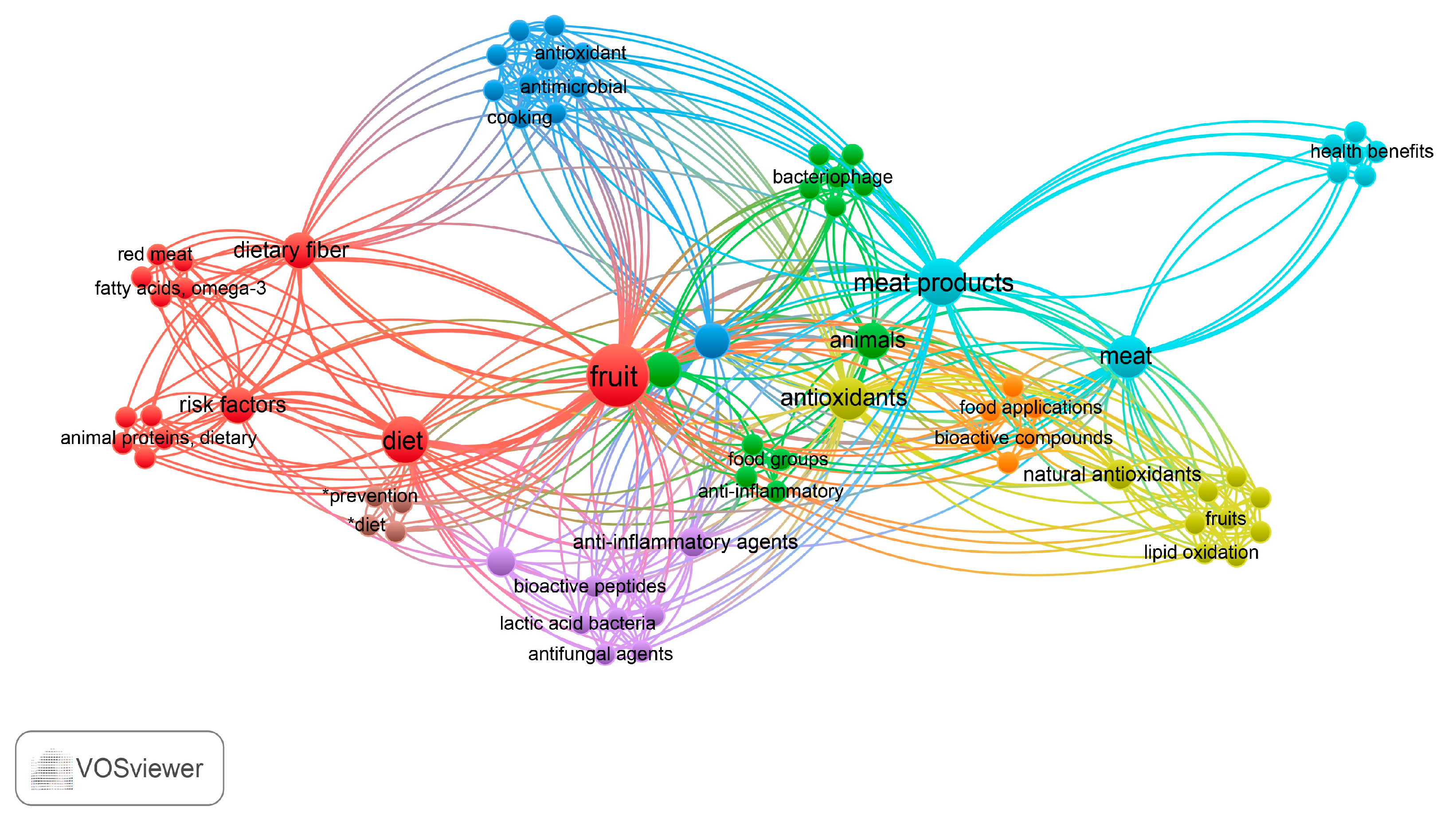
3. Bibliometric Analysis of the Topic in the Literature
4. The Phytochemical Composition of Fruits and Their Biological Properties
| a. Flavonoids | |||
Catechin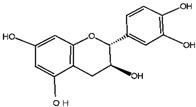 | Quercetin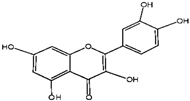 | Cyanidin | |
| b. Phenolic acids | |||
Caffeic Acid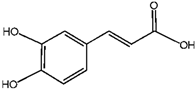 | Chlorogenic Acid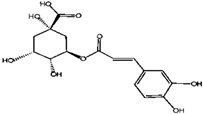 | ||
| c. Alkaloids | |||
Caffeine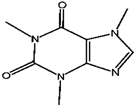 | Theobromine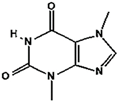 | Theophylline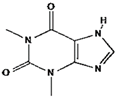 | |
| d. Essential oils | |||
Limonene | β-pinene and α-pinene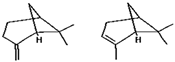 | γ-terpinene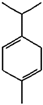 | Geranial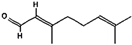 |
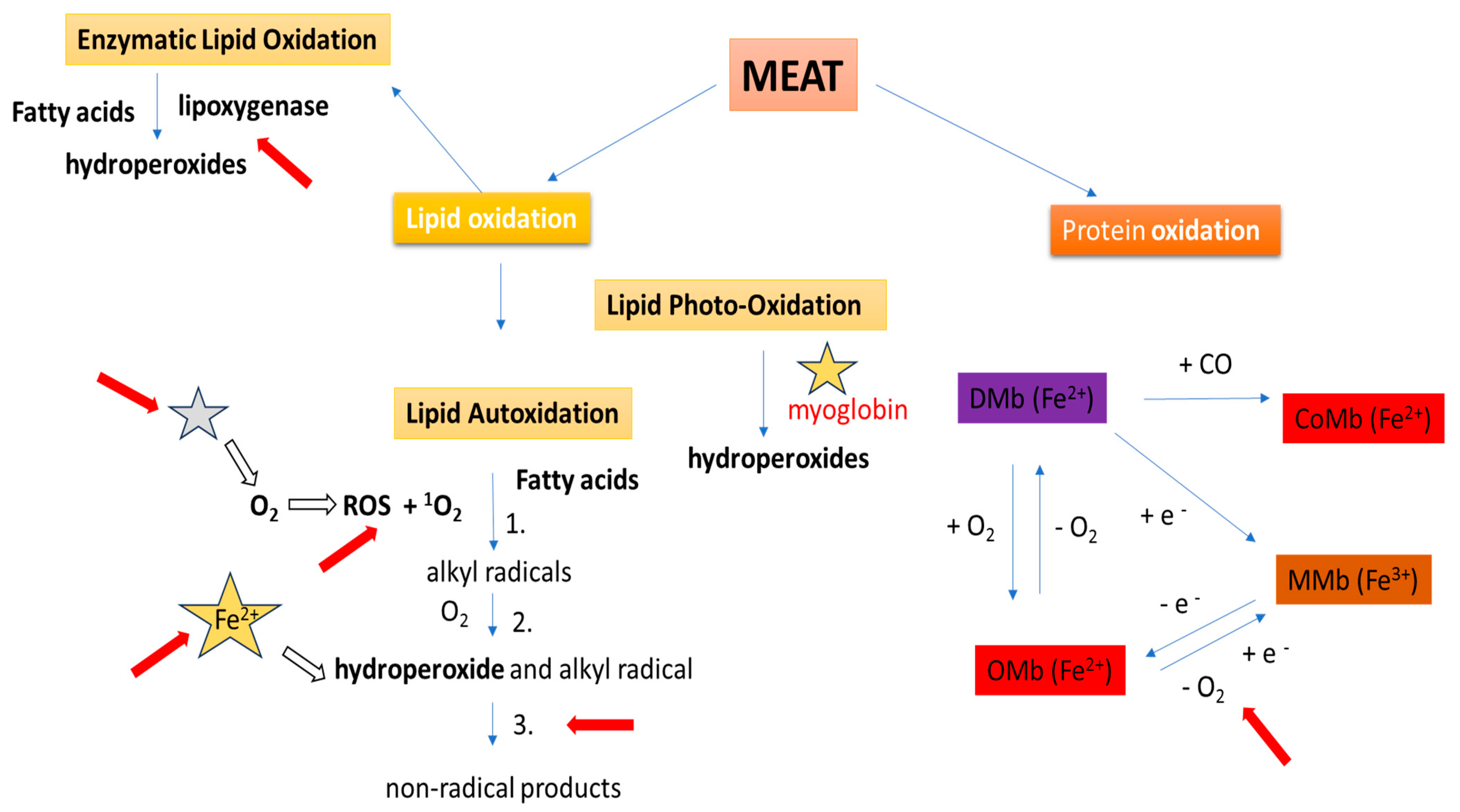
5. Fruits as Antioxidant Agents in Meat Products
6. Fruits as Antimicrobial Agents in Meat
7. The Aroma and Coloring Properties of Fruits
8. The Limitations Associated with the Use of Fruits and Their By-Products in the Food Industry
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickson-Spillmann, M.; Siegrist, M.; Keller, C. Attitudes toward Chemicals Are Associated with Preference for Natural Food. Food Qual. Prefer. 2011, 22, 149–156. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Valorization of Fruit Wastes for Circular Bioeconomy: Current Advances, Challenges, and Opportunities. Bioresour. Technol. 2022, 359, 127459. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Ahmad, S.; Azad, Z.R.A.A.; Adnan, M.; Ashraf, S.A. Incorporating Dietary Fiber from Fruit and Vegetable Waste in Meat Products: A Systematic Approach for Sustainable Meat Processing and Improving the Functional, Nutritional and Health Attributes. PeerJ 2023, 11, e14977. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Elshebrawy, H.A.; Imre, K.; Morar, A.; Herman, V.; Pașcalău, R.; Sallam, K.I. Antioxidant and Antibacterial Effect of Fruit Peel Powders in Chicken Patties. Foods 2022, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Comp. Rev. Food Sci. Food Safe 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural Antioxidants against Lipid–Protein Oxidative Deterioration in Meat and Meat Products: A Review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Devatkal, S.K.; Thorat, P.; Manjunatha, M. Effect of Vacuum Packaging and Pomegranate Peel Extract on Quality Aspects of Ground Goat Meat and Nuggets. J. Food Sci. Technol. 2014, 51, 2685–2691. [Google Scholar] [CrossRef]
- AL-Hijazeen, M.A.; AL-Rawashdeh, M.S.; Al-Rabadi, G.J. Cooked Broiler Meat Quality Affected by Different Mediterranean Medicinal Plants in the Diet. Anim. Biosci. 2022, 35, 290–298. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Afshari, A. Using Natural Antioxidants in Meat and Meat Products as Preservatives: A Review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef]
- Armenteros, M.; Morcuende, D.; Ventanas, S.; Estévez, M. Application of Natural Antioxidants from Strawberry Tree (Arbutus unedo L.) and Dog Rose (Rosa canina L.) to Frankfurters Subjected to Refrigerated Storage. J. Integr. Agric. 2013, 12, 1972–1981. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of Guarana (Paullinia cupana) Seed and Pitanga (Eugenia uniflora L.) Leaf Extracts on Lamb Burgers with Fat Replacement by Chia Oil Emulsion during Shelf Life Storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of Pomegranate Peel Extract on Lipid and Protein Oxidation in Beef Meatballs during Refrigerated Storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef]
- Lee, M.-A.; Choi, J.-H.; Choi, Y.-S.; Kim, H.-Y.; Kim, H.-W.; Hwang, K.-E.; Chung, H.-K.; Kim, C.-J. Effects of Kimchi Ethanolic Extracts on Oxidative Stability of Refrigerated Cooked Pork. Meat Sci. 2011, 89, 405–411. [Google Scholar] [CrossRef]
- Biswas, A.K.; Chatli, M.K.; Sahoo, J. Antioxidant Potential of Curry (Murraya koenigii L.) and Mint (Mentha spicata) Leaf Extracts and Their Effect on Colour and Oxidative Stability of Raw Ground Pork Meat during Refrigeration Storage. Food Chem. 2012, 133, 467–472. [Google Scholar] [CrossRef]
- Badr, H.M.; Mahmoud, K.A. Antioxidant Activity of Carrot Juice in Gamma Irradiated Beef Sausage during Refrigerated and Frozen Storage. Food Chem. 2011, 127, 1119–1130. [Google Scholar] [CrossRef]
- Tian, Y.; Karhu, S.; Virtanen, M.; Linderborg, K.M.; Yang, B.; Laaksonen, O. Variation of Chemical and Sensory Profiles of Blackcurrant (Ribes nigrum) Juices Produced from Different Cultivars of European Origins. LWT 2023, 173, 114353. [Google Scholar] [CrossRef]
- Yıldız-Turp, G.; Serdaroglu, M. Effects of Using Plum Puree on Some Properties of Low Fat Beef Patties. Meat Sci. 2010, 86, 896–900. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Fraqueza, M.J.; Fernandes, M.H.; Moldão-Martins, M.; Alves, V.D. Application of Edible Alginate Films with Pineapple Peel Active Compounds on Beef Meat Preservation. Antioxidants 2020, 9, 667. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Sadr, S.; Hosseinzadeh, A.; Gholamine, B.; Shahbazi, A.; FallahHuseini, H.; Ghaznavi, H. Anticonvulsant Activity of the Ethanolic Extract of Punica granatum L. Seed. Neurol. Res. 2015, 37, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, E.; Ponce-Alquicira, E.; Jaramillo-Flores, M.E.; Guerrero Legarreta, I. Antioxidant Effect Rosemary (Rosmarinus officinalis L.) and Oregano (Origanum vulgare L.) Extracts on TBARS and Colour of Model Raw Pork Batters. Meat Sci. 2009, 81, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, A.; Chouaibi, M.; Ben Haj Koubaier, H.; Bouzouita, N. Encapsulation of Tunisian Thyme Essential Oil in O/W Nanoemulsions: Application for Meat Preservation. Meat Sci. 2022, 188, 108785. [Google Scholar] [CrossRef]
- Lee, M.-A.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Shim, S.-Y.; Chung, H.-K.; Kim, C.-J. The Antioxidative Properties of Mustard Leaf (Brassica juncea) Kimchi Extracts on Refrigerated Raw Ground Pork Meat against Lipid Oxidation. Meat Sci. 2010, 84, 498–504. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s New in Biopotential of Fruit and Vegetable by-Products Applied in the Food Processing Industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural Antimicrobial/Antioxidant Agents in Meat and Poultry Products as Well as Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2016, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Oyom, W.; Tahergorabi, R. Utilization of Fruits and Vegetable Processing Wastes for Meat Analog Products. In Handbook of Plant-Based Meat Analogs; Elsevier: Amsterdam, The Netherlands, 2024; pp. 187–202. ISBN 978-0-443-21846-0. [Google Scholar]
- Balawejder, M.; Piechowiak, T.; Kapusta, I.; Chęciek, A.; Matłok, N. In Vitro Analysis of Selected Antioxidant and Biological Properties of the Extract from Large-Fruited Cranberry Fruits. Molecules 2023, 28, 7895. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Struck, S.; Rohm, H. Fruit Processing By-Products as Food Ingredients. In Valorization of Fruit Processing by-Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. ISBN 978-0-12-817106-6. [Google Scholar]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fruit and Vegetable Processing By-Products as Functional Meat Product Ingredients—A Chance to Improve the Nutritional Value. LWT 2023, 189, 115442. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Mtibaa, A.C.; Fourati, M.; Sellem, I.; Elhadef, K.; Ennouri, K.; Mellouli, L. Pomegranate Peel as Phenolic Compounds Source: Advanced Analytical Strategies and Practical Use in Meat Products. Meat Sci. 2019, 158, 107914. [Google Scholar] [CrossRef]
- Parafati, L.; Palmeri, R.; Trippa, D.; Restuccia, C.; Fallico, B. Quality Maintenance of Beef Burger Patties by Direct Addiction or Encapsulation of a Prickly Pear Fruit Extract. Front. Microbiol. 2019, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Chanshotikul, N.; Hemung, B.-O. Encapsulation of Gac Powder Extract and Its Application in Low-Nitrite Chicken Sausage. Int. J. Food Eng. 2019, 5, 146–151. [Google Scholar] [CrossRef]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; De Godoy, S.H.S.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; De Lima, C.G.; et al. Microencapsulated Jabuticaba (Myrciaria cauliflora) Extract Added to Fresh Sausage as Natural Dye with Antioxidant and Antimicrobial Activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef]
- Dos Santos Silva, M.E.; De Oliveira, R.L.; Sousa, T.C.D.A.; Grisi, C.V.B.; Ferreira, V.C.D.S.; Porto, T.S.; Madruga, M.S.; Silva, S.P.D.; Silva, F.A.P.D. Microencapsulated Phenolic-Rich Extract from Juice Processing Grape Pomace (Vitis labrusca. Isabella Var): Effects on Oxidative Stability of Raw and Pre-Cooked Bovine Burger. Food Biosci. 2022, 50, 102212. [Google Scholar] [CrossRef]
- Eghbal, N.; Liao, W.; Dumas, E.; Azabou, S.; Dantigny, P.; Gharsallaoui, A. Microencapsulation of Natural Food Antimicrobials: Methods and Applications. Appl. Sci. 2022, 12, 3837. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Karama, M.; Hadjichralambous, C.; Sechi, P.; Grispoldi, L. Is EU Regulation on the Use of Antioxidants in Meat Preparation and in Meat Products Still Cutting Edge? Eur. Food Res. Technol. 2020, 246, 661–668. [Google Scholar] [CrossRef]
- Mann, B.; Sharma, R. Legislation. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 674–681. ISBN 978-0-12-818767-8. [Google Scholar]
- Feiner, G. Additives. In Salami; Elsevier: Amsterdam, The Netherlands, 2016; pp. 59–88. ISBN 978-0-12-809598-0. [Google Scholar]
- Surendran Nair, M.; Nair, D.V.T.; Kollanoor Johny, A.; Venkitanarayanan, K. Use of Food Preservatives and Additives in Meat and Their Detection Techniques. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–213. ISBN 978-0-12-819233-7. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and Nitrate in Meat Processing: Functions and Alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of Citrus Co-Products: Recovery of Bioactive Compounds and Application in Meat and Meat Products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Lu, X.-H.; Sun, D.-Q.; Wu, Q.-S.; Liu, S.-H.; Sun, G.-M. Physico-Chemical Properties, Antioxidant Activity and Mineral Contents of Pineapple Genotypes Grown in China. Molecules 2014, 19, 8518–8532. [Google Scholar] [CrossRef] [PubMed]
- De Ancos, B.; Sánchez-Moreno, C.; González-Aguilar, G.A. Pineapple Composition and Nutrition. In Handbook of Pineapple Technology; Lobo, M.G., Paull, R.E., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 221–239. ISBN 978-1-118-96738-6. [Google Scholar]
- Haagen-Smit, A.J.; Kirchner, J.G.; Prater, A.N.; Deasy, C.L. Chemical Studies of Pineapple (Ananas sativus Lindl). I. The Volatile Flavor and Odor Constituents of Pineapple. J. Am. Chem. Soc. 1945, 67, 1646–1650. [Google Scholar] [CrossRef]
- Agarry, S. Batch Equilibrium and Kinetic Studies of Simultaneous Adsorption and Biodegradation of Phenol by Pineapple Peels Immobilized Pseudomonas aeruginosa NCIB 950. BBJ 2012, 2, 26–48. [Google Scholar] [CrossRef]
- Bartolomé, A.P.; Rupérez, P.; Fúster, C. Pineapple Fruit: Morphological Characteristics, Chemical Composition and Sensory Analysis of Red Spanish and Smooth Cayenne Cultivars. Food Chem. 1995, 53, 75–79. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S.; Demirci, A.Ş.; Gümüş, T.; Ekinci, N. Evaluation of Antioxidant and Antimicrobial Potential of Strawberry Tree (Arbutus unedo L.) Leaf. Food Sci. Biotechnol. 2011, 20, 1249–1256. [Google Scholar] [CrossRef]
- Orak, H.; Aktas, T.; Yagar, H.; İsbilir, S.S.; Ekinci, N.; Sahin, F.H. Effects of Hot Air and Freeze Drying Methods on Antioxidant Activity, Colour and Some Nutritional Characteristics of Strawberry Tree (Arbutus unedo L.) Fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Santo, D.E.; Galego, L.; Gonçalves, T.; Quintas, C. Yeast Diversity in the Mediterranean Strawberry Tree (Arbutus unedo L.) Fruits’ Fermentations. Food Res. Int. 2012, 47, 45–50. [Google Scholar] [CrossRef]
- Miguel, M.; Faleiro, M.; Guerreiro, A.; Antunes, M. Arbutus unedo L.: Chemical and Biological Properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef]
- Ferreira, S.; Santos, J.; Duarte, A.; Duarte, A.P.; Queiroz, J.A.; Domingues, F.C. Screening of Antimicrobial Activity of Cistus ladanifer and Arbutus unedo Extracts. Nat. Prod. Res. 2012, 26, 1558–1560. [Google Scholar] [CrossRef]
- Afkir, S.; Nguelefack, T.B.; Aziz, M.; Zoheir, J.; Cuisinaud, G.; Bnouham, M.; Mekhfi, H.; Legssyer, A.; Lahlou, S.; Ziyyat, A. Arbutus unedo Prevents Cardiovascular and Morphological Alterations in L-NAME-Induced Hypertensive Rats. J. Ethnopharmacol. 2008, 116, 288–295. [Google Scholar] [CrossRef]
- Hoang, Y.T.H.; Vu, A.T.L. Sodium Benzoate and Potassium Sorbate in Processed Meat Products Collected in Ho Chi Minh City, Vietnam. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 477. [Google Scholar] [CrossRef]
- Navarro-García, N.; Morte, A.; Pérez-Tornero, O. In Vitro Adventitious Organogenesis and Histological Characterization from Mature Nodal Explants of Citrus limon. Vitro Cell. Dev. Biol.-Plant 2016, 52, 161–173. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus Limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’hallewin, G.; Zaru, M.; et al. Chemical Characterization of Citrus limon Var. Pompia and Incorporation in Phospholipid Vesicles for Skin Delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef]
- Abid, M.M.; Hussain, T.K.; Hwaidi, E.H.; Kadhim, H.I. Effect of Aromatic Oil of Three Plants of Labiatae Gene on Microorganism by Using in Preservation of Minced Meat. J. Pharm. Negat. Results 2022, 13, 686–689. [Google Scholar] [CrossRef]
- Mesquita Júnior, G.A.D.; Da Costa, Y.F.G.; Mello, V.D.; Costa, F.F.; Rodarte, M.P.; Costa, J.D.C.D.; Alves, M.S.; Vilela, F.M.P. Chemical Characterisation by UPLC-Q-ToF-MS/MS and Antibacterial Potential of Coffea arabica L. Leaves: A Coffee By-product. Phytochem. Anal. 2022, 33, 1036–1044. [Google Scholar] [CrossRef]
- Cavatte, P.C.; Rodríguez-López, N.F.; Martins, S.C.V.; Mattos, M.S.; Sanglard, L.M.V.P.; DaMatta, F.M. Functional Analysis of the Relative Growth Rate, Chemical Composition, Construction and Maintenance Costs, and the Payback Time of Coffea arabica L. Leaves in Response to Light and Water Availability. J. Exp. Bot. 2012, 63, 3071–3082. [Google Scholar] [CrossRef]
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef]
- Gallardo-Ignacio, J.; Santibáñez, A.; Oropeza-Mariano, O.; Salazar, R.; Montiel-Ruiz, R.M.; Cabrera-Hilerio, S.; Gonzáles-Cortazar, M.; Cruz-Sosa, F.; Nicasio-Torres, P. Chemical and Biological Characterization of Green and Processed Coffee Beans from Coffea arabica Varieties. Molecules 2023, 28, 4685. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Santos, E.A.; Regis, W.C.B.; Ferreira, I.C.F.R. The Powerful in Vitro Bioactivity of Euterpe oleracea Mart. Seeds and Related Phenolic Compounds. Ind. Crops Prod. 2015, 76, 318–322. [Google Scholar] [CrossRef]
- Brunschwig, C.; Leba, L.-J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.-C. Chemical Composition and Antioxidant Activity of Euterpe oleracea Roots and Leaflets. Int. J. Mol. Sci. 2016, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.R.; Do Amaral, F.R.L.; Brum, F.L.; Mohana-Borges, R.; De Moura, S.S.T.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.S.; Figueiredo, N.G.; Silva, A.S.D. Chemical Characterization, Antioxidant and Antimicrobial Activities of Açaí Seed (Euterpe oleracea Mart.) Extracts Containing A- and B-Type Procyanidins. LWT 2020, 132, 109830. [Google Scholar] [CrossRef]
- Galotta, A.L.Q.A.; Boaventura, M.A.D.; Lima, L.A.R.S. Antioxidant and Cytotoxic Activities of “açaí” (Euterpe precatoria Mart.). Quím. Nova 2008, 31, 1427–1430. [Google Scholar] [CrossRef]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea Mart.)—A Phytochemical and Pharmacological Assessment of the Species’ Health Claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.; Moon, J.Y.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Comparative Antioxidant and Antiproliferative Activities of Red and White Pitayas and Their Correlation with Flavonoid and Polyphenol Content. J. Food Sci. 2011, 76, C38–C45. [Google Scholar] [CrossRef]
- Joshi, M.; Prabhakar, B. Phytoconstituents and Pharmaco-therapeutic Benefits of Pitaya: A Wonder Fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef]
- Quispe Lupuche, E.; Chávez Pérez, J.A.; Medina-Pizzali, M.L.; Loayza Gutiérrez, L.; Apumayta Suárez, E. Chemical Characterization, Polyphenol Content and Antioxidant Capacity of Two Pitahaya Ecotypes (Hylocereus spp.). Rev. Fac. Nac. Agron. Medellín 2021, 74, 9723–9734. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Effectiveness of Submicron Chitosan Dispersions in Controlling Anthracnose and Maintaining Quality of Dragon Fruit. Postharvest Biol. Technol. 2013, 86, 147–153. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical Composition and in Vitroevaluation of the Cytotoxic and Antioxidant Activities of Supercritical Carbon Dioxide Extracts of Pitaya (Dragon Fruit) Peel. Chem. Cent. J. 2014, 8, 1. [Google Scholar] [CrossRef]
- Attar, Ş.H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional Analysis of Red-Purple and White-Fleshed Pitaya (Hylocereus) Species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef] [PubMed]
- Do, T.V.T.; Fan, L.; Suhartini, W.; Girmatsion, M. Gac (Momordica cochinchinensis Spreng) Fruit: A Functional Food and Medicinal Resource. J. Funct. Foods 2019, 62, 103512. [Google Scholar] [CrossRef]
- Aamir, M.; Jittanit, W. Ohmic Heating Treatment for Gac Aril Oil Extraction: Effects on Extraction Efficiency, Physical Properties and Some Bioactive Compounds. Innov. Food Sci. Emerg. Technol. 2017, 41, 224–234. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Encapsulation of Carotenoid-Rich Oil from Gac Peel: Optimisation of the Encapsulating Process Using a Spray Drier and the Storage Stability of Encapsulated Powder. Powder Technol. 2019, 344, 373–379. [Google Scholar] [CrossRef]
- Kubola, J.; Meeso, N.; Siriamornpun, S. Lycopene and Beta Carotene Concentration in Aril Oil of Gac (Momordica cochinchinensis Spreng) as Influenced by Aril-Drying Process and Solvents Extraction. Food Res. Int. 2013, 50, 664–669. [Google Scholar] [CrossRef]
- Jung, K.; Lee, D.; Yu, J.S.; Namgung, H.; Kang, K.S.; Kim, K.H. Protective Effect and Mechanism of Action of Saponins Isolated from the Seeds of Gac (Momordica cochinchinensis Spreng.) against Cisplatin-Induced Damage in LLC-PK1 Kidney Cells. Bioorganic Med. Chem. Lett. 2016, 26, 1466–1470. [Google Scholar] [CrossRef]
- Le, A.V.; Huynh, T.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines 2018, 5, 104. [Google Scholar] [CrossRef]
- Alezandro, M.R.; Dubé, P.; Desjardins, Y.; Lajolo, F.M.; Genovese, M.I. Comparative Study of Chemical and Phenolic Compositions of Two Species of Jaboticaba: Myrciaria jaboticaba (Vell.) Berg and Myrciaria cauliflora (Mart.) O. Berg. Food Res. Int. 2013, 54, 468–477. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Granato, D.; Maciel, L.G.; Weinert, P.L.; Prado-Silva, L.D.; Alvarenga, V.O.; De Souza Sant’Ana, A.; Bataglion, G.A.; Eberlin, M.N.; Rosso, N.D. Jabuticaba (Myrciaria cauliflora) Seeds: Chemical Characterization and Extraction of Antioxidant and Antimicrobial Compounds. J. Food Sci. 2016, 81, C2206–C2217. [Google Scholar] [CrossRef]
- Estévez, M. Critical Overview of the Use of Plant Antioxidants in the Meat Industry: Opportunities, Innovative Applications and Future Perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant Potential of Rat Plasma by Administration of Freeze-Dried Jaboticaba Peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.D.; Barbosa, J.M.G.; Ribeiro Da Silva, A.J.; Ferri, P.H.; Santos, S.C. Polyphenol and Ellagitannin Constituents of Jabuticaba (Myrciaria cauliflora) and Chemical Variability at Different Stages of Fruit Development. J. Agric. Food Chem. 2017, 65, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.V.D.N.; Cordeiro, M.F.; E Lins, T.U.L.; Sampaio, M.C.P.D.; De Mello, G.S.V.; Da Costa, V.D.C.M.; Marques, L.L.M.; Klein, T.; De Mello, J.C.P.; Cavalcanti, I.M.F. Evaluation of Antibacterial, Antineoplastic, and Immunomodulatory Activity of Paullinia cupana Seeds Crude Extract and Ethyl-Acetate Fraction. Evid.-Based Complement. Altern. Med. 2016, 2016, 1203274. [Google Scholar] [CrossRef]
- Da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; De Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; De Jesus, R.M. Chemical Profiling of Guarana Seeds (Paullinia cupana) from Different Geographical Origins Using UPLC-QTOF-MS Combined with Chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef]
- De Oliveira Campos, M.P.; Riechelmann, R.; Martins, L.C.; Hassan, B.J.; Casa, F.B.A.; Giglio, A.D. Guarana (Paullinia cupana) Improves Fatigue in Breast Cancer Patients Undergoing Systemic Chemotherapy. J. Altern. Complement. Med. 2011, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, G.I.; Marino, F.; Cosentino, M. Effects of a Commercial Product Containing Guaraná on Psychological Well-Being, Anxiety and Mood: A Single-Blind, Placebo-Controlled Study in Healthy Subjects. J. Negat. Results BioMed. 2013, 12, 9. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; Gelain, D.P.; Kolling, E.A.; Rybarczyk-Filho, J.L.; Ambrosi, P.; Resende Terra, S.; Pires, A.S.; Da Rocha, J.B.T.; Antônio Behr, G.; Fonseca Moreira, J.C. Major Components of Energy Drinks (Caffeine, Taurine, and Guarana) Exert Cytotoxic Effects on Human Neuronal SH-SY5Y Cells by Decreasing Reactive Oxygen Species Production. Oxidative Med. Cell. Longev. 2013, 2013, 791795. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Röhrig, T.; Richling, E.; Wang, X.; Wink, M. Anti-Aging and Antioxidant Potential of Paullinia cupana Var. Sorbilis: Findings in Caenorhabditis Elegans Indicate a New Utilization for Roasted Seeds of Guarana. Medicines 2017, 4, 61. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Plum (Prunus domestica): Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Springer International Publishing: Cham, Switzerland, 2021; pp. 169–179. ISBN 978-3-030-75501-0. [Google Scholar]
- Michalska, A.; Wojdyło, A.; Łysiak, G.; Figiel, A. Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef]
- Nergiz, C.; Yıldız, H. Research on Chemical Composition of Some Varieties of European Plums (Prunus domestica) Adapted to the Aegean District of Turkey. J. Agric. Food Chem. 1997, 45, 2820–2823. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Tešić, Ž.; Kalaba, M.; Ćirić, I.; Pezo, L.; Lončar, B.; Gašić, U.; Dojčinović, B.; Tosti, T.; Meland, M. Breakthrough Analysis of Chemical Composition and Applied Chemometrics of European Plum Cultivars Grown in Norway. Horticulturae 2023, 9, 477. [Google Scholar] [CrossRef]
- Vio-Michaelis, S.; Feucht, W.; Gómez, M.; Hadersdorfer, J.; Treutter, D.; Schwab, W. Histochemical Analysis of Anthocyanins, Carotenoids, and Flavan-3-Ols/Proanthocyanidins in Prunus domestica L. Fruits during Ripening. J. Agric. Food Chem. 2020, 68, 2880–2890. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant Capacities, Phenolic Compounds, Carotenoids, and Vitamin C Contents of Nectarine, Peach, and Plum Cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Poonam, V.; Raunak; Kumar, G.; Reddy L., C.S.; Jain, R.; Sharma, S.K.; Prasad, A.K.; Parmar, V.S. Chemical Constituents of the Genus Prunus and Their Medicinal Properties. Curr. Med. Chem. 2011, 18, 3758–3824. [Google Scholar] [CrossRef]
- Zezulová, E.; Ondrášek, I.; Kiss, T.; Nečas, T. Qualitative and Nutritional Characteristics of Plum Cultivars Grown on Different Rootstocks. Horticulturae 2022, 8, 1123. [Google Scholar] [CrossRef]
- Lozano, M.; Vidal-Aragón, M.C.; Hernández, M.T.; Ayuso, M.C.; Bernalte, M.J.; García, J.; Velardo, B. Physicochemical and Nutritional Properties and Volatile Constituents of Six Japanese Plum (Prunus salicina Lindl.) Cultivars. Eur. Food Res. Technol. 2009, 228, 403–410. [Google Scholar] [CrossRef]
- Şengül, M.; Karataş, N.; Zor, M.; Topdas, E.F.; Yilmaz, B. Screening of the Chemical Composition and Antioxidant Activity of the Prunus salicina Pestil. Erzincan Üniversitesi Fen Bilim. Enstitüsü Derg. 2020, 13, 1317–1333. [Google Scholar] [CrossRef]
- Liu, G.; Wei, P.; Tang, Y.; Pang, Y.; Sun, J.; Li, J.; Rao, C.; Wu, C.; He, X.; Li, L.; et al. Evaluation of Bioactive Compounds and Bioactivities in Plum (Prunus salicina Lindl.) Wine. Front. Nutr. 2021, 8, 766415. [Google Scholar] [CrossRef]
- Bei, M.F.; Apahidean, A.I.; Budău, R.; Rosan, C.A.; Popovici, R.; Memete, A.R.; Domocoș, D.; Vicas, S.I. An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry. Horticulturae 2023, 10, 29. [Google Scholar] [CrossRef]
- Karakas, N.; Okur, M.E.; Ozturk, I.; Ayla, S.; Karadağ, A.E.; Polat, D.Ç. Antioxidant Activity and Cytotoxic Effects of Prunus spinosa L. Fruit Extract on Various Cancer Cell Lines. Medeni. Med. J. 2019, 39, 297–304. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic Compounds of Blackthorn (Prunus spinosa L.) and Influence of in Vitro Digestion on Their Antioxidant Capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Condello, M.; Meschini, S. Role of Natural Antioxidant Products in Colorectal Cancer Disease: A Focus on a Natural Compound Derived from Prunus spinosa, Trigno Ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules 2022, 27, 3302. [Google Scholar] [CrossRef] [PubMed]
- Coppari, S.; Colomba, M.; Fraternale, D.; Brinkmann, V.; Romeo, M.; Rocchi, M.B.L.; Di Giacomo, B.; Mari, M.; Guidi, L.; Ramakrishna, S.; et al. Antioxidant and Anti-Inflammaging Ability of Prune (Prunus spinosa L.) Extract Result in Improved Wound Healing Efficacy. Antioxidants 2021, 10, 374. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of Polyphenolic Profile and Antibacterial Activity of Pomegranate Peel (Punica granatum) Flour Obtained from Co-Product of Juice Extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Salah El Dine, R.; Ma, Q.; Kandil, Z.A.; El-Halawany, A.M. Triterpenes as Uncompetitive Inhibitors of α-Glucosidase from Flowers of Punica granatum L. Nat. Prod. Res. 2014, 28, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate Juice, Total Pomegranate Ellagitannins, and Punicalagin Suppress Inflammatory Cell Signaling in Colon Cancer Cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S. Antimicrobial Activity of Pomegranate (Punica granatum L.) Fruit Peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Gironi, G.; Servili, M. Chemical Composition, Antioxidant Activity, and Sensory Characterization of Commercial Pomegranate Juices. Antioxidants 2021, 10, 1381. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Singh, S.; Verma, R.; Sharma, H. Exploring the Therapeutic Potential and Bioactive Compounds in Pyrus Species. Pharmacol. Res. —Mod. Chin. Med. 2024, 10, 100342. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical Composition and Antioxidant Capacity of Different Anatomical Parts of Pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Öztürk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic Compounds and Chemical Characteristics of Pears (Pyrus communis L.). Int. J. Food Prop. 2015, 18, 536–546. [Google Scholar] [CrossRef]
- Kırca, L.; Kırca, S.; Aygün, A. Organic Acid, Phenolic Compound and Antioxidant Contents of Fresh and Dried Fruits of Pear (Pyrus communis L.) Cultivars. Erwerbs-Obstbau 2023, 65, 677–691. [Google Scholar] [CrossRef]
- Akagić, A.; Oras, A.; Gaši, F.; Meland, M.; Drkenda, P.; Memić, S.; Spaho, N.; Žuljević, S.O.; Jerković, I.; Musić, O.; et al. A Comparative Study of Ten Pear (Pyrus communis L.) Cultivars in Relation to the Content of Sugars, Organic Acids, and Polyphenol Compounds. Foods 2022, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Laaksonen, O.; Tian, Y.; Haikonen, T.; Yang, B. Chemical Composition of Juices Made from Cultivars and Breeding Selections of European Pear (Pyrus communis L.). J. Agric. Food Chem. 2022, 70, 5137–5150. [Google Scholar] [CrossRef]
- Tian, Y.; Laaksonen, O.; Haikonen, H.; Vanag, A.; Ejaz, H.; Linderborg, K.; Karhu, S.; Yang, B. Compositional Diversity among Blackcurrant (Ribes nigrum) Cultivars Originating from European Countries. J. Agric. Food Chem. 2019, 67, 5621–5633. [Google Scholar] [CrossRef]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verrall, S.; Johansson, E.; Rumpunen, K. Phenolic Compounds in Blackcurrant (Ribes nigrum L.) Leaves Relative to Leaf Position and Harvest Date. Food Chem. 2015, 172, 135–142. [Google Scholar] [CrossRef]
- Linnamaa, P.; Nieminen, K.; Koulu, L.; Tuomasjukka, S.; Kallio, H.; Yang, B.; Tahvonen, R.; Savolainen, J. Black Currant Seed Oil Supplementation of Mothers Enhances IFN -γ and Suppresses IL -4 Production in Breast Milk. Pediatr. Allergy Immunol. 2013, 24, 562–566. [Google Scholar] [CrossRef]
- Cortez, R.E.; Gonzalez De Mejia, E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef]
- Wójciak, M.; Mazurek, B.; Tyśkiewicz, K.; Kondracka, M.; Wójcicka, G.; Blicharski, T.; Sowa, I. Blackcurrant (Ribes nigrum L.) Seeds—A Valuable Byproduct for Further Processing. Molecules 2022, 27, 8679. [Google Scholar] [CrossRef] [PubMed]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S. Chemical Composition of Fruits in Some Rose (Rosa spp.) Species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Deliorman Orhan, D.; Hartevioğlu, A.; Küpeli, E.; Yesilada, E. In Vivo Anti-Inflammatory and Antinociceptive Activity of the Crude Extract and Fractions from Rosa canina L. Fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef]
- Živković, J.; Stojković, D.; Petrović, J.; Zdunić, G.; Glamočlija, J.; Soković, M. Rosa canina L.—New Possibilities for an Old Medicinal Herb. Food Funct. 2015, 6, 3687–3692. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A.; Martinez Zapater, J.M.; De Pascual-Teresa Fernandez, S. Composition and Biological Activity of the Algerian Plant Rosa canina L. by HPLC-UV-MS. Arab. J. Chem. 2020, 13, 1105–1119. [Google Scholar] [CrossRef]
- Ozkan, G.; Stübler, A.; Aganovic, K.; Draeger, G.; Esatbeyoglu, T.; Capanoglu, E. A Comparative Study on Physicochemical Properties and in Vitro Bioaccessibility of Bioactive Compounds in Rosehip (Rosa canina L.) Infusions Treated by Non-thermal and Thermal Treatments. Food Process. Preserv. 2022, 46, e16096. [Google Scholar] [CrossRef]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium oxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef]
- Šedbarė, R.; Sprainaitytė, S.; Baublys, G.; Viskelis, J.; Janulis, V. Phytochemical Composition of Cranberry (Vaccinium oxycoccos L.) Fruits Growing in Protected Areas of Lithuania. Plants 2023, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Shareef, S.M.; Khaleel, R.A.; Maryoosh, T.M. Nephroprotective Effect of Cranberry (Vaccinium oxycoccos) in Streptozocin-Induced Diabetic Nephropathy in Mice. Drug Metab. Pers. Ther. 2024, 39, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shamilov, A.A.; Bubenchikova, V.N.; Chernikov, M.V.; Pozdnyakov, D.I.; Garsiya, E.R. Vaccinium vitis-idaea L.: Chemical Contents, Pharmacological Activities. Pharm. Sci. 2020, 26, 344–362. [Google Scholar] [CrossRef]
- Amundsen, M.; Hykkerud, A.L.; Kelanne, N.; Tuominen, S.; Schmidt, G.; Laaksonen, O.; Yang, B.; Martinussen, I.; Jaakola, L.; Aaby, K. Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages. Foods 2023, 12, 2154. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, P.; Gradinarska, D.; Dobreva, V.; Ivanov, I.; Petkova, N. Effects of Lingonberry Extract (Vaccinium vitis-idaea L.) on the Antioxidant, Physicochemical and Sensory Characteristics of Ice Cream. BIO Web Conf. 2022, 45, 01008. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Nie, C.; Wang, T.; Qin, X.; Yang, J.; Chang, Y.; Nie, S.; Fu, Y. Comprehensive Phytochemical Analysis of Lingonberry (Vaccinium vitis-idaea L.) from Different Regions of China and Their Potential Antioxidant and Antiproliferative Activities. RSC Adv. 2023, 13, 29438–29449. [Google Scholar] [CrossRef] [PubMed]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Kurt-Celebi, A.; Colak, N.; Hayirlioglu-Ayaz, S.; Kostadinović Veličkovska, S.; Ilieva, F.; Esatbeyoglu, T.; Ayaz, F.A. Accumulation of Phenolic Compounds and Antioxidant Capacity during Berry Development in Black ‘Isabel’ Grape (Vitis vinifera L. × Vitis labrusca L.). Molecules 2020, 25, 3845. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Vélez, M.D.; Llano-Ramirez, M.A.; Ramón, C.; Rojas, J.; Bedoya, C.; Arango-Varela, S.; Santa-González, G.A.; Gil, M. Antioxidant Capacity and Cytotoxic Effect of an Optimized Extract of Isabella Grape (Vitis labrusca) on Breast Cancer Cells. Heliyon 2023, 9, e16540. [Google Scholar] [CrossRef]
- Ghafoor, K.; AL Juhaimi, F.; Yong, A.; Choi, H. Effects of Grape (Vitis labrusca B.) Peel and Seed Extracts on Phenolics, Antioxidants and Anthocyanins in Grape Juice. Pak. J. Bot. 2011, 43, 1581–1586. [Google Scholar]
- Yamamoto, L.Y.; Koyama, R.; Assis, A.M.D.; Borges, W.F.S.; Oliveira, I.R.D.; Roberto, S.R. Color of Berry and Juice of “Isabel” Grape Treated with Abscisic Acid in Different Ripening Stages. Pesq. Agropec. Bras. 2015, 50, 1160–1167. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic Composition of the Edible Parts (Flesh and Skin) of Bordô Grape (Vitis labrusca) Using HPLC–DAD–ESI-MS/MS. J. Agric. Food Chem. 2011, 59, 13136–13146. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical Composition of Grape Stalks of Vitis vinifera L. from Red Grape Pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical Composition and Bioactive Compounds of Grape Pomace (Vitis vinifera L.), Benitaka Variety, Grown in the Semiarid Region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef]
- De Moura, R.S.; Viana, F.S.C.; Souza, M.A.V.; Kovary, K.; Guedes, D.C.; Oliveira, E.P.B.; Rubenich, L.M.S.; Carvalho, L.C.R.M.; Oliveira, R.M.; Tano, T.; et al. Antihypertensive, Vasodilator and Antioxidant Effects of a Vinifera Grape Skin Extract. J. Pharm. Pharmacol. 2010, 54, 1515–1520. [Google Scholar] [CrossRef]
- Cuevas, V.M.; Calzado, Y.R.; Guerra, Y.P.; Yera, A.O.; Despaigne, S.J.; Ferreiro, R.M.; Quintana, D.C. Effects of Grape Seed Extract, Vitamin C, and Vitamin E on Ethanol- and Aspirin-Induced Ulcers. Adv. Pharmacol. Sci. 2011, 2011, 740687. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.-L.; Haroutounian, S.A. Grape Stem Extracts: Polyphenolic Content and Assessment of Their in Vitro Antioxidant Properties. LWT—Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Eckhardt, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Coffee Cherry (Cascara) Fruit Products for Flour Replacement and Other Alternative Food Uses. Molecules 2022, 27, 8435. [Google Scholar] [CrossRef]
- Gil-Gómez, J.A.; Florez-Pardo, L.M.; Leguizamón-Vargas, Y.C. Valorization of Coffee By-Products in the Industry, a Vision towards Circular Economy. Discov. Appl. Sci. 2024, 6, 480. [Google Scholar] [CrossRef]
- Magar, S.; Thagunna, B.; Lamichhane, B.; Pokhrel, B.; Kau, J. Study on Coffee Cherry Its Chemical Characteristics, Processing and Its By-Product Utilization in Food: A Review. Malays. J. Halal Res. (MJHR) 2023, 6, 34–38. [Google Scholar] [CrossRef]
- ChemDraw. Available online: https://revvitysignals.com/products/research/chemdraw (accessed on 20 October 2024).
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-Based Natural Antioxidants in Meat and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef]
- Wang, S.Y. Maximizing Antioxidants in Fruits. Acta Hortic. 2010, 877, 81–93. [Google Scholar] [CrossRef]
- Wang, S.Y. Effect of Pre-Harvest Conditions on Antioxidant Capacity in Fruits. Acta Hortic. 2006, 712, 299–306. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Franco, D. Advances in Natural Antioxidants for Food Improvement. Antioxidants 2022, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Martuscelli, M.; Esposito, L.; Mastrocola, D. The Role of Coffee Silver Skin against Oxidative Phenomena in Newly Formulated Chicken Meat Burgers after Cooking. Foods 2021, 10, 1833. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Dos Santos, J.M.; Carvalho, L.T.; Borgonovi, T.F.; Lorenzo, J.M.; Silva-Barretto, A.C.D. Açaí Extract Powder as Natural Antioxidant on Pork Patties during the Refrigerated Storage. Meat Sci. 2022, 184, 108667. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, E.R.B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Da Silva Barretto, A.C. Red Pitaya Extract as Natural Antioxidant in Pork Patties with Total Replacement of Animal Fat. Meat Sci. 2021, 171, 108284. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; Do Amaral Sobral, P.J.; et al. Guarana Seed Extracts as a Useful Strategy to Extend the Shelf Life of Pork Patties: UHPLC-ESI/QTOF Phenolic Profile and Impact on Microbial Inactivation, Lipid and Protein Oxidation and Antioxidant Capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Basanta, M.F.; Rizzo, S.A.; Szerman, N.; Vaudagna, S.R.; Descalzo, A.M.; Gerschenson, L.N.; Pérez, C.D.; Rojas, A.M. Plum (Prunus salicina) Peel and Pulp Microparticles as Natural Antioxidant Additives in Breast Chicken Patties. Food Res. Int. 2018, 106, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Mandic, S.; Savanovic, D.; Velemir, A.; Kalaba, V.; Savanovic, J.; Jokanovic, V. Effect of Incorporating Blackthorn Fruit (Prunus spinosa L.) Extract in Natural Casing on Quality of Kranjska Sausage. Meat Technol. 2018, 59, 80–90. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Zhang, Z.-H.; Li, L.; Xiong, W.; Shi, J.-Y.; Zhao, T.-R.; Fan, J. Antioxidant Effect of Pomegranate Rind Powder Extract, Pomegranate Juice, and Pomegranate Seed Powder Extract as Antioxidants in Raw Ground Pork Meat. Food Sci. Biotechnol. 2013, 22, 1063–1069. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of Pomegranate Peel Nanoparticles on Quality Attributes of Meatballs during Refrigerated Storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant Activity of Black Currant (Ribes nigrum L.) Extract and Its Inhibitory Effect on Lipid and Protein Oxidation of Pork Patties during Chilled Storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef]
- Özalp Özen, B.; Soyer, A. Effect of Plant Extracts on Lipid and Protein Oxidation of Mackerel (Scomber scombrus) Mince during Frozen Storage. J. Food Sci. Technol. 2018, 55, 120–127. [Google Scholar] [CrossRef]
- Lee, C.; Reed, J.D.; Richards, M.P. Ability of Various Polyphenolic Classes from Cranberry To Inhibit Lipid Oxidation in Mechanically Separated Turkey and Cooked Ground Pork. J. Muscle Foods 2006, 17, 248–266. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; Barretto, A.C.D.S. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods 2019, 8, 579. [Google Scholar] [CrossRef]
- Tatiyaborworntham, N.; Oz, F.; Richards, M.P.; Wu, H. Paradoxical Effects of Lipolysis on the Lipid Oxidation in Meat and Meat Products. Food Chem. X 2022, 14, 100317. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-Lipoxygenases by Flavonoids: Structure–Activity Relations and Mode of Action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.A.; Lorenzo, J.M.; Trindade, M.A. Fruit and Agro-Industrial Waste Extracts as Potential Antimicrobials in Meat Products: A Brief Review. Foods 2021, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-Starch Film Containing Pomegranate Peel Extract and Thymus kotschyanus Essential Oil Can Prolong the Shelf Life of Beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Stobnicka, A.; Gniewosz, M. Antimicrobial Protection of Minced Pork Meat with the Use of Swamp Cranberry (Vaccinium oxycoccos L.) Fruit and Pomace Extracts. J. Food Sci. Technol. 2018, 55, 62–71. [Google Scholar] [CrossRef]
- Gadang, V.P.; Hettiarachchy, N.S.; Johnson, M.G.; Owens, C. Evaluation of Antibacterial Activity of Whey Protein Isolate Coating Incorporated with Nisin, Grape Seed Extract, Malic Acid, and EDTA on a Turkey Frankfurter System. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants 2022, 11, 602. [Google Scholar] [CrossRef]
- Aini, N.V.; Elfidasari, D.; Sugoro, I. The Effectiveness of Lime (Citrus aurantifolia) Solution on Quality of Pterygoplichthys pardalis Flesh from Ciliwung River, Jakarta, Indonesia. Int. J. Biosci. Biotech. 2022, 9, 11. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, N.; Agnihotri, V.; Singh, K.K.; Pandey, A. Antioxidant, Antimicrobial Activity and Bioactive Compounds of Bergenia ciliata Sternb.: A Valuable Medicinal Herb of Sikkim Himalaya. J. Tradit. Complement. Med. 2017, 7, 152–157. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Cao, Y. Characterization of Potent Odorants Causing Meaty Odor Reduction in Thermal Process Flavorings with Beef-like Odor by the Sensomics Approach. Food Chem. 2023, 426, 136649. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, A.; Cho, C.; Baritugo, K.A.; Kim, B.; Ullah, Q.; Rahman, A.; Park, S. Production and Analytical Aspects of Natural Pigments to Enhance Alternative Meat Product Color. Foods 2023, 12, 1281. [Google Scholar] [CrossRef] [PubMed]
- Sarewicz, M.; Pintscher, S.; Pietras, R.; Borek, A.; Bujnowicz, Ł.; Hanke, G.; Cramer, W.A.; Finazzi, G.; Osyczka, A. Catalytic Reactions and Energy Conservation in the Cytochrome Bc1 and b6f Complexes of Energy-Transducing Membranes. Chem. Rev. 2021, 121, 2020–2108. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Ağaoğlu, S. Antioxidant, Antimicrobial and Metmyoglobin Reducing Activity of Artichoke (Cynara scolymus) Powder Extract-Added Minced Meat during Frozen Storage. Molecules 2021, 26, 5494. [Google Scholar] [CrossRef]
- Palanisamy, S.; Singh, A.; Zhang, B.; Zhao, Q.; Benjakul, S. Effects of Different Phenolic Compounds on the Redox State of Myoglobin and Prevention of Discoloration, Lipid and Protein Oxidation of Refrigerated Longtail Tuna (Thunnus tonggol) Slices. Foods 2024, 13, 1238. [Google Scholar] [CrossRef]
- Jayanthi Antonisamy, A.; Marimuthu, S.; Malayandi, S.; Rajendran, K.; Lin, Y.-C.; Andaluri, G.; Lee, S.L.; Ponnusamy, V.K. Sustainable Approaches on Industrial Food Wastes to Value-Added Products—A Review on Extraction Methods, Characterizations, and Its Biomedical Applications. Environ. Res. 2023, 217, 114758. [Google Scholar] [CrossRef]
- Hoa, V.-B.; Cho, S.-H.; Seong, P.-N.; Kang, S.-M.; Kim, Y.-S.; Moon, S.-S.; Choi, Y.-M.; Kim, J.-H.; Seol, K.-H. The Significant Influences of pH, Temperature and Fatty Acids on Meat Myoglobin Oxidation: A Model Study. J. Food Sci. Technol. 2021, 58, 3972–3980. [Google Scholar] [CrossRef]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Promoting Anti-listeria Activity of Lemongrass Oil on Pork Loin by Cold Nitrogen Plasma Assist. J. Food Saf. 2017, 37, e12316. [Google Scholar] [CrossRef]
| Scientific/Common Name | Photos * | Chemical Composition | Overall Biological Properties |
|---|---|---|---|
| Ananas sativus Schult. & Schult.f./Pineapple |  | Phenolic acids: gallic acid, syringic acid, ferulic acid, vanillin, sinapic acid, coumaric acid, chlorogenic acid, arbutin; Nutrients: Sucrose, glucose, fructose, vitamin C, vitamin D, vitamin E, calcium, iron, zinc, citric, malic; Other: bromelain [46,47,48]. | Antioxidant, anti-inflammatory, antibacterial, antifungal, and anticancer activities [49,50]. |
| Arbutus unedo L./Strawberry |  | Phenolic acids: ellagic acid, cholorogenic acid; Flavanols: catechin, procyanidin; Anthocyanins: cyanidin-3-glucoside; Fatty acids: α-Linolenic, linoleic acid (ω-6); Nutrients: vitamin E, vitamin C [51,52,53]. | Antibacterial, antiallergic, hepatoprotective, antithrombotic, antiviral, urinary antiseptic, anti-inflammatory, anti-diarrheal, anti-hypertension, anti-diabetic, anticarcinogenic, and vasodilator effects [54,55,56]. |
| Citrus limon (L.) Osbeck/Lemon |  | Flavanones: eriodictyol, hesperidin, hesperetin, naringin; Flavones: apigenin, diosmin; flavonols: quercetin, lymphocitrin; Essential oils: limonene, β-pinene, γ-terpinene, α-pinene, myrcene, sabinene, geranial; Nutrients: copper, iron, magnesium, zinc, vitamin C, citric acid, malic acid, oxalic acid, malonic acid [57,58,59]. | Antimicrobial, antiparasitic, antibacterial, antifungal, anti-inflammatory, anticancer, hepatoprotective, and cardioprotective activities [60,61]. |
| A combination of Coffea arabica L. and Coffea canephora Pierre ex A.Froehner/Coffee cherry |  | Phenolic acids: vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, gentosic acid, caftaric acid; Flavonols: quercetin, kaempferol, isoquercitrin, patuletin; Flavones: apigenin, luteolin; Flavanols: (+)-catechin, (−)-epicatechin; Anthocyanins: cyanidin-3-o-glucoside, cyanidin-3-o-rutinoside; Alkaloids: caffeine, theobromine, theophyline; Fatty acids: linoleic acid (ω-6), palmitic acid; Nutrients: riboflavin (vitamin B2), niacin (vitamin B3), magnesium, potassium [62,63]. | Antibacterial, anti-inflammatory, analgesic, vasodilator, anti-allergic, and anticancer effects [64,65]. |
| Euterpe oleracea Mart./Açai |  | Phenolic acids: vanillic acid, syringic acid, protocatechuic acid, ferulic acid; Flavanols: (+)-catechin, (−)-epicatechin; Flavones: apigenin, luteolin; Anthocyanins: cyanidin 3-rutinoside, cyanidin 3-glucoside; Fatty acids: palmitic acid, linoleic acid (ω-6); Nutrients: vitamin C, vitamin E, calcium, magnesium, potassium, manganese [66,67,68]. | Antioxidant activity, anti-nociceptive, anticancer, anti-proliferative, anti-inflammatory, and cardioprotective activities [69,70,71]. |
| Hylocereus monacanthus (Lem.) Britton & Rose/Dragon fruit |  | Phenolic acids: gallic, vanillic, syringic, protocatechuic, p-hydroxybenzoic, p-coumaric, and caffeic acids; Flavonols: quercetin-3-O-b-D-rutinoside, kaempferol-3-neohespedridosoide; Alkaloid: betacyanin; Carotenoid: lycopene; Fatty acids: linoleic acid (ω-6), linolenic acid; Nutrients: magnesium, potassium, phosphorus, vitamin C [72,73,74]. | Antioxidant, antiproliferative, anti-inflammatory, chemopreventive, and anti-diabetic properties [75,76,77]. |
| Momordica cochinchinensis Spreng./Gac |  | Phenolic acids: gallic acid, p-hydroxybenzoic acid; Carotenoids: lycopene, beta-carotene; Saponins: gypsogenin 3-O-β-d-galactopyranosyl(1→2)-[α-l-rhamnopyranosyl(1→3)]-β-d-glucuronopyranoside, quillaic acid 3-O-β-d-galactopyranosyl(1→2)-[α-l-rhamnopyranosyl(1→3)]-β-d-glucuronopyranoside, momordica saponin I; Fatty acids: palmitic and linoleic acids; Nutrients: α-tocopherol, vitamin C [78,79,80]. | Anti-inflammatory, antioxidant, anticancer, and neuroprotective effects [81,82,83]. |
| Myrciaria cauliflora (Mart.) O.Berg/ Jaboticaba |  | Phenolic acids: ellagic acid, chlorogenic acid, sinapic acid; Flavonols: quercetin, quercetin-glucoside; Tannins: pedunculagin, castalagin, vescalagin, cauliflorin; Anthocyanins: cyanidin-3-O-glucoside, delphinidin-3-O-glucoside; Essential oils: β-pinene, linalool; Nutrients: fructose, vitamin C, copper, manganese [84,85,86]. | Antioxidant, antimicrobial, antiproliferative, anti-inflammatory, anticancer, and anti-diabetic effects [87,88]. |
| Paullinia cupana Kunth/Guarana |  | Phenolic acids: gallic acid, ellagic acid; Flavanols: catechin, epicatechin, B-type procyanidin dimer; Alkaloids: caffeine, theobromine, theophylline; Nutrients: polysaccharides, vitamin C, vitamin D, manganese, rubidium, nickel, strontium [89,90,91]. | Anxiolytic, antioxidant, antidepressant, anti-aging, neuroprotective, anticancer, and antimicrobial activities [92,93,94]. |
| Prunus domestica L./Plum |  | Phenolic acids: caffeic acid, 3-O-caffeoylquinic (neochloro-genic acid), 5-O-caffeoylquinic (chlorogenic acid), 4-O-caffeoylquinic (crypto-chlorogenic acid); Fatty acids: linoleic (ω-6), linolenic; Nutrients: disaccharides (sucrose), vitamin C, vitamin E, K, A, thiamine, riboflavin [95,96,97]. | Antioxidant, antimicrobial, hepatoprotective, anti-inflammatory, anti-diabetic, and cytotoxic activity and anticancer properties [98,99,100]. |
| Prunus salicina Lindl./Japanese Plum |  | Phenolic acids: 5-O-caffeoylquinic acid; Flavanols: catechin; Flavones: apigenin; Flavonols: rutin; Anthocyanins: cyanidin-3-monoglucoside, cyanidin-3-rhamnoglucosid; Nutrients: glucose, fructose, sucrose, sorbitol, vitamins (A, C, and E) [98,101,102]. | Antioxidant, anticancer, antihyperglycemic, antihypertensive, anti-allergic, laxative, and diuretic effects [103,104,105]. |
| Prunus spinosa L./Blackthorn |  | Phenolic acids: 3-p-Coumaroylquinic acid, caffeic acid hexoside, 3-Feruloylquinic acid; Flavanols: catechin, epicatechin; Flavonols: quercetin pentoside 2, quercetin-3-glucoside, kaempferol pentoside hexoside; Anthocyanins: Cyanidin pentoside, pelargonidin-3-glucoside, peonidin-3-rutinoside [106,107,108]. | Antibacterial, antioxidant, neurodegenerative, cardiovascular, anti-diabetic, and anticancer properties [109,110,111]. |
| Punica granatum L./Pomegranate |  | Phenolic acids: gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, ferulic acid, cinnamic acid; Flavonols: quercetin, kaempferol; Flavanols: catechin; Tannins: ellagic acid, 1,2,4-tri-O-galloyl-β-glucopyranose; Anthocyanins: delphinidin, cyanidin, pelargonidin; Nutrients: punicic acid, ascorbic acid, malic acid, oxalic acid, tartaric, succinic, quinic acid, potassium, phosphorus, calcium, iron, manganese, zinc, copper [21,112,113]. | Antioxidant, antimicrobial, antiviral, anti-inflammatory, cardiovascular, and anticancer activities [114,115,116,117]. |
| Pyrus communis Thunb./Pear |  | Phenolic acids: 5-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, coumaroylquinic acid isomer, chlorogenic acid, arbutin; Flavanols: catechin, epicatechin; Flavonols: quercetin, kaempferol; Nutrients: fructose, retinol, vitamin C, E, B complex, iron, fiber, copper, potassium, malic acid, succinic acid, citric acid, quinic acid, oxalic acid, tartaric acid [118,119,120]. | Antioxidant, antibacterial, antimutagenic, anticarcinogenic, antifungal, and enzyme-inhibiting effects [121,122,123]. |
| Ribes nigrum L./ Black currant |  | Flavanols: catechin, epicatechin; Flavonols: quercetin, myricetin, kaempferol, isorhamnetin; Anthocyanins: delphinidin-3-O-rutinoside, cyanidin-3-O-rutinoside; Fatty acids: linoleic (ω-6), linolenic; Nutrients: ascorbic acid, calcium, magnesium, phosphorus, zinc; Others: phytoestrogens: naringenin, resveratrol, estrone, β-estradiol [18,124,125]. | Antioxidants, antimicrobials, anti-inflammatory, hypocholesterolemic, phytoestrogenic, and immunomodulating activities [126,127,128]. |
| Rosa canina L./ Dog rose |  | Phenolic acids: caffeic acid, chlorogenic acid, homovanilic acid hexoside, 4-O-caffeoyl-quinic acid, phloretin-C-diglycoside; Flavanones: naringenin; Flavonols: quercetin, myricetin, kaempferol, rutin; Anthocyanins: cyanidin-3-glucoside; Carotenoids: lycopene, β-cryptoxanthin, β-carotene; Fatty acids: linoleic acid (ω-6), linolenic acid; Nutrients: quinic acid, citric acid, vitamin C, calcium, chloride, chromium, cobalt [129,130,131]. | Antioxidant, antimicrobial, anti-inflammatory, cardioprotective, antiulcerogenic, antimutagenic, and anticancerogenic effects [132,133,134]. |
| Vaccinium oxycoccos L./Swamp cranberry |  | Phenolic acids: p-coumaric, sinapic, caffeic, and ferulic acids; Flavonols: quercetin 3-O-rhamnoside, myricetin 3-O-arabinoside, quercetin 3-O-galactoside, myricetin 3-O-galactoside, kaempferol; Flavanols: catechin, (epi) gallocatechins; Anthocyanins: cyanidin-3-O-arabinoside, cyanidin-3-O-galactoside; Nutrients: glucose, fructose, sucrose, citric acid, malic, vitamin C, vitamin E, vitamin K [28,135,136]. | Antioxidant, antibacterial, antifungal, anticancer, antiangiogenic, and anti-inflammatory properties [136,137,138]. |
| Vaccinium vitis-idaea L./Lingonberry or Cranberry |  | Phenolic acids: caffeic acid, cinnamic acid, ferulic acid; Flavanols: epicatechin; procyanidin B1, B3, B7, proanthocyanidin A2; Flavonols: quercetin-3-O-β-galactoside, quercetin-3-O-β-glucoside, kaempferol-pentoside, kaempferol-3-O-glucuronide, avicularin; Anthocyanins: cyaniding-3-glucoside, cyaniding-3-galactoside, cyaniding-3-arabinoside; Saponins: oleanolic acid, ursolic acid; Nutrients: citric acid, mallic acid, tartaric acid, fumaric [139,140,141]. | Antioxidant, antiviral, antifungal, astringent, antiseptic, diuretic, anti-fever, hipoglycemic, and anti-inflammatory effects [142,143,144]. |
| Vitis labrusca Isabella L./Fox grape |  | Phenolic acids: p-coumaric, caffeic, sinapic and ferulic acids; Flavanols: catechin; Flavonols: quercetin, kaempferol, myricetin; Anthocyanins: malvidin, cyanidin, peonidin, delphinidin, pelargonidin, petunidin; Fatty acids: linoleic acid (ω-6), linolenic acid Nutrients: vitamin C, E [145,146,147]. | Antioxidant, antimicrobial, anti-inflammatory, anticancer, antiviral, cardioprotective, neuroprotective, and hepatoprotective activities [148,149,150]. |
| Vitis vinifera L./ Common grape |  | Phenolic acids: caffeic acid, gallic acid, vanillin acid; Flavanols: catechin, epicatechin; Flavonols: quercetin 3-O-rhamnoside, myricetin, quercetin-4-glucoside; Anthocyanins: malvidin-3-O-glucoside, cyanidin-3-glucoside; Fatty acids: palmitic acid, stearic acid; Nutrients: proteins, fiber, malic acid, citric acid, vitamin C; Other: resveratrol [151,152,153]. | Antioxidant, anticancerous, antibacterial, and anti-diabetic effects, cardiovascular diseases, antimicrobial, antihypertensive, and anti-ulcer activities [154,155,156]. |
| Scientific Plant Name/Common Name | Type of Anatomical Part Used/Form Used | Type of Meat Used | Quantity Used | Storage Conditions | Phenolic Content and Antioxidant Capacity | Main Outcomes | References |
|---|---|---|---|---|---|---|---|
| Ananas sativus Schult. & Schult.f./Pineapple | Pineapple peel/ alginate-based edible films | Beef meat | 5% | 5 days at 4 °C | TPC: 3.77 ± 0.02 mg GAE/g dry particles; FRAP: 0.35 ± 0.04 µmol; FeSO4.7H2O/mg dry films | ↑ antioxidant capacity ↓ TBARS values | [20] |
| Arbutus unedo L./Strawberry and Rosa canina L./ Dog rose | Whole fruits/direct in formula incorporation | Frankfurter sausage | 30 g of each fruit | 30 days 4 °C | TPC: 1175 ± 222 mg GAE 100 g−1 and 428 ± 61 mg GAE 100 g−1 | ↑ antioxidant capacity ↓ level of TBARS ↓ protein carbonyl | [12] |
| Coffea arabica L. and Coffea canephora Pierre ex A.Froehner/Coffee cherry | Coffee silver skin/ direct in formula incorporation | Chicken burgers | 1.5%, 3.0% | 3 days at 4 °C | Nd | ↓ level of TBARS ↓ lipid oxidation | [165] |
| Euterpe oleracea Mart./Açai | Fuit powder extract/direct in formula incorporation | Pork patty | 0.025, 0.05, and 0.075% | Packed in nylon–polyethylene bags without vacuum for 10 days in the dark at 2 °C | Nd | ↓ level of TBARS | [166] |
| Hylocereus monacanthus (Lem.) Britton & Rose/Dragon fruit | Whole fruit/pulsed electric field extraction | Pork patties | 250, 500, or 1000 mg/kg | 18 days at 2 °C | TPC: 268.13 mg GAE/100 g; FRAP: 825.40 Fe+2/100 g; DPPH: 229 mg TE/100 g | ↑ antioxidant capacity ↓ level of TBARS ↑ protein carbonyl | [167] |
| Momordica cochinchinensi Spreng./Gac | Aril and pulp powder/encapsulation in maltodextrin | Chicken sausage | 1 g | 7 days at 10 °C | DPPH: 21.05 ± 4.89 (%) | ↑ antioxidant capacity ↓ level of TBARS | [35] |
| Myrciaria cauliflora (Mart.) O.Berg/Jabuticaba | Whole fruit/microencapsulated in spray dryer | Fresh pork sausages | 2 and 4% | 15 days at 1 °C | TPC: 15.63 mg GAE/g; anthocyanin content: 7.21 mg CE/g; FRAP: 20.51 µmol TE/g; DPPH: 52.90 mmol TE/g | ↑ antioxidant capacity ↓ level of TBARS ↑ texture and flavor | [36] |
| Paullinia cupana Kunth/Guarana | Whole fruit/hydro ethanolic extract | Lamb burgers | 250 mg/kg | 18 days at 2 °C | TPC: 258 mg GAE/g; DPPH: 0.3 g/L; TEAC: 2072 μmol TE/g | ↑ antioxidant capacity ↓ level of TBARS ↑ protein carbonyl | [13] |
| Paullinia cupana Kunth/Guarana | Seed powder extract/direct in formula incorporation | Pork patties | 0.025, 0.05, and 0.10% | Modified atmosphere (80% O2 and 20% CO2); under light for 18 days at 2 ± 1 °C | Nd | ↓ level of TBARS ↑ protein carbonyl | [168] |
| Prunus domestica L./Plum | Puree/direct in formula incorporation | Beef patties | 5%, 10%, or 15% | 45 days at −18 °C | Nd | ↓ level of TBARS ↑ juiciness and texture | [19] |
| Prunus salicina Lindl./Japanese plum | Peel and pulp/polyethylene films (oxygen permeability) | Chicken breast patties | 2.0% | 10 days at 4.0 ± 0.5 °C | FRAP: 9–12 mM Fe2+/kg | ↑ antioxidant capacity ↓ level of TBARS | [169] |
| Prunus spinosa L./Blackthorn | Whole fruit/aqueous and ethanol extract | Kranjska sausages | 40 g/kg blackthorn fruit extract, 10 g/kg blackthorn fruit extract | 60 days at 4 °C | Nd | ↓ level of TBARS ↓ level of peroxide | [170] |
| Punica granatum L./Pomegranate | Rind powder extract, juice and seed powder/direct in formula incorporation | Ground pork meat | 20 mg equivalent phenolics/ 100 g meat | 12 days 4 ± 1 °C | Nd | ↓ lipid oxidation ↓ level of peroxide | [171] |
| Punica granatum L./Pomegranate | Peel extract/concentrated lyophilized water extract | Beef meatballs | 0.5 and 1% | 8 days at 4 °C | TPC: 165.4 mg GAE/g; FRSA: 5720 mM TE/g | ↑ antioxidant capacity ↓ level of TBARS ↓ level of peroxide | [14] |
| Punica granatum L./Pomegranate | Peel nanoparticles/ direct in formula incorporation | Freshly minced beef meat | 1% and 1.5% | 15 days at 4 °C | TPC: 215.2 ± 2.23 mg GAE/g; TFC: 70.4 ± 2.69 mg QE/g | ↑ antioxidant capacity ↓ lipid oxidation ↓ level of TBARS | [172] |
| Ribes nigrum L./Black currant | Fruits extract/direct in formula incorporation | Pork patties | 5 g/kg; 10 g/kg 20 g/kg | 9 days at 4 °C | Nd | ↓ lipid oxidation ↓ level of TBARS ↓ protein carbonyls sulfhydryl content | [173] |
| Vitis labrusca Isabella L. Var./Fox grape | Pomace/microencapsulated extract (maltodextrin) | Raw and precooked bovine burger | 6.7 g/kg | 15 days at 4 °C | TPC: 16.6 ± 0.05 mg GAE/g | ↑ antioxidant capacity ↓ level of TBARS ↑ total carbonyl compounds | [37] |
| Vitis vinifera L./Common grape | Seeds and leaves extract/direct in formula incorporation | Fish (Scomber scombrus) mince | 0.01% | 6 months frozen, storage at −18 ± 1 °C | Nd | ↓ level of TBARS ↓ level of peroxide protein carbonyls sulphydryls | [174] |
| Vaccinium vitis-idaea L./ Lingonberry or Cranberry | Concentrate juice powder/50.0% aqueous ethanol (v/v) | Turkey and cooked ground pork | 10% | 14 days at 2 °C | Nd | ↓ level of TBARS | [175] |
| Scientific Plant Name/Common Name | Type of Anatomical Part Used/Form Used | Type of Meat Used | Quantity Used | Storage Conditions | Antimicrobial Effect | References |
|---|---|---|---|---|---|---|
| Ananas sativus Schult. & Schult.f./ Pineapple | Pineapple peel/ alginate-based edible films/ | Beef meat | 5% | 5 days at 4 °C | Pseudomonas spp. | [20] |
| Citrus limon (L.) Osbeck/Lemon | Essential oil/direct in formula incorporation | Minced beef meat | 0.06 and 0.312 mg/g | 10 days storage at 4 °C | Listeria monocytogenes | [182] |
| Punica granatum L./Pomegranate | Peel/films based on corn starch and chitosan | Beef | 0.5 and 1% | 21 days at 4 °C | Pseudomonas spp., lactic acid bacteria, and L. monocytogenes | [183] |
| Pyrus communis Thunb./Pear | Pulp-picked encapsulation in sodium alginate/direct application | Beef burger patties | 0.5% | 8 days at 4 °C | Enterobacteriaceae and Pseudomonas spp. | [34] |
| Vaccinium oxycoccos L./Swamp cranberry | Fruit and pomace extracts/direct in formula incorporation | Minced pork meat | 2.5% | 6 days at 4 °C | Staphylococcus aureus, Listeria monocytogenes, Salmonella Enteritidis, and E. coli | [184] |
| Vitis vinifera L./Common grape | Seed extract/incorporated in whey protein isolate | Turkey frankfurters | 0.5% | 28 days at 4 °C | Escherichia coli, L. monocytogenes, and S. typhimurium, | [185] |
| Fruit By-Product | Meat Product | Color Parameters | References | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| Ananas sativus Schult. & Schult.f. | Beef meat | ↓ | ↑ | - | [20] |
| Arbutus unedo L. and Rosa canina L. | Frankfurter sausage | ↓ | ↑ | ↑ | [12] |
| Coffea arabica L. and Coffea canephora Pierre ex A.Froehner/Coffee | Chicken burger | ↓ | ↑ | - | [165] |
| Euterpe oleracea Mart. | Pork patty | ↓ | ↓ | ↓ | [166] |
| Hylocereus monacanthus (Lem.) Britton & Rose | Pork patties | ↑ | ↓ | ↓ | [167] |
| Momordica cochinchinensi Spreng. | Chicken sausage | ↓ | ↑ | ↑ | [35] |
| Myrciaria cauliflora (Mart.) O.Berg | Fresh pork sausages | ↓ | ↓ | ↓ | [36] |
| Paullinia cupana Kunth | Lamb burgers | ↓ | ↓ | - | [13] |
| Paullinia cupana Kunth | Pork patties | ↓ | ↓ | ↓ | [168] |
| Prunus domestica L. | Beef patties | ↓ | ↑ | ↓ | [19] |
| Prunus salicina Lindl. | Breast chicken patties | ↓ | ↑ | ↓ | [169] |
| Prunus spinosa L. | Kranjska sausages | ns | ns | ↓ | [170] |
| Punica granatum L. | Ground pork meat | ↓ | ↑ | ↓ | [171] |
| Punica granatum L. | Beef meatballs | ↓ | ↑ | ↓ | [14] |
| Ribes nigrum L. | Pork patties | ↓ | ↓ | - | [173] |
| Vitis labrusca Isabella Var L. | Raw and pre-cooked bovine burger | ↑ | ↓ | ↑ | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orădan, A.C.; Tocai, A.C.; Rosan, C.A.; Vicas, S.I. Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants. Processes 2024, 12, 2756. https://doi.org/10.3390/pr12122756
Orădan AC, Tocai AC, Rosan CA, Vicas SI. Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants. Processes. 2024; 12(12):2756. https://doi.org/10.3390/pr12122756
Chicago/Turabian StyleOrădan, Adrian Cristian, Alexandra Cristina Tocai (Moțoc), Cristina Adriana Rosan, and Simona Ioana Vicas. 2024. "Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants" Processes 12, no. 12: 2756. https://doi.org/10.3390/pr12122756
APA StyleOrădan, A. C., Tocai, A. C., Rosan, C. A., & Vicas, S. I. (2024). Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants. Processes, 12(12), 2756. https://doi.org/10.3390/pr12122756







