Photocatalytic Composites Based on Biochar for Antibiotic and Dye Removal in Water Treatment
Abstract
1. Introduction
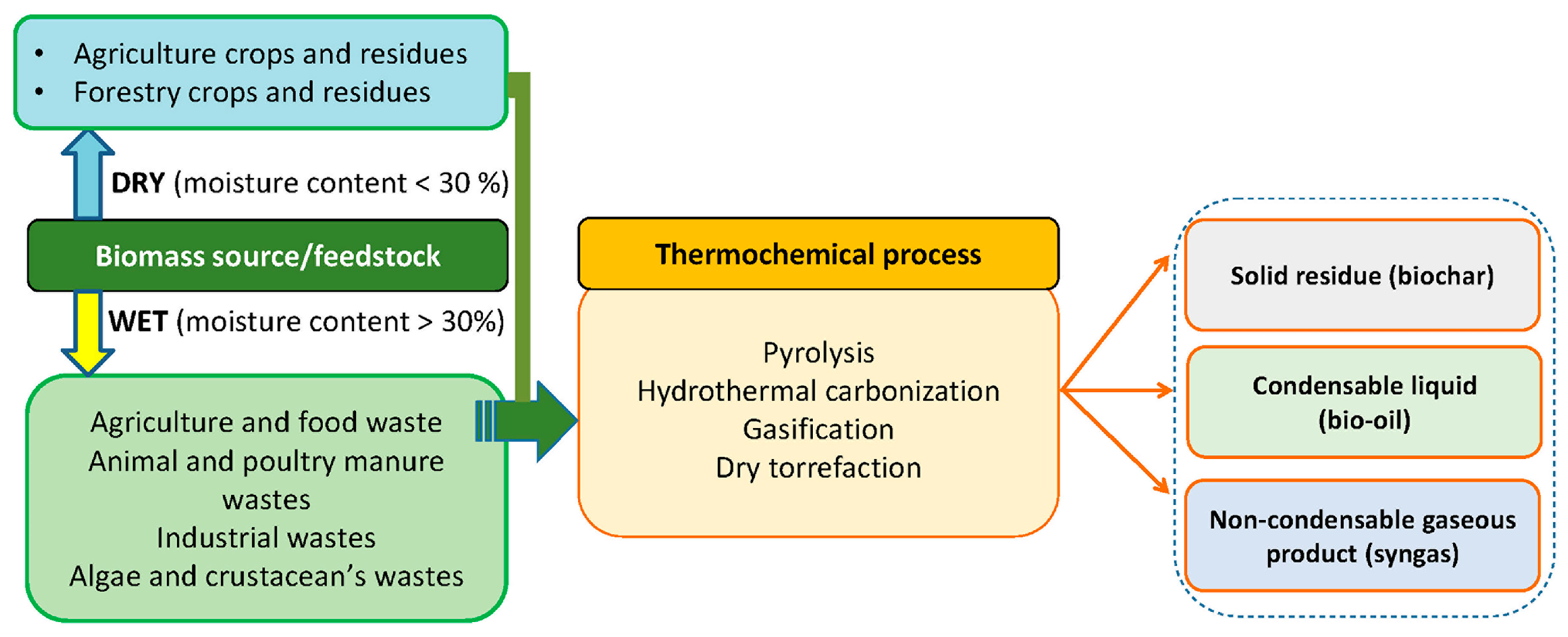
2. Biochar
2.1. Biochar Preparation
2.1.1. Pyrolysis
2.1.2. Hydrothermal Carbonization
2.1.3. Gasification
2.1.4. Dry Torrefaction
2.2. Biochar Sources
2.2.1. Agriculture and Food Waste
2.2.2. Animal and Poultry Manure Wastes
2.2.3. Industrial Wastes
2.2.4. Algae and Crustacean’s Wastes
2.3. Biochar Structure and Physico-Chemical Properties
3. Preparation of Biochar-Based Photocatalytic Composites
3.1. Sol–Gel Synthesis
3.2. Ultrasound-Assisted Synthesis
3.3. Thermal Polycondensation
3.4. Solvothermal Synthesis
3.5. Hydrothermal Synthesis
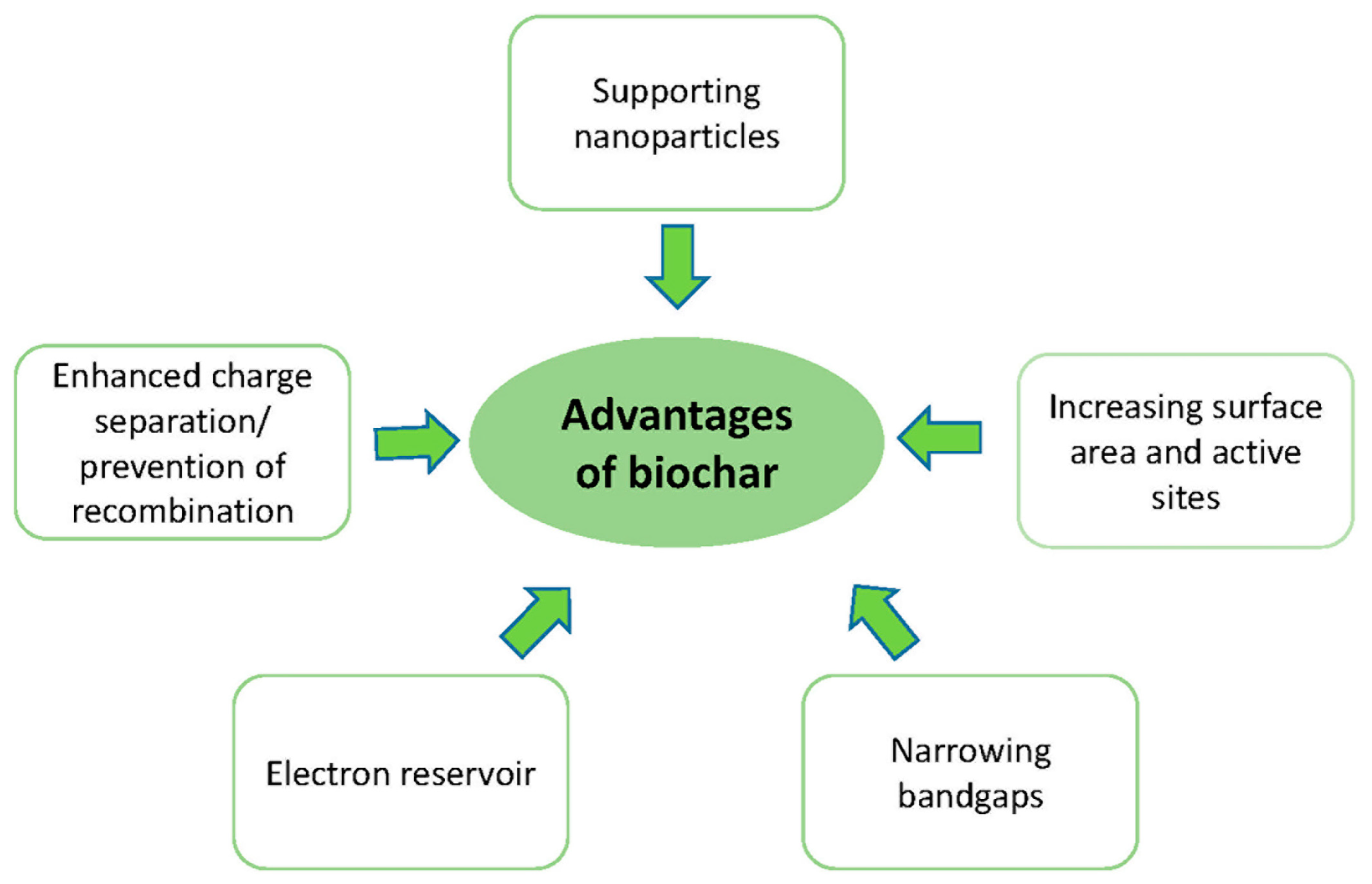
4. Advantages of Biochar in Photocatalytic Degradation of Pollutants
4.1. Supporting Nanoparticles
4.2. Increasing Surface Area and Active Sites
4.3. Narrowing Bandgap
4.4. Electron Reservoir (Sink)
4.5. Enhanced Charge Separation/Prevention Recombination
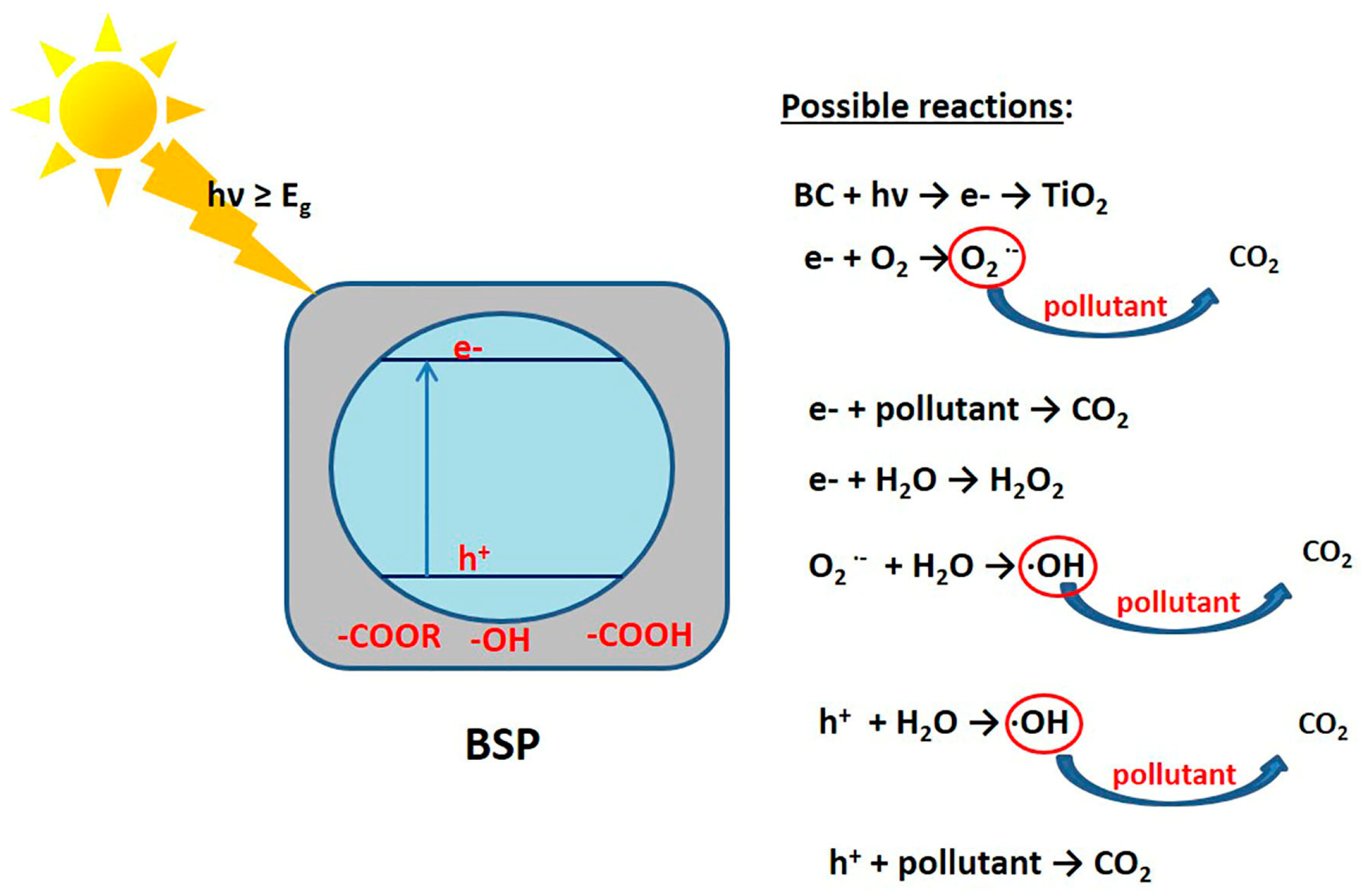
5. Photodegradation Mechanism of Biochar-Supported Photocatalyst
5.1. Adsorption
5.2. Photodegradation
6. Application of Photocatalytic Composites Based on Biochar for the Removal of Organic Contaminants
6.1. Antibiotics
6.2. Dyes
7. Discussion and Future Prospective
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Freshwater Crisis, National Geographic. Available online: https://www.nationalgeographic.com/environment/article/freshwater-crisis (accessed on 10 July 2024).
- Water Scarcity. Available online: https://www.worldwildlife.org/threats/water-scarcity (accessed on 10 July 2024).
- Subramaniam, M.N.; Wu, Z.; Goh, P.S.; Zhou, S. The state-of-the-art development of biochar based photocatalyst for removal of various organic pollutants in wastewater. J. Clean. Prod. 2023, 429, 139487. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Q.; Tan, C.; Lim, T.T.; Huang, L.; Zhang, H. Carbonbased sorbents with three-dimensional architectures for water remediation. Small 2015, 11, 3319–3336. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Water Development Report; Wastewater: The Untapped Resource; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Global Antibiotic Production Market—Industry Trends and Forecast to 2029, 22 Jully 2022, Data Bridge Market Research. Available online: https://www.databridgemarketresearch.com/reports/global-antibiotic-production-market (accessed on 11 July 2024).
- Dyestuff and Pigments Market Trends 2023—Global Outlook. Available online: https://www.linkedin.com/pulse/dyestuff-pigments-market-2023-2030-global-industry-gathf (accessed on 11 July 2024).
- Dyes And Pigments Market Size, Share & Trends Analysis Report By Product (Dyes (Reactive, Vat, Acid, Direct, Disperse), Pigment (Organic, Inorganic)), by Application, by Region, and Segment Forecasts, 2023–2030. Market Analysis Report. Available online: https://www.grandviewresearch.com/industry-analysis/dyes-and-pigments-market (accessed on 11 July 2024).
- Progress Towards the Sustainable Development Goals, 2024, UN, A/79/79-E/2024/54. Available online: https://unstats.un.org/sdgs/files/report/2024/SG-SDG-Progress-Report-2024-advanced-unedited-version.pdf (accessed on 24 October 2024).
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar—A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, F.; Li, H.; Cui, J.; Ren, Y.; Yu, X. Recent Progress in Biochar-Based Photocatalysts for Wastewater Treatment: Synthesis, Mechanisms, and Applications. Appl. Sci. 2020, 10, 1019. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Cao, H.; Milan, Y.J.; Mood, S.H.; Ayiania, M.; Zhang, S.; Gong, X.; Lora, S.E.E.; Yuan, Q.; Garcia-Perez, M. A novel elemental composition based prediction model for biochar aromaticity derived from machine learning. Artif. Intell. Agric. 2021, 5, 133–141. [Google Scholar] [CrossRef]
- Rathnayake, D.M.; Schmidt, H.-P.; Leifeld, J.; Mayer, J.; Epper, C.A.; Bucheli, T.D.; Hagemann, N. Biochar from animal manure: A critical assessment on technical feasibility, economic viability, and ecological impact. GCB Bioenergy 2023, 15, 1078–1104. [Google Scholar] [CrossRef]
- Lee, J.; Sarmah, A.K.; Kwon, E.E. Production and Formation of Biochar. In Biochar from Biomass and Waste: Fundamentals and Applications; Daniel, Y.S.O., Tsang, C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Pecha, B.; Hills, K.M.; Garcia-Pérez, M.; Hunt, J.; Miles, T.R.; Wilson, K.; Amonette, J.E. Biochar Production. In Biomass to Biochar: Maximizing the Carbon Value; Amonette, J.E., Archuleta, J.G., Fuchs, M.R., Hills, K.M., Yorgey, G.G., Flora, G., Hunt, J., Han, H.-S., Jobson, B.T., Miles, T.R., et al., Eds.; Report by Center for Sustaining Agriculture and Natural Resources; Washington State University: Pullman, WA, USA, 2021; pp. 149–155. [Google Scholar]
- Ishak, F.A.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 10013. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, S.; Chen, B.; Wu, P.; Feng, N.; Deng, F.; Wang, Z. Visible-light photocatalysis degradation of enrofloxacin by crawfish shell biochar combined with g-C3N4: Effects and mechanisms. J. Environ. Chem. Eng. 2023, 11, 109693. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Liu, T.; Fu, W.; Li, B. A review of biochar prepared by microwave-assisted pyrolysis of organic wastes. Sustain. Energ. Technol. 2022, 50, 101873. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T. A review of the synthesis of activated carbon for biodiesel production: Precursor, preparation, and modification. Energy Convers. Manag. X 2022, 13, 100152. [Google Scholar] [CrossRef]
- Fernandez, E.; Santamaria, L.; Amutio, M.; Artetxe, M.; Arregi, A.; Lopez, G.; Bilbao, J.; Olazar, M. Role of temperature in the biomass steam pyrolysis in a conical spouted bed reactor. Energy 2022, 238, 122053. [Google Scholar] [CrossRef]
- Grifoni, M.; Pedron, F.; Rosellini, I.; Petruzzelli, G. 26—From waste to resource: Sorption properties of biological and industrial sludge. In Industrial and Municipal Sludge; Prasad, M.N.V., de Campos Favas, P.J., Vithanage, M., Mohan, S.V., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 595–621. [Google Scholar] [CrossRef]
- Sierra, I.; Epelde, E.; Ayastuy, J.L.; Iriarte-Velasco, U. Production of sludge biochar by steam pyrolysis and acid treatment: Study of the activation mechanism and its impact on physicochemical properties. J. Anal. Appl. Pyrolysis 2024, 180, 106545. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, G.; Chen, H.; Li, X. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment. Chemosphere 2018, 191, 64–71. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Hydochar and biochar: Production, physicochemical properties and techno-economic analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Gupta, D.; Mahajani, S.M.; Garg, A. Investigation on hydrochar and macromolecules recovery opportunities from food waste after hydrothermal carbonization. Sci. Total. Environ. 2020, 749, 142294. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment—A sustainability assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment-conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Meng, F.; Wang, D. Effects of vacuum freeze drying pretreatment on biomass and biochar properties. Renew. Energy 2020, 155, 1–9. [Google Scholar] [CrossRef]
- Li, Q.; Wei, G.; Duan, G.; Zhang, L.; Li, Z.; Yan, F. Valorization of ball-milled waste red mud into heterogeneous catalyst as effective peroxymonosulfate activator for tetracycline hydrochloride degradation. J. Environ. Manag. 2022, 324, 116301. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Bhaskar, T. High surface area biochar from Sargassum tenerrimum as potential catalyst support for selective phenol hydrogenation. Environ. Res. 2020, 186, 109533. [Google Scholar] [CrossRef]
- Ahmaruzzaman Biochar based nanocomposites for photocatalytic degradation of emerging organic pollutants from water and wastewater. Mater. Res. Bull. 2021, 140, 111262. [CrossRef]
- Bhattacharjee, B.; Ahmaruzzaman, M. Photocatalytic degradation of pharmaceuticals: Insights into biochar modification and degradation mechanism. Next Mater. 2024, 5, 100238. [Google Scholar] [CrossRef]
- Skenderović, D.; Terihaj, L.; Rezić, T.; Vrsalović Presečki, A. Biopolimeri hitin i hitozan—Svojstva i priprava. Kem. Ind. 2023, 72, 103–112. [Google Scholar] [CrossRef]
- Sutar, S.; Otari, S.; Jadhav, J. Biochar based photocatalyst for degradation of organic aqueous waste: A review. Chemosphere 2022, 287, 132200. [Google Scholar] [CrossRef]
- Moyo, G.G.; Hu, Z.; Getahun, M.D. Decontamination of xenobiotics in water and soil environment through potential application of composite maize stover/rice husk (MS/RH) biochar—A review. Environ. Sci. Pollut. Res. 2020, 27, 28679–28694. [Google Scholar] [CrossRef]
- Ruan, Z.; Wang, X.; Liu, Y.; Liao, W. Chapter 3—Corn. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 59–72. [Google Scholar] [CrossRef]
- Singh, B. 13—Rice husk ash. In Woodhead Publishing Series in Civil and Structural Engineering, Waste and Supplementary Cementitious Materials in Concrete; Siddique, R., Cachim, P., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 417–460. [Google Scholar] [CrossRef]
- Günal, H.; Bayram, O.; Günal, E.; Erdem, H. Characterization of soil amendment potential of 18 different biochar types produced by slow pyrolysis. Eurasian J. Soil Sci. 2019, 8, 329–339. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hoslett, J.; Ghazal, H.; Katsou, E.; Jouhara, H. The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci. Total Environ. 2021, 751, 141755. [Google Scholar] [CrossRef]
- Chang, J.; Shen, Z.; Hu, X.; Schulman, E.; Cui, C.; Guo, Q.; Tian, H. Adsorption of tetracycline by shrimp Shell waste from aqueous solutions: Adsorption isotherm, kinetics modeling, and mechanism. ACS Omega 2020, 5, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Wijayanti, H.K.; Ramanda, G.D.; Tamyiz, M.; Doong, R.-A.; Sagadevan, S. Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation. Molecules 2022, 27, 6871. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Wade, P.; Bolan, N. Assessment of the fertilizer potential of biochars produced from slow pyrolysis of biosolid and animal manures. JAAP 2021, 155, 105043. [Google Scholar] [CrossRef]
- Saady, N.M.C.; Rezaeitavabe, F.; Ruiz Espinoza, J.E. Chemical methods for hydrolyzing dairy manure fiber: A concise review. Energies 2021, 14, 6159. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Almutairi, A.A.; Ahmad, M.; Rafique, M.I.; Al-Wabel, M.I. Variations in composition and stability of biochars derived from different feedstock types at varying pyrolysis temperature. J. Saudi Soc. Agric. Sci. 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Sikder, S.; Joardar, J.C. Biochar production from poultry litter as management approach and effects on plant growth. Int. J. Recycl. Org. Waste Agric. 2019, 8, 47–58. [Google Scholar] [CrossRef]
- Shen, T.; Tang, Y.; Lu, X.-Y.; Meng, Z. Mechanisms of copper stabilization by mineral constituents in sewage sludge biochar. J. Clean. Prod. 2018, 193, 185–193. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using sewage sludge with high ash content for biochar production and Cu(II) sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gu, Z.; Wu, M.; Ma, Z.; Lim, H.R.; Khoo, K.S.; Show, P.L. Advancement pathway of biochar resources from macroalgae biomass: A review. Biomass-Bioenergy 2022, 167, 106650. [Google Scholar] [CrossRef]
- Kumar Mondal, A.; Hinkley, C.; Krishnan, L.; Ravi, N.; Akter, F.; Ralph, P.; Kuzhiumparambil, U. Macroalgae-based biochar: Preparation and characterization of physicochemical properties for potential applications. RSC Sustain. 2024, 2, 1828–1836. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Kang, H.-J.; Ahn, K.-H. Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresour. Technol. 2016, 211, 108–116. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Ur Rehman, M.S.; Faisal, A.; Rehman, F. Integrating Adsorption and Photocatalysis: A cost effective Strategy for Textile Wastewater Treatment using Hybrid Biochar-TiO2 Composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef]
- Fryda, L.; Visser, R. Biochar for Soil Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Chen, T.; Sun, J.; Ma, X.; Wang, Y.; Wang, J.; Xie, Z. Preparation of TiO2-modified Biochar and its Characteristics of Photo-catalysis Degradation for Enrofloxacin. Sci. Rep. 2020, 10, 6588. [Google Scholar] [CrossRef]
- Lou, Z.; Li, Y.; Han, H.; Ma, H.; Wang, L.; Cai, J.; Yang, L.; Yuan, C.; Zou, J. Synthesis of Porous 3D Fe/C Composites from Waste Wood with Tunable and Excellent Electromagnetic Wave Absorption Performance. ACS Sustain. Chem. Eng. 2018, 6, 15598–15607. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumara, P.; Varjanic, S.; Saravanand, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-F.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar–based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process. Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Kim, J.R.; Kan, E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef]
- Milkowski, K.; Clark, J.H.; Doi, S. New materials based on renewable resources: Chemically modified highly porous starches and their composites with synthetic monomers. Green Chem. 2004, 6, 189–190. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, V.; Lisovytskiy, V.; Kamińska, A.; Łomot, D. Dual Functionality of TiO2/Biochar Hybrid Materials: Photocatalytic Phenol Degradation in the Liquid Phase and Selective Oxidation of Methanol in the Gas Phase. ACS Sustain. Chem. Eng. 2017, 5, 6274–6287. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Grzonka, J.; Kurzydłowski, K. Design and Fabrication of TiO2/Lignocellulosic Carbon Materials: Relevance of Low-temperature Sonocrystallization to Photocatalysts Performance. ChemCatChem 2018, 10, 3469–3480. [Google Scholar] [CrossRef]
- Meng, L.R.; Yin, W.H.; Wang, S.S.; Wu, X.G.; Hou, J.H.; Yin, W.Q.; Feng, K.; Ok, Y.S.; Wang, X.Z. Photocatalytic behavior of biochar-modified carbon nitride with enriched visible-light reactivity. Chemosphere 2020, 239, 124713. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, L.; Liu, Y.; Wang, T.; Bai, Y.; Guan, Y. Facile assembled N, S-codoped corn straw biochar loaded Bi2WO6 with the enhanced electron-rich feature for the efficient photocatalytic removal of ciprofloxacin and Cr(VI). Chemosphere 2021, 263, 127988. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Makrigianni, V.; Giannakas, A.; Daikopoulos, C.; Deligiannakis, Y.; Konstantinou, I. Preparation, characterization and photocatalytic performance of pyrolytic-tire-char/TiO2 composites, toward phenol oxidation in aqueous solutions. Appl. Catal. B Environ. 2015, 174–175, 244–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Gan, L.; Lian, H.; Pan, M. S-doped carbon nanosheets supported ZnO with enhanced visible-light photocatalytic performance for pollutants degradation. J. Clean. Prod. 2021, 319, 128803. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; de Lima Brombilla, V.; Silvestri, S.; Foletto, E.L. Conversion of spent coffee grounds to biochar as promising TiO2 support for effective degradation of diclofenac in water. Appl. Organomet. Chem. 2020, 34, e6001. [Google Scholar] [CrossRef]
- Peng, X.; Wang, M.; Hu, F.; Qiu, F.; Dai, H.; Cao, Z. Facile fabrication of hollow biochar carbon-doped TiO2/CuO composites for the photocatalytic degradation of ammonia nitrogen from aqueous solution. J. Alloys Compd. 2019, 770, 1055–1063. [Google Scholar] [CrossRef]
- Umrao, S.; Chakraborty, S.; Ahuja, R.; Popp, J.; Dietzek, B.; Srivastava, A. A possible mechanism for the emergence of an additional band gap due to a Ti–O–C bond in the TiO2—Graphene hybrid system for enhanced photodegradation of methylene blue under visible light. RSC Adv. 2014, 4, 59890–59901. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.; Zhang, Y. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- McKay, G.; Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M. 12—Dye removal using biochars. In Sustainable Biochar for Water and Wastewater Treatment; Mohan, D., Pittman, C.U., Mlsna, T.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 429–471. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S.; Johir, M.A.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Dorraj, M.; Alizadeh, M.; Sairi, N.A.; Basirun, W.J.; Goh, B.T.; Woi, P.M.; Alias, Y. Enhanced visible light photocatalytic activity of copper-doped titanium oxide–zinc oxide heterojunction for methyl orange degradation. Appl. Surf. Sci. 2017, 414, 251–261. [Google Scholar] [CrossRef]
- Amdeha, E. Biochar-based nanocomposites for industrial wastewater treatment via adsorption and photocatalytic degradation and the parameters affecting these processes. Biomass-Convers. Biorefinery 2024, 14, 23293–23318. [Google Scholar] [CrossRef]
- Bratovcic, A. Photocatalytic Degradation of Plastic Waste: Recent Progress and Future Perspectives. ANP Adv. Nanoparticles 2024, 13, 61–78. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water: An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Mao, L.; Yang, Z. Dependence of kinetics and pathway of acetaminophen photocatalytic degradation on irradiation photon energy and TiO2 crystalline. Chem. Eng. J. 2017, 330, 1091–1099. [Google Scholar] [CrossRef]
- Chang, C.-T.; Wang, J.-J.; Ouyang, T.; Zhang, Q.; Jing, Y.-H. Photocatalytic degradation of acetaminophen in aqueous solutions by TiO2/ZSM-5 zeolite with low energy irradiation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2015, 196, 53–60. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Bedia, J.; Rodriguez, J.J.; Belver, C. Effect of Activating Agent on the Properties of TiO2/Activated Carbon Heterostructures for Solar Photocatalytic Degradation of Acetaminophen. Materials 2019, 12, 378. [Google Scholar] [CrossRef]
- Hosseini-Monfared, H.; Mohammadi, Y.; Montazeri, R.; Gasemzadeh, S.; Varma, R.S. Effect of biochar on the photocatalytic activity of nitrogen-doped titanium dioxide nanocomposite in the removal of aqueous organic pollutants under visible light illumination. Nanochem. Res. 2021, 6, 79–93. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted Degradation of Dye Pollutants. V. Self-Photosensitized Oxidative Transformation of Rhodamine B under Visible Light Irradiation in Aqueous TiO2 Dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Shen, T.; Hidaka, H.; Pelizzetti, E.; Serpone, N. TiO2-assisted photodegradation of dye pollutants II. Adsorption and degradation kinetics of eosin in TiO2 dispersions under visible light irradiation. Appl. Catal. B Environ. 1998, 15, 147–156. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Photochemistry on surfaces: Photodegradation of 1,3-diphenylisobenzofuran over metal oxide particles. J. Phys. Chem. 1992, 96, 5053–5059. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Zang, L.; Shen, T.; Hidaka, H.; Pelizzetti, E.; Serpone, N. Photoassisted degradation of dye pollutants in aqueous TiO2 dispersions under irradiation by visible light. J. Mol. Catal. A Chem. 1997, 120, 173–178. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Qu, K.; Huang, L.; Hu, S.; Liu, C.; Yang, Q.; Liu, L.; Li, K.; Zhao, Z.; Wang, Z. TiO2 supported on rice straw biochar as an adsorptive and photocatalytic composite for the efficient removal of ciprofloxacin in aqueous matrices. J. Environ. Chem. Eng. 2022, 11, 109430. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Chen, M.; Feng, Q.; Zhang, X.; Wang, S.; Zhao, R.; Jiang, T. Adsorption and photocatalytic degradation of quinolone antibiotics from wastewater using functionalized biochar. Environ. Pollut. 2023, 336, 122409. [Google Scholar] [CrossRef]
- Wang, T.; Cai, J.; Zheng, J.; Fang, K.; Hussain, I.; Husein, D.Z. Facile synthesis of activated biochar/BiVO4 heterojunction photocatalyst to enhance visible light efficient degradation for dye and antibiotics: Applications and mechanisms. J. Mater. Res. Technol. 2022, 19, 5017–5036. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Hu, J.; Wang, K.; Zhai, Y.; Chen, Y.; Duan, Y.; Wang, Y.; Zhang, W. Photocatalytic property correlated with microstructural evolution of the biochar/ZnO composites. J. Mater. Res. Technol. 2021, 11, 1308–1321. [Google Scholar] [CrossRef]
- de Lima Brombilla, V.; Sarmento Lazarotto, J.; Silvestri, S.; Anschau, F.K.; Dotto, G.L.; Foletto, E.L. Biochar derived from yerba-mate (Ilex paraguariensis) as an alternative TiO2 support for enhancement of photocatalytic activity toward Rhodamine-B degradation in water. Chem. Eng. Commun. 2022, 209, 1334–1347. [Google Scholar] [CrossRef]
- Pinna, M.; Binda, G.; Altomare, M.; Marelli, M.; Dossi, C.; Monticelli, D.; Spanu, D.; Recchia, S. Biochar Nanoparticles over TiO2 Nanotube Arrays: A Green Co-Catalyst to Boost the Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1048. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X. Treatment of wastewater containing Reactive Brilliant Blue KN-R using TiO2/BC composite as heterogeneous photocatalyst and adsorbent. Chemosphere 2018, 206, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Wu, R.; Liu, W.; Bai, R.; Zheng, D.; Tian, X.; Lin, W.; Ke, Q.; Li, L. Waste Biomass-Mediated Synthesis of TiO2/P, K-Containing Grapefruit Peel Biochar Composites with Enhanced Photocatalytic Activity. Molecules 2024, 29, 2090. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Abouseada, M.; El-Azazy, M. TiO2-functionalized biochar from pistachio nutshells: Adsorptive removal and photocatalytic decolorization of methyl orange. Appl. Water Sci. 2023, 13, 227. [Google Scholar] [CrossRef]
| Thermochemical Process | BC Yields, wt.% | Pyrolysis Temperature, °C | Limitations/ Residence Time | Ref. |
|---|---|---|---|---|
Pyrolysis
|
| 300–900 300–900 900–1300 | absence of O2, >1 h, and 0.5–10 s <0.5 s | [18] [19] |
| Gasification | very low (5–10) | >700 | in the presence of air, O2, or steam | [31] |
| Hydrothermal carbonization | carbon content of the biochar is greater than that of the pyrolysis | <250 | from minutes to several hours | [29] |
| Dry torrefaction | high (70–80) | 200 to 300 | absence of air; 30 min–4 h | [32] |
| Active Species/ Composite | Biomass/Pyrolysis Temp. °C/Time | Mass of Photocatalyst | Adsorption Eq. Time | Light Source/ Irradiation Time | Pollutant | Removal Efficiency % | Ref. |
|---|---|---|---|---|---|---|---|
| BC/CN-2% | Crawfish shells 550 °C/2 h | 1.0 g/L | 30 min | Vis light/ 8 h | (ENR) | 90% | [20] |
| Biochar/TiO2 | Flash carbonization of corn cob at 600 °C | 5 g/L | 30 min | UVC light 3 h | 100 mL of 10 mg/L (SMX) pH = 4 | 75% 0.6–2.4 mg SMX per g biochar/TiO2 | [67] |
| Biochar/ TiO2 | Flash carbonization of corn cob at 600 °C | 5 g/L | 30 min | UVC light 6 h | 100 mL of 10 mg/L (SMX) pH = 4 | 91% | [67] |
| Bi2 WO6/NSBC | Corn straw/550 °C/2 h | 1.0 gL−1 | 30 min | 500 W Xe lamp 75 min | 100 mL of 5 mg/L (CIP) | 90.33% | [73] |
| B1T1 (Biochar–TiO2 (1:1)) TiO2 | coffee grounds 650 °C/2 h | 1 g/L 0.1 g | 60 min | 125 W mercury vapor lamp UV irrad 120 min | DCF pH = 6.15 20 mg/L | 90% 40% | [77] |
| TiO2 Ti-RSB | Rice straw 800 °C/1 h | 0.5 gL−1 | 30 min | UV light 150 min | 10 mgL−1 (CIP) pH = 5 | 83.82% | [98] |
| Fe/Ti biochar composite (Fe/Ti-MBC) | Wood chip | 0.25 g/L | 12 h | 6 h sunlight, xenon lamp, ultraviolet lamp | 40 mg/L CIP NOR pH = 6 | 88.4% 88.0% | [99] |
| TiO2/activated carbon | Lignin reed straw | 0.25 g/L | 16 h | Suntest solar simulator, Xe lamp 6 h | 5 mg/L ACE pH = 6.9 | 92% | [91] |
| BC-TiO2 (300) acid pre-treated | Reed straw | 1.25 g/L | 30 min | 50 W xenon lamp UV 3 h | 10 mg/L SMX pH = 4 | 91.27% | [101] |
| Photoactive Specie/ Composite | Biomass/Pyrolysis Temp. °C/Time | Mass of Photocatalyst | Adsorption Eq. Time | Light Source/ Irradiation Time | Pollutant | Decolorization, %/Mineralization, % | Ref. |
|---|---|---|---|---|---|---|---|
| BTiO2 | yerba mate/650 | 0.1 g/L | UV lamp (F15T8/B, GE 15 W, λ = 380–445 nm/ 120 min | (RhB) pH = 6.5 | 96%/75% | [103] | |
| TiO2 nanotubes 5-NBC-TiO2 | commercially available feedstocks: nutshells (NBC) and the microalgae Nannochloropsis sp. (MBC)/350/1 h | 0.1 g/L | UV LED 40 mW/cm2 λ = 365 nm 3 h | (MB) 10 mg/L | 5-MBC-TiO2 complete mineralization, 48 h 90% | [104] | |
| 5-MBC-TiO2 | 1 g/L | 100 mW/cm2 λ = 365 nm 3 h | 10 mL (MB) 10 mg/L | 90% | |||
| 0.2:1 biochar: Ti ratio, labeled as CT0.2/1 | walnut shells | 1 g/L | 60 min | A mercury lamp, 500 W, λ = 360 nm | MO 20 mg/L | 96.88% 83.23% | [74] |
| N-TiO2 | barley straw | 1 g/L | 30 min | Vis LED lamp 150 min | 10 ppm RhB | 99% | [92] |
| N-TiO2/with 3% C | barley straw | 1 g/L | 30 min | 105 min | RhB | 97% | [92] |
| Calcinated N-TiO2/with 3% C | barley straw | 1 g/L | 30 min | 180 min | RhB | 22% | [92] |
| TiO2/BC | coconut shell | 6 g/L | not reported | 60 min | RBB KN-R 30 mg/L | pH = 1 (99.71%) pH = 11 (96.99%) | [105] |
| hybrid biochar-TiO2 (BCT) | macroalgae | 2 g/L | 60 min | 3 h Vis light | MB 50 mg/L | 99.40%(BCT-4) 41.30 | [59] |
| Ag/TiO2/ biochar (CTAg1) | walnut shell WB700 | 0.25 g/L | 60 min | 1 h 500 W mercury-vapor lamp | MO 20 mg/L | 97.48% DE 85.38% ME | [106] |
| PCT-400–550 | grapefruit Peel | 0.5 g/L | 30 min | 30 min 300 W mercury lamp | RhB, 10 mg/L 100 mL pH = 4.9 MO, MB | 99.07% | [107] |
| 3% TiO2@PNS-BC | pistachio biochar (PNS-BC) | 0.5 g | Not reported | 30 min UV lamp | MO 150 mL 100 ppm pH = 6 | 99.47% | [108] |
| 2% TiO2@PNS-BC | 88.44% | ||||||
| 81.38% | |||||||
| 1% TiO2@PNS-BC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratovčić, A.; Tomašić, V. Photocatalytic Composites Based on Biochar for Antibiotic and Dye Removal in Water Treatment. Processes 2024, 12, 2746. https://doi.org/10.3390/pr12122746
Bratovčić A, Tomašić V. Photocatalytic Composites Based on Biochar for Antibiotic and Dye Removal in Water Treatment. Processes. 2024; 12(12):2746. https://doi.org/10.3390/pr12122746
Chicago/Turabian StyleBratovčić, Amra, and Vesna Tomašić. 2024. "Photocatalytic Composites Based on Biochar for Antibiotic and Dye Removal in Water Treatment" Processes 12, no. 12: 2746. https://doi.org/10.3390/pr12122746
APA StyleBratovčić, A., & Tomašić, V. (2024). Photocatalytic Composites Based on Biochar for Antibiotic and Dye Removal in Water Treatment. Processes, 12(12), 2746. https://doi.org/10.3390/pr12122746









