Evaluation of the In Vitro Disinfection Potential of the Phytochemicals Linalool and Citronellal Against Biofilms Formed by Escherichia coli and Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Biofilm Cell and Biomass Reduction Assessment
2.4. Biofilm Cell Respiratory Chain Dehydrogenase Activity
2.5. The Integrity of Bacterial Cell Membrane
2.6. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration
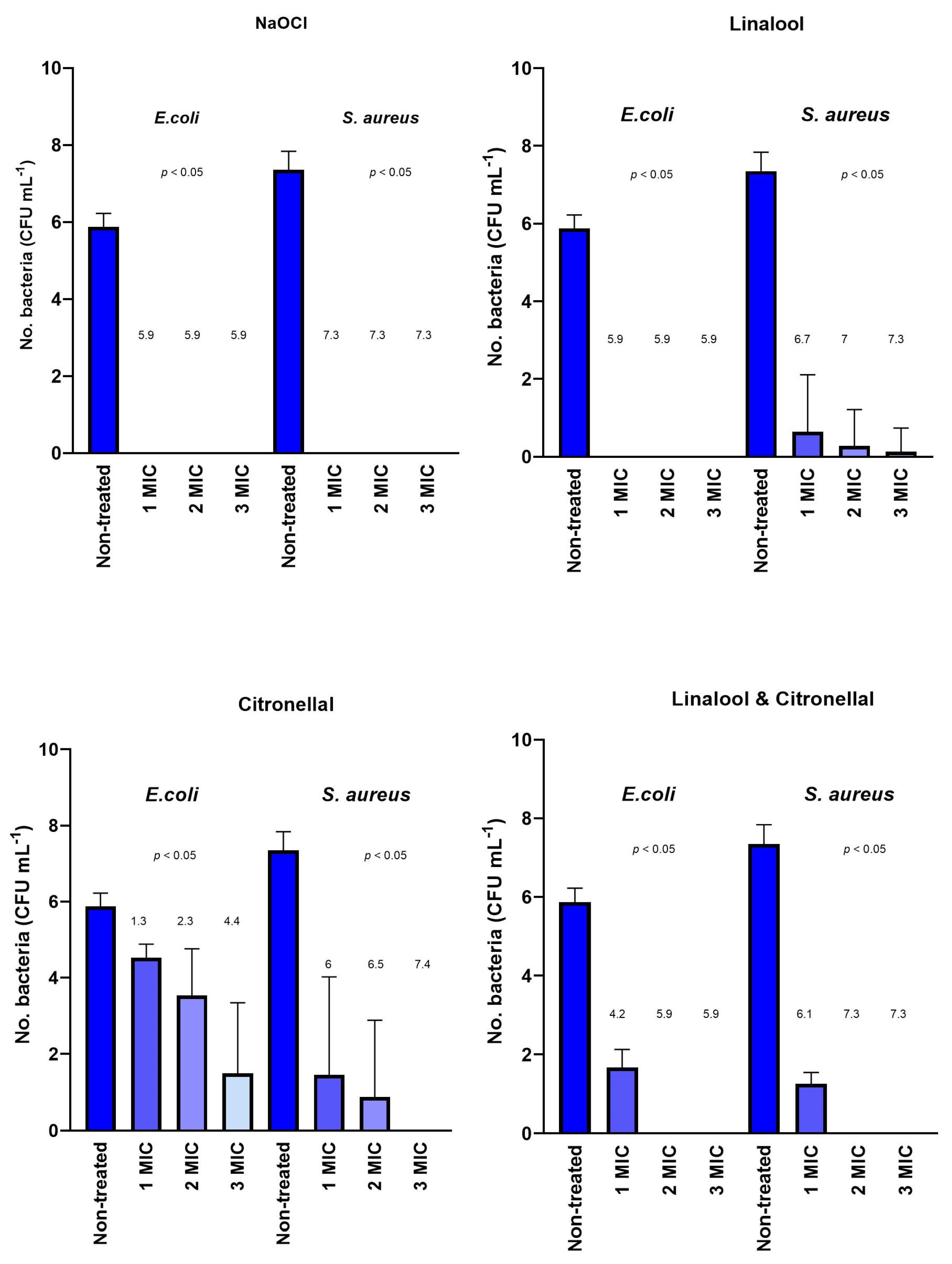
3.2. Biofilm Cell and Biomass Reduction Assessment
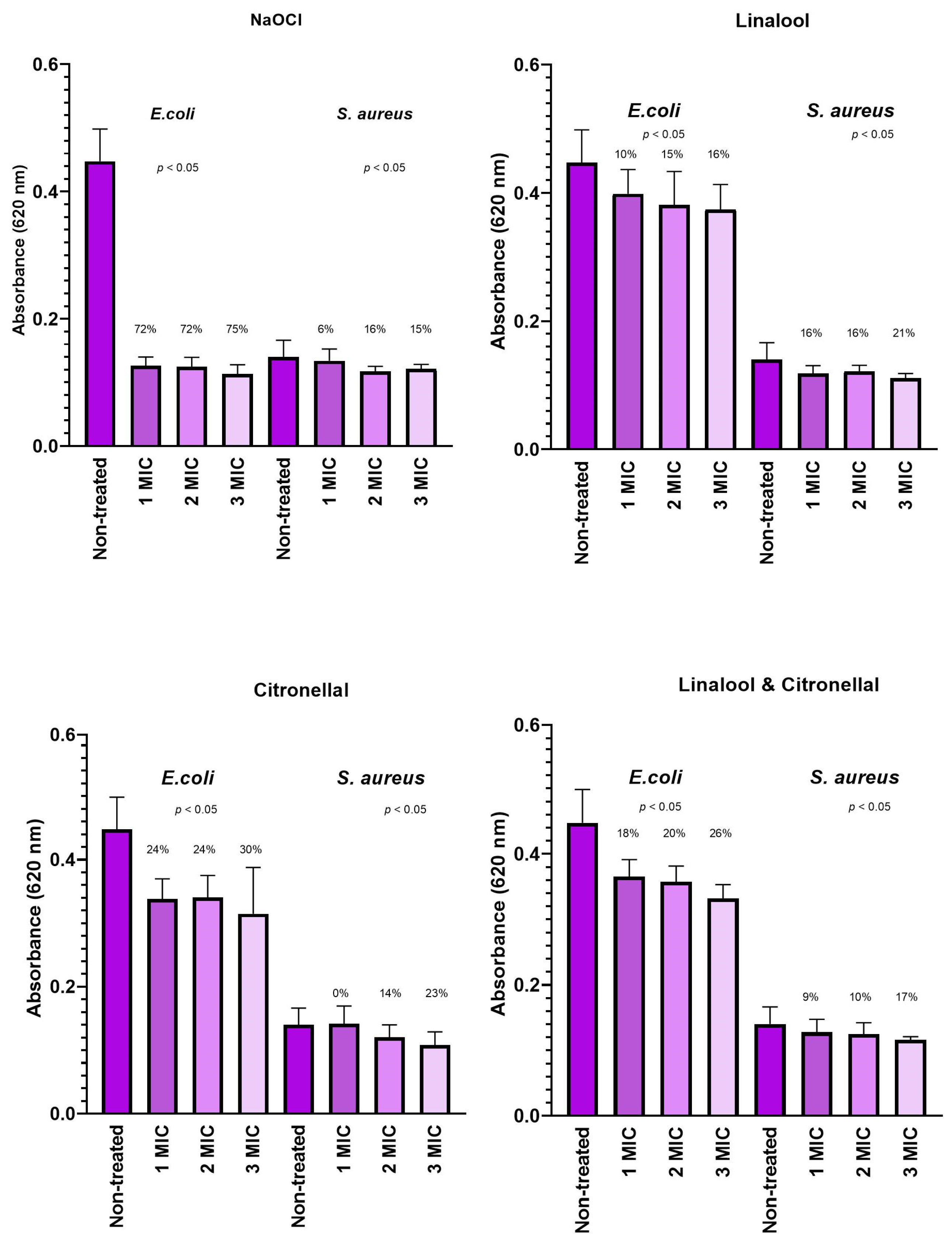
3.3. Biofilm Cell Respiratory Chain Dehydrogenase Activity
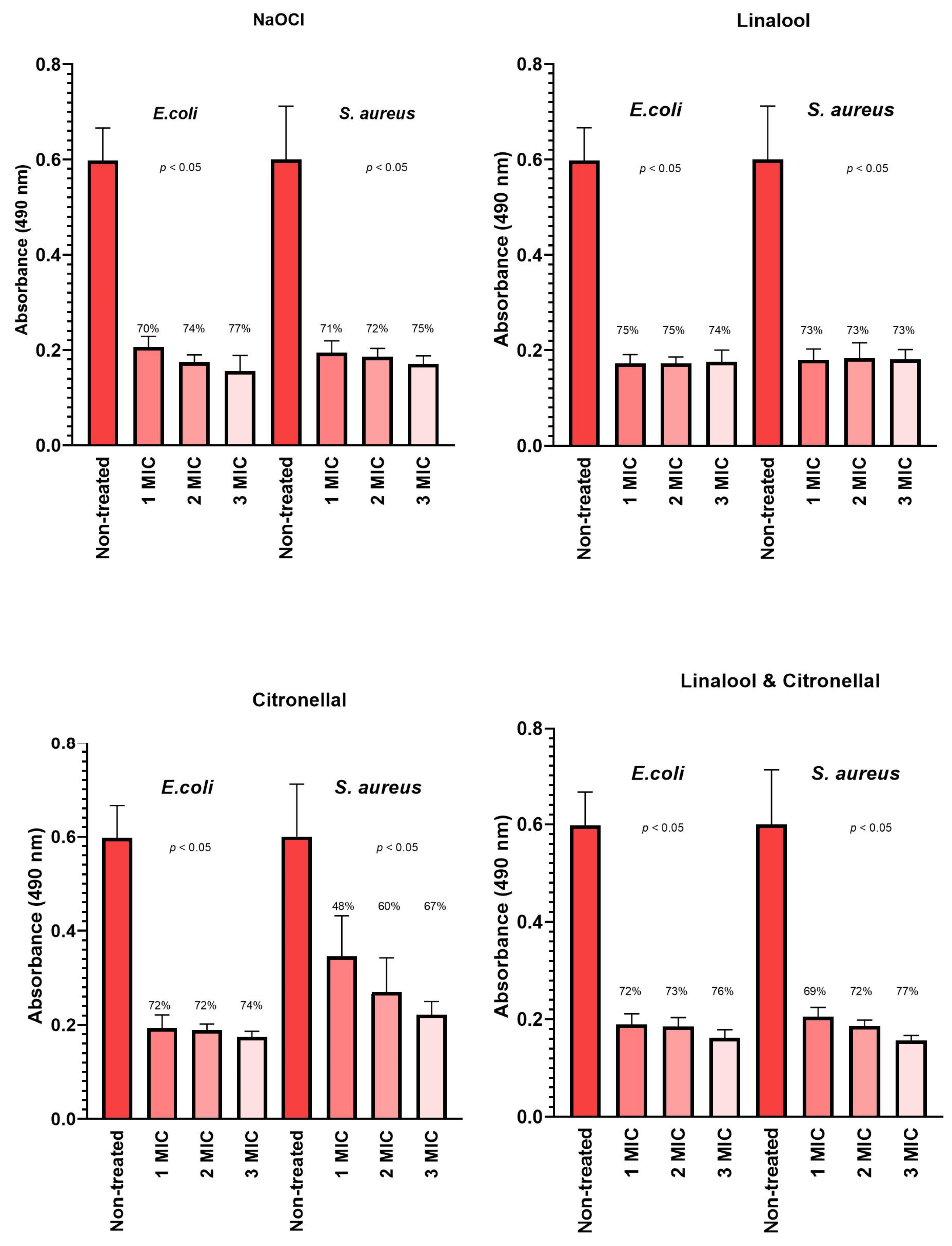
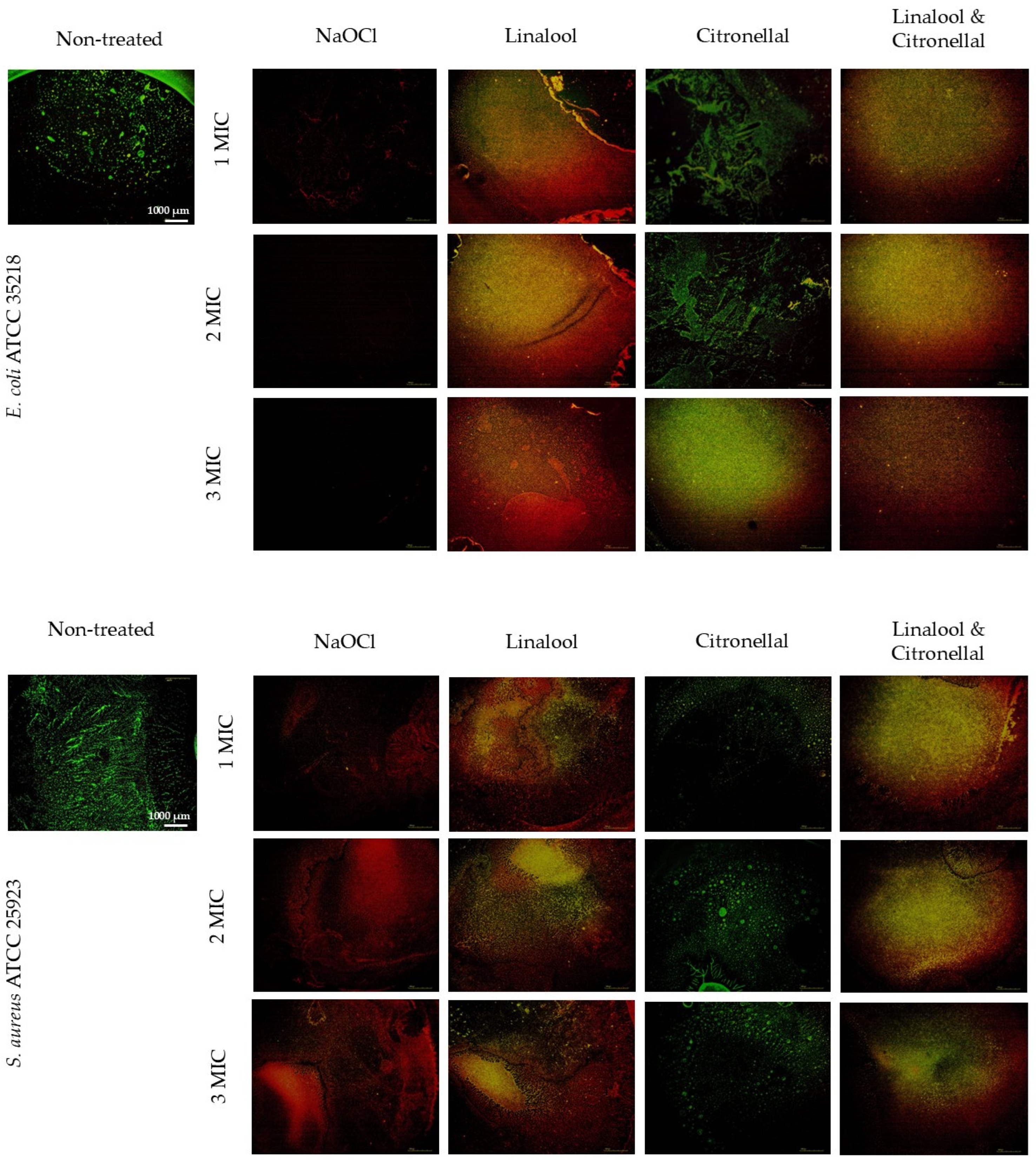
3.4. The Integrity of the Bacterial Cell Membrane
4. Discussion
4.1. Minimum Inhibitory Concentration
4.2. Biofilm Cell and Biomass Reduction Assessment
4.3. Biofilm Cell Respiratory Chain Dehydrogenase Activity
4.4. The Integrity of Bacterial Cell Membrane
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maillard, J.-Y.; Pascoe, M. Disinfectants and Antiseptics: Mechanisms of Action and Resistance. Nat. Rev. Microbiol. 2023, 22, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.; Wang, Z.; Oder, M.; Brooks, B.W. Balancing Chemical Function with Reduced Environmental Health Hazards: A Joint Probability Approach to Examine Antimicrobial Product Efficacy and Mammalian Toxicity. J. Clean. Prod. 2020, 262, 121323. [Google Scholar] [CrossRef]
- Arya, S.; Gupta, S.; Thakur, S.; Mishra, A.K. Impacts of Sodium Hypochlorite on Humans and Environment. Int. J. Environ. Clim. Chang. 2023, 13, 3200–3217. [Google Scholar] [CrossRef]
- Di Marzio, W.D.; Cifoni, M.; Sáenz, M.E.; Galassi, D.M.; Di Lorenzo, T. The Ecotoxicity of Binary Mixtures of Imazamox and Ionized Ammonia on Freshwater Copepods: Implications for Environmental Risk Assessment in Groundwater Bodies. Ecotoxicol. Environ. Saf. 2018, 149, 72–79. [Google Scholar] [CrossRef]
- McGinley, H.R.; Enzien, M.; Hancock, G.; Gonsior, S.; Miksztal, M. Glutaraldehyde: An Understanding of Its Ecotoxicity Profile and Environmental Chemistry. Presented at the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009. NACE-09405. [Google Scholar]
- Zhang, H.; Tang, W.; Chen, Y.; Yin, W. Disinfection Threatens Aquatic Ecosystems. Science 2020, 368, 146–147. [Google Scholar] [CrossRef]
- Dewey, H.M.; Jones, J.M.; Keating, M.R.; Budhathoki-Uprety, J. Increased Use of Disinfectants during the COVID-19 Pandemic and Its Potential Impacts on Health and Safety. ACS Chem. Health Saf. 2021, 29, 27–38. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Fink, R. Good Hygiene Practices and Their Prevention of Biofilms in the Food Industry; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; ISBN 1-5275-3677-7. [Google Scholar]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of Essential Oil as a Sustained Release Preparation in Food Packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent Updates on Bioactive Properties of Linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef]
- Api, A.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.; Buschmann, J.; Cancellieri, M.; Dagli, M.; Date, M.; Dekant, W. Update to RIFM Fragrance Ingredient Safety Assessment, Linalool, CAS Registry Number 78-70-6. Food Chem. Toxicol. 2022, 159, 112687. [Google Scholar] [CrossRef]
- Lenardao, E.J.; Botteselle, G.V.; de Azambuja, F.; Perin, G.; Jacob, R.G. Citronellal as Key Compound in Organic Synthesis. Tetrahedron 2007, 63, 6671–6712. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Alternat. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Gilling, D.H.; Ravishankar, S.; Bright, K.R. Antimicrobial Efficacy of Plant Essential Oils and Extracts against Escherichia coli. J. Environ. Sci. Health Part A 2019, 54, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Dumas, E.; Ismail, A.; Karam, L. Essential Oils and Their Active Components Applied as: Free, Encapsulated and in Hurdle Technology to Fight Microbial Contaminations. A Review. Heliyon 2022, 8, e12472. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 20776-1:2020; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases (ISO 20776-1:2019, Including Corrected Version 2019-12). CEN-CENELEC Management Centre: Brussels, Belgium, 2020.
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; ISBN 1-68440-104-6. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, K.; Dražić, G.; Fink, R.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič-Torkar, K.; Raspor, P. Available Surface Dictates Microbial Adhesion Capacity. Int. J. Adhes. Adhes. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Stiefel, P.; Schneider, J.; Amberg, C.; Maniura-Weber, K.; Ren, Q. A Simple and Rapid Method for Optical Visualization and Quantification of Bacteria on Textiles. Sci. Rep. 2016, 6, 39635. [Google Scholar] [CrossRef]
- Benov, L. Improved Formazan Dissolution for Bacterial MTT Assay. Microbiol. Spectr. 2021, 9, e01637-21. [Google Scholar] [CrossRef]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.-U.; Egli, T. Assessment and Interpretation of Bacterial Viability by Using the LIVE/DEAD BacLight Kit in Combination with Flow Cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef]
- Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Ghanbar, S.; Fumakia, M.; Ho, E.A.; Liu, S. A New Strategy for Battling Bacterial Resistance: Turning Potent, Non-Selective and Potentially Non-Resistance-Inducing Biocides into Selective Ones. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 471–481. [Google Scholar] [CrossRef]
- Fu, Y.; Deering, A.J.; Bhunia, A.K.; Yao, Y. Biofilm of Escherichia coli O157: H7 on Cantaloupe Surface Is Resistant to Lauroyl Arginate Ethyl and Sodium Hypochlorite. Int. J. Food Microbiol. 2017, 260, 11–16. [Google Scholar] [CrossRef]
- Kim, S.-G.; Choi, M.-H.; Park, S.-N.; Kook, J.-K. Antimicrobial Effects of Linalool and α-Terpineol against Methicillin-Resistant Staphylococcus aureus Isolated from Korean. Int. J. Oral. Biol. 2013, 38, 51–54. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wei, Y.; Li, X.; Shan, A.; Wang, J. Antimicrobial Peptides in Combination with Citronellal Efficiently Kills Multidrug Resistance Bacteria. Phytomedicine 2023, 120, 155070. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of Diffusion and Dilution Methods to Determine the Antibacterial Activity of Plant Extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Van der Plas, M.; Mörgelin, M.; Sonesson, A. Antibacterial and Antibiofilm Effects of Sodium Hypochlorite against Staphylococcus aureus Isolates Derived from Patients with Atopic Dermatitis. Br. J. Dermatol. 2017, 177, 513–521. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Galvão, J.A.; Mazine, M.R.; Gloria, E.M.; Oetterer, M. Control of Staphylococcus aureus Biofilms by the Application of Single and Combined Treatments Based in Plant Essential Oils. Int. J. Food Microbiol. 2018, 286, 128–138. [Google Scholar] [CrossRef]
- Borges, A.; Lopez-Romero, J.; Oliveira, D.; Giaouris, E.; Simões, M. Prevention, Removal and Inactivation of Escherichia coli and Staphylococcus aureus Biofilms Using Selected Monoterpenes of Essential Oils. J. Appl. Microbiol. 2017, 123, 104–115. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Sousa, L.G.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic Effects of Carvacrol, α-Terpinene, γ-Terpinene, ρ-Cymene and Linalool against Gardnerella Species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Prakash, A.; Vadivel, V. Citral and Linalool Nanoemulsions: Impact of Synergism and Ripening Inhibitors on the Stability and Antibacterial Activity against Listeria Monocytogenes. J. Food Sci. Technol. 2020, 57, 1495–1504. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. The Effects of Eugenol, Trans-Cinnamaldehyde, Citronellol, and Terpineol on Escherichia coli Biofilm Control as Assessed by Culture-Dependent and-Independent Methods. Molecules 2020, 25, 2641. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.; Brebu, M.; Horhogea, C.; Miron, A. Coriander Essential Oil and Linalool–Interactions with Antibiotics against Gram-positive and Gram-negative Bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef] [PubMed]
| Bacteria | Citronellal | Linalool | Citronellal/Linalool | NaOCl |
|---|---|---|---|---|
| E. coli | 37.5 | 9.4 | 4.7 | 4.7 |
| S. aureus | 18.8 | 18.8 | 4.7 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krapež, P.; Lunder, M.; Oder, M.; Fink, R. Evaluation of the In Vitro Disinfection Potential of the Phytochemicals Linalool and Citronellal Against Biofilms Formed by Escherichia coli and Staphylococcus aureus. Processes 2024, 12, 2743. https://doi.org/10.3390/pr12122743
Krapež P, Lunder M, Oder M, Fink R. Evaluation of the In Vitro Disinfection Potential of the Phytochemicals Linalool and Citronellal Against Biofilms Formed by Escherichia coli and Staphylococcus aureus. Processes. 2024; 12(12):2743. https://doi.org/10.3390/pr12122743
Chicago/Turabian StyleKrapež, Patricija, Manca Lunder, Martina Oder, and Rok Fink. 2024. "Evaluation of the In Vitro Disinfection Potential of the Phytochemicals Linalool and Citronellal Against Biofilms Formed by Escherichia coli and Staphylococcus aureus" Processes 12, no. 12: 2743. https://doi.org/10.3390/pr12122743
APA StyleKrapež, P., Lunder, M., Oder, M., & Fink, R. (2024). Evaluation of the In Vitro Disinfection Potential of the Phytochemicals Linalool and Citronellal Against Biofilms Formed by Escherichia coli and Staphylococcus aureus. Processes, 12(12), 2743. https://doi.org/10.3390/pr12122743









