Cyanoremediation of Polluted Seawater in the Arabian Gulf: Risks and Benefits to Human Health
Abstract
1. Introduction
1.1. Roles of Phytoplankton in Marine and Freshwater Ecosystems
1.2. Disadvantages of Phytoplankton
2. Methods and Mechanisms of Phycoremediation
3. Biodiversity and Functions of Cyanobacteria Under Pollution Stress
| Species | Family | Features | Roles | References |
|---|---|---|---|---|
| Amphanizomenon sp. | Aphanizomenonaceae | Unicellular organisms that consolidate into linear (non-branching) chains called trichomes. Inhabits freshwater lakes and can cause dense blooms. | Fixes nitrogen, remediates petroleum hydrocarbons, produces anticancer agents, and develops blooms; produces toxic metabolites such as hepatotoxins, neurotoxins, and cytotoxins. | [56,57,58] |
| Anabaena spp. (2 species) | Nostocaceae | Found in all types of water, including rivers, streams, lakes, and ponds; filamentous, cylindrical, barrel-shaped, or spherical. | Fixes nitrogen, remediate crude oil. Water extracts have anticancer activity against breast cancer. Forms symbiotic relationships with some plants; produces neurotoxins; forms blooms. | [59,60,61] |
| Calothrix spp. (3 species) | Rivulariaceae | Found in marine, fresh waters, and terrestrial environments; grow in high-salinity conditions, and form relationships with the roots of plants like rice, tomato, and wheat. Form a black, slick surface on rocks and mud and produce a gelatinous outer layer to prevent drying. | Fixes nitrogen, possible remediation of industrial wastewater. Calothrixins are produced; used to cure many diseases including cytotoxicity in cancer; Aplysiatoxins might be produced that cause serious health issues including various dermatitis and promoting tumors. | [62,63] |
| Chroococcus sp. | Chroococcaceae | Unicellular ovoid or rod-shaped organisms, irregular to roughly spherical, forming colonies with gelatinous texture, found in water reserves. | Uses large amounts of atmospheric CO2 for photosynthesis; produces O2. These were some of the first organisms to use water as asource of electrons and hydrogens for photosynthesis which enables the evolution of other organisms. Remediation of polluted water and soil is very possible; do not fix nitrogen and might produce anticancer agents. | [64,65,66] |
| Croococcidiopsis sp. | Chroococcidiopsidaceae | Unicellular with survival in extreme environments; salinity and desiccation, high antioxidant, develop thick mucilage envelopes. | Fixes nitrogen, it resists very extreme environments (e.g., endurance to a Mars-like environment), phycoremedes wastewater and contaminated soil, might be used as food and oxygen producers in space, Mars, for example. | [67,68,69] |
| Dermocarpella sp. | Dermocarpellaceae | Gram-negative, thickened ovoid aggregate uniquely exhibits polarity; motile baeocytes result from multiple fission of apical cells; undergo binary and multiple fission. | Needs to be tested for nitrogen fixation; might have toxic impact on the ecosystem. Possible role in phycoremediation. | [54,70,71] |
| Euhalothece sp. | Cyanobacteriaceae | Unicellular, halophilic, not pathogenic, and not known to produce toxins. High concentration of mycosporine-like amino acids, carotenoid-binding proteins, and C-phycocyanin subunits. | Needs to be tested for nitrogen fixation; primary producer in hypersaline environments; strong antioxidant and some oxidant compounds protect its cellular machinery from UV-induced oxidative stress; it has β-car. and Zea-dependent ROS scavenging systems to help cells cope with salt stress, helps in biotechnology applications; possible role in phycoremediation. | [71,72] |
| Geitlerinema sp. | Coleofasciculaceae | Filamentous, thin, delicate thallus, bright blue–green, violet, or brown; has motile trichomes, active oxygenic and anoxygenic, rich in proteins and phycobiliproteins, contains polyphosphates, starch, and carboxysomes. | Fixes nitrogen, oxygenic, and anoxygenic photosynthetic capabilities, biomass feedstock, produces biofuels, produces pro-inflammatory cytokines and adhesion molecules, develops glucocorticoid-resistant asthma, increases the phagocytic ability of monocytes, possible role in phycoremediation. | [71,73,74] |
| Geminocystis sp. | Geminocystaceae | Small, spherical cells, found in colonies, photosynthetically adapted to aquatic environments, can be found in freshwater ecosystems. | Needs to be tested for nitrogen fixation and can adjust the wavelengths of light they absorb by remodeling photosynthetic antennae—possible role in phycoremediation. | [72,75] |
| Lyngbya sp. | Oscillatoriaceae | Filamentous, non-branched or pseudo-branched; macroscopic layered or stratified and brownish-colored sheaths; musty or foul odor, cell division occurs crosswise with reproduction by hormogonium formation; found in high alkaline water. | Fixes nitrogen, a rich source of marine natural products; produces extracellular sunscreen scytonemin, indole alkaloid, source of food for some grass carp, forming blooms, possible remediation of heavy metals, and petroleum hydrocarbons. | [76,77] |
| Leptolyngbya sp. | Lyptolyngbyaceae | Filamentous cells divide by symmetrical crosswise binary fission, produce hormogonia, tolerate severe environmental conditions, have antioxidant activity, scavenge free radicals, act as an iron-chelating agent; applications in food and pharmaceuticals; high in lipids. | Some species fix nitrogen, phycoremediation of contaminated of water and soil, and phycoremediation petroleum hydrocarbons. | [78,79,80] |
| Merismopedia sp. | Microcystaceae | Found in fresh and salt water, ovoid or spherical, arranged in rows and flats, forming rectangular colonies held together by a mucilaginous matrix; non-nitrogen-fixing bacteria. | Not fixing nitrogen; remediation efficiency needs to be tested; produces lipopolysaccharides; can cause skin irritation and gastrointestinal distress. | [81] |
| Microcystis wesenbergii | Microcystaceae | Single cell; cells are small (a few micrometers in diameter) and spherical or hemispherical; cells are light blue–green in color, but appear brown or dark due to the presence of gas-filled vesicles; forms colonies surrounded by a thick mucilage; colonies begin spherical but become irregular over time. | Does not fix nitrogen; possible pollution remediation; freshwater forms harmful bloom and hepatotoxins such as microcystin and cyanopeptolin; communities are often a mix of toxin-producing and nonproducing isolates; scum formation with pollution. | [6,82,83] |
| Nodularia spumigera | Aphanizomenonaceae | Form solitary filaments or groups of filaments; unipolar, straight, or curved; yellowish, olive green, or blue–green in color; reproduced by the formation of hormogonia, filament breakage, akinetes, salinity, and temperature stress reduce toxin production. | Fixes nitrogen and forms blooms; can be toxic to ecosystems and water quality; possible remediation of pollutants produces nodularin (liver toxin) and beta-methylamino L-alanine (nerve toxin), stress might affect toxin production. | [80,84,85] |
| Nostoc linekia | Nostocaceae | Cylindrical, barrel-shaped, or spherical, thick cell wall; peptidoglycan, various pigments (chlorophyll, phycocyanin, and phycoerythrin); found in a variety of environments, grows symbiotically with plants providing nitrogen. | Fixes nitrogen and remediates petroleum hydrocarbons. | [81,86,87] |
| Oscillatoria spp. (3 species) | Oscillatoriaceae | Filamentous; form bright blue–green mats; motile with a slow rhythmic oscillation motion. | Fixes nitrogen, remediates sewage wastewater and crude oil polluted water; heavy metals are eliminated. | [81,88,89,90] |
| Phormidium sp. | Oscillatoriaceae | Has curved trichomes and unbranched filaments; cells divide crosswise perpendicular to the long axis of the trichome; cells are isodiametric; cells of the filament with rounded or pointed apical cells. | Fixes nitrogen, remediates petroleum hydrocarbons and heavy metals. | [91] |
| Richelia sp. | Nostocaceae | Filamentous trichomes; heterocystous associated within the cell membrane and cell wall of diatoms; exist as an epiphyte and an endophyte. | Fixes nitrogen, possible remediation of industrial wastewater. | [92,93] |
| Stanieria sp. | Dermocarpellaceae | Coccoid sessile on shells or epiphytic on algae and other cyanobacteria, coccoid microfossils, PTB-microbialite, and Permian Triassic. Boundary: calcified sheaths of the extant unicellular endospores are used for identification; anoxia is favorable to the preservation of Stanieria as fossils. | Needs to be tested for nitrogen fixation, potential bioremediation of petroleum hydrocarbons. | [94,95,96] |
| Synechococcus sp. | Synechococcaceae | Unicellular, spherical, ellipsoidal, rod-shaped, marine environments and fresh water; motile without flagellates; it moves by oscillating its cell surface and grows in a wide range of light intensities; prefers neutral to slightly alkaline pH; contains phycoerthrin, cell wall is peptidoglycan and polysaccharides; can tolerate long periods without nutrient supply. | Some species fix nitrogen; remediates petroleum hydrocarbons such as kerosene and other oil and gas compounds. | [80,97] |
| Trichodesmium erythraeum | Microcoleaceae | Found in tropical and subtropical oceans; straight or curved individual filaments form spherical aggregates and large blooms; the red pigments responsible for the color of the red sea. | Fixes nitrogen and carbon while undergoing photosynthesis; the close physical contact between genetically identical cells allows cell specialization; and metabolizes some organic compounds and related components. | [98,99,100,101] |
3.1. Nitrogen Fixation
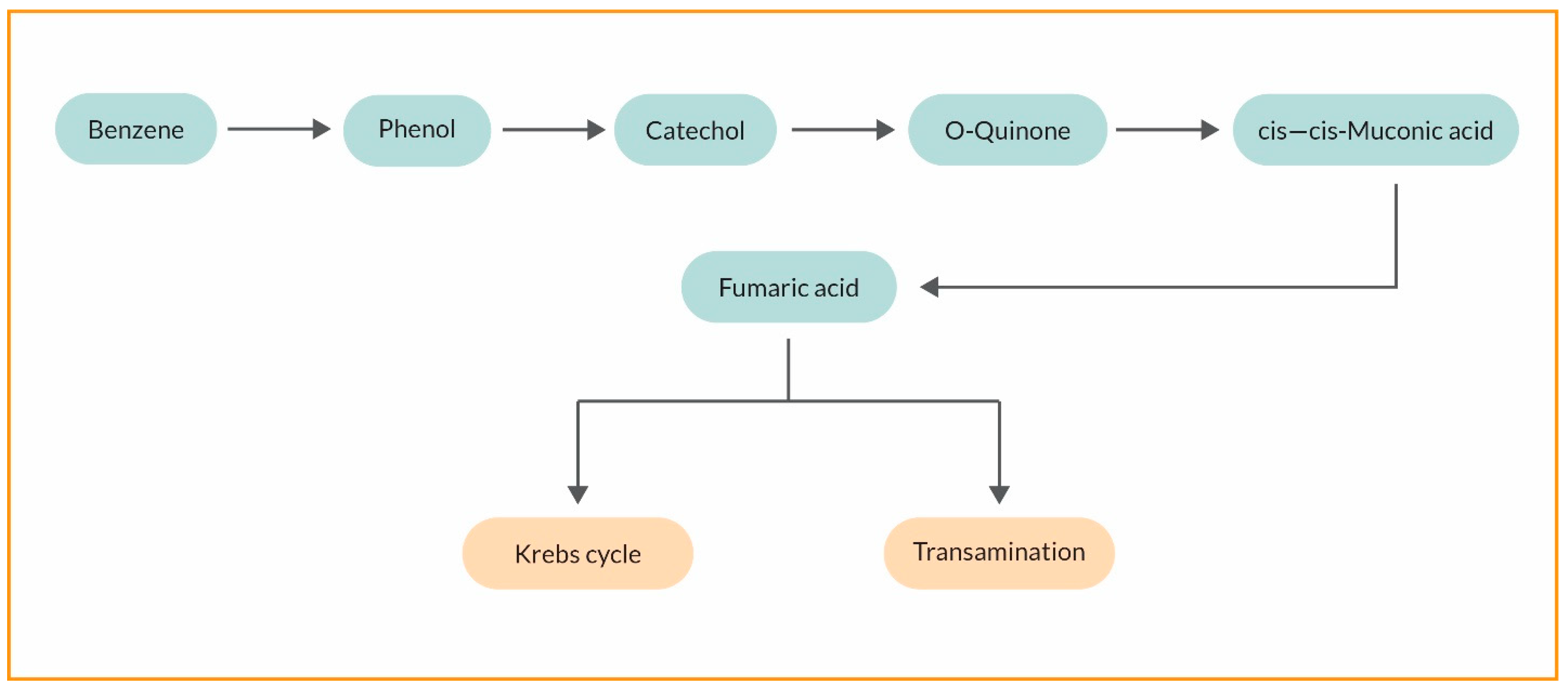
3.2. Cyanoremediation of Oil and Gas Components
3.3. Source of Industrial Biotechnological and Health Applications
3.4. Bloom Formation
4. Bioactive Compounds in the Arabian Gulf
5. Challenges and Future Perspectives
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abulfatih, H.A.; Abdel-Bari, E.M.; Alsubaey, A.; Ibrahim, Y.M. Vegetation of Qatar; Scientific and Applied Research Center (SARC), University of Qatar: Doha, Qatar, 2001. [Google Scholar]
- Yasseen, B.T.; Abulfatih, H.A.; Nasher, A.K.; Abid, K.Y.; Al-Mofti, M.B. Preliminary assessment of pollution due to faulty sewage system in north of Sana’a, Republic of Yemen. Dirasat Eng. Sci. 2001, 28, 89–96. [Google Scholar]
- Abulfatih, H.A.; Al-Thani, R.F.; Al-Naimi, I.S.; Swelleh, J.A.; Elhag, E.A.; Kardousha, M.M. Ecology of Wastewater Ponds in Qatar, Doha; Scientific and Applied Research Centre (SARC), University of Qatar: Doha, Qatar, 2002. [Google Scholar]
- Al-Thani, R.F.; Yasseen, B.T. Perspectives of future water sources in Qatar by phytoremediation: Biodiversity at ponds and modern approach. Int. J. Phytoremediat. 2021, 23, 866–889. [Google Scholar] [CrossRef]
- Jahan, S.; Singh, A. The Role of phytoplanktons in the environment and in human life, a review. Bas. J. Sci. 2023, 41, 379–398. [Google Scholar] [CrossRef]
- Wang, X.; Yin, Z.; Chen, J.; Liu, J. Phytoplankton carbon utilization strategies and effects on carbon fixation. Water 2023, 15, 2137. [Google Scholar] [CrossRef]
- Available online: https://oceanservice.noaa.gov/facts/ocean-oxygen.html (accessed on 11 June 2024).
- Baumas, C.; Bizic, M. A focus on different types of organic matter particles and their significance in the open ocean carbon cycle. Prog. Oceanogr. 2024, 224, 103233. [Google Scholar] [CrossRef]
- Litchman, E.; Pinto, P.T.; Edwards, K.F.; Klausmeier, C.A.; Kremer, C.T.; Thomas, M.K. Global biogeochemical impacts of phytoplankton: A trait-based perspective. J. Ecol. 2015, 103, 1384–1396. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://earthobservatory.nasa.gov/features/Phytoplankton (accessed on 14 August 2024).
- Jackson, R.; Gabric, A. Climate change impacts on the marine cycling of biogenic sulfur: A review. Microorganisms 2022, 10, 1581. [Google Scholar] [CrossRef]
- Irwin, A.J.; Finkel, Z.V.; Müller-Karger, F.E.; Ghinaglia, L.T. Phytoplankton adapt to changing ocean environments. Proc. Natl. Acad. Sci. USA 2015, 112, 5762–5766. [Google Scholar] [CrossRef]
- Vallina, S.M.; Cermeno, P.; Dutkiewicz, S.; Loreau, M.; Montoya, J.M. Phytoplankton functional diversity increases ecosystem productivity and stability. Ecol. Model. 2017, 361, 184–196. [Google Scholar] [CrossRef]
- Sauterey, B.; Le Gland, G.; Cermeño, P.; Aumont, O.; Lévy, M.; Sergio, M.; Vallina, S.M. Phytoplankton adaptive resilience to climate change collapses in case of extreme events-A modeling study. Ecol. Model. 2023, 483, 110437. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, D.; Gu, T.; Jiang, T.; Qu, K.; Sun, J.; Cui, Z. Different responses of phytoplankton and zooplankton communities to current changing coastal environments. Environ. Res. 2022, 215, 114426. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Ki, J.S. Phytoplankton toxins and their potential therapeutic applications: A journey toward the quest for potent pharmaceuticals. Mar. Drugs 2022, 20, 271. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms (HABs): Paradigm shifts and new technologies for research, monitoring and management. Ann. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Whalen, J.K.; Cai, C.; Shan, K.; Zhou, H. Harmful cyanobacteria-diatom/dinoflagellate blooms and their cyanotoxins in freshwaters: A non-eligible chronic health and ecological hazard. Water Res. 2023, 233, 119807. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Probyn, T.A. Suffocating phytoplankton, suffocating waters-red tides and anoxia. Front. Mar. Sci. 2016, 3, 186. [Google Scholar] [CrossRef]
- Gauns, M.; Mochemadkar, S.; Pratihary, A.; Shirodkar, G.; Narvekar, P.V.; Naqvi, S.W.A. Phytoplankton associated with seasonal oxygen depletion in waters of the western continental shelf of India. J. Mar. Syst. 2020, 204, 103308. [Google Scholar] [CrossRef]
- Devlin, M.; Fernand, L.; Collingridge, K. Concentrations of dissolved oxygen near the seafloor in the greater North Sea, Celtic Sea and Bay of Biscay and Iberian coast. In OSPAR, 2023: The 2023 Quality Status Report for the North-East Atlantic; OSPAR Commission: London, UK, 2022; Available online: https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicator-assessments/seafloor-dissolved-oxygen (accessed on 21 July 2024).
- Available online: https://www.globalseafood.org/advocate/phytoplankton-impact-water-quality/ (accessed on 18 July 2024).
- Amorim, C.A.; Moura, A.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Hintz, N.H.; Schulze, B.; Wacker, A.; Striebel, M. Ecological impacts of photosynthetic light harvesting in changing aquatic environments: A systematic literature map. Ecol. Evol. 2022, 12, e8753. [Google Scholar] [CrossRef]
- Käse, L.; Geuer, J.K. Phytoplankton responses to marine climate change-An introduction. In YOUMARES 8—Oceans Across Boundaries: Learning from Each Other; Jungblut, S., Liebich, V., Bode, M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Cho, R. Plankton Are Central to Life on Earth. How Is Climate Change Affecting Them? State of the Planet; Colombia Climate School: New York, NY, USA, 2023; Available online: https://news.climate.columbia.edu/2023/08/23/plankton-are-central-to-life-on-earth-how-is-climate-change-affecting-them/ (accessed on 1 July 2024).
- Al-Thani, R.F.; Yasseen, B.T. Phytoremediation of polluted soils and waters by native Qatari plants: Future perspectives. Environ. Pollut. 2020, 259, 113694. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Microbial ecology of Qatar, the Arabian Gulf: Possible roles of microorganisms. Front. Mar. Sci. 2021, 8, 697269. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Possible future risks of pollution consequent to the expansion of oil and gas operations in Qatar. Environ. Pollut. 2023, 12, 12–52. [Google Scholar] [CrossRef]
- Gupta, P.K.; Ranjan, S.; Gupta, S.K. Phycoremediation of petroleum hydrocarbon-polluted sites: Application, challenges, and future prospects. In Application of Microalgae in Wastewater Treatment; Gupta, K., Bux, F., Eds.; Springer Nature: Cham, Switzerland, 2019; p. 20195. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Sarma, U.; Hoque, M.E.; Thekkangil, A.; Venkatarayappa, N.; Rajagopal, S. Microalgae in removing heavy metals from wastewater-An advanced green technology for urban wastewater treatment. J. Hazard. Mater. Adv. 2024, 15, 100444. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Griebe, T.; Mayer, C. Physico-Chemical Properties of Biofilms. In Biofilms: Recent Advances in their Study and Control; Evans, L.V., Ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-9058230935. [Google Scholar]
- Al-Thani, R.F.; Yasseen, B.T. Solutes in native plants in the Arabian Gulf region and the role of microorganisms: Future research. J. Plant Ecol. 2018, 11, 671–684. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, S.; Wei, Y.; Liu, Y.; Xu, Y.; Yang, Y.; Zhang, P.; Zhou, Q.; Wang, J.J. The role of extracellular polymeric substances (EPS) in chemical-degradation of persistent organic pollutants in soil: A review. Sci. Total Environ. 2024, 912, 168877. [Google Scholar] [CrossRef]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Fan, X.; Lu, X.; Yu, B.; Zuo, L.; Fan, P.; Yang, Y.; Zhuang, S.; Liu, H.; Qin, Q. Risk and sources of heavy metals and metalloids in dust from university campuses: A case study of Xi’an, China. Environ. Res. 2021, 202, 111703. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Nayak, R.; Patra, S.; Behera, C.; Ki, J.S.; Ragusa, A.; Lukatkin, A.S.; Jena, M. Microalgal phycoremediation: A glimpse into a sustainable environment. Toxics 2022, 10, 525. [Google Scholar] [CrossRef]
- Tong, Y.; Kneer, R.; Zhu, Y.G. Vacuolar compartmentalization: A second-generation approach to engineering plants for phytoremediation. Trends Plant Sci. 2004, 9, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Ankit; Bauddh, K.; Korstad, J. Phycoremediation: Use of algae to sequester heavy metals. Hydrobiology 2022, 1, 288–303. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M.O. Factors and mechanisms regulating heavy metal phycoremediation in polluted water. Discov. Water 2023, 3, 14. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Bharadvaja, N. Phycoremediation of effluents containing dyes and its prospects for value-added products: A review of opportunities. J. Water Process Eng. 2021, 41, 102080. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Sun, G.-X.; Yin, X.-X.; Rensing, C.; Zhu, Y.-G. Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere 2013, 93, 47–53. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Xu, L.; Ma, A.; Zhuang, G.; Huo, S.; Zou, B.; Qian, J.; Cui, Y. Selenium volatilization in plants, microalgae, and microorganisms. Heliyon 2024, 10, e26023. [Google Scholar] [CrossRef]
- Yasseen, B.; Al-Thani, R.F. Endophytes and halophytes to remediate industrial wastewater and saline soils: Perspectives from Qatar. Plants 2022, 11, 1497. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Methods using marine aquatic photoautotrophs along the Qatari coastline to remediate oil and gas industrial water. Toxics 2024, 12, 625. [Google Scholar] [CrossRef]
- Freije, A.M. Heavy metal, trace element and petroleum hydrocarbon pollution in the Arabian Gulf. J. Assoc. Arab. Univ. Basic Appl. Sci. 2015, 17, 90–100. [Google Scholar] [CrossRef]
- John, D.M.; Al-Thani, R.F. Benthic marine algae of the Arabian Gulf: A critical review and analysis of distribution and diversity patterns. Nova Hedwig. 2014, 98, 341–392. [Google Scholar] [CrossRef]
- Dorgham, M.M.; Al-Muftah, A.M. Plankton studies in the Arabian Gulf. I. Preliminary list of phytoplankton species in Qatari waters. Arab Gulf J. Scient. Res. Agric. Biol. Sci. 1986, 4, 421–436. [Google Scholar]
- Rizk, A.M.; Al-Easa, H.S.; Kornprobst, J.M. The Phytochemistry of the Macro and Blue-Green Algae of the Arabian Gulf; Faculty of Science, Doha Modern Printing Press Ltd.: Doha, Qatar, 1999; ISBN 999921-46-64-8. [Google Scholar]
- Al-Khelaifi, F.A. Phylogenetic Diversity of Cynobacteria from Qatar Coastal Waters. Master’s Thesis, Qatar University, College of Arts and Sciences, Doha, Qatar, 2014. [Google Scholar]
- Al-Khelaifi, F.; Yilmaz, M.; Saadaoi, I.; Ben-Hamadou, R. Phylogenic diversity of cyanobacteria from Qatar. In Science Proceedings, Qatar University Life Science Symposium-QULSS 2015 Global Changes: The Arabian Gulf Ecosystem; Hamad bin Khalifa University Press (HBKU Press): Doha, Qatar, 2015; Volume 28. [Google Scholar] [CrossRef]
- Quesada, A.; Moreno, E.; Carrasco, D.; Paniagua, T.; Wormer, L.; Hoyos, C.; Sukenik, A. Toxicity of Aphanizomenon ovalisporum (Cyanobacteria) in a Spanish water reservoir. Eur. J. Phycol. 2006, 41, 39–45. [Google Scholar] [CrossRef]
- Jacques, N.R. Remediation of Light Extractable Petroleum Hydrocarbons in Water by Algae. Master’s Thesis, Royal Roads University, Colwood, BC, Canada, 2008. [Google Scholar]
- Furniturewalla, A.; Barve, K. Marine antioxidants in the management of atherosclerosis, Chapter 20. In Marine Antioxidants: Preparations, Syntheses, and Applications; Academic Press: Cambridge, MA, USA, 2023; pp. 273–284. [Google Scholar] [CrossRef]
- Atyah, B.S.; Al-Mayaly, I.K. Biodegradation of crude oil by Anabaena variabilis isolated from Al-Dora Refinery. IOSR J. Pharm. Biol. Sci. 2018, 13, 51–56. [Google Scholar] [CrossRef]
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as natural therapeutics and pharmaceutical potential: Role in antitumor activity and as nano-vectors. Molecules 2021, 26, 247. [Google Scholar] [CrossRef]
- Das, S.; Banik, R.D. Microbial action to remediate petroleum-hydrocarbon contaminated soil. J. Adv. Microbiol. Res. 2024, 5, 27–32. [Google Scholar] [CrossRef]
- Yadav, P.; Gupta, R.K.; Singh, R.P.; Yadav, P.K.; Alam, J.; Patel, A.K.; Pandy, K.D. Role of Cyanobacteria in Green Remediation, Chapter 9. In Sustainable Environhental Clean-Up; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Melzi, A.; Zecchin, S.; Gomarasca, S.; Abruzzese, A.; Cavalca, L. Ecological indicators and biological resources for hydrocarbon rhizoremediation in a protected area. Front. Bioeng. Biotechnol. 2024, 12, 1379947. [Google Scholar] [CrossRef]
- Goshtasbi, H.; Khiavi, M.A.; Safary, A.; Movafeghi, A.; Omidi, Y. In vitro anticancer activity of methanolic extract of Chroococcus sp.; Kani Barazan International Wetland. In Proceedings of the 3rd International Congress and 4th National Conference on Biotechnology of Medicinal Plants and Mushrooms, Zanjan, Iran, 17 May 2021. [Google Scholar]
- Duarte, I.F.; de Souza Ribeiro, V.; dos Santos, M.I.G.R.; Costa, T.A.D.; Santana, M.B.; Oliveira, A.C.V.; Marques, I.M.; Nanez, K.B.; Moreira Icaro, T.A. Remediation mechanisms of polycyclic aromatic petroleum hydrocarbons using microalgae and cyanobacteria with emphasis on circular bioeconomy. Res. Soc. Dev. 2021, 10, e512101119954. [Google Scholar] [CrossRef]
- Kalita, N.; Baruah, P.P. Cyanobacteria as a potent platform for heavy metals biosorption: Uptake, responses, and removal mechanisms. J. Hazard. Mater. Adv. 2023, 11, 100349. [Google Scholar] [CrossRef]
- Hart, R. Cyanobacteria Biocomposites for In Situ Treatment of Domestic Wastewater. Master’s Thesis, School of Natural and Environmental Sciences, The University of Newcastle, Newcastle, UK, 2020. [Google Scholar]
- Li, C.; Zhang, X.; Ye, T.; Li, X.; Wang, G. Protection and damage repair mechanisms contributed to the survival of Chroococcidiopsis sp. exposed to a Mars-like near space environment. Microbiol Spectr. 2022, 10, e0344022. [Google Scholar] [CrossRef]
- Baldanta, S.; Arnal, R.; Blanco-Rivero, A.; Guevara, G.; Navarro Llorens, J.M. First characterization of cultivable extremophile Chroococcidiopsis isolates from a solar panel. Front. Microbiol. 2023, 14, 982422. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, C.; Vipparty, V.; David, J.J.; Chandramohan, D. Degradation of crude oil by marine cyanobacteria. Appl. Microbiol. Biot. 2001, 57, 433–436. [Google Scholar] [CrossRef]
- Mekonnen, B.A.; Aragaw, T.A.; Genet, M.B. Bioremediation of petroleum hydrocarbon contaminated soil: A review on principles, degradation mechanisms, and advancements. Front. Environ. Sci. 2024, 12, 1354422. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Al-Kharusi, S.; Prigent, S.; Headley, T. Diversity, distribution, and hydrocarbon biodegradation capabilities of microbial communities in oil-contaminated cyanobacterial mats from a constructed wetland. PLoS ONE 2014, 9, e114570. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, R.; Rao, P.; Wu, B.; Yan, L.; Hu, L.; Park, S.; Ryu, M.; Zhou, X. Bioremediation of petroleum hydrocarbons using Acinetobacter sp. SCYY-5 isolated from contaminated oil sludge: Strategy and effectiveness study. Int. J. Environ. Res. Public Health 2021, 18, 819. [Google Scholar] [CrossRef]
- Hirose, Y.; Misawa, N.; Yonekawa, C.; Nagao, N.; Watanabe, M.; Ikeuchi, M.; Eki, T. Characterization of the genuine type 2 chromatic acclimation in the two Geminocystis cyanobacteria. DNA Res. 2017, 24, 387–396. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Kiran, B.; Thanasekaran, K. Metal tolerance of an indigenous cyanobacterial strain, Lyngbya putealis. Int. Biodeterior. Biodegrad. 2011, 65, 1128–1132. [Google Scholar] [CrossRef]
- Panah, B.A. Biodegradation ability and physiological responses of cyanobacterium Leptolyngbya sp. ISC 25 under naphthalene. Algologia 2015, 25, 125–134. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Niknam, V.; Soltani, N.; Ebrahimzadeh, H. Leptolyngbya fragilis ISC 108 is the most effective strain for dodecane biodegradation in contaminated soils. Int. J. Phytoremediat. 2019, 21, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Alhumairi, A.M.; Saddiq, A.A. Simultaneous bioremediation of petroleum hydrocarbons and production of biofuels by the micro-green alga, cyanobacteria, and its consortium. Heliyon 2023, 9, e16656. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, W.J.; Mary, A. Remediation of crude oil polluted river using Nostoc and Oscillatoria spp. J. Biol. Gen. Res. 2018, 4, 1–9. [Google Scholar]
- Vasconcelos, V.M.; Sivonen, K.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. Hepatotoxic microcystin diversity in cyanobacterial blooms collected in Portuguese freshwaters. Water Res. 1996, 30, 2377–2384. [Google Scholar] [CrossRef]
- Kataokam, T.; Ohbayashi, K.; Kobayashi, Y.; Takasu, H.; Nakano, S.I.; Kondo, R.; Hodoki, Y. Distribution of the harmful bloom-forming cyanobacterium, Microcystis aeruginosa, in freshwater environments across Japan. Microbes Environ. 2020, 35, ME19110. [Google Scholar] [CrossRef]
- Henriksen, P. Estimating nodularin content of cyanobacterial blooms from abundance of Nodularia spumigena and its characteristic pigments—A case study from the Baltic entrance area. Harmful Algae 2005, 4, 167–178. [Google Scholar] [CrossRef]
- Konkel, R.; Toruńska-Sitarz, A.; Cegłowska, M.; Ežerinskis, Z.; Šapolaitė, J.; Mažeika, J.; Mazur-Marzec, H. Blooms of toxic cyanobacterium Nodularia spumigena in Norwegian Fjords during Holocene warm periods. Toxins 2020, 12, 257. [Google Scholar] [CrossRef]
- Pimda, W.; Bunnag, S. Biodegradation of used motor oil by single and mixed cultures of cyanobacteria. Afr. J. Biotechnol. 2012, 11, 9074–9078. [Google Scholar] [CrossRef]
- Trentin, G.; Piazza, F.; Carletti, M.; Zorin, B.; Khozin-Goldberg, I.; Bertucco, A.; Sforza, E. Fixing N2 into cyanophycin: Continuous cultivation of Nostoc sp. PCC 7120. Appl. Microbiol. Biotechnol. 2023, 107, 97–110. [Google Scholar] [CrossRef]
- Akl, F.M.A.; Ahmed, S.I.; El-Sheekh, M.M.; Makhlof, M.E.M. Bioremediation of n-alkanes, polycyclic aromatic hydrocarbons, and heavy metals from wastewater using seaweeds. Environ. Sci. Pollut. Res. Int. 2023, 30, 104814–104832. [Google Scholar] [CrossRef]
- Ugboma, C.J.; Sampson, T.; Tamunoiboroma, J.E. Bioremediation potentials of halotolerant Oscillatoria sp. in the remediation of crude oil polluted water. J. Adv. Microbiol. Res. (JAMR) 2023, 4, 45–52. [Google Scholar]
- Almutairi, H.H. Microbial communities in petroleum refinery effluents and their complex functions. Saudi J. Biol. Sci. 2024, 31, 104008. [Google Scholar] [CrossRef]
- Pimda, W.; Bunnag, S. Impact of inorganic nutrients and heavy metals present as co-contaminants on biodegradation of petroleum hydrocarbons by Phormidium ambiguum strain TISTR 8296. Water Air Soil Pollut. 2017, 228, 58. [Google Scholar] [CrossRef]
- Flores, E.; Romanovicz, D.K.; Nieves-Morión, M.; Foster, R.A.; Villareal, T.A. Adaptation to an intracellular lifestyle by a nitrogen-fixing, heterocyst-forming cyanobacterial endosymbiont of a diatom. Front. Microbiol. 2022, 13, 799362. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yu, J.; Fang, K.; Dong, P.; Li, Z.; Zhang, W.; Liu, M.; Xiang, L.; Cai, J. Microbial removal of petroleum hydrocarbons from contaminated soil under arsenic stress. Toxics 2023, 11, 143. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Yu, G.-L.; Jiang, H.-X.; Liu, L.-J.; Zhao, R. Role and lifestyle of calcified cyanobacteria (Stanieria) in Permian–Triassic boundary microbialites. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 448, 39–47. [Google Scholar] [CrossRef]
- Xu, S.; Nijampatnam, B.; Dutta, S.; Velu, S.E. Cyanobacterial metabolite calothrixins: Recent advances in synthesis and biological evaluation. Mar. Drugs 2016, 14, 17. [Google Scholar] [CrossRef]
- Borah, D.; Agarwal, K.; Khataniar, A.; Konwar, D.; Gogoi, S.B.; Kallel, M. A newly isolated strain of Serratia sp. from an oil spillage site of Assam shows excellent bioremediation potential. 3 Biotech 2019, 9, 283. [Google Scholar] [CrossRef]
- Al-Kaabi, N.; Al-Ghouti, M.A.; Jaoua, S.; Zouari, N. Potential for native hydrocarbon-degrading bacteria to remediate highly weathered oil-polluted soils in Qatar through self-purification and bioaugmentation in biopiles. Biotechnol. Rep. 2020, 28, e00543. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Harvey, H.R.; Fry, B.; Capone, D.G. Biogeochemical tracers of the marine cyanobacterium Trichodesmium. Deep Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 27–38. [Google Scholar] [CrossRef]
- Bergman, B.; Sandh, G.; Lin, S.; Larsson, J.; Carpenter, E.J. Trichodesmium-A widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev. 2013, 37, 286–302. [Google Scholar] [CrossRef]
- Gosselin, K.M.; Nelson, R.K.; Spivak, A.C.; Sylva, S.P.; Van Mooy, B.A.S.; Aeppli, C.; Sharpless, C.M.; O’Neil, G.W.; Arrington, E.C.; Reddy, C.M.; et al. Production of two highly abundant 2-methyl-branched fatty acids by blooms of the globally significant marine Cyanobacteria Trichodesmium erythraeum. ACS Omega 2021, 6, 22803–22810. [Google Scholar] [CrossRef]
- Gardner, J.J.; Hodge, B.-M.S.; Boyle, N.R. Investigating the unique ability of Trichodesmium to fix carbon and nitrogen simultaneously using MiMoSA. mSystems 2023, 8, e00601-20. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Lea, P.J.; Leegood, R.C. Plant Biochemistry & Molecular Biology; John Wiley & Sons: New York, NY, USA, 1994; ISBN 0 471 93313 9. [Google Scholar]
- Yasseen, B.T. Fundamentals of Plant Physiology; Department of Biological Sciences, Qatar University: Doha, Qatar, 2001; ISBN 8-81-46-99921. (In Arabic) [Google Scholar]
- Magnuson, A. Heterocyst thylakoid bioenergetics. Life 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 2, a000315. [Google Scholar] [CrossRef] [PubMed]
- Ashore, M.M. Sabkhas in the Peninsula of Qatar—Geomorphologic and Geological and Biological Studies—University of Qatar; Centre of Documentation and Humanitarian Studies: Doha, Qatar, 1991. [Google Scholar]
- Redfield, E.; Barns, S.M.; Belnap, J.; Daane, L.L.; Kuske, C.R. Comparative diversity and composition of cyanobacteria in three predominant soil crusts of the Colorado Plateau. FEMS Microbiol. Ecol. 2002, 40, 55–63. [Google Scholar] [CrossRef][Green Version]
- Al-Thani, R.F.; Yasseen, B.T. Halo-thermophilic bacteria and heterocyst cyanobacteria found adjacent to halophytes at Sabkhas—Qatar: Preliminary study and possible roles. Afr. J. Microbiol. Res. 2017, 11, 1346–1354. [Google Scholar]
- Gao, H.; Wu, M.; Liu, H.; Xu, Y.; Liu, Z. Effect of petroleum hydrocarbon pollution levels on the soil microecosystem and ecological function. Environ. Pollut. 2022, 293, 118511. [Google Scholar] [CrossRef]
- Ou, Y.; Wu, M.; Yu, Y.; Liu, Z.; Zhang, T.; Zhang, X. Low dose phosphorus supplementation is conducive to remediation of heavily petroleum-contaminated soil-From the perspective of hydrocarbon removal and ecotoxicity risk control. Sci. Total Environ. 2024, 929, 172478. [Google Scholar] [CrossRef]
- Chaillan, F.; Gugger, M.; Saliot, A.; Couté, A.; Oudot, J. Role of cyanobacteria in the biodegradation of crude oil by a tropical cyanobacterial mat. Chemosphere 2006, 62, 1574–1582. [Google Scholar] [CrossRef]
- Sandermann, H. Pestizid-Rückstande in Nahrungspflanzen: Die Rolle des pflanzlichen Metabolismus (Translation: Pesticide residues in food crops: The role of plant metabolism). Naturwissenschaften 1987, 74, 573–578. [Google Scholar] [CrossRef]
- Ohtsubo, Y.; Kudo, T.; Tsuda, M.; Nagata, Y. Strategies for bioremediation of polychlorinated biphenyls. Appl. Microbiol. Biotechnol. 2004, 65, 250–258. [Google Scholar] [CrossRef]
- Van Aken, B. Transgenic plants for phytoremediation: Helping nature to clean up environmental pollution. Trends Biotechnol. 2008, 26, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, W.; Zhang, Y.; Qiao, Q.; Zhang, X. Are fish fed with cyanobacteria safe, nutritious, and delicious? A laboratory study. Sci. Rep. 2015, 5, 15166. [Google Scholar] [CrossRef]
- Epstein, E. Crops tolerant to salinity and other mineral stresses. In Better Crops for Food; Nugent, J., O’Connor, M., Eds.; Ciba Foundation Symposium 97; Pitman Books Ltd.: London, UK, 1983; pp. 61–82. [Google Scholar]
- Lasat, M.M. The Use of Plants for the Removal of Toxic Metals from Contaminated Soil. 2000. Available online: https://clu-in.org/download/remed/lasat.pdf (accessed on 16 March 2013).
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Available online: https://thepeninsulaqatar.com/article/11/12/2018/Amir-inaugurates-largest-water-security-mega-reservoirs-project-in-the-world (accessed on 26 March 2024).
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria-A review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Perera, R.M.T.D.; Herath, K.H.I.N.M.; Sanjeewa, K.K.A.; Jayawardena, T.U. Recent reports on bioactive compounds from marine cyanobacteria in relation to human health applications. Life 2023, 13, 1411. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Metabolic transformation of cyanobacteria for biofuel production. Chemosphere 2022, 299, 134342. [Google Scholar] [CrossRef]
- Nowruzi, B.; Sarvari, G.; Blanco, S. The cosmetic application of cyanobacterial secondary metabolites. Algal Res. 2020, 49, 101959. [Google Scholar] [CrossRef]
- Robles-Bañuelos, B.; Durán-Riveroll, L.M.; Rangel-López, E.; Pérez-López, H.I.; González-Maya, L. Marine cyanobacteria as sources of lead anticancer compounds: A review of families of metabolites with cytotoxic, antiproliferative, and antineoplastic effects. Molecules 2022, 27, 4814. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakrim, S.; Chamkhi, I.; Taha, D.; El Omari, N.; El Mneyiy, N.; El Hachlafi, N.; El-Shazly, M.; Khalid, A.; Abdalla, A.N.; et al. Bioactive substances of cyanobacteria and microalgae: Sources, metabolism, and anticancer mechanism insights. Biomed. Pharmacother. 2024, 170, 115989. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria-from the oceans to the potential biotechnological and biomedical applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.L.; Chaudhuri, R.G.; Dutta, S. A critical review on phycoremediation of pollutants from wastewater-a novel algae-based secondary treatment with the opportunities of production of value-added products. Environ. Sci. Pollut. Res. Int. 2023, 30, 114844–114872. [Google Scholar] [CrossRef] [PubMed]
- Chelsea, A.; Weirich, B.S.; Todd, R.; Miller, P.D. Freshwater harmful algal blooms: Toxins and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 2–24. [Google Scholar] [CrossRef]
- Hu, C. Detection, biosynthesis, and biofunctions of microcystins in the freshwater bloom-forming cyanobacterium Microcystis, Chapter 10. In Cyanobacterial Physiology (Fundamentals to Biotechnology); Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–135. [Google Scholar] [CrossRef]
- Teikari, J.E.; Hou, S.; Wahlsten, M.; Hess, W.; Sivonen, K. Comparative genomics of Beltic sea toxic cyanobacteria Nodularia spumigena UHCC 0039 and its response to varying salinity. Front. Microbiol. 2018, 9, 356. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Al Muftah, A.; Selwood, A.I.; Foss, A.J.; Al-Jabri, H.M.S.J.; Potts, M.; Yilmaz, M. Algal toxins and producers in the marine waters of Qatar, Arabian Gulf. Toxicon 2016, 122, 54–66. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Xiao, Y.; Zhang, Y.; Yu, Y.; Zheng, Z.; Liu, Y.; Li, Q. The impact of cyanobacteria blooms on the aquatic environment and human health. Toxins 2022, 14, 658. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Hassanshahian, M.; Amirinejad, N.; Behzadi, M.A. Crude oil pollution and biodegradation at the Persian Gulf: A comprehensive and review study. J. Environ. Health Sci. Eng. 2020, 18, 1415–1435. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, R.; Liang, D.; Wang, F.; Han, Y.; Luo, Y.; Gao, P.; Wang, Q.; Wang, Q.; Yu, C.; et al. Comparison of the advantages and disadvantages of algae removal technology and its development status. Water 2023, 15, 1104. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nano-formulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Karan, T.; Aydin, A. Anticancer potential and cytotoxic effect of some freshwater cyanobacteria. Trop. J. Pharm. Res. 2018, 17, 2183–2188. [Google Scholar] [CrossRef]
- Ding, J.; Wu, B.; Chen, L. Application of marine microbial natural products in cosmetics. Front. Microbiol. 2022, 13, 892505. [Google Scholar] [CrossRef] [PubMed]
- Bechelli, J.; Coppage, M.; Rosell, K.; Liesveld, J. Cytotoxicity of algae extracts on normal and malignant cells. Leuk. Res. Treat. 2011, 2011, 373519. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal biorefinery concepts’ developments for biofuel and bioproducts: Current perspective and bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef]
- Mandhata, C.P.; Bishoyi, A.K.; Sahoo, C.R.; Maharana, S.; Padhy, R.N. Insight to biotechnological utility of phy-cochemicals from cyanobacterium Anabaena sp.: An overview. Fitoterapia 2023, 169, 105594. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Sharma, Y.C. Biodiesel synthesis from microalgae (Anabaena PCC 7120) by using barium titanium oxide (Ba2TiO4) solid base catalyst. Bioresour. Technol. 2019, 287, 121357. [Google Scholar] [CrossRef]
- Zhu, S.; Higa, L.; Barela, A.; Lee, C.; Chen, Y.; Du, Z.-Y. Microalgal consortia for waste treatment and valuable bioproducts. Energies 2023, 16, 884. [Google Scholar] [CrossRef]
- Rickards, R.W.; Rothschild, J.M.; Willis, A.C.; Chazal, N.M.de.; Kirk, J.; Kirk, K.; Saliba, K.J.; Geoffrey, D.; Smith, G.D. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513–13520. [Google Scholar] [CrossRef]
- Matharasi, A.; Anahas, P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel pro-duction based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Phyco-cosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Narayanan, I.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Biodiesel production from microalgae: A comprehensive review on influential factors, transesterification processes, and challenges. Fuel 2024, 367, 131547. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Vicente, A.A.; Vasconcelos, V. Cyanobacteria-based bioprocess for cosmetic products-Cyanobium sp. as a novel source of bioactive pigments. Phycology 2023, 3, 47–64. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; Abdul-Quadir, M.; Thaher, M.I.; Hawari, A.H.; Alshamri, N.; Al-Ghasal, G.; Al-Jabri, H.M.J. Biocrude oil production from a self-settling marine cyanobacterium, Chroococcidiopsis sp., using a biorefinery approach. Renew. Energy 2023, 203, 1–9. [Google Scholar] [CrossRef]
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial metabolites as a source of sunscreens and moisturizes: A comparison with current synthetic compounds. Eur. J. Phycol. 2016, 52, 43–56. [Google Scholar] [CrossRef]
- Nozzi, N.E.; Oliver, J.W.K.; Atsumi, S. Cyanobacteria as a platform for biofuel production. Front. Bioeng. Biotechnol. 2013, 1, 7. [Google Scholar] [CrossRef]
- Mogany, T.; Swalaha, F.M.; Allam, M.; Mtshali, P.S.; Ismail, A.; Kumari, S.; Faizal Bux, F. Phenotypic and genotypic characterization of an unique indigenous hypersaline unicellular cyanobacterium, Euhalothece sp. nov. Microbiol. Res. 2018, 211, 47–56. [Google Scholar] [CrossRef]
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Šantek, M.I. Bioprospecting of microalgae isolated from the Adriatic Sea: Characterization of biomass, pigment, lipid and fatty acid composition, and an-tioxidant and antimicrobial activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef]
- Srivastava, A.; Tiwari, R.; Srivastava, V.; Singh, T.B.; Asthana, R.K. Fresh water cyanobacteria Geitlerinema sp. CCC728 and Arthrospira sp. CCC729 as an anticancer drug resource. PLoS ONE 2015, 10, e0136838. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Soares, A.T.; D’Alessandro, N.C.d.O.; Filho, N.R.A. Potential use of a thermal water cyanobacte-rium as raw material to produce biodiesel and pigments. Bioprocess Biosyst. Eng. 2019, 42, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Oliveira, R.; Dias, A.C.P. Microalgae and cyanobacteria as sources of bioactive compounds for cosmetic applications: A systematic review. Algal Res. 2023, 76, 103287. [Google Scholar] [CrossRef]
- Shishido, T.K.; Popin, R.V.; Jokela, J.; Wahlsten, M.; Fiore, M.F.; Fewer, D.P.; Herfindal, L.; Sivonen, K. Dereplication of natural products with antimicrobial and anticancer activity from Brazilian Cyanobacteria. Toxins 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Pittman, K.; Edmundson, S.; Huesemann, M.; Greer, M.; Louie, W.; Chen, P.; Nobles, D.; Benemann, J.; Crowe, B. A newly isolated alkaliphilic cyanobacterium for biomass production with direct air CO2 capture. J. CO2 Util. 2023, 69, 102399. [Google Scholar] [CrossRef]
- Adejimi, O.E.; Sadhasivam, G.; Schmilovitch, Z.; Shapiro, O.H.; Herrmann, I. Applying hyperspectral transmittance for inter-genera classification of cyanobacterial and algal cultures. Algal Res. 2023, 71, 103067. [Google Scholar] [CrossRef]
- Swain, S.S.; Padhy, R.N.; Singh, P.K. Anticancer compounds from cyanobacterium Lyngbya species—A review. Antonie Van Leeuwenhoek 2015, 108, 223–265. [Google Scholar] [CrossRef]
- Kushwaha, D.; Srivastava, N.; Prasad, D.; Mishra, P.K.; Upadhyay, S.N. Biobutanol production from hydrolysates of cyanobacteria Lyngbya limnetica and Oscillatoria obscura. Fuel 2020, 271, 117583. [Google Scholar] [CrossRef]
- Abbas, A.M.; Elkheralla, R.J.; Ali, A.T.A.; Jebur, A.S.; Shwayel, I.H.; Jayp, M.R. An experimental study of the production of biofuel from Lyngbya sp. algae. Univ. Thi-Qar J. Sci. 2024, 11, 121–123. [Google Scholar] [CrossRef]
- Fuentes-Tristan, S.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J. Photochem. Photobiol. B Biol. 2019, 201, 111684. [Google Scholar] [CrossRef]
- Gara-Ali, M.; Zili, F.; Hosni, K.; Ben-Ouada, H.; Ben-Mahrez, K. Lipophilic extracts of the thermophilic cyanobacterium Leptolyngbya sp. And chlorophyte Graesiella sp. and their potential use as food and anticancer agents. Algal Res. 2021, 60, 102511. [Google Scholar] [CrossRef]
- Singh, J.; Tripathi, R.; Thakur, I.S. Characterization of endolithic cyanobacterial strain, Leptolyngbya sp. ISTCY101, for prospective recycling of CO2 and biodiesel production. Bioresour. Technol. 2014, 166, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Thakur, I.S. Evaluation of cyanobacterial endolith Leptolyngbya sp. ISTCY101, for integrated wastewater treatment and biodiesel production: A toxicological perspective. Algal Res. 2015, 11, 294–303. [Google Scholar] [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria secondary metabolites as biotechnological ingredients in natural anti-aging cosmetics: Potential to overcome hyper-pigmentation, loss of skin density and UV radia-tion-deleterious effects. Mar. Drugs 2022, 20, 183. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine cyanobacteria and microalgae metabolites-A rich source of potential anticancer drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Kaushalendra; Verma, S.; Lalnunpuii, R.; Rajan, J.P. Current issues and developments in cyanobacte-ria-derived biofuel as a potential source of energy for sustainable future. Sustainability 2023, 15, 10439. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Morais, J.; Neves, J.; Vasconcelos, V.; Martins, R. Cosmetic application of cyanobacteria extracts with a sustainable vision to skincare: Role in the antioxidant and antiaging process. Mar. Drugs 2022, 20, 761. [Google Scholar] [CrossRef]
- Madusanka, D.A.T.; Manage, P.M. Potential utilization of Microcystis sp. for biodiesel production: Green solution for future energy crisis. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 19, 143–149. [Google Scholar]
- Yarkent, C.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Sharif, S.K.; Rao, B.N.; Jagadish, D. Comparative performance and emission studies of the CI engine with Nodularia Spumigena microalgae biodiesel versus different vegetable oil derived biodiesel. SN Appl. Sci. 2020, 2, 858. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Oliveira, B.; Vasconcelos, V.; Martins, R. Cyanobacteria in cosmetics: A natural alternative for anti-aging ingredients, Chapter 9. In The Pharmacological Potential of Cyanobacteria; Academic Press: Cambridge, MA, USA, 2022; pp. 257–286. [Google Scholar] [CrossRef]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus Nostoc: A review. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential antioxidant and anticancer activities of secondary metabolites of Nostoc linckia cultivated under Zn and Cu Stress Conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Kamaraj, B.; Chi, N.T.L.; Cornejo, P. Cultivation of Nostoc sp. LS04 in municipal wastewater for biodiesel production and their de-oiled biomass cellular extracts as bio-stimulants for Lactuca sativa growth improvement. Chemosphere 2021, 280, 130644. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Yeh, H.-Y.; Liao, Z.-H.; Hung, S.-W.; Chen, B.; Lee, P.-T.; Nan, F.-H.; Shih, W.-L.; Chang, C.-C.; Lee, M.-C. An in vitro study shows the potential of Nostoc commune (Cyanobacteria) polysaccharides extract for wound-healing and anti-allergic use in the cosmetics industry. J. Funct. Foods 2021, 87, 104754. [Google Scholar] [CrossRef]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef]
- Nainangu, P.; Perianaika, A.; Antonyraj, M.; Subramanian, K.; Kaliyaperumal, S.; Gopal, S.; Renuka, S.P.; Aruni, A.W. In vitro screening of antimicrobial, antioxidant, cytotoxic activities, and characterization of bioactive substances from freshwater cyanobacteria Oscillatoria sp. SSCM01 and Phormidium sp. SSCM02. Biocatal. Agric. Biotechnol. 2020, 29, 101772. [Google Scholar] [CrossRef]
- Siqueira, S.F.; Francisco, E.; Queiroz, M.I.; Menezes, C.; Zepka, L.Q.; Jacob-Lopes, E. Third generation biodiesel production from microalgae Phormidium autumnale. Braz. J. Chem. Eng. 2016, 33, 427–433. [Google Scholar] [CrossRef]
- McAllister, T.G.; Wood, S.A.; Hawes, I. The rise of toxic benthic Phormidium proliferations: A review of their taxonomy, distribution, toxin content and factors regulating prevalence and increased severity. Harmful Algae 2016, 55, 282–294. [Google Scholar] [CrossRef]
- Lupette, J.; Maréchal, E. Phytoplankton glycerolipids: Challenging but promising prospects from biomedicine to green chemistry and biofuels. In Blue Biotechnology: Production and Use of Marine Molecules; La Barre, S., Bates, S.S., Eds.; Wiley: Chichester, UK, 2018; pp. 191–215. [Google Scholar]
- Yanti, N.W.K.E.; Rochmanto, A.T.; Damacena, R.; Prihantini, N.B. Utilization of local fertilizer and spring water com-bination for growth medium of Stanieria HS-48 (cyanobacteria) as a biofuel feedstock. AIP Conf. Proc. 2020, 2255, 030010. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Sarrafzadeh, M.H.; Tavakoli, O.; Soltani, N.; Faramarzi, M.A. Potential for biodiesel production and carbon capturing from Synechococcus Elongatus: An isolation and evaluation study. Biocatal. Agric. Biotechnol. 2017, 9, 230–235. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Spungin, D.; Schuster, G.; Berman-Frank, I.; Adir, N. Trichodesmium erythraeum produces a higher pho-tocurrent than other cyanobacterial species in bio-photo electrochemical cells. Biochim. Biophys. Acta (BBA) Bioenerg. 2022, 1863, 148910. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F. Ecophysiology of wild plants and conservation perspectives in the State of Qatar. In Agricultural Chemistry; Stoytcheva, M., Zlatev, R., Eds.; InTech: Rijeka, Croatia, 2013; pp. 37–70. [Google Scholar]
- Al-Thani, R.F.; Al-Mohannadi, A.; Deyab, D.; Al-Yafei, F.A.; Ashfaq, M.Y.; Yasseen, B.T. Properties of Streptomyces bacteria from the rhizosphere of some halophytes at north-east of Qatar: Al Ghariya case study. Front. Environ. Microbiol. 2023, 9, 34–51. [Google Scholar] [CrossRef]



| Genera | Nitrogen Fixation | Cyanoremediation | Production of Agents | ||
|---|---|---|---|---|---|
| Anticancer | Biofuels | Cosmetics | |||
| Amphanizomenon | + | + | +: [141] | Needs test | P: [140,142] |
| Anabaena | + | + | +: [143] | +: [144] | +: [140,145] |
| Calothrix | + | P | +: [146] | VP: [142,147] | P: [140,148] |
| Chroococcus | − | VP | +: [64] | +: [149] | P: [150] |
| Croococcidiopsis | + | + | Needs test | +: [151] | P: [152] |
| Dermocarpella | Needs test | P | Needs test: [60] | P: [153] | Needs test |
| Euhalothece | Needs test | P | Needs test: [154] | P: [155] | P: [124] |
| Geitlerinema | + | P | +: [156] | +: [157] | P: [140,158] |
| Geminocystis | Needs test | P | P: [159] | P: [160] | P: [161] |
| Lyngbya | + | P | +: [162] | +: [163,164] | +: [140,165] |
| Leptolyngbya | Some + | + | P: [125,166] | +: [167,168] | P: [140,169] |
| Merismopedia | − | Needs test | P: [126,170] | P: [171] | P: [140,172] |
| Microcystis | − | P | P: [130] | +: [173] | P: [140,174] |
| Nodularia | + | P | P: [60] | +: [175] | P: [176] |
| Nostoc | + | + | +: [177,178] | +: [179] | +: [172,176,180] |
| Oscillatoria | + | + | +: [181,182] | +: [163] | +: [148] |
| Phormidium | + | + | +: [182] | +: [183] | +: [172,176,184] |
| Richelia | + | P | P: [125] | P: [145] | P: [10,185] |
| Stanieria | Needs test | + | +: [60] | +: [186] | P: [172,176] |
| Synechococcus | Some + | + | +: [60] | +: [187] | P: [150,172] |
| Trichodesmium | + | + | Needs test: [99] | +: [188] | P: [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Thani, R.F.; Yasseen, B.T. Cyanoremediation of Polluted Seawater in the Arabian Gulf: Risks and Benefits to Human Health. Processes 2024, 12, 2733. https://doi.org/10.3390/pr12122733
Al-Thani RF, Yasseen BT. Cyanoremediation of Polluted Seawater in the Arabian Gulf: Risks and Benefits to Human Health. Processes. 2024; 12(12):2733. https://doi.org/10.3390/pr12122733
Chicago/Turabian StyleAl-Thani, R. F., and B. T. Yasseen. 2024. "Cyanoremediation of Polluted Seawater in the Arabian Gulf: Risks and Benefits to Human Health" Processes 12, no. 12: 2733. https://doi.org/10.3390/pr12122733
APA StyleAl-Thani, R. F., & Yasseen, B. T. (2024). Cyanoremediation of Polluted Seawater in the Arabian Gulf: Risks and Benefits to Human Health. Processes, 12(12), 2733. https://doi.org/10.3390/pr12122733






