Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Response Surface Methodology and Experiment Design
2.3. Extraction Conditions Provided by Statistical Optimization
2.4. Total Polyphenol (TPC) and Flavonoid (TFC) Content
2.5. Free Radical Scavenging Capacity
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. Identification of Sugars and Organic Acids
2.7. Statistical Analysis
3. Results and Discussion
3.1. Central Composite Design (CCD) and Validation of the Statistical Methodology
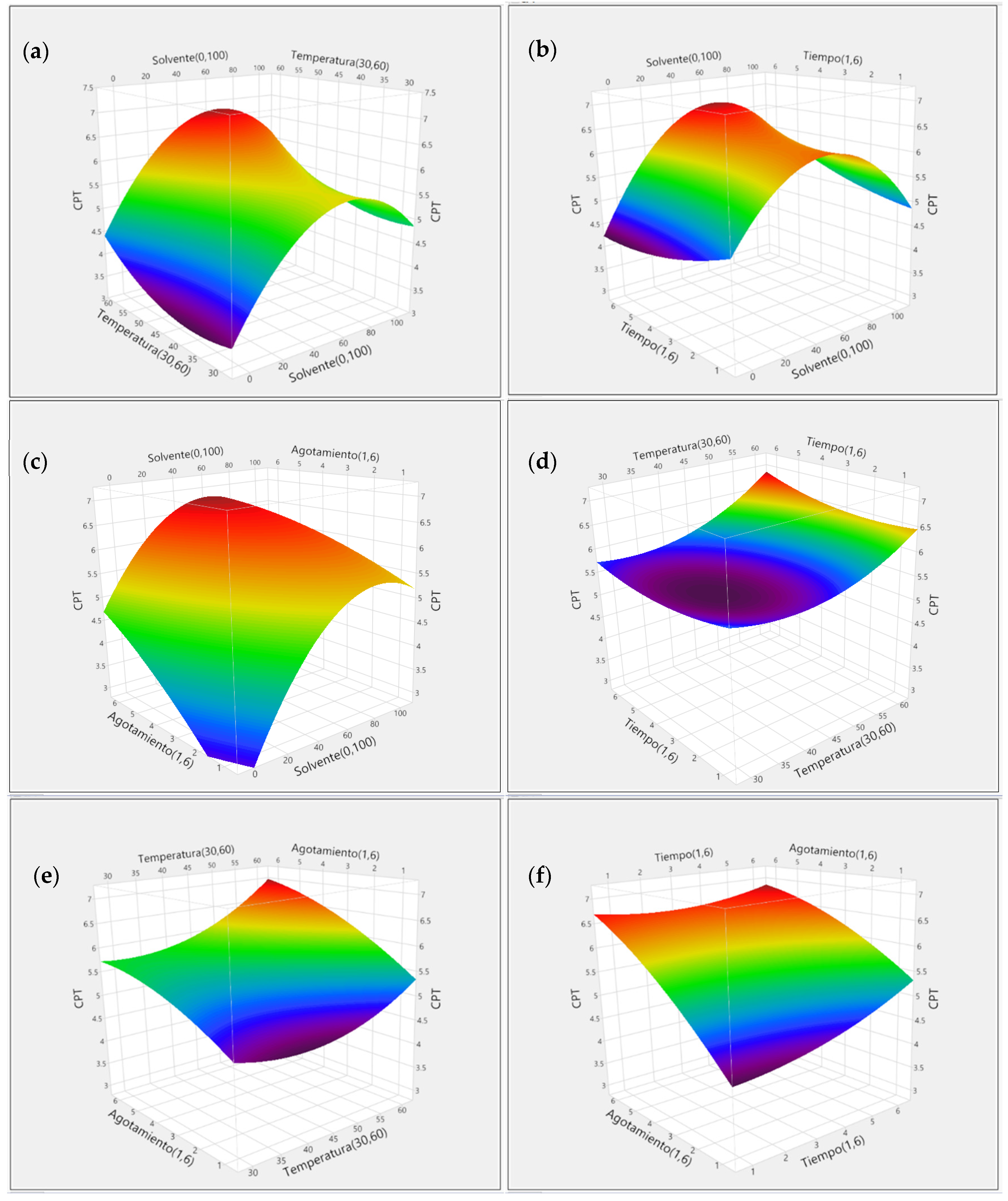
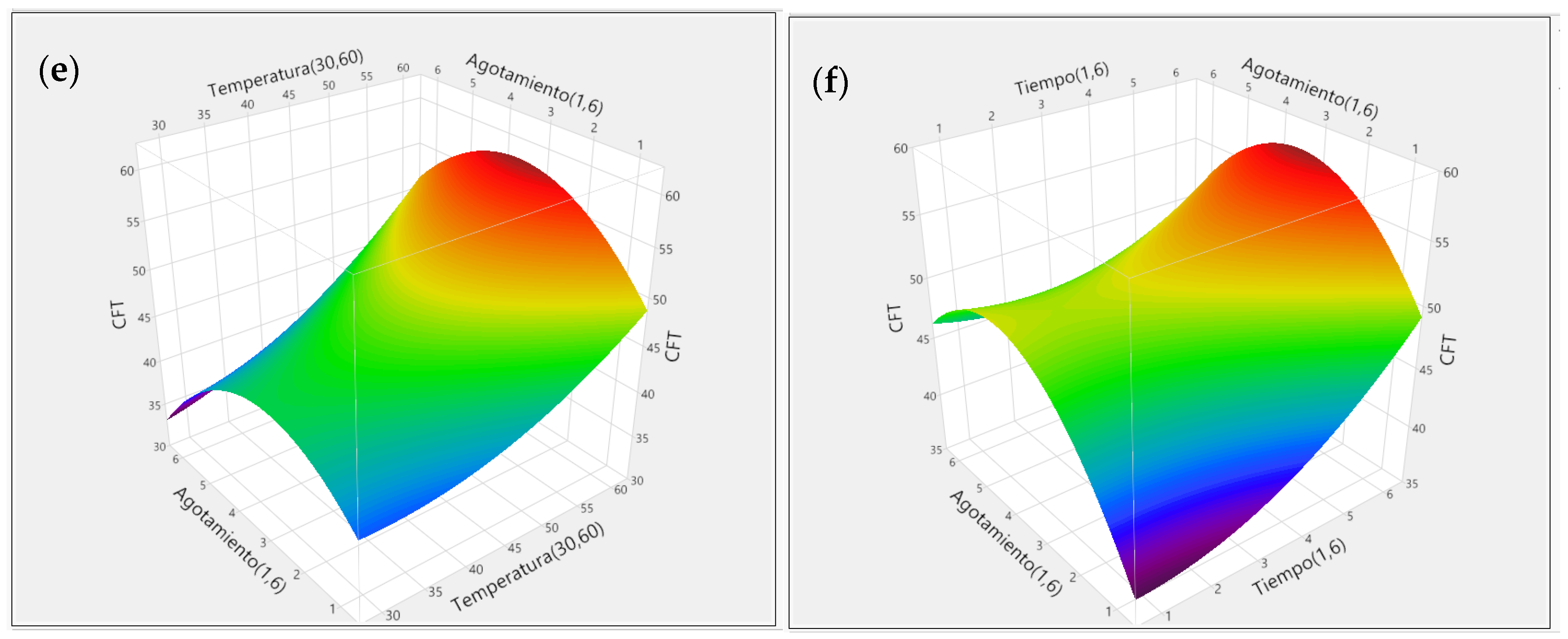
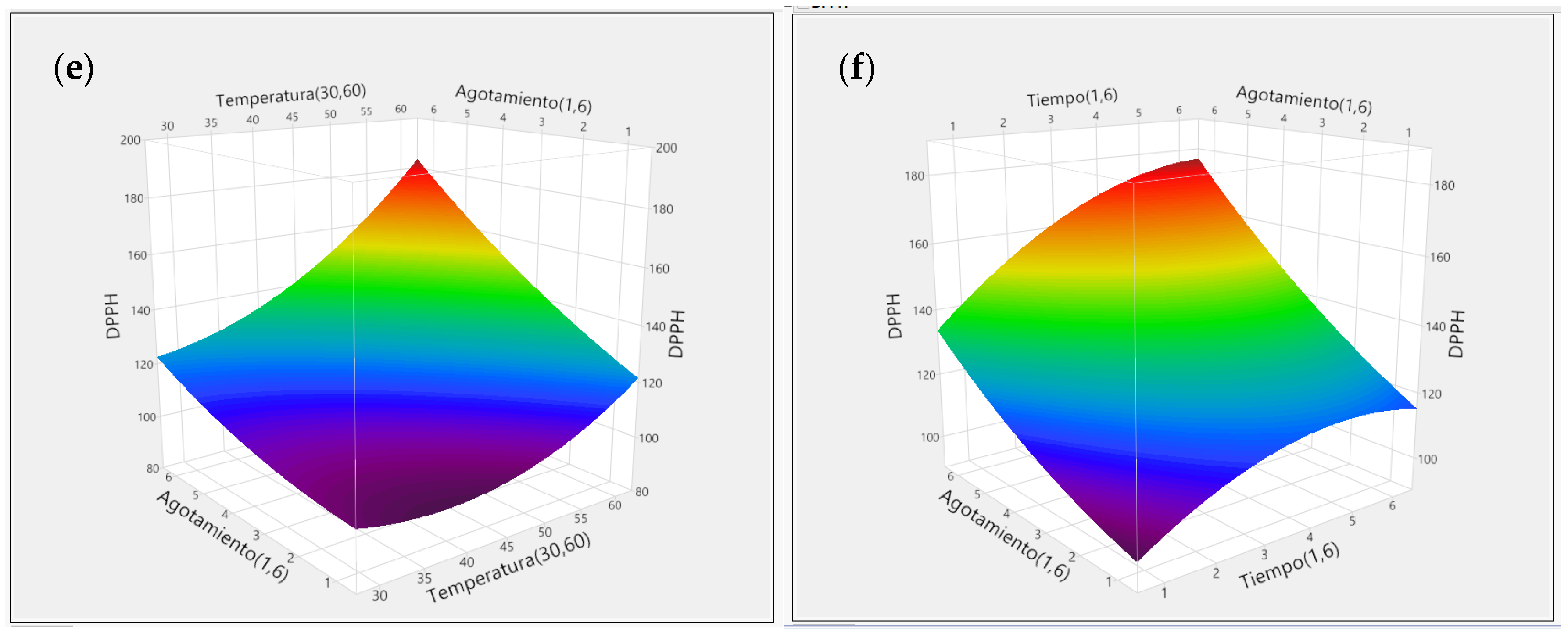
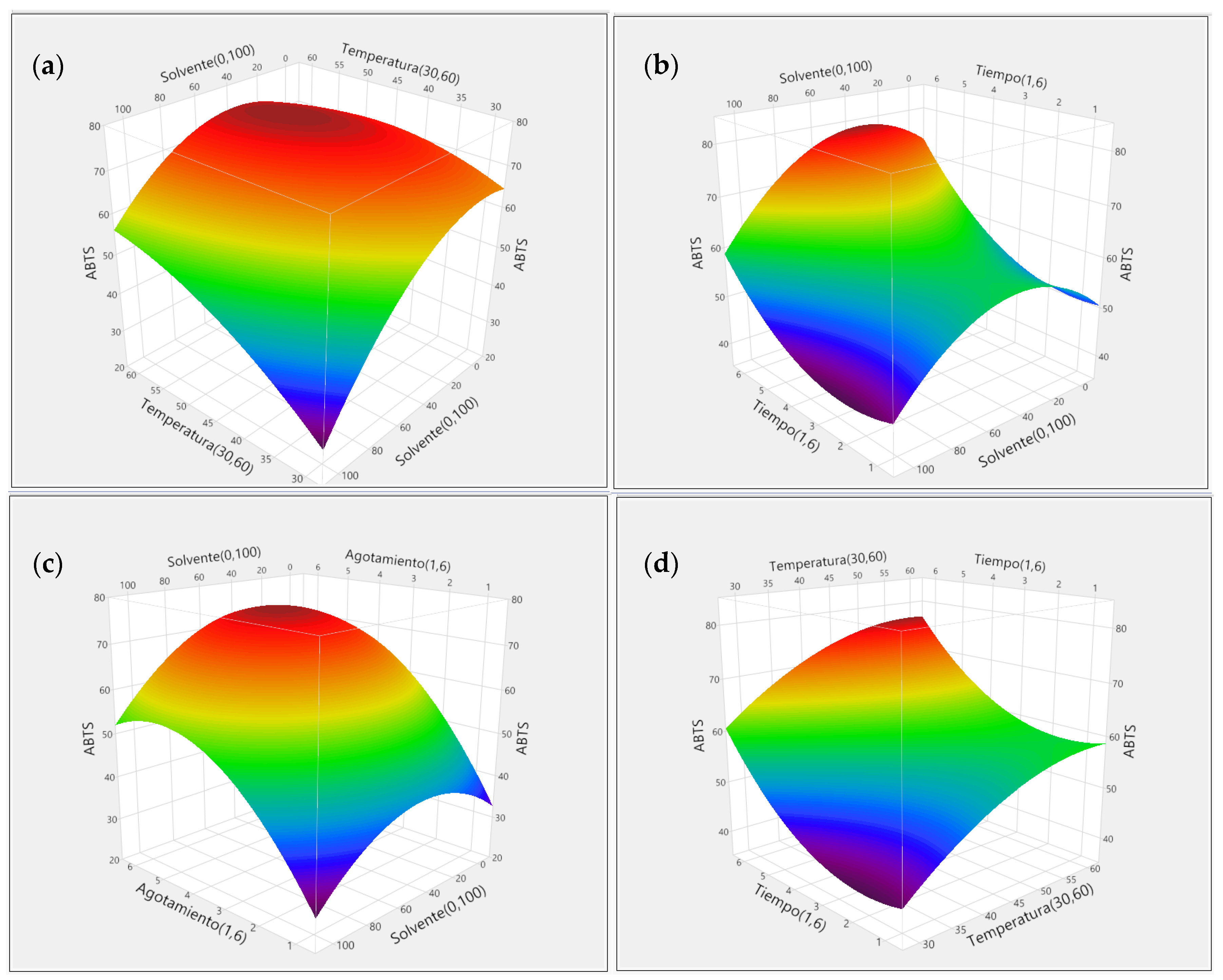
3.2. Response Surface Methodology (RSM)
3.2.1. Optimization of Papaya Seed Extraction
3.2.2. Effect of Extraction Variables on TPC and TFC
3.2.3. Effect of Extraction Method for Free Radical DPPH and ABTS Scavenging Capacity Using RSM
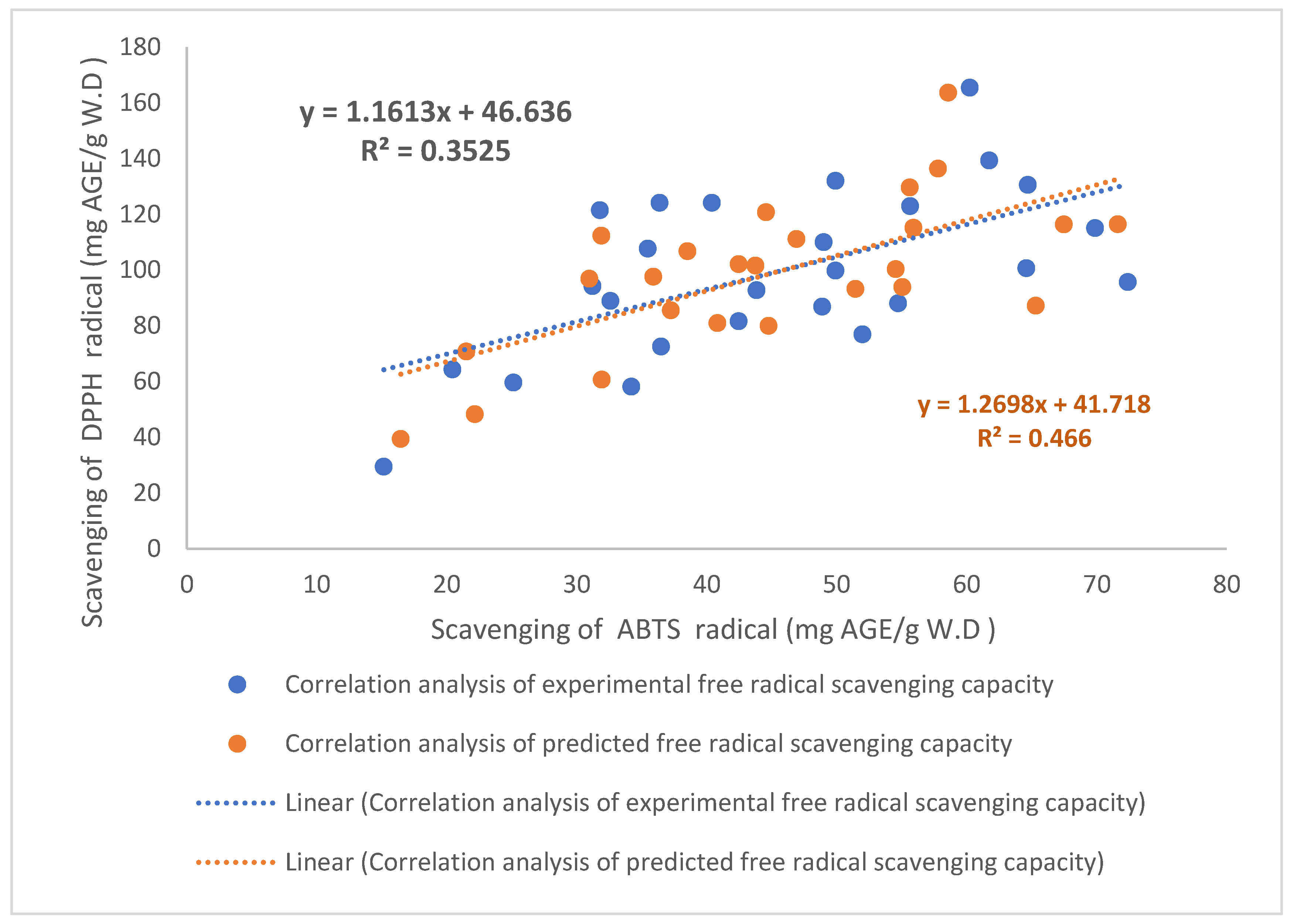
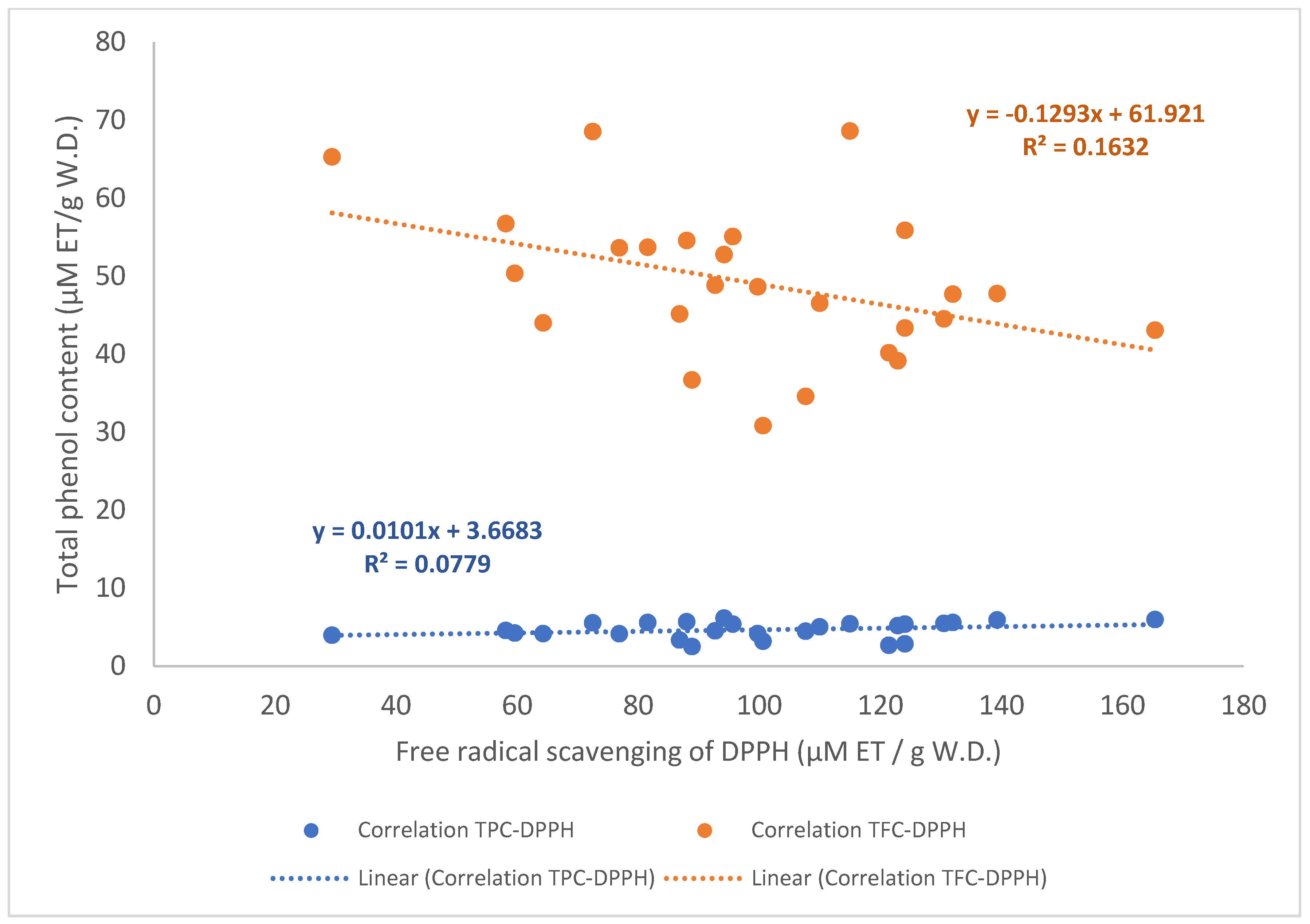
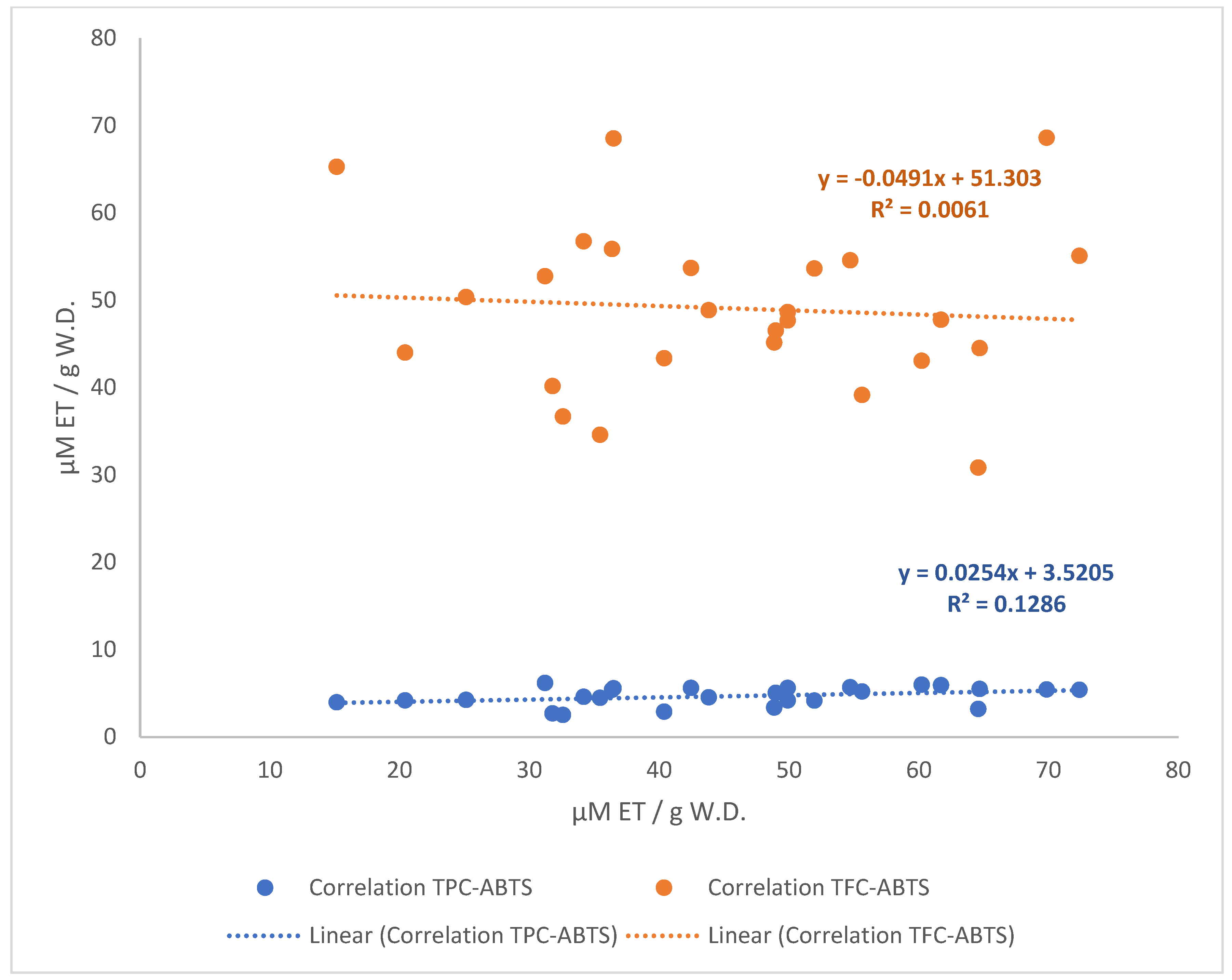
3.3. Correlation of the Free Radical Scavenging Activity and Polyphenol Compound
3.4. Identification of Sugars and Organic Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Mazza, G. Functional food. In The Wiley Encyclopedia of Science & Technology, 2nd ed.; Francis, F.J., Ed.; Wiley: New York, NY, USA, 2000; pp. 1176–1182. [Google Scholar]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fiber concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit juice industry wastes as a source of bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.Z.; Taher, S.; Khan, A.; Sultana, N.; Irfan, M.F.; Haq, B.; Razzak, S.A. Experimental study and modeling approach of response surface methodology coupled with crow search algorithm for optimizing the extraction conditions of papaya seed waste oil. Arab. J. Sci. Eng. 2020, 45, 7371–7383. [Google Scholar] [CrossRef]
- Nyorere, O.; Oluka, S.I. Impact of optimization on carica papaya seeds oil extraction. Explor. J. Eng. Technol. 2024, 5, 34–41. [Google Scholar]
- Castro-Vargas, H.I.; Baumann, W.; Ferreira, S.R.S.; Parada-Alfonso, F. Valorization of papaya (Carica papaya L.) agroindustrial waste through the recovery of phenolic antioxidants by supercritical fluid extraction. J. Food Sci. Technol. 2019, 56, 3055–3066. [Google Scholar] [CrossRef]
- Li, M.; Dunwell, J.M.; Zhang, H.; Wei, S.; Li, Y.; Wu, J.; Zhang, S. Network analysis reveals the co-expression of sugar and aroma genes in the Chinese white pear (Pyrus bretschneideri). Gene 2018, 677, 370–377. [Google Scholar] [CrossRef]
- González-Vega, R.I.; Cárdenas-López, J.L.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [Google Scholar] [CrossRef]
- Tan, C.X.; Tan, S.T.; Tan, S.S. Bioactive phytochemicals from papaya seed oil processing by-products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2023; pp. 391–402. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.R.L.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Al-Jadidi, H.S.; Hossain, M.A. Studies on total phenolics, total flavonoids and antimicrobial activity from the leaves crude extracts of neem traditionally used for the treatment of cough and nausea. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 93–98. [Google Scholar]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT-Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Gurnani, N.; Gupta, M.; Mehta, D.; Mehta, B.K. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). J. Taibah Univ. Sci. 2016, 10, 462–470. [Google Scholar] [CrossRef]
- Ang, Y.K.; Sia, W.C.; Khoo, H.E.; Yim, H.S. Antioxidant potential of carica papaya peel and seed. Foc. Mod. Food Ind. 2012, 1, 11–16. [Google Scholar]
- Ornelas-Paz, J.J.; Yahia, E.M.; Pérez-Martínez, J.D.; Escalante-Minakata, M.P.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa Duch, Cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Extraction of curcuminoids by using ethyl lactate and its optimisation by response surface methodology. J. Pharm. Biomed. Anal. 2018, 149, 89–95. [Google Scholar] [CrossRef]
- Mäkelä, M. Experimental design and response surface methodology in energy. Ene. Conv. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Bilgin, M.; Elhussein, E.; Özyürek, M.; Güçlü, K.; Şahin, S. Optimizing the extraction of polyphenols from Sideritis montana L. using response surface methodology. J. Pharmac. Biom. Anal 2018, 158, 137–143. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G. Extraction optimization of saffron nutraceuticals through response surface methodology. Food Anal. Methods 2015, 8, 2273–2285. [Google Scholar] [CrossRef]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crops Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Khor, E.S.; Wong, N.K. Potential antioxidant and cytotoxic properties of secondary metabolite extracts from carica papaya fruits and seeds. Int. J. Pharm. Pharm. Sci. 2014, 7, 220–224. [Google Scholar]
- Hall, R.M.; Mayer, D.A.; Mazzutti, S.; Ferreira, S.R. Simulating large scale SFE applied to recover bioactive compounds from papaya seeds. J. Supercrit. Fluids 2018, 140, 302–309. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, K.Y. Optimization of ultrasound-assisted deep eutectic solvent extraction of bioactive compounds from pomegranate peel using response surface methodology. Food Sci. Biotechnol. 2023, 32, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purifi. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Sood, A.; Gupta, M. Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Nieto-Calvache, J.; Cueto, M.; Farroni, A.; de Escalada Pla, M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith Apple. Food Chem. 2011, 128, 520–529. [Google Scholar] [CrossRef]
- Ali, A.; Lim, X.Y.; Chong, C.H.; Mah, S.H.; Chua, B.L. Optimization of ultrasound-assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT-Food Sci. Technol. 2018, 89, 681–688. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Serna-Cock, L.; Belmares-Cerda, R.; Aguilar, C.N. Extraction of antioxidants from mango seed kernel: Optimization assisted by microwave. Food Bioprod. Process. 2017, 105, 188–196. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Application of response surface methodology to acidified water extraction of black soybeans for improving anthocyanin content, total phenols content and antioxidant activity. Food Chem. 2018, 261, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Wissam, Z.; Ghada, B.; Wassim, A.; Warid, K. Effective extraction of polyphenols and proanthocyanidins from Pomegranate’s peel. International. J. Pharm. Pharm. Sci. 2012, 4, 675–682. [Google Scholar]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Parniakov, O.; Roselló-Soto, E.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. New approaches for the effective valorization of papaya seeds: Extraction of proteins, phenolic compounds, carbohydrates, and isothiocyanates assisted by pulsed electric energy. Food Res. Int. 2015, 77, 711–717. [Google Scholar] [CrossRef]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biom. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Gubbuk, H.; Gunes, E. Comparative evaluation of volatiles, phenolics, sugars, organic acids and antioxidant properties of Sel-42 and Tainung papaya varieties. Food Chem. 2015, 173, 912–919. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, G.; Li, Y. Bread characteristics and antioxidant activities of Maillard reaction products of white pan bread containing various sugars. LWT-Food Sci. Technol. 2018, 95, 308–315. [Google Scholar] [CrossRef]
- Kayashima, T.; Katayama, T. Oxalic acid is available as a natural antioxidant in some systems. Biochim. Biophy. Acta Gen. Subj. 2002, 1573, 1–3. [Google Scholar] [CrossRef]
- Pereira, D.M.; Faria, J.; Gaspar, L.; Ferreres, F.; Valentão, P.; Sottomayor, M.; Andrade, P.B. Exploiting catharanthus roseus roots: Source of antioxidants. Food Chem. 2010, 121, 56–61. [Google Scholar] [CrossRef]




| Treatment | Factors | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 Solvent (%) | X2 (°C) | X3 (h) | X4 (Time) | TPC (mg AGE/g WD) | TFC (mg QE/g WD) | DPPH (µM ET/g WD) | ABTS (µM ET/g WD) | |
| 1 | 100 | 60 | 6 | 6 | 5.970 | 43.058 | 165.393 | 60.230 |
| 2 | 100 | 60 | 6 | 1 | 5.559 | 68.526 | 72.508 | 36.487 |
| 3 | 100 | 60 | 1 | 6 | 5.391 | 55.864 | 124.078 | 36.372 |
| 4 | 100 | 60 | 1 | 1 | 4.583 | 56.733 | 58.150 | 34.192 |
| 5 | 100 | 45 | 3.5 | 4 | 4.181 | 48.629 | 99.758 | 49.907 |
| 6 | 100 | 30 | 6 | 6 | 6.168 | 52.753 | 94.191 | 31.210 |
| 7 | 100 | 30 | 6 | 1 | 4.177 | 43.998 | 64.303 | 20.428 |
| 8 | 100 | 30 | 1 | 6 | 4.247 | 50.365 | 59.615 | 25.131 |
| 9 | 100 | 30 | 1 | 1 | 3.960 | 65.270 | 29.434 | 15.151 |
| 10 | 50 | 60 | 3.5 | 4 | 5.925 | 47.761 | 139.315 | 61.722 |
| 11 | 50 | 45 | 6 | 4 | 5.391 | 55.068 | 95.656 | 72.389 |
| 12 | 50 | 45 | 3.5 | 6 | 5.488 | 44.505 | 130.524 | 64.704 |
| 13 | 50 | 45 | 3.5 | 4 | 4.526 | 48.846 | 92.726 | 43.828 |
| 14 | 50 | 45 | 3.5 | 4 | 5.612 | 47.688 | 131.990 | 49.907 |
| 15 | 50 | 45 | 3.5 | 1 | 4.481 | 34.592 | 107.669 | 35.454 |
| 16 | 50 | 45 | 1 | 4 | 5.206 | 39.151 | 122.906 | 55.642 |
| 17 | 50 | 30 | 3.5 | 4 | 5.047 | 46.531 | 110.013 | 48.989 |
| 18 | 0 | 60 | 6 | 6 | 5.440 | 68.599 | 114.995 | 69.866 |
| 19 | 0 | 60 | 6 | 1 | 2.878 | 43.347 | 124.078 | 40.387 |
| 20 | 0 | 60 | 1 | 6 | 5.687 | 54.562 | 88.037 | 54.725 |
| 21 | 0 | 60 | 1 | 1 | 2.521 | 36.691 | 88.916 | 32.587 |
| 22 | 0 | 45 | 3.5 | 4 | 4.163 | 53.621 | 76.903 | 51.972 |
| 23 | 0 | 30 | 6 | 6 | 3.205 | 30.830 | 100.637 | 64.589 |
| 24 | 0 | 30 | 6 | 1 | 3.351 | 45.156 | 86.865 | 48.875 |
| 25 | 0 | 30 | 1 | 6 | 5.607 | 53.694 | 81.591 | 42.451 |
| 26 | 0 | 30 | 1 | 1 | 2.693 | 40.164 | 121.441 | 31.784 |
| Treatment | Factors | Predicted Formula | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 Solvent (%) | X2 (°C) | X3 (h) | X4 (Time) | TPC (mg AGE/g WD) | TFC (mg QE/g WD) | DPPH (µM ET/g WD) | ABTS (µM ET/g WD) | |
| 1 | 100 | 60 | 6 | 6 | 5.970 | 43.058 | 165.393 | 60.230 |
| 2 | 100 | 60 | 6 | 1 | 5.559 | 68.526 | 72.508 | 36.487 |
| 3 | 100 | 60 | 1 | 6 | 5.391 | 55.864 | 124.078 | 36.372 |
| 4 | 100 | 60 | 1 | 1 | 4.583 | 56.733 | 58.150 | 34.192 |
| 5 | 100 | 45 | 3.5 | 4 | 4.181 | 48.629 | 99.758 | 49.907 |
| 6 | 100 | 30 | 6 | 6 | 6.168 | 52.753 | 94.191 | 31.210 |
| 7 | 100 | 30 | 6 | 1 | 4.177 | 43.998 | 64.303 | 20.428 |
| 8 | 100 | 30 | 1 | 6 | 4.247 | 50.365 | 59.615 | 25.131 |
| 9 | 100 | 30 | 1 | 1 | 3.960 | 65.270 | 29.434 | 15.151 |
| 10 | 50 | 60 | 3.5 | 4 | 5.925 | 47.761 | 139.315 | 61.722 |
| 11 | 50 | 45 | 6 | 4 | 5.391 | 55.068 | 95.656 | 72.389 |
| 12 | 50 | 45 | 3.5 | 6 | 5.488 | 44.505 | 130.524 | 64.704 |
| 13 | 50 | 45 | 3.5 | 4 | 4.526 | 48.846 | 92.726 | 43.828 |
| 14 | 50 | 45 | 3.5 | 4 | 5.612 | 47.688 | 131.990 | 49.907 |
| 15 | 50 | 45 | 3.5 | 1 | 4.481 | 34.592 | 107.669 | 35.454 |
| 16 | 50 | 45 | 1 | 4 | 5.206 | 39.151 | 122.906 | 55.642 |
| 17 | 50 | 30 | 3.5 | 4 | 5.047 | 46.531 | 110.013 | 48.989 |
| 18 | 0 | 60 | 6 | 6 | 5.440 | 68.599 | 114.995 | 69.866 |
| 19 | 0 | 60 | 6 | 1 | 2.878 | 43.347 | 124.078 | 40.387 |
| 20 | 0 | 60 | 1 | 6 | 5.687 | 54.562 | 88.037 | 54.725 |
| 21 | 0 | 60 | 1 | 1 | 2.521 | 36.691 | 88.916 | 32.587 |
| 22 | 0 | 45 | 3.5 | 4 | 4.163 | 53.621 | 76.903 | 51.972 |
| 23 | 0 | 30 | 6 | 6 | 3.205 | 30.830 | 100.637 | 64.589 |
| 24 | 0 | 30 | 6 | 1 | 3.351 | 45.156 | 86.865 | 48.875 |
| 25 | 0 | 30 | 1 | 6 | 5.607 | 53.694 | 81.591 | 42.451 |
| 26 | 0 | 30 | 1 | 1 | 2.693 | 40.164 | 121.441 | 31.784 |
| Analysis | Equation |
|---|---|
| Source | GF | Sum of Squares | Mean Squares | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 14 | 23.4534 | 1.6752 | 3.3585 | 0.0250 | * |
| Solvent | 1 | 4.1963 | 4.1963 | 8.4127 | 0.0144 | * |
| Temperature | 1 | 1.6799 | 1.6799 | 3.3679 | 0.0936 | |
| Time | 1 | 0.2798 | 0.2798 | 0.5608 | 0.4696 | |
| Exhaust | 1 | 9.3889 | 9.3889 | 18.8227 | 0.0012 | ** |
| Solvent * Temperature | 1 | 0.1026 | 0.1026 | 0.2056 | 0.6590 | |
| Solvent * Time | 1 | 1.7736 | 1.7736 | 3.5556 | 0.0860 | |
| Solvent * Exhaust | 1 | 1.5619 | 1.5619 | 3.1312 | 0.1045 | |
| Temperature * Time | 1 | 0.1010 | 0.1010 | 0.2024 | 0.6615 | |
| Temperature * Exhaust | 1 | 0.2259 | 0.2259 | 0.4528 | 0.5149 | |
| Time * Exhaust | 1 | 0.3472 | 0.3472 | 0.6961 | 0.4218 | |
| Solvent * Solvent | 1 | 2.4547 | 2.4547 | 4.9212 | 0.0485 | * |
| Temperature * Temperature | 1 | 0.2873 | 0.2873 | 0.5761 | 0.4638 | |
| Time * Time | 1 | 0.0557 | 0.0557 | 0.1116 | 0.7446 | |
| Exhaust * Exhaust | 1 | 0.0710 | 0.0710 | 0.1424 | 0.7131 | |
| Lack of fit | 10 | 4.8972 | 0.4897 | 0.8305 | 0.7018 | |
| Residual | 11 | 5.4869 | 0.4988 | |||
| pure error | 1 | 0.5897 | 0.5897 | |||
| Total Correlation | 25 | 28.9403 |
| Source | GF | Sum of Squares | Mean Squares | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 14 | 1232.5598 | 88.0400 | 0.9380 | 0.5530 | |
| Solvent | 1 | 190.3331 | 190.3331 | 2.0278 | 0.1822 | |
| Temperature | 1 | 119.5058 | 119.5058 | 1.2732 | 0.2832 | |
| Time | 1 | 0.0746 | 0.0746 | 0.0008 | 0.9780 | |
| Exhaust | 1 | 21.6767 | 21.6767 | 0.2309 | 0.6402 | |
| Solvent * Temperature | 1 | 29.0521 | 29.0521 | 0.3095 | 0.5891 | |
| Solvent * Time | 1 | 32.2567 | 32.2567 | 0.3437 | 0.5696 | |
| Solvent * Exhaust | 1 | 349.8209 | 349.8209 | 3.7269 | 0.0797 | |
| Temperature * Time | 1 | 199.0639 | 199.0639 | 2.1208 | 0.1733 | |
| Temperature * Exhaust | 1 | 35.2005 | 35.2005 | 0.3750 | 0.5527 | |
| Time * Exhaust | 1 | 28.6600 | 28.6600 | 0.3053 | 0.5916 | |
| Solvent * Solvent | 1 | 93.7275 | 93.7275 | 0.9986 | 0.3391 | |
| Temperature * Temperature | 1 | 10.9805 | 10.9805 | 0.1170 | 0.7388 | |
| Time * Time | 1 | 10.5968 | 10.5968 | 0.1129 | 0.7432 | |
| Exhaust * Exhaust | 1 | 78.2275 | 78.2275 | 0.8334 | 0.3809 | |
| Lack of fit | 10 | 1031.8272 | 103.183 | 153.8934 | 0.0627 | |
| Residual | 11 | 1032.4977 | 93.8634 | |||
| pure error | 1 | 0.6705 | 0.6700 |
| Source | GF | Sum of Squares | Mean Squares | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 14 | 17,983.086 | 1284.51 | 3.4314 | 0.0232 | * |
| Solvent | 1 | 747.9809 | 747.981 | 1.9982 | 0.1852 | |
| Temperature | 1 | 2872.3147 | 2872.315 | 7.6731 | 0.0182 | * |
| Time | 1 | 1159.3397 | 1159.34 | 3.0971 | 0.1062 | |
| Exhaust | 1 | 2350.6253 | 2350.625 | 6.2794 | 0.0292 | * |
| Solvent * Temperature | 1 | 1352.2903 | 1352.29 | 3.6125 | 0.0839 | |
| Solvent * Time | 1 | 385.4154 | 385.415 | 1.0296 | 0.3321 | |
| Solvent * Exhaust | 1 | 4061.5766 | 4061.577 | 10.8501 | 0.0072 | ** |
| Temperature * Time | 1 | 255.0249 | 255.025 | 0.6813 | 0.4267 | |
| Temperature * Exhaust | 1 | 824.5512 | 824.551 | 2.2027 | 0.1658 | |
| Time * Exhaust | 1 | 324.7384 | 324.738 | 0.8675 | 0.3716 | |
| Solvent * Solvent | 1 | 1969.6883 | 1969.688 | 5.2618 | 0.0425 | * |
| Temperature * Temperature | 1 | 189.4341 | 189.434 | 0.5061 | 0.4917 | |
| Time * Time | 1 | 117.809 | 117.809 | 0.3147 | 0.5860 | |
| Exhaust * Exhaust | 1 | 23.5595 | 23.559 | 0.0629 | 0.8065 | |
| Lack of fit | 10 | 3346.8716 | 334.687 | 0.4342 | 0.8399 | |
| Residual | 11 | 4117.7020 | 374.3400 | |||
| pure error | 1 | 770.8308 | 770.8310 |
| Source | GF | Sum of Squares | Mean Squares | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 14 | 5197.8154 | 371.273 | 7.0527 | 0.0012 | ** |
| Solvent | 1 | 912.0436 | 912.044 | 17.3251 | 0.0016 | ** |
| Temperature | 1 | 533.1201 | 533.12 | 10.1271 | 0.0087 | ** |
| Time | 1 | 753.0563 | 753.056 | 14.305 | 0.0030 | ** |
| Exhaust | 1 | 1316.4094 | 1316.409 | 25.0064 | 0.0004 | *** |
| Solvent * Temperature | 1 | 268.0997 | 268.1 | 5.0928 | 0.0454 | * |
| Solvent * Time | 1 | 38.0103 | 38.01 | 0.722 | 0.4136 | |
| Solvent * Exhaust | 1 | 61.2815 | 61.281 | 1.1641 | 0.3037 | |
| Temperature * Time | 1 | 0.1389 | 0.139 | 0.0026 | 0.9599 | |
| Temperature * Exhaust | 1 | 57.7486 | 57.749 | 1.097 | 0.3174 | |
| Time * Exhaust | 1 | 75.4857 | 75.486 | 1.4339 | 0.2563 | |
| Solvent * Solvent | 1 | 162.6148 | 162.615 | 3.089 | 0.1066 | |
| Temperature * Temperature | 1 | 32.3204 | 32.32 | 0.614 | 0.4498 | |
| Time * Time | 1 | 66.8067 | 66.807 | 1.2691 | 0.2839 | |
| Exhaust * Exhaust | 1 | 199.6319 | 199.632 | 3.7922 | 0.0775 | |
| Lack of fit | 10 | 560.59397 | 56.0594 | 3.034 | 0.4214 | |
| Residual | 11 | 579.0711 | 52.643 | |||
| pure error | 1 | 18.47712 | 18.4771 |
| Sugar | mg/g DW | Organic Acids | mg/g DW |
|---|---|---|---|
| Saccharose | 0.35 c ± 0.029 | Oxalic | 12.9793 ± 0.3086 |
| Glucose | 14.49 a ± 0.564 | Citric | 48.7583 ± 1.7241 |
| Fructose | 5.72 b ± 0.215 | Tartaric | 2.9542 ± 0.2187 |
| Malic | 6.3562 ± 0.3227 | ||
| Ascorbic | ND | ||
| Quinic | 6.3213 ± 0.0873 | ||
| Succinic | 16.2580 ± 0.6597 | ||
| Fumaric | 3.8212 ± 0.1612 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-Apodaca, S.M.; González-Vega, R.I.; Ruíz-Cruz, S.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A.; Márquez-Ríos, E.; Del-Toro-Sánchez, C.L.; Ornelas-Paz, J.d.J.; Suárez-Jiménez, G.M.; Ocaño-Higuera, V.M. Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes 2024, 12, 2729. https://doi.org/10.3390/pr12122729
Robles-Apodaca SM, González-Vega RI, Ruíz-Cruz S, Estrada-Alvarado MI, Cira-Chávez LA, Márquez-Ríos E, Del-Toro-Sánchez CL, Ornelas-Paz JdJ, Suárez-Jiménez GM, Ocaño-Higuera VM. Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes. 2024; 12(12):2729. https://doi.org/10.3390/pr12122729
Chicago/Turabian StyleRobles-Apodaca, Silvia Mitzel, Ricardo Iván González-Vega, Saúl Ruíz-Cruz, María Isabel Estrada-Alvarado, Luis A. Cira-Chávez, Enrique Márquez-Ríos, Carmen Lizette Del-Toro-Sánchez, José de Jesús Ornelas-Paz, Guadalupe M. Suárez-Jiménez, and Víctor Manuel Ocaño-Higuera. 2024. "Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology" Processes 12, no. 12: 2729. https://doi.org/10.3390/pr12122729
APA StyleRobles-Apodaca, S. M., González-Vega, R. I., Ruíz-Cruz, S., Estrada-Alvarado, M. I., Cira-Chávez, L. A., Márquez-Ríos, E., Del-Toro-Sánchez, C. L., Ornelas-Paz, J. d. J., Suárez-Jiménez, G. M., & Ocaño-Higuera, V. M. (2024). Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes, 12(12), 2729. https://doi.org/10.3390/pr12122729






