Numerical Simulation Study on the Diffusion Characteristics of High-Pressure Hydrogen Gas Leakage in Confined Spaces
Abstract
:1. Introduction
2. Numerical Calculation Model
2.1. Model Assumption
- (1)
- The hydrogen–air mixture can be treated as an ideal gas and follows the ideal gas law;
- (2)
- The pressure remains constant during the hydrogen leakage process;
- (3)
- Hydrogen does not undergo any chemical reactions with other gasses in the environment during the leakage process, and the ambient temperature is maintained at 298.15 K with no heat exchange with the surroundings;
- (4)
- The hydrogen exhibits turbulent flow during the leakage process.
2.2. Governing Equation
- (1)
- Continuity Equation
- (2)
- Momentum equation
- (3)
- Energy equation
- (4)
- Compositional equation
2.3. Turbulence Model
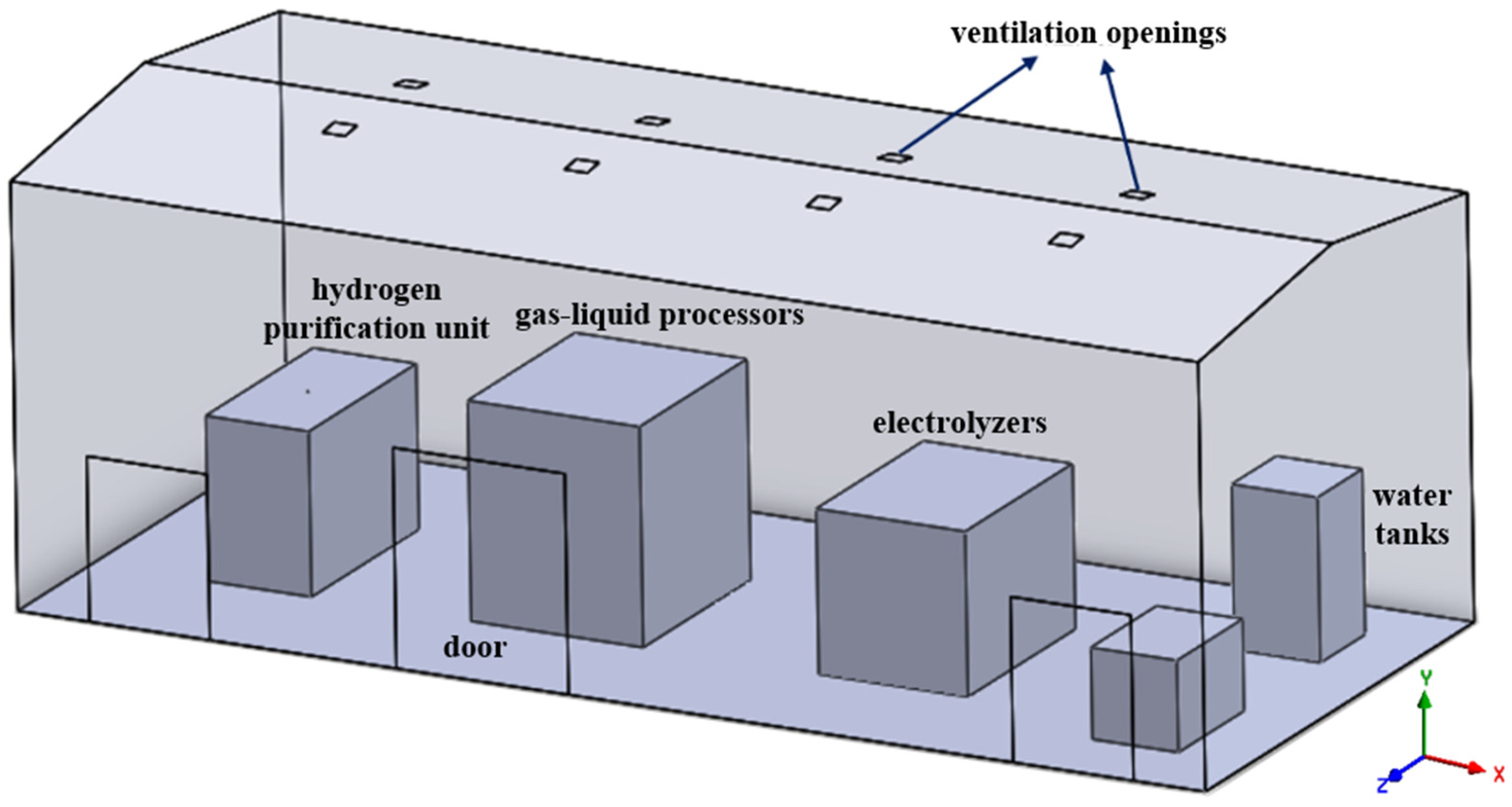
2.4. Geometric Model
2.5. Simulation Setup
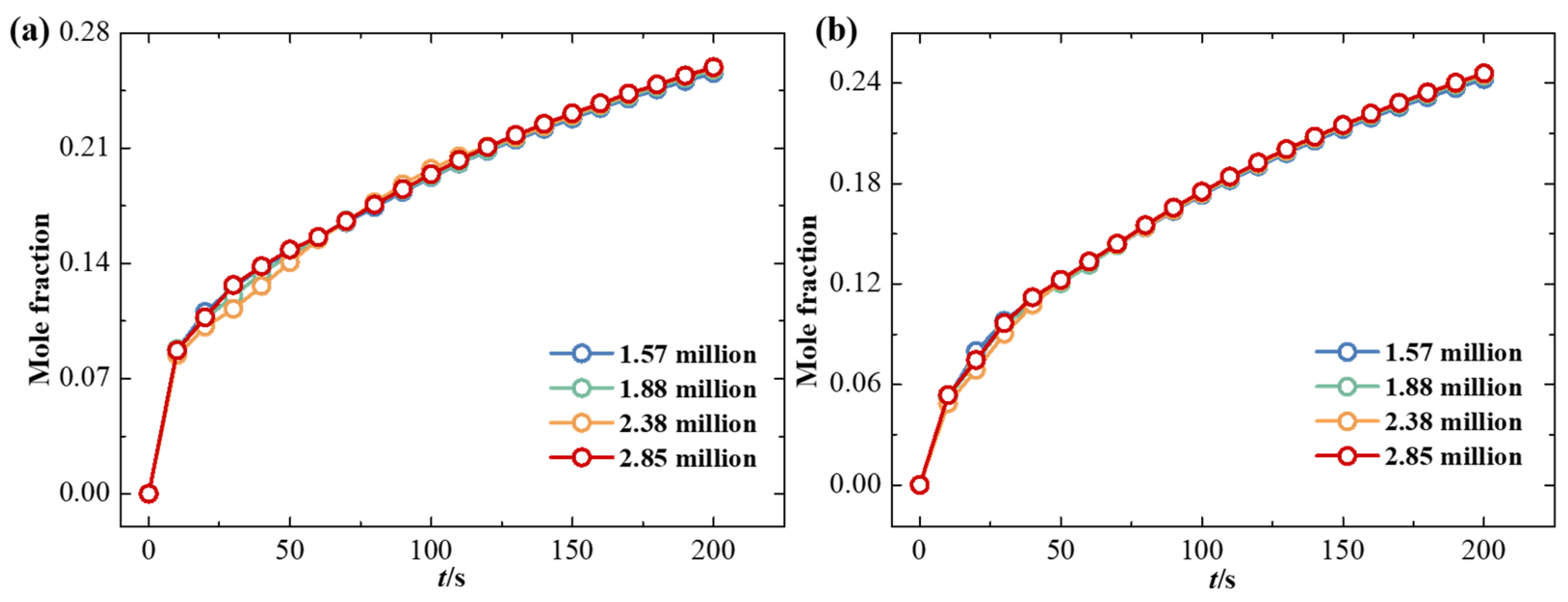
2.6. Grid Division and Independence Assessment
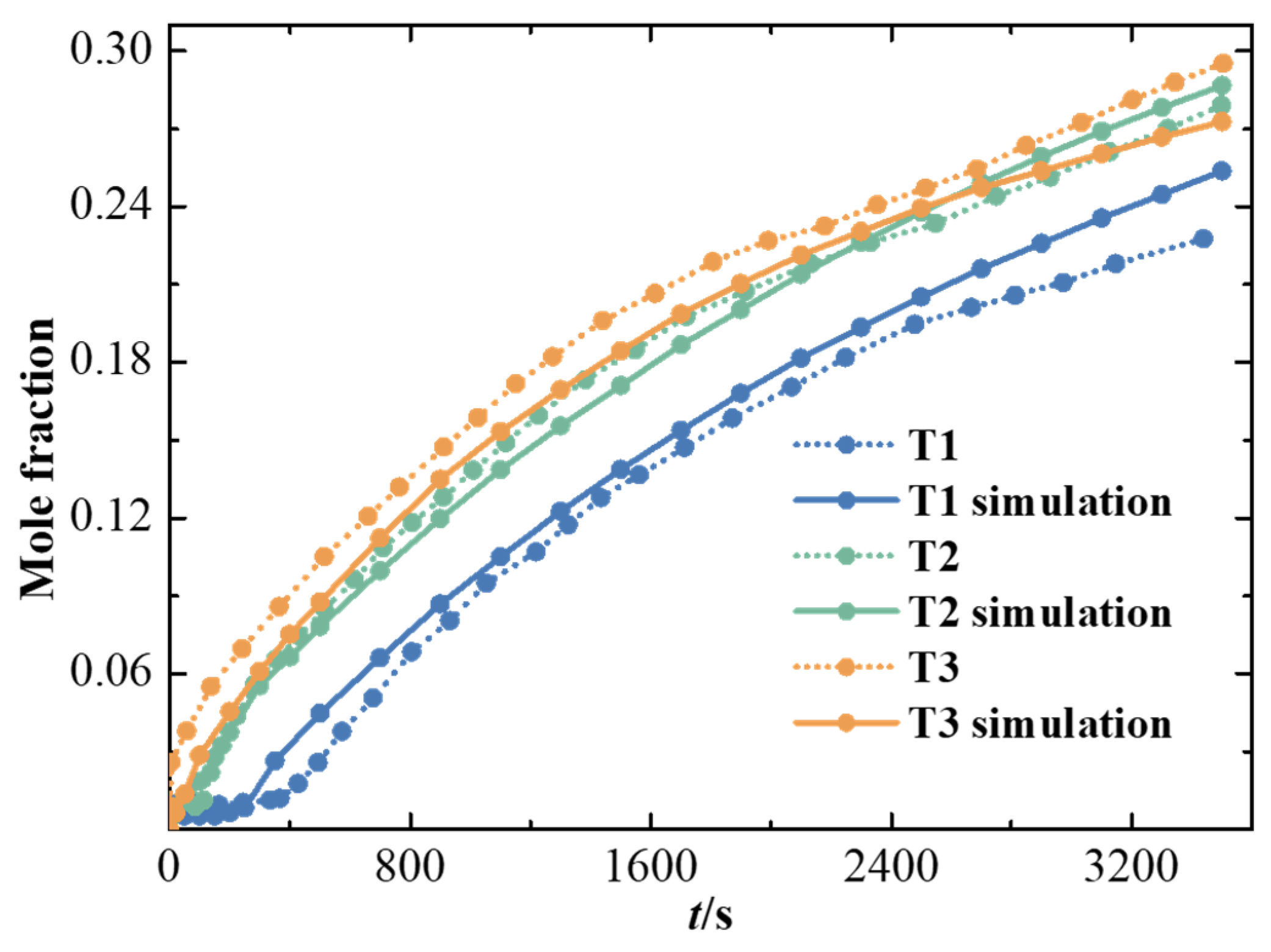
2.7. Hydrogen Leakage Concentration Field Simulation Verification
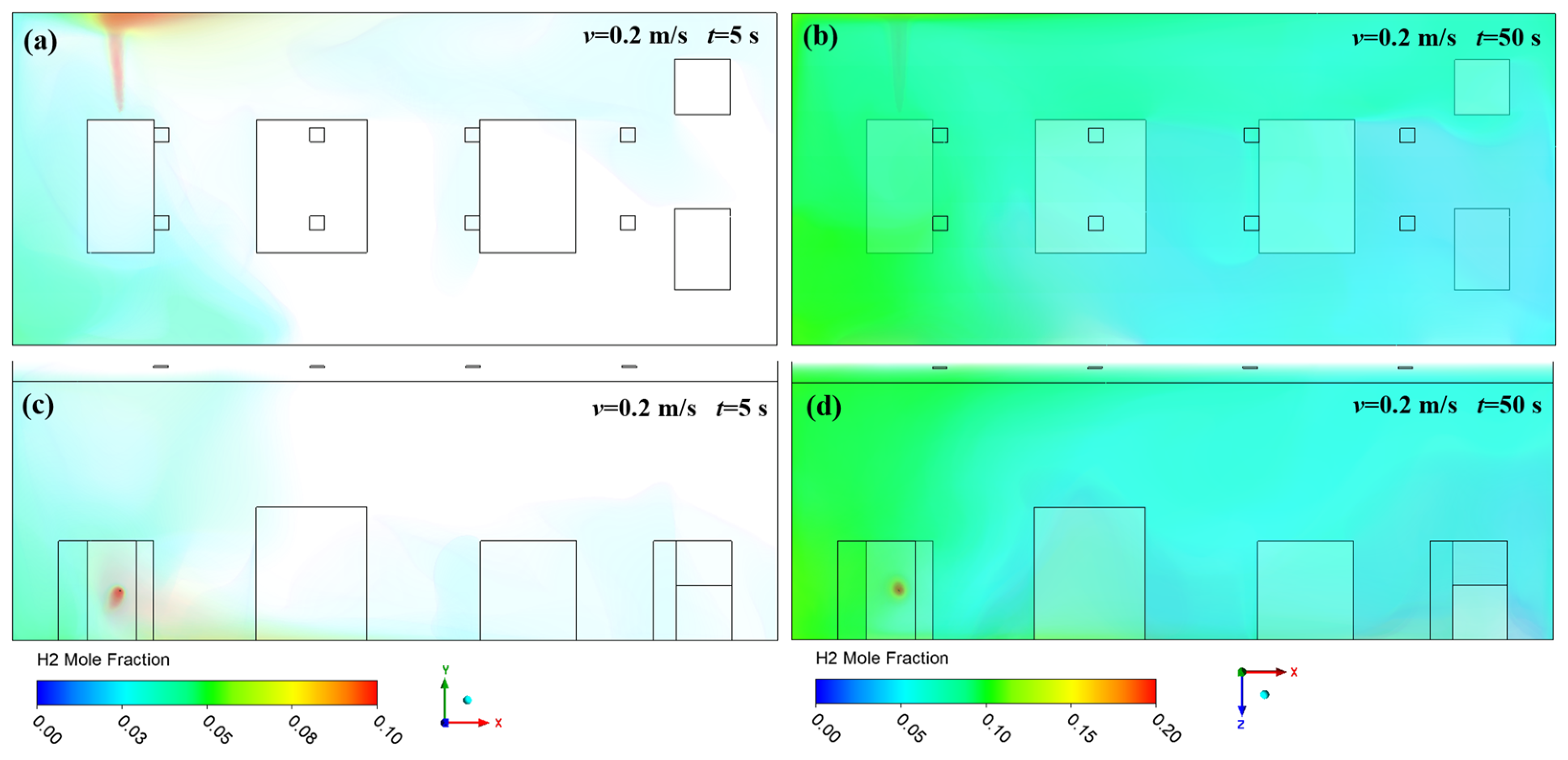
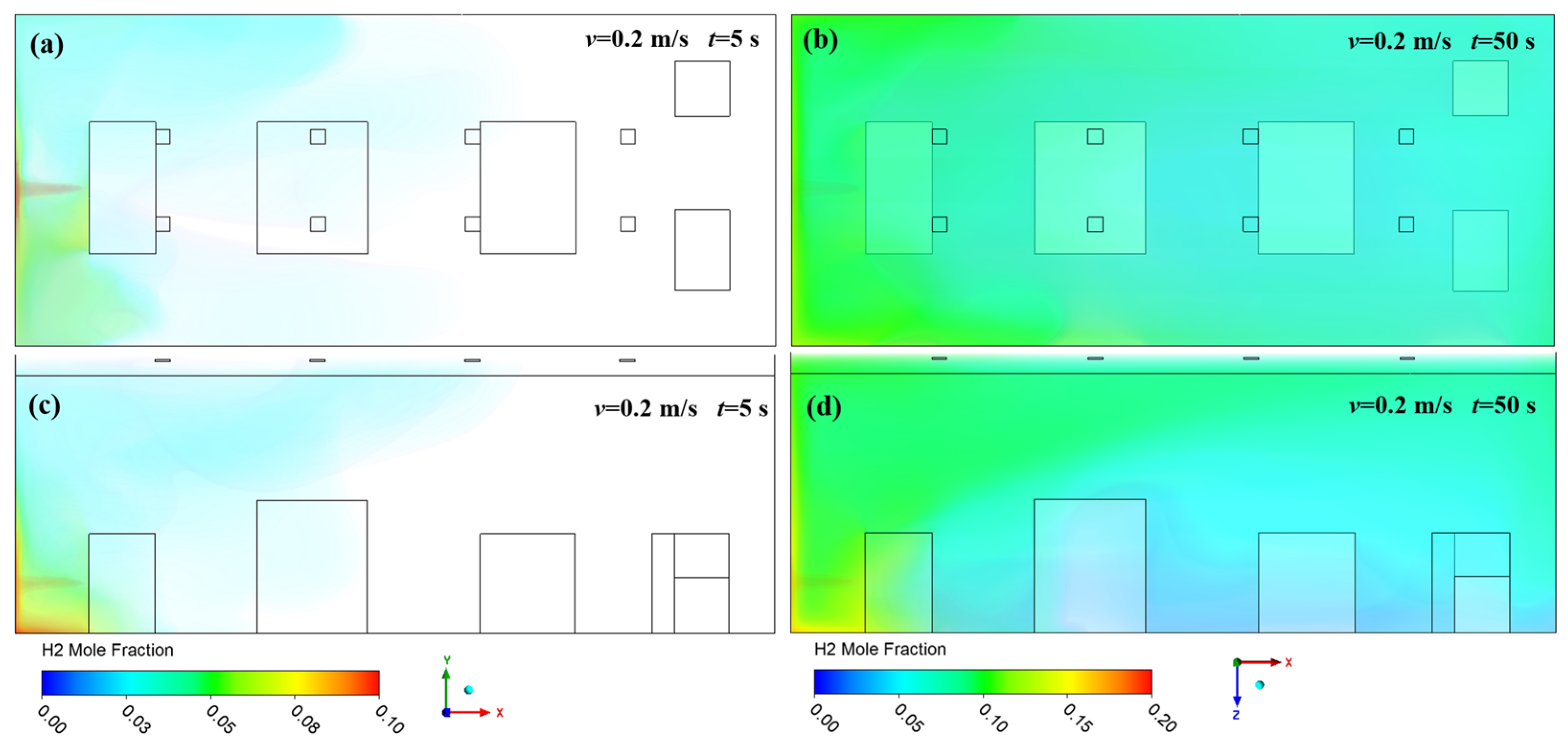
3. Results and Discussion
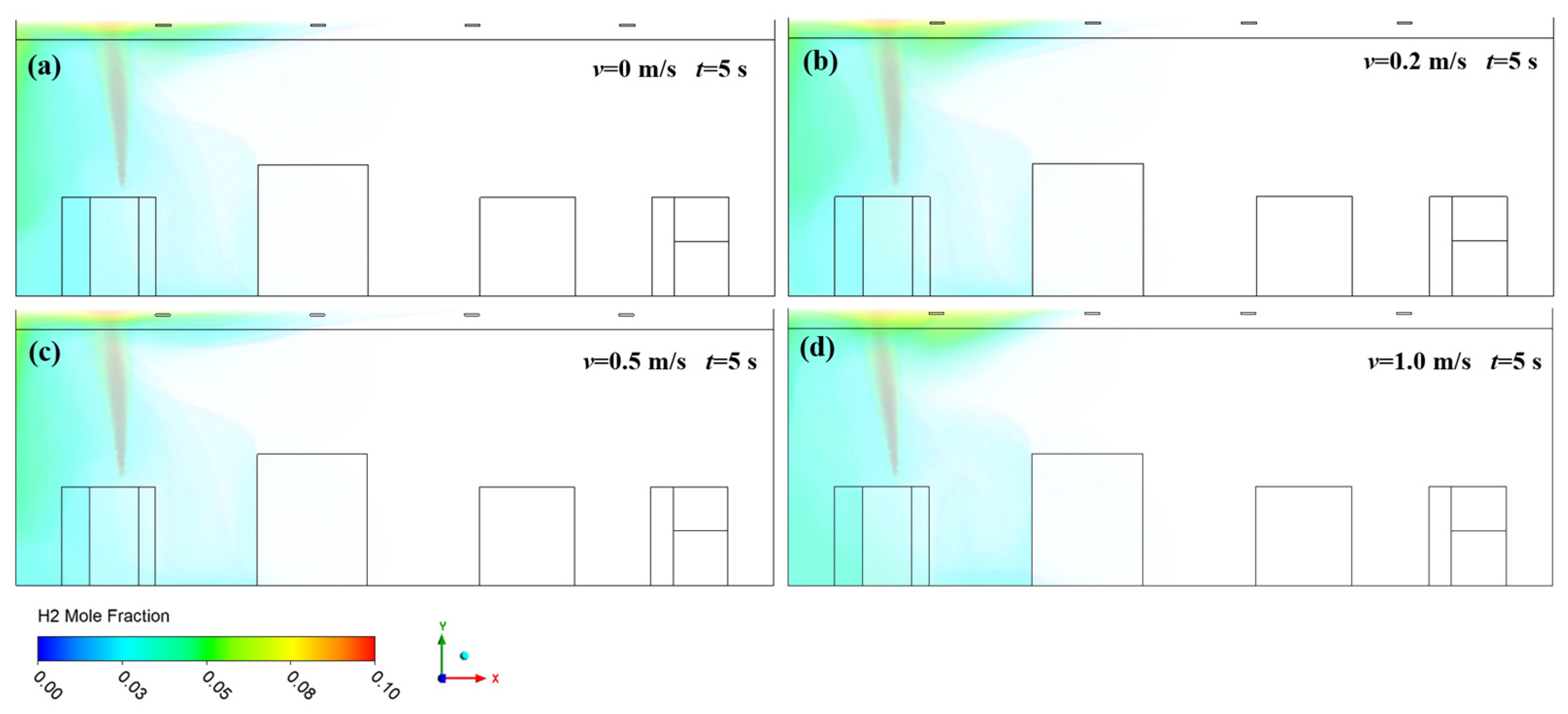
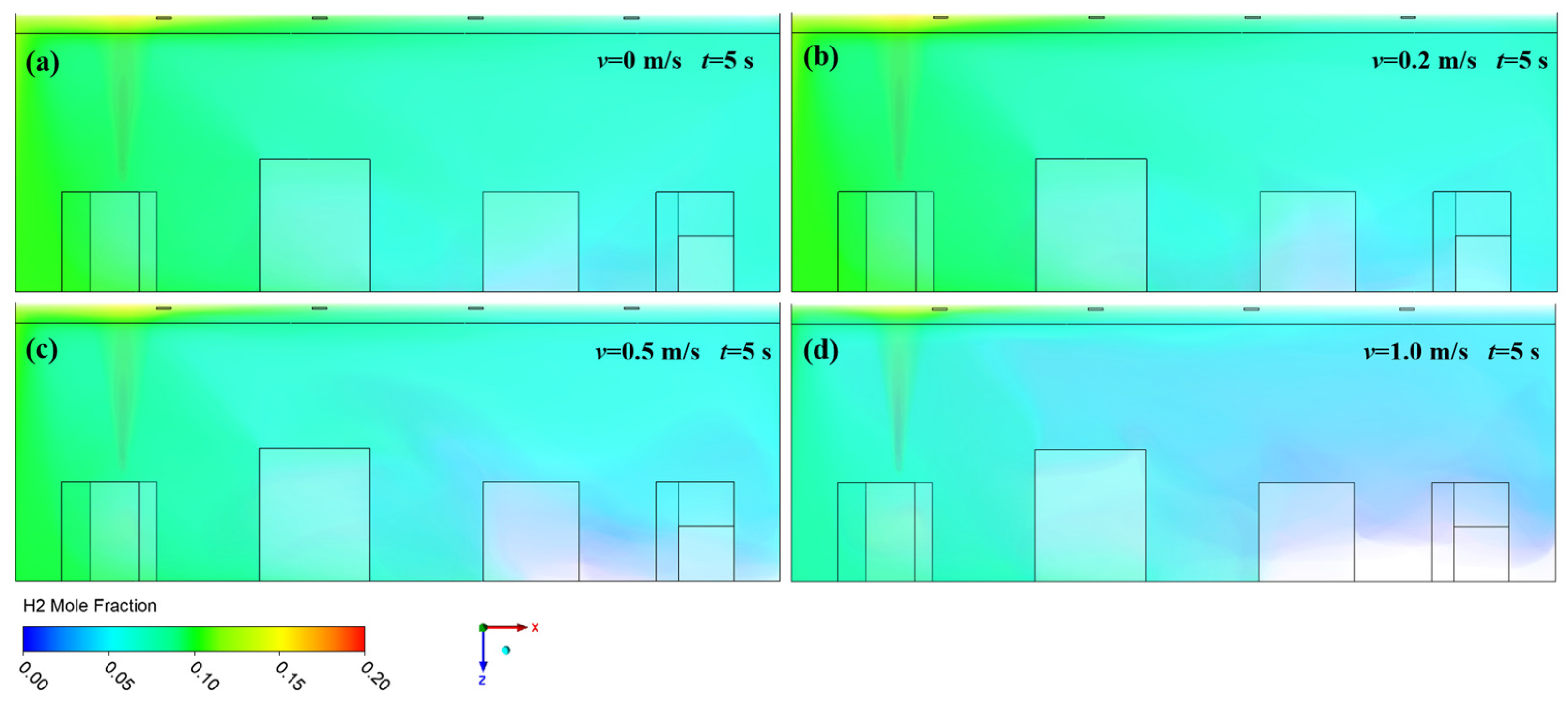
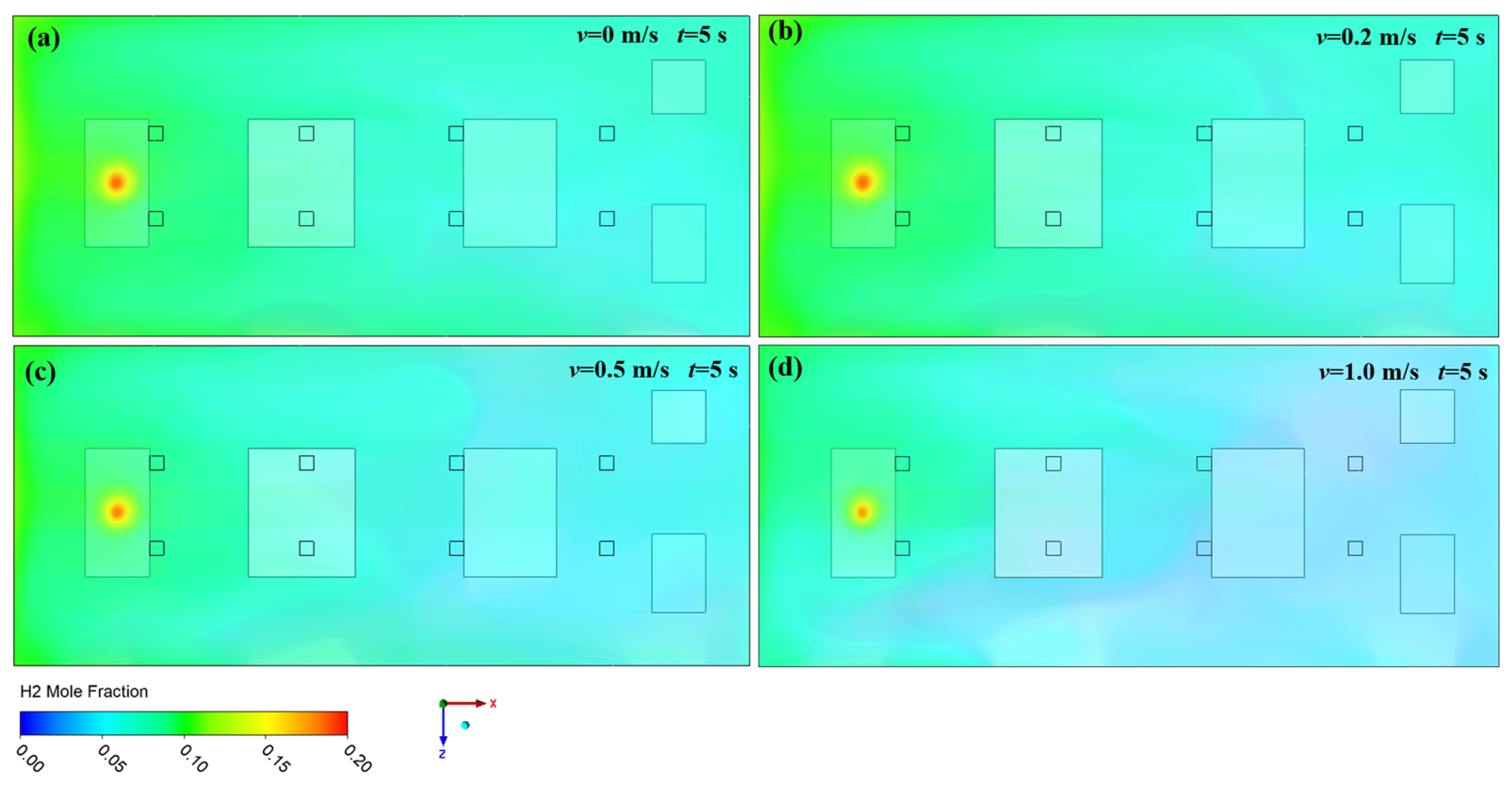
3.1. Effect of Ventilation on Hydrogen Diffusion
3.2. Effect of Leakage Direction on Hydrogen Diffusion
4. Conclusions
- (1)
- High-pressure hydrogen primarily diffuses rapidly along the walls of the facility, with local mole fractions near the leak point quickly reaching the explosive limit of 4%. Increasing the inlet wind speed effectively suppresses the rapid rise in hydrogen mole fraction in localized areas, with greater wind speeds yielding better suppression. In practical scenarios, it is essential to consider the leakage location and install exhaust fans along the walls to create airflow, thereby enhancing ventilation.
- (2)
- The study revealed that hydrogen tends to accumulate at wall corners regardless of the leakage direction before gradually diffusing to other walls. This is due to a change in the flow direction of hydrogen at the corners, which reduces the diffusion speed. This phenomenon emphasizes the necessity of placing hydrogen monitoring devices at wall junctions for timely detection of hydrogen leaks. Additionally, because hydrogen has a lower density than air, installing monitoring equipment at the top of the facility helps to detect hydrogen leaks early, thereby mitigating potential safety risks.
- (3)
- In terms of recommendations for standards and regulations, hydrogen leakage tests should be conducted under operating conditions to evaluate the leakage characteristics of equipment during normal operation, establish permissible emission limits, and ensure that hydrogen diffusion does not pose potential hazards. Additionally, it is recommended that hydrogen leakage tests are performed under parking conditions, particularly considering the risk of rapid hydrogen accumulation in confined spaces due to buoyancy effects.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Al-Jiboory, A.K.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. The renewable energy role in the global energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Bampaou, M.; Panopoulos, K. An overview of hydrogen valleys: Current status, challenges and their role in increased renewable energy penetration. Renew. Sustain. Energy Rev. 2025, 207, 114923. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q.P. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Ishimoto, J.; Shimada, S. Coupled computing for reactive hydrogen leakage phenomena with crack propagation in a pressure tank. Int. J. Hydrogen Energy 2022, 47, 2735–2758. [Google Scholar] [CrossRef]
- Yang, F.; Wang, T.; Deng, X.; Dang, J.; Huang, Z.; Hu, S.; Li, Y.; Ouyang, M. Review on hydrogen safety issues: Incident statistics, hydrogen diffusion, and detonation process. Int. J. Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar] [CrossRef]
- Yang, N.; Deng, J.; Wang, C.; Bai, Z.; Qu, J. High pressure hydrogen leakage diffusion: Research progress. Int. J. Hydrogen Energy 2024, 50, 1029–1046. [Google Scholar] [CrossRef]
- Campari, A.; Ustolin, F.; Alvaro, A.; Paltrinieri, N. A review on hydrogen embrittlement and risk-based inspection of hydrogen technologies. Int. J. Hydrogen Energy 2023, 48, 35316–35346. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Kang, H.; Zhou, W.; Zhang, C. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Ramirez, J.P.B.; Halm, D.; Grandidier, J.-C.; Villalonga, S.; Nony, F. 700 bar type IV high pressure hydrogen storage vessel burst–Simulation and experimental validation. Int. J. Hydrogen Energy 2015, 40, 13183–13192. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, D.; Wang, F.; Qin, C.; Chen, X.; Tian, Y. Quantitative analysis of hydrogen leakage and diffusion processes based on real-time concentration measurement at different spatial positions in a customized chamber. Int. J. Hydrogen Energy 2024, 66, 231–241. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, G.; Xie, M.; Li, X.; Zhao, Y.; Su, S.; Li, S. Experimental and numerical studies on hydrogen leakage and dispersion evolution characteristics in space with large aspect ratios. J. Clean. Prod. 2024, 438, 140467. [Google Scholar] [CrossRef]
- Hajji, Y.; Bouteraa, M.; Elcafsi, A.; Belghith, A.; Bournot, P.; Kallel, F. Natural ventilation of hydrogen during a leak in a residential garage. Renew. Sustain. Energy Rev. 2015, 50, 810–818. [Google Scholar] [CrossRef]
- Yu, X.; Wang, C.; He, Q. Numerical study of hydrogen dispersion in a fuel cell vehicle under the effect of ambient wind. Int. J. Hydrogen Energy 2019, 44, 22671–22680. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Hou, Y. Effects of leakage location and ventilation condition on hydrogen leakage during shipping of fuel cell vehicles. Int. J. Hydrogen Energy 2024, 54, 1532–1543. [Google Scholar] [CrossRef]
- Hou, X.; Lan, H.; Zhao, Z.; Li, J.; Hu, C.; Li, Y. Effect of obstacle location on hydrogen dispersion in a hydrogen fuel cell bus with natural and mechanical ventilation. Process. Saf. Environ. Prot. 2023, 171, 995–1008. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Li, X.-J.; Gao, Z.-X.; Jin, Z.-J. A numerical study of hydrogen leakage and diffusion in a hydrogen refueling station. Int. J. Hydrogen Energy 2020, 45, 14428–14439. [Google Scholar] [CrossRef]
- Pitts, W.M.; Yang, J.C.; Blais, M.; Joyce, A. Dispersion and burning behavior of hydrogen released in a full-scale residential garage in the presence and absence of conventional automobiles. Int. J. Hydrogen Energy 2012, 37, 17457–17469. [Google Scholar] [CrossRef]
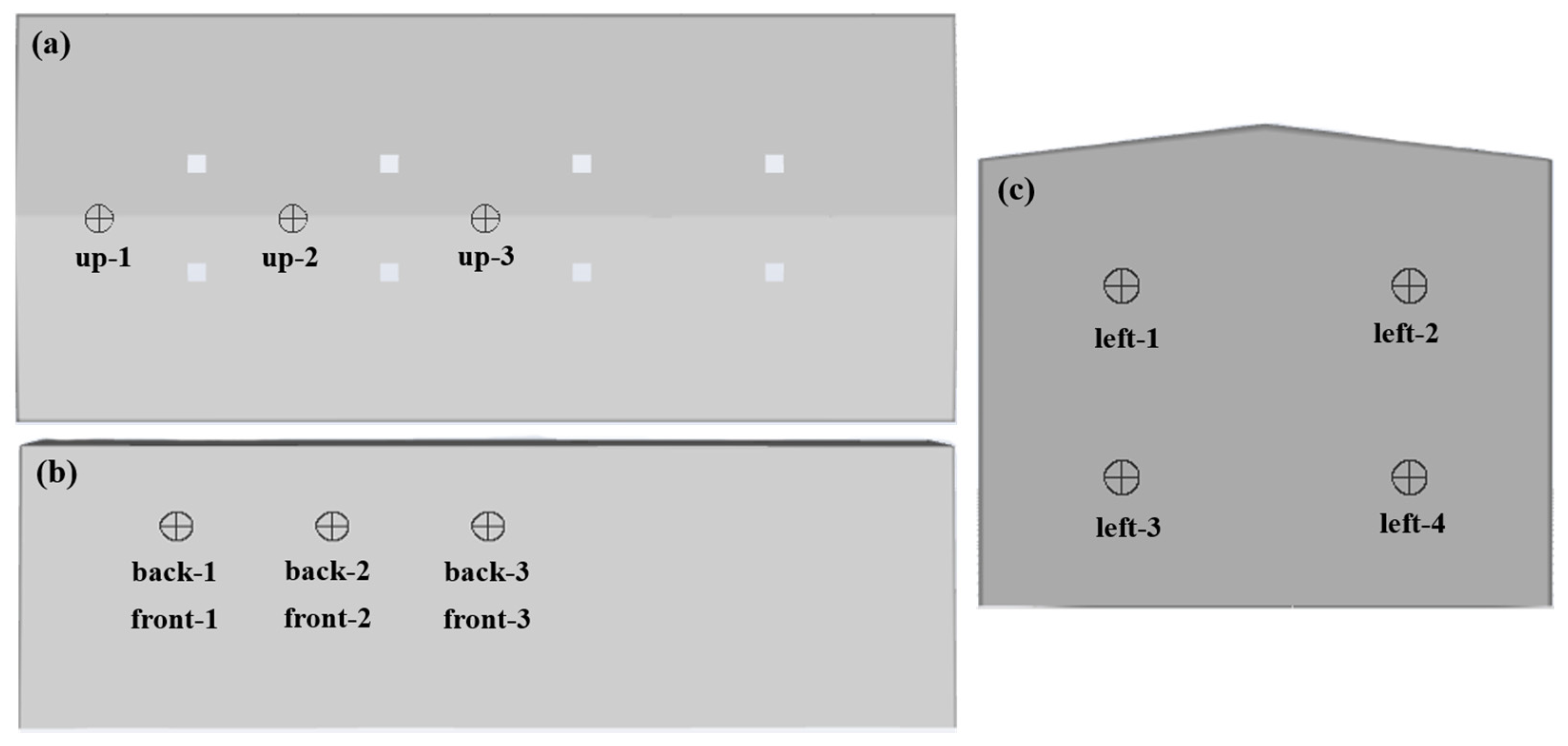

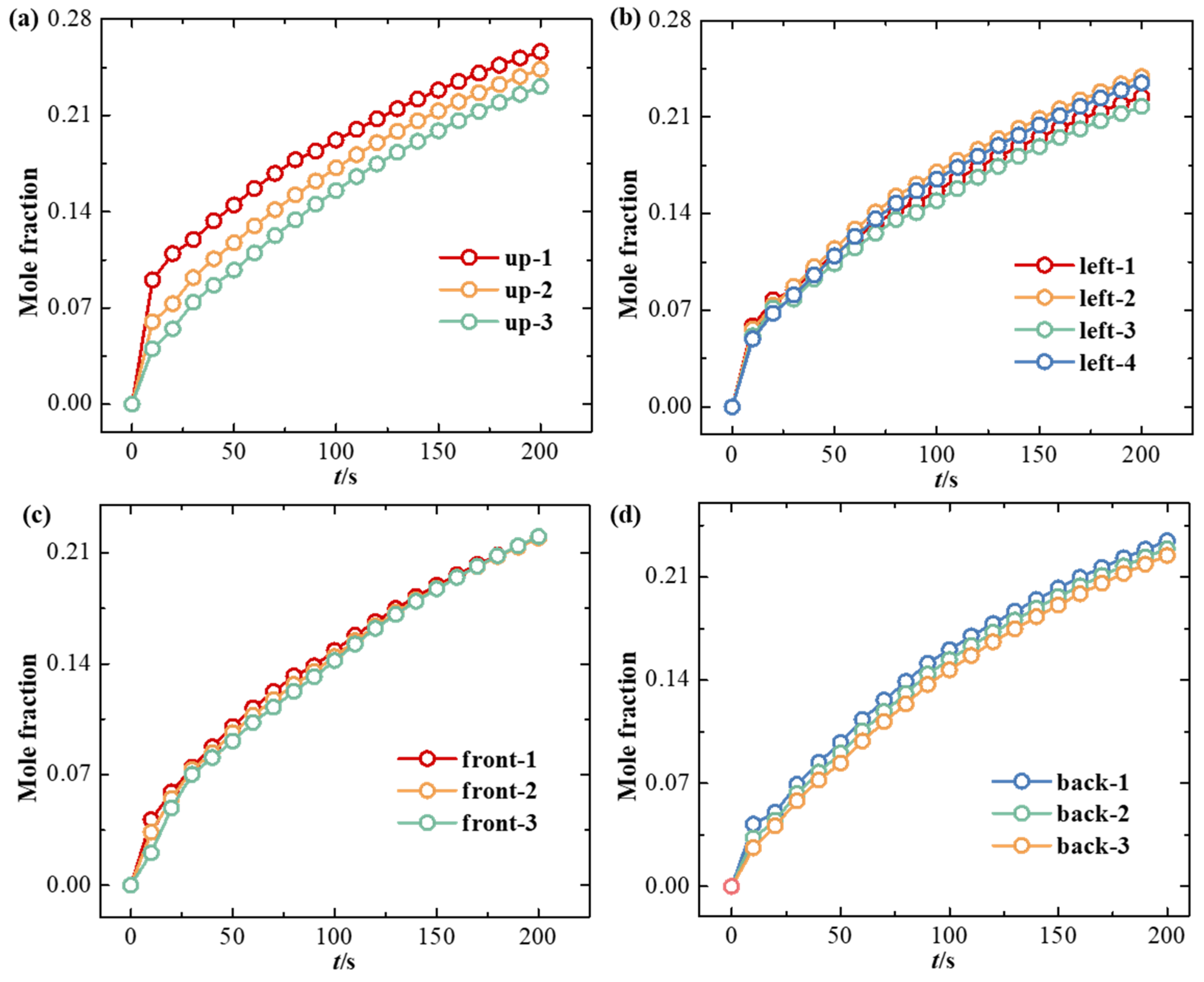

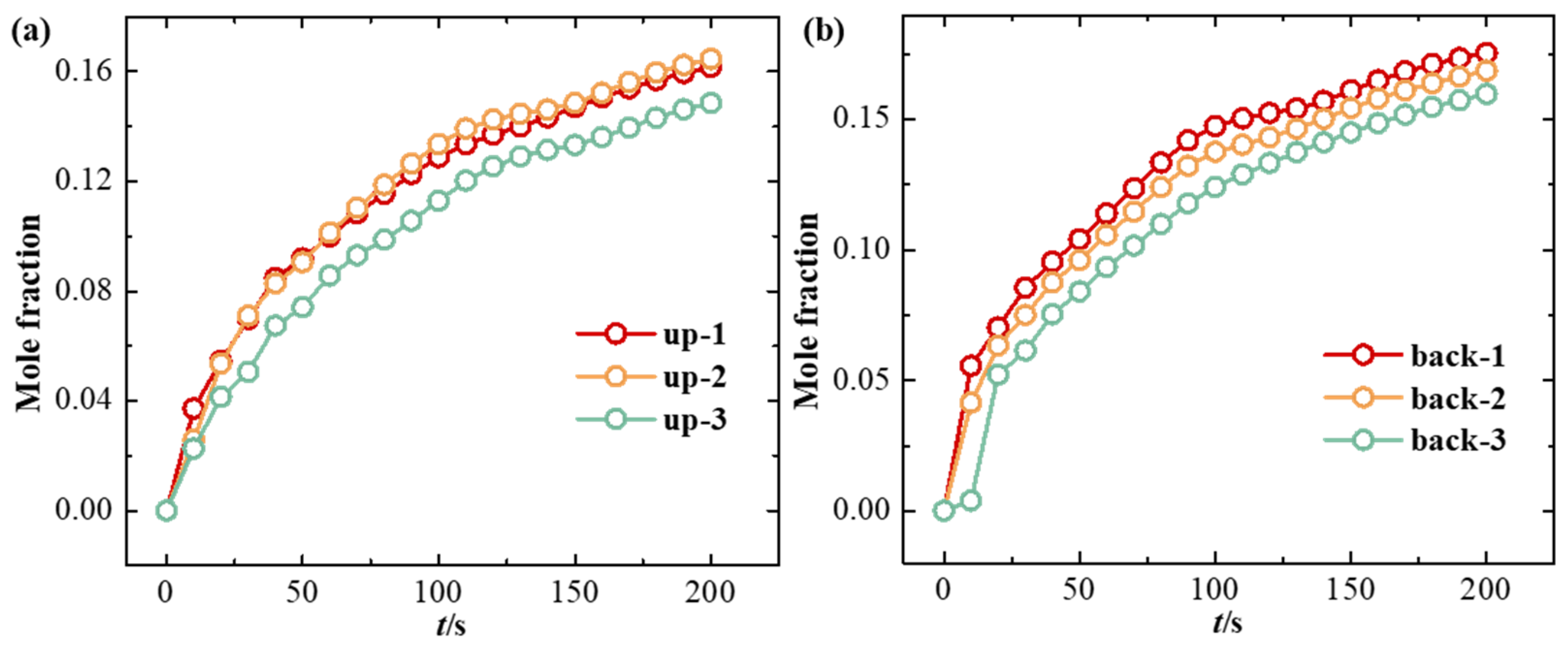

| Station Number | Coordinate | Station Number | Coordinate |
|---|---|---|---|
| up-1 | (1.91, 7.5, 0) | back-1 | (3.45, 5, 4.5) |
| up-2 | (6.13, 7.5, 0) | back-2 | (6.9, 5, 4.5) |
| up-3 | (10.35, 7.5, 0) | back-3 | (10.35, 5, 4.5) |
| front-1 | (3.45, 5, −4.5) | left-1 | (0, 5, 2.25) |
| front-2 | (6.9, 5, −4.5) | left-2 | (0, 5, −2.25) |
| front-3 | (10.35, 5, −4.5) | left-3 | (0, 2, 2.25) |
| left-4 | (0, 2, −2.25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.-M.; Zhang, Y.; Wei, Z.-N.; Yuan, L.; Li, J.-D.; Gong, L.; Zhu, C.-Y. Numerical Simulation Study on the Diffusion Characteristics of High-Pressure Hydrogen Gas Leakage in Confined Spaces. Processes 2024, 12, 2711. https://doi.org/10.3390/pr12122711
Li F-M, Zhang Y, Wei Z-N, Yuan L, Li J-D, Gong L, Zhu C-Y. Numerical Simulation Study on the Diffusion Characteristics of High-Pressure Hydrogen Gas Leakage in Confined Spaces. Processes. 2024; 12(12):2711. https://doi.org/10.3390/pr12122711
Chicago/Turabian StyleLi, Feng-Ming, Yuan Zhang, Zheng-Nan Wei, Lin Yuan, Jia-Dong Li, Liang Gong, and Chuan-Yong Zhu. 2024. "Numerical Simulation Study on the Diffusion Characteristics of High-Pressure Hydrogen Gas Leakage in Confined Spaces" Processes 12, no. 12: 2711. https://doi.org/10.3390/pr12122711
APA StyleLi, F.-M., Zhang, Y., Wei, Z.-N., Yuan, L., Li, J.-D., Gong, L., & Zhu, C.-Y. (2024). Numerical Simulation Study on the Diffusion Characteristics of High-Pressure Hydrogen Gas Leakage in Confined Spaces. Processes, 12(12), 2711. https://doi.org/10.3390/pr12122711





