Copper Anode Slime Processing with a Focus on Gold Recovery: A Review of Traditional and Recent Technologies
Abstract
1. Introduction
2. Copper Anode Slime
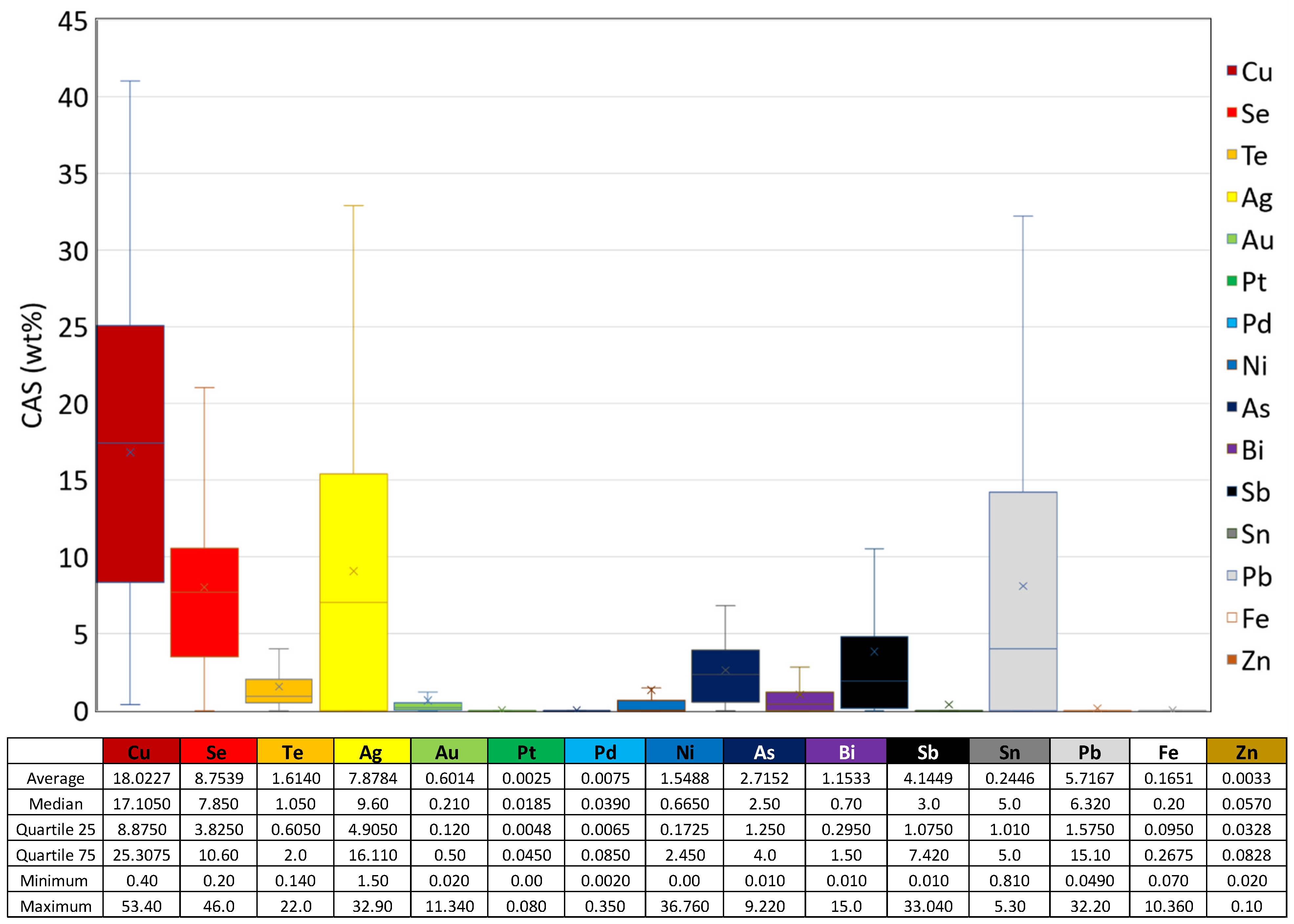
2.1. Composition of Copper Anode Slime
2.2. Overview of Copper Anode Slime Processing Routes
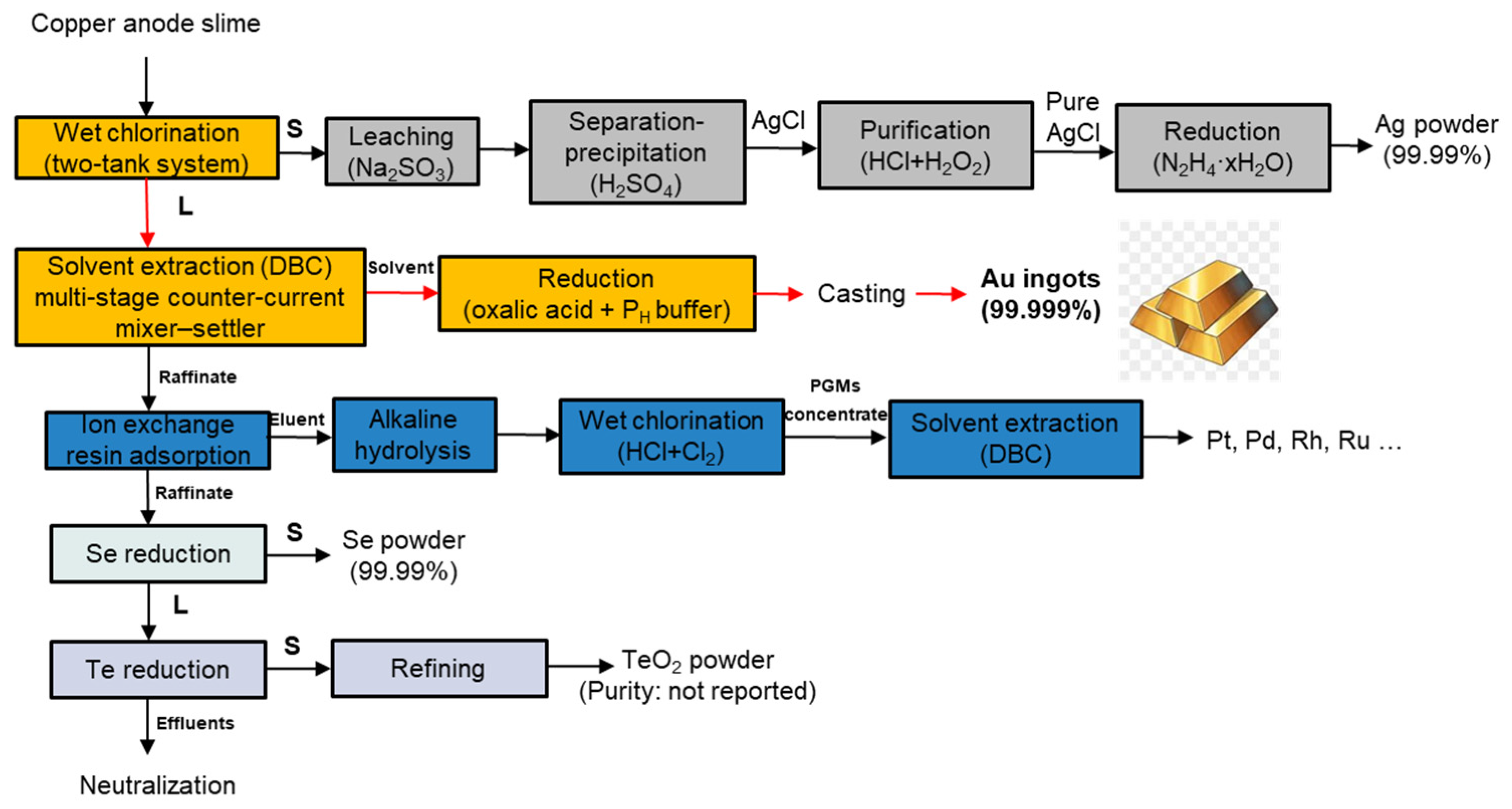
2.2.1. Hydrometallurgical Routes
2.2.2. Hybrid Routes
3. Industrial Flow Sheets
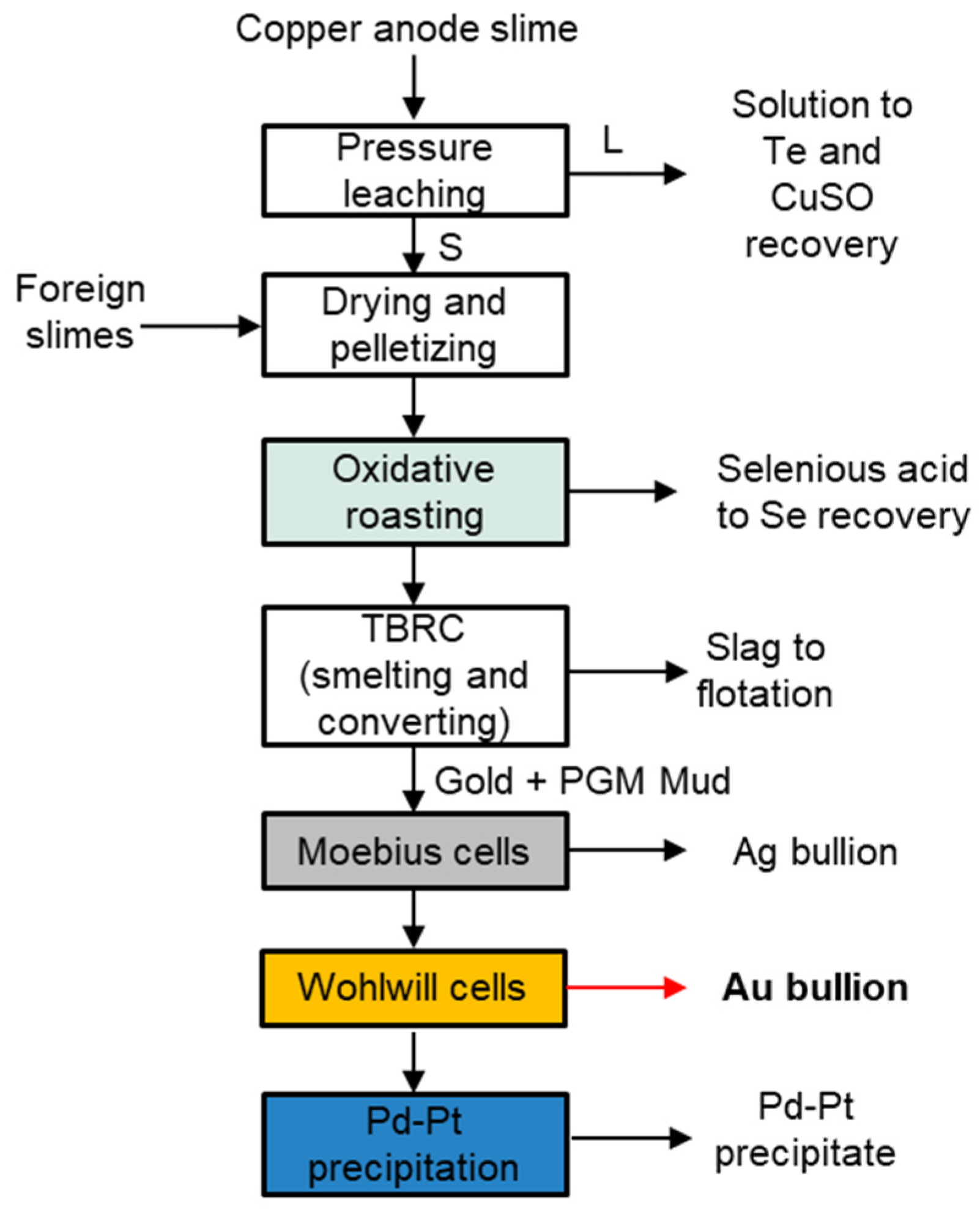
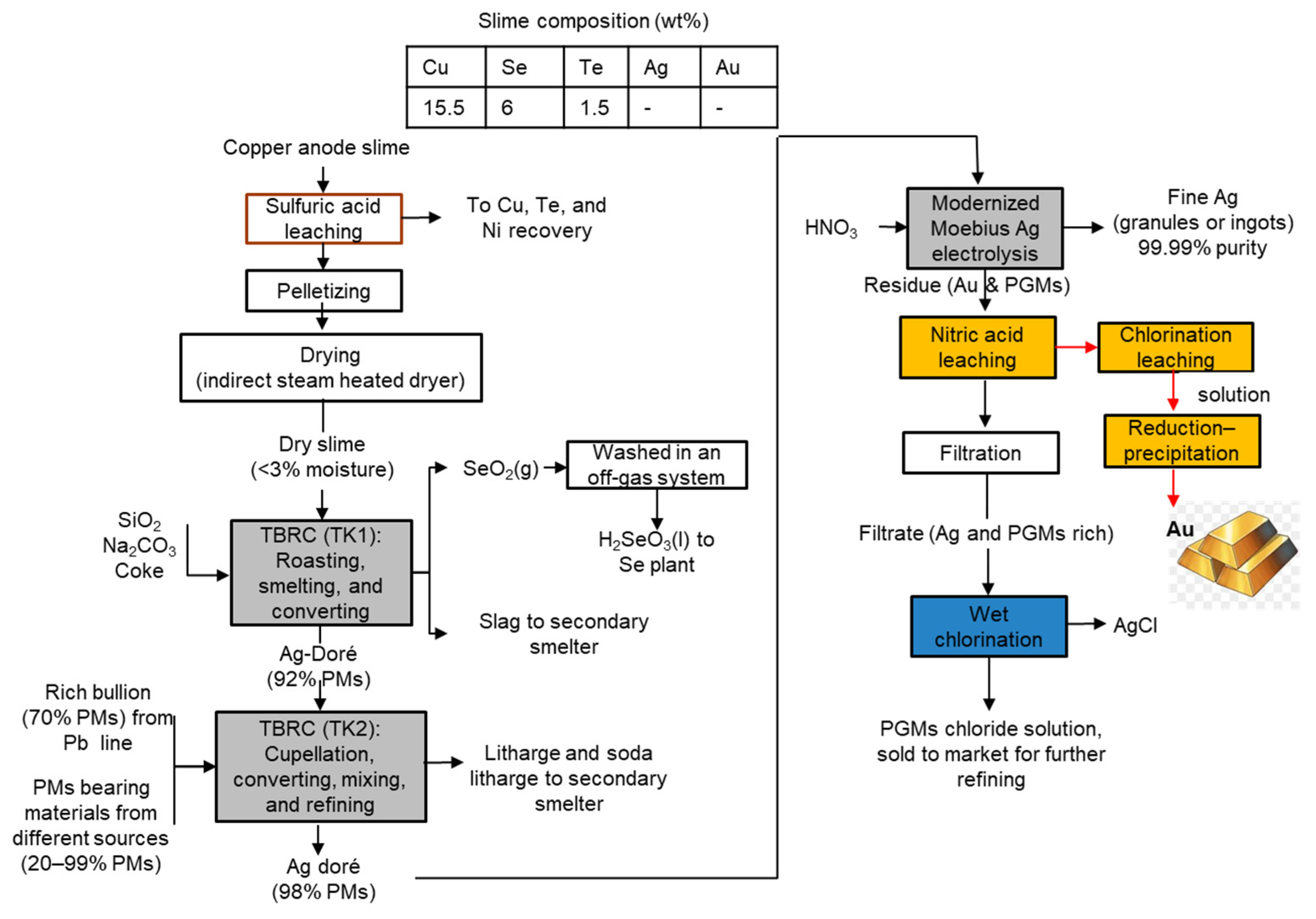
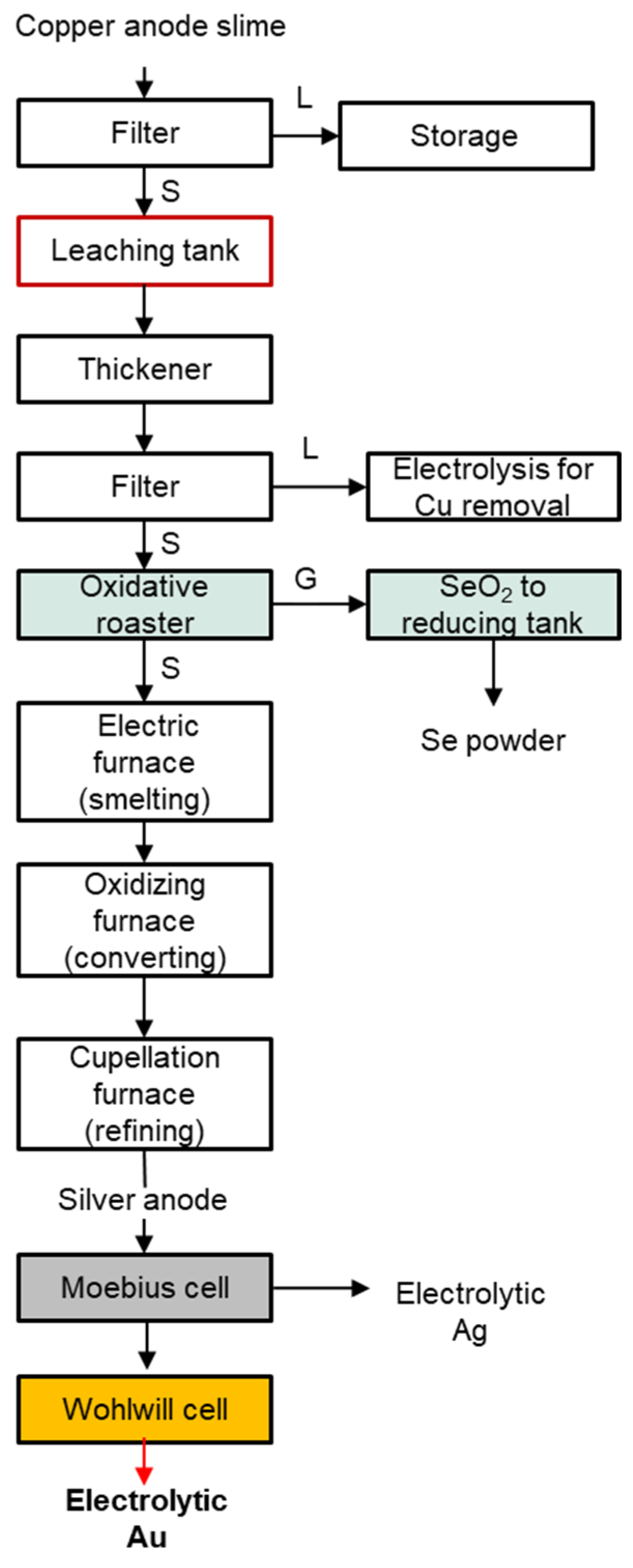
3.1. Sumitomo Metal Mining Co., Ltd., Japan
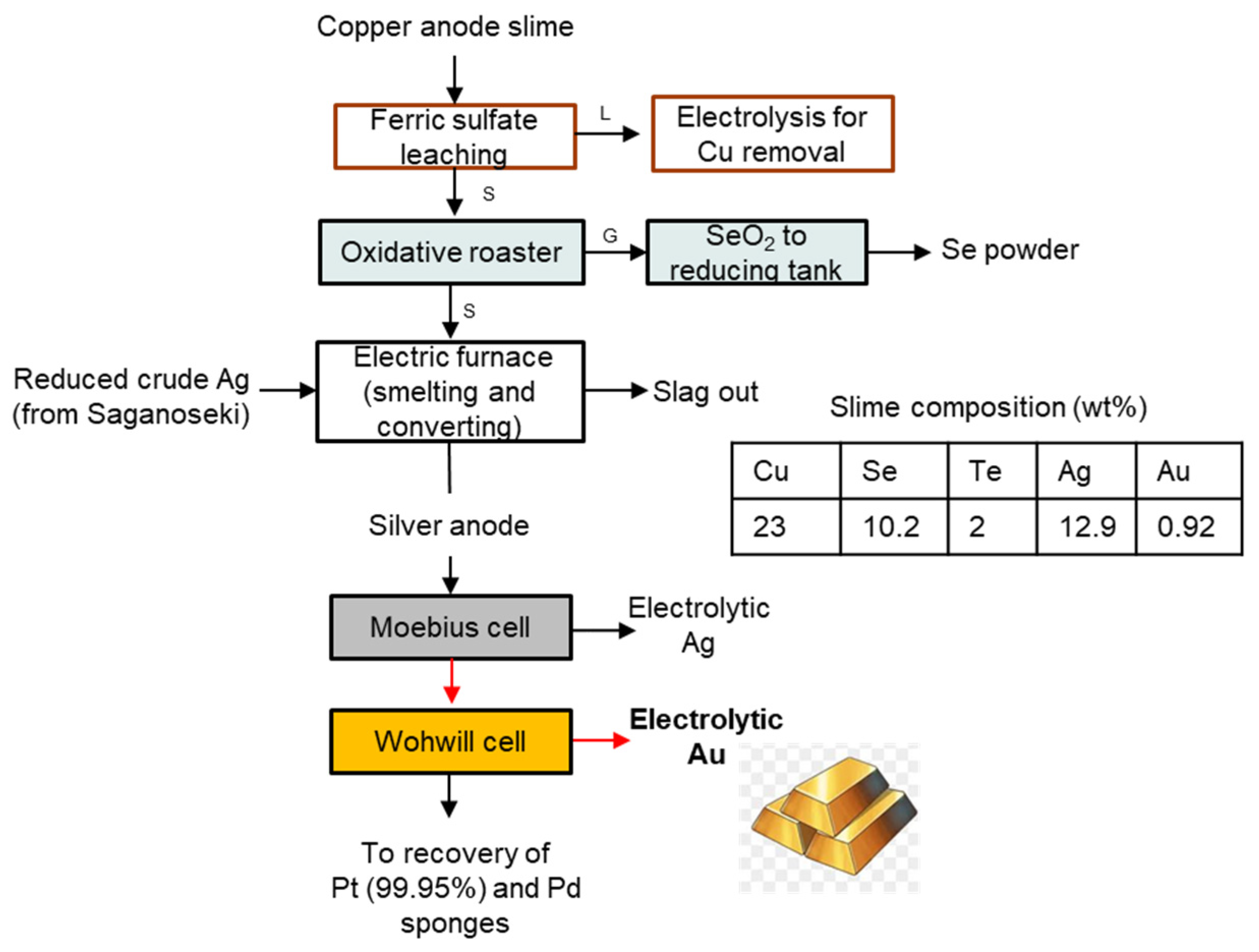
3.2. Saganoseki Smelter and Refinery of Pan Pacific Copper Co., Ltd., Japan
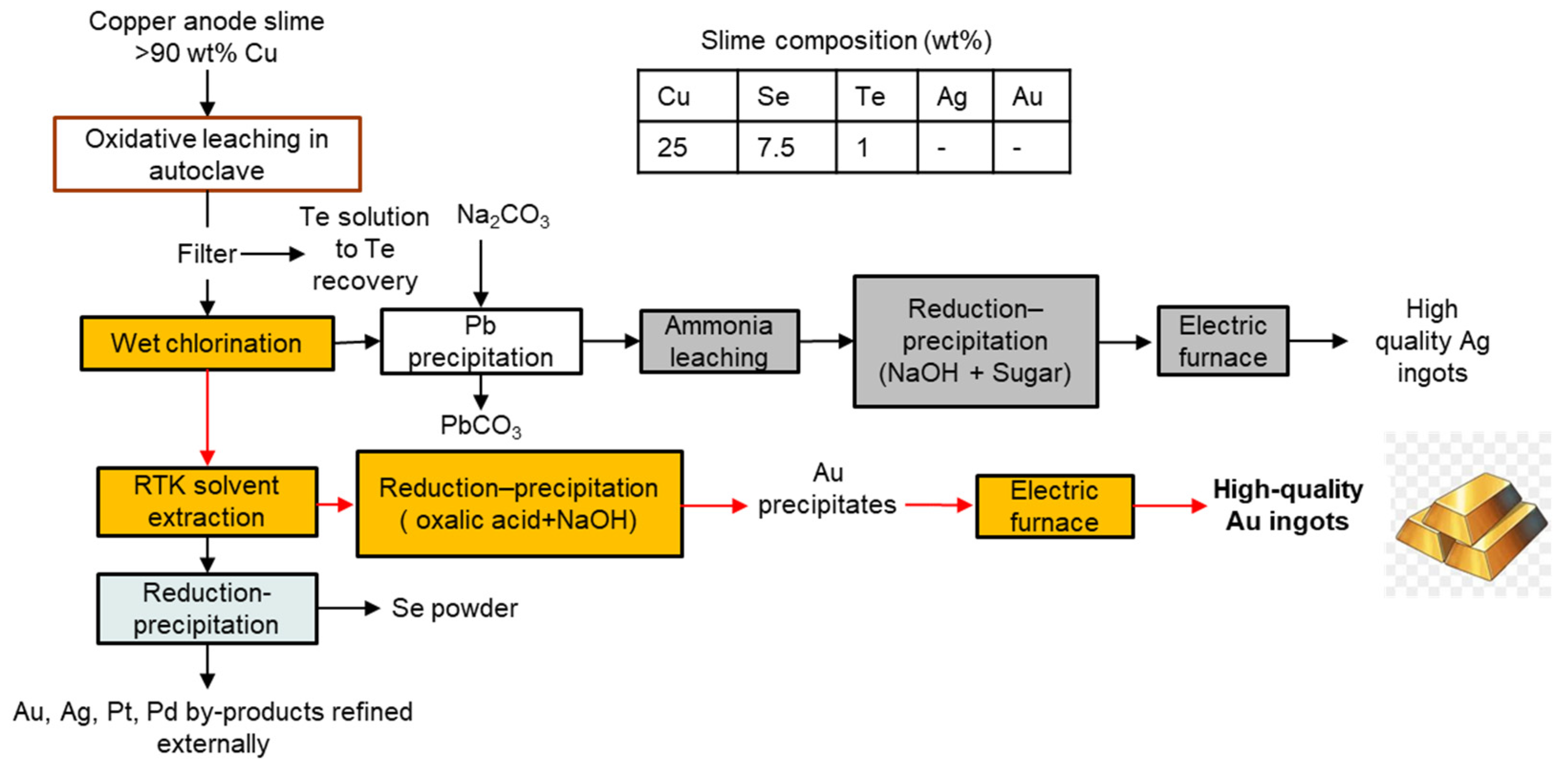
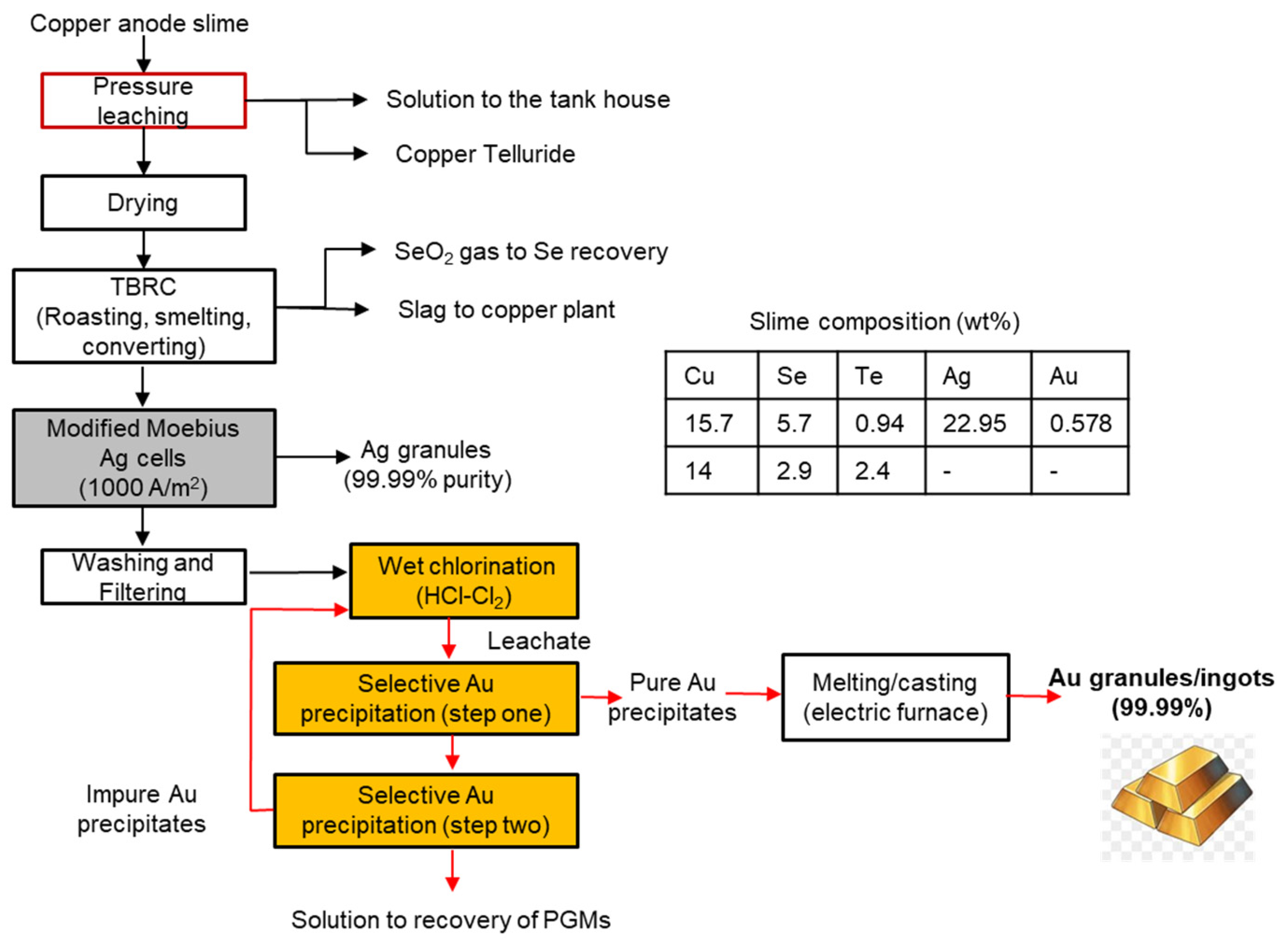
3.3. Rio Tinto Kennecott, USA
3.4. Phelps Dodge El Paso Refinery, USA
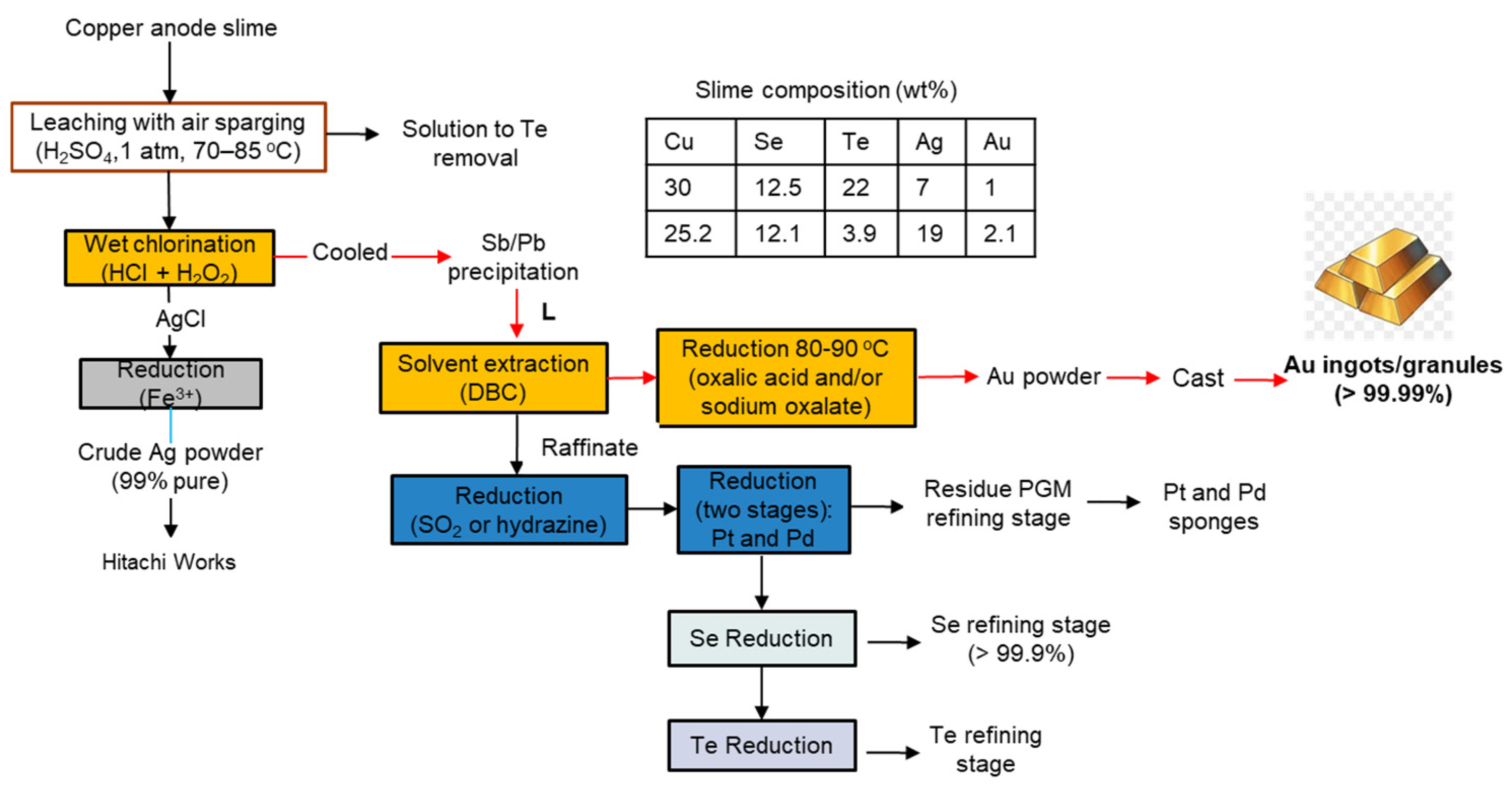
3.5. Hitachi Works, Mitsubishi Heavy Industries, Ltd., Japan
3.6. Aurubis Hamburg, Germany
3.7. Boliden
3.8. Glencore Canada Copper Refinery
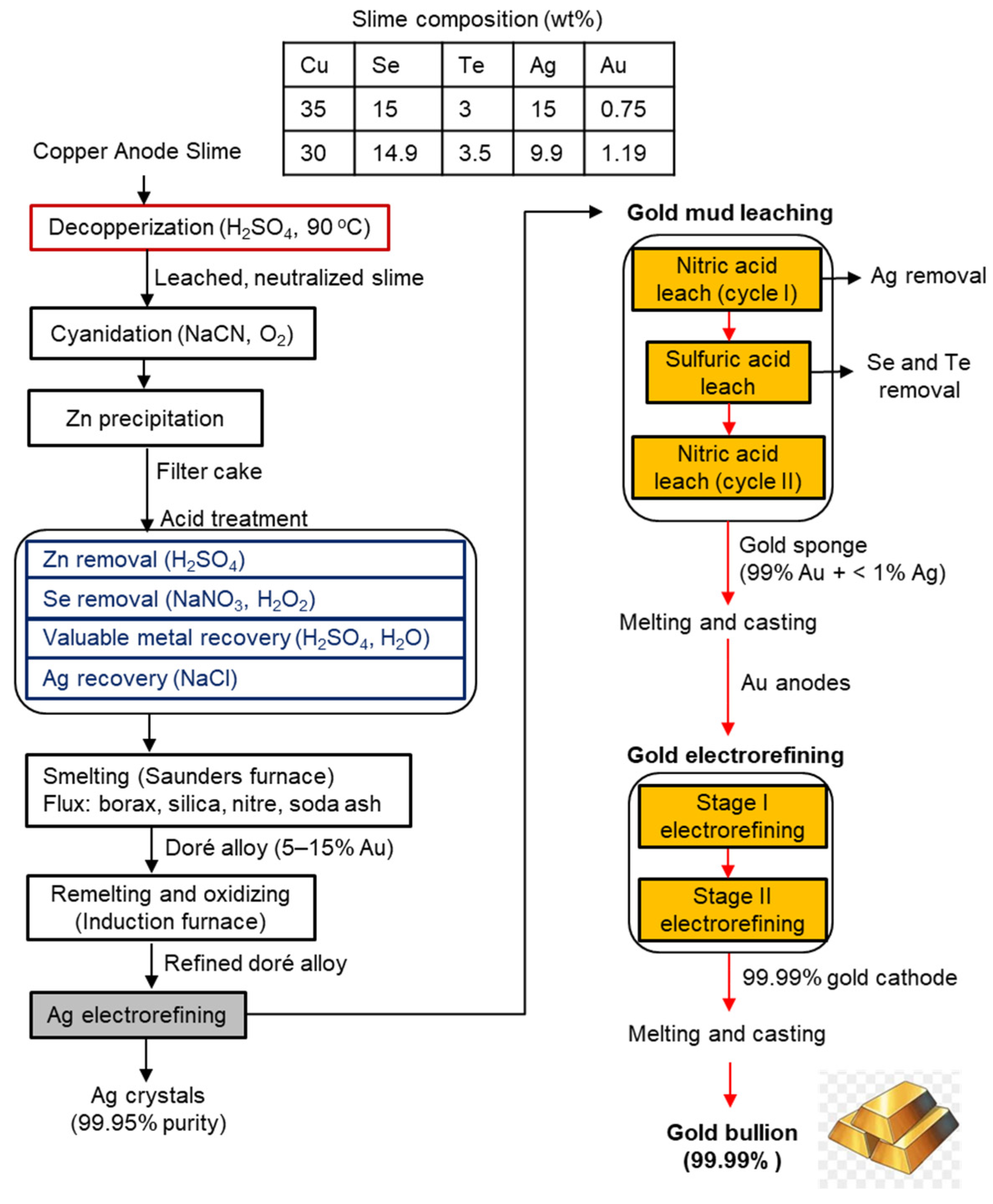
3.9. Olympic Dam
3.10. Outukumpu Pori Refinery
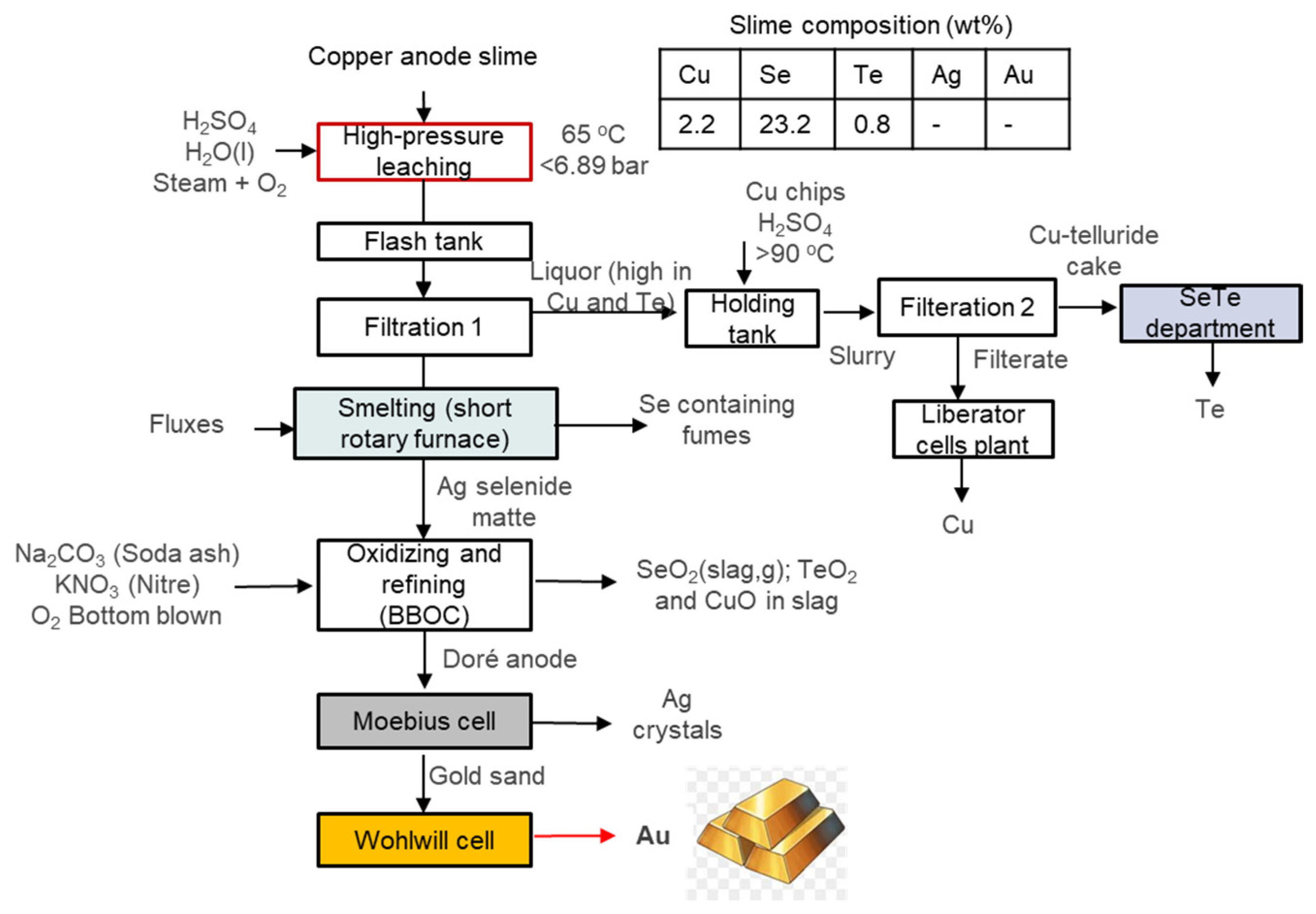
3.11. Asarco Amarillo
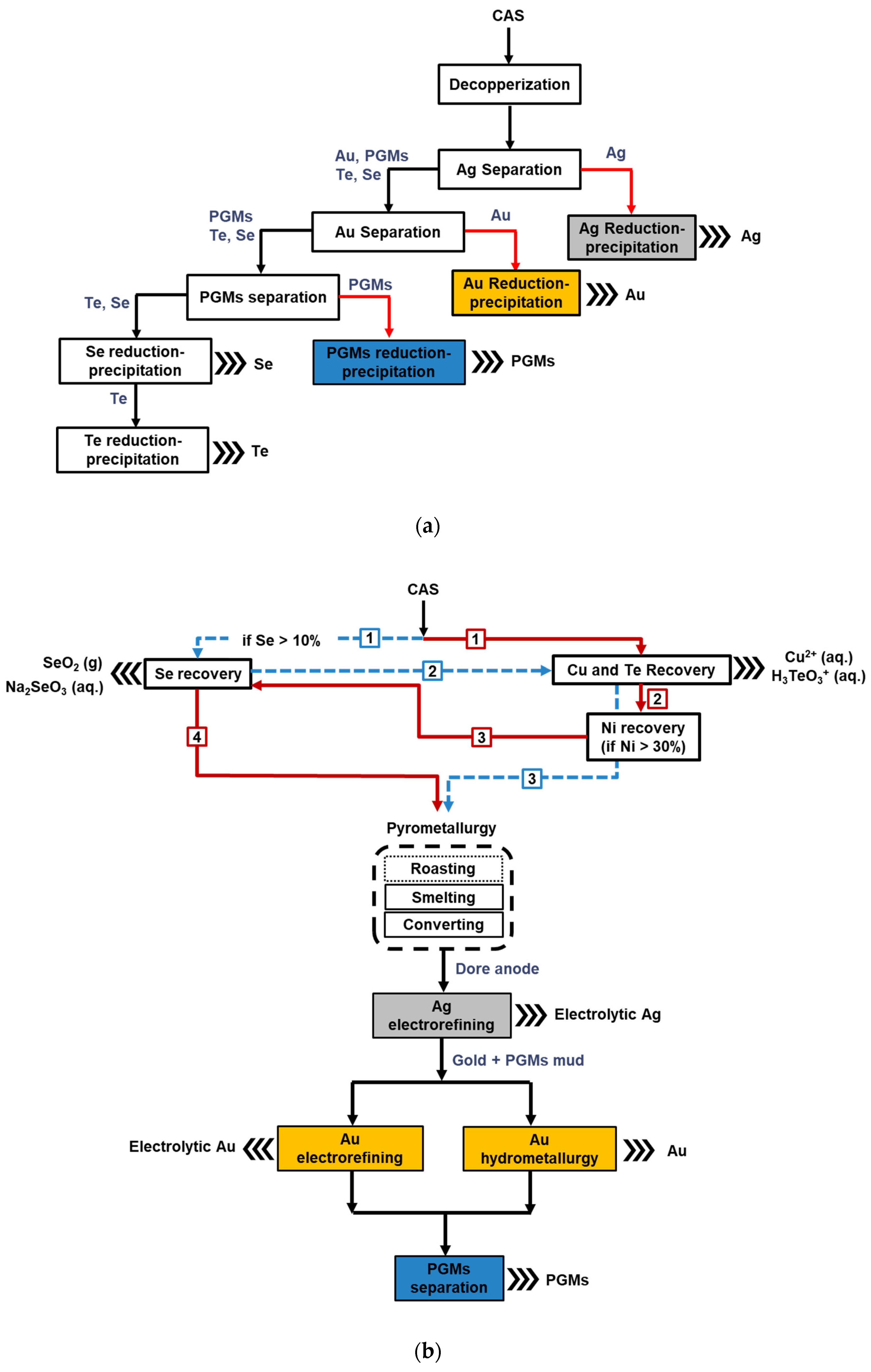
4. Analysis of Hybrid Routes
4.1. Pyrometallurgical Technologies
4.1.1. Reverberatory Doré Furnace
4.1.2. Short Rotary Furnace
4.1.3. TBRC
4.1.4. BBOC
4.2. Innovations in Hybrid Processing
5. Analysis of Hydrometallurgical Routes
6. Comparison of Hybrid and Hydrometallurgical Routes
7. Conclusions
- Hydrometallurgical advantage: Hydrometallurgical processes excel in gold recovery from various aspects. They achieve a first-pass recovery rate of 99%. These methods minimize gold in-process hold-up and facilitate its rapid recovery at the early stages of the copper anode slime treatment flow sheet, with an overall duration of approximately 7–8 days.
- Shift to hydrometallurgy: Analysis of various industrial flow sheets for copper anode slime treatment indicates a notable trend toward transitioning from hybrid routes to purely hydrometallurgical routes, which demonstrate greater promise in terms of efficiency, recovery rates, and environmental benefits, including reduced energy consumption and lower emissions. These advancements could point to hydrometallurgy as the more sustainable and effective method for CAS treatment moving forward.
- Challenges of hybrid routes: Hybrid routes result in longer processing times—reported to be up to 45 days—where gold recovery occurs at later stages. These methods face challenges such as lower gold first-pass recovery rates due to losses in slag (which can contain 10–30% of the precious metal value), higher retention of gold within the process (in the form of gold anodes, cathodes, and electrolytes containing 60–100 g/L of gold), and additional work-in-progress inventory. This can potentially add 30–90 days of gold in-process hold-up to the overall treatment time.
- Drawbacks of pyrometallurgy: Pyrometallurgical routes, often used as intermediate steps in hybrid processes, are associated with higher operational and environmental costs, significant emissions, and reduced efficiency in precious metal recovery compared to purely hydrometallurgical methods.
- Improvements in hybrid routes: Various enhancements have been implemented in hybrid routes to increase gold first-pass recovery rates and reduce hold-up time. Notable improvements include the adoption of high-pressure leaching for copper removal, replacing gold electrorefining with leaching methods, and the implementation of more efficient high-temperature reactors. The bottom-blown oxygen converter, in particular, has emerged as the most efficient option, offering shorter production cycle times (18 h), lower gold loss, and up to 100% oxygen utilization, significantly enhancing energy efficiency. Therefore, the most viable hybrid route appears to be the combination of high-pressure Cu leaching, bottom-blown oxygen converting, and gold leaching.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Refinery | Country | Composition (wt%) | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu * | Se | Te | Ag | Au | Pt | Pd | Ni | As | Bi | Sb | Sn | Pb | Fe | Zn | |||
| Noranda | Canada | 18.7 | 10 | 1.2 | 19.5 | 0.18 | - | - | - | 1.14 | 0.77 | 1.68 | - | 8 | - | - | [13] |
| Chuquicamata | Chile | 27 | 4 | - | 12 | 0.07 | - | - | - | 5 | - | 4 | - | - | - | - | [11] |
| Hibi Kyodo, Tamano | Japan | 21.5 | - | - | 9.6 | 0.5 | - | - | - | - | - | - | - | 22.7 | - | - | [13] |
| IMI Refinery | UK | 14 | 2 | 0.6 | 5.5 | 0.07 | 0 | 0.01 | 9 | 3.5 | 0.5 | 3.5 | 5 | 22 | - | - | |
| INCO | USA | 21 | 8.4 | 1.8 | 6.37 | 0.12 | - | - | 17 | 5 | 0.14 | 0.9 | - | 1.7 | - | - | |
| Indian Copper Complex | India | 12.3 | 10.5 | 3.38 | 1.54 | 0.1 | - | - | 36.8 | 0.04 | - | 0.01 | - | 0.16 | 0.3 | - | [11] |
| Kidd Creek | USA | 26 | 19.5 | 0.5 | 12.7 | 0.15 | - | - | 0.03 | 0.45 | 0.28 | 0.09 | - | 22 | - | - | [13] |
| Phelps Dodge | USA | 27.1 | 8.8 | 3.1 | 12.2 | 0.12 | 0.01 | 0.01 | 0.65 | 1.7 | - | 0.66 | - | 4.65 | 0.1 | - | |
| Sukuysan | Turkey | 26.7 | 7.6 | 2.5 | 4.73 | 0.1 | - | - | 0.82 | 3.6 | - | 1.3 | - | 1.7 | - | - | |
| Southwire | Georgia | 10 | 0.6 | - | 4.1 | 0.02 | 0.08 | 0.35 | 8.4 | 1.4 | 0.5 | 9.4 | 5.3 | 13.1 | - | - | |
| Freeport, Atlantic Copper | Spain | 25.4 | 8.23 | 1.27 | 14.54 | 0.382 | - | - | 0.45 | 3.41 | 0.76 | 33 | - | 2.89 | - | - | [14] |
| Outukumpu | Finland | 8.5 | 46 | - | 16.8 | 0.3 | - | - | 5.7 | - | - | - | - | 8 | - | - | [11] |
| Saganoseki | Japan | 30 | 12.5 | 22 | 7 | 1 | - | - | - | - | - | - | - | 1 | - | - | [13] |
| Balkhash | Kazakhstan | 3.9 | 6.6 | 1.2 | 20 | 1.2 | - | - | - | - | - | 19 | - | 11.9 | - | - | [14] |
| La Caridad | Mexico | 12.7 | 4.2 | 0.37 | 15.4 | 0.04 | - | - | 0 | 5 | 1.2 | 5 | - | 14.8 | - | - | |
| Jinchuan | China | 17.9 | 4.65 | 1.35 | 11.05 | 2.18 | - | - | 1.46 | 2.28 | 0.66 | 1.93 | - | 9.79 | - | - | |
| Baiyin | China | 34.5 | 13.2 | 0.62 | 8.33 | 0.27 | - | - | - | - | - | - | - | 3.41 | - | - | |
| Ronnskar | Sweden | 15.7 | 9.69 | 5.17 | 22.95 | 0.578 | - | - | 5.09 | 0.94 | 3.21 | 0.78 | - | 9.69 | - | - | |
| Daye Nonferrous | China | 11.9 | 5.22 | 0.58 | 10.45 | 0.21 | 0.03 | - | - | 4.1 | - | 5.09 | 1.01 | 16.2 | - | - | [13] |
| Yunnan | China | 17.8 | 8.9 | 3.17 | 22.25 | 0.37 | - | - | 0.97 | 6.8 | 2.55 | 8.52 | 0.81 | 18.6 | - | - | |
| Jiangxi | China | 18.4 | 10 | - | 4.81 | 0.208 | - | - | - | - | - | - | - | 5.94 | - | - | |
| El Salvador | Chile | 5 | 21 | - | 24 | 1.4 | - | - | - | 0.7 | - | 3 | - | - | - | - | [10] |
| Fundición Hernán Videla | Chile | 19.8 | 3.9 | 0.77 | - | 7.74 | - | - | 0.43 | 1.51 | 0.19 | 0.06 | - | 32.2 | 0.3 | 0.1 | |
| El Teniente | Chile | 28.8 | 9.3 | 0.14 | - | 0.47 | - | - | 0.12 | 9.2 | 0.29 | 8.06 | - | 1.76 | 0.1 | 0 | |
| Ventanas | Chile | 12.1 | 3.14 | 0.82 | - | 11.34 | - | - | 0.68 | 2 | 0.12 | 0.18 | - | 23.3 | 0.2 | 0.1 | |
| Ventanas | Chile | 24.3 | 7.9 | 0.8 | 14.77 | 5.4 | - | - | 0.1 | 6.2 | 0.3 | 5.5 | - | 8.1 | 0.1 | - | |
| Potrerillos | Chile | 7.8 | 8.65 | 0.66 | 15.42 | 0.47 | - | - | 0.02 | 9.22 | 0.41 | 10.5 | - | 1.16 | 0.2 | - | |
| Northern | Chile | 1.49 | - | - | 5 | 0.2 | - | - | - | 0.1 | - | - | - | 0.05 | 10 | 0 | |
| ER&S | Australia | 13 | 5.8 | 0.2 | 9 | 0.1 | 0.09 | 2 | 1.2 | 0.3 | 3 | 5 | 31 | - | - | [16] | |
| Palabora | Africa | 53.4 | 3.6 | 2.2 | 7.8 | 0.33 | 0.05 | 0.07 | 4.5 | 0.15 | 0.01 | - | - | - | - | - | |
| Minero | Peru | 41 | 11 | 1.1 | 20 | 0.04 | - | - | - | - | - | - | - | - | - | - | |
| Rabak | Turkey | 24.7 | 7.9 | 3 | 4.8 | 0.11 | 0 | 0 | 0.05 | 2.3 | 0.5 | - | - | - | - | - | |
| Sarkuysan | Turkey | 26.7 | 7.6 | 2.5 | 4.73 | 0.1 | - | - | 0.82 | 3.6 | - | 1.3 | - | 1.7 | - | - | |
| Codelco | Chile | 2.53 | 11.5 | 0.73 | 32.9 | 0.17 | - | - | - | 5 | 0.13 | 10.5 | - | 0.71 | - | - | [17] |
| Townsville | Australia | 21 | 3 | 0.5 | 6.1 | 0.6 | - | - | 0.3 | 3.9 | 0.7 | 0.6 | - | 9.8 | - | - | [18] |
| Amarillo | USA | 19.6 | 14.1 | 1.4 | 18.6 | 0.1 | - | - | 0.7 | 2.9 | 0.4 | 2.9 | - | 0.1 | - | - | |
| Kayserie | Turkey | 18 | 1 | 0.4 | 1.5 | 0.08 | - | - | 0.2 | 0.3 | 0.04 | 0.4 | - | 16.9 | - | - | |
| Boliden | Sweden | 15.7 | 5.2 | 0.9 | 22.9 | 0.6 | - | - | 5.1 | 2.5 | 0.8 | 16 | - | 9.7 | - | - | |
| CCR | Canada | 15 | 5 | 2 | 29 | 1.4 | - | - | 3.8 | 1.5 | 1.5 | 4.5 | - | 16 | - | - | |
| Bahia | Brazil | 4.4 | 11.5 | 2.6 | 7.6 | 0.4 | - | - | 0.1 | 3.8 | 0.7 | 4.1 | - | 6.7 | - | - | |
| El Paso | USA | 1 | 20 | 0.4 | 22 | 0.2 | - | - | 0.05 | 2 | 0.7 | 4 | - | 5 | - | - | |
| Luenen | Germany | 1 | 0.7 | 0.7 | 8 | 0.2 | - | - | 1 | 2.5 | 0.4 | 9 | - | 30 | - | - | |
| Potrerillios | Chile | 7.8 | 8.6 | 0.7 | 15.4 | 0.5 | - | - | 0.02 | 9.2 | 0.4 | 10.4 | - | 1.2 | - | - | |
| Sarcheshmeh | Iran | 7.4 | 15.3 | 0.7 | 8 | 0.2 | - | - | 0.6 | 0.04 | 1.9 | - | 4.3 | ||||
| Montanwerke | Austria | 1 | 0.2 | - | - | - | - | - | - | 0.8 | 1.5 | 3 | - | - | - | - | [19] |
| Metallo Chimique | Belgium | 19 | 0.5 | 0.5 | - | - | - | - | - | 2.5 | 1.5 | 16 | - | - | - | - | |
| Aurubis Bulgaria | Bulgaria | 24.3 | 9.4 | 1.4 | - | - | - | - | - | 4.7 | 2.4 | 2.5 | - | - | - | - | |
| Glencore CCR | Canada | 16.3 | 6.1 | 2.1 | - | - | - | - | - | 2.1 | 1.2 | 1.9 | - | - | - | - | |
| Las ventanas | Chile | 1.54 | 13.4 | 0.8 | - | - | - | - | - | 3.4 | 0.6 | 9.7 | - | - | - | - | |
| Jinlong Copper | China | 25 | 10 | 2 | - | - | - | - | - | 2 | 1 | 1 | - | - | - | - | |
| Xianguang | China | 11.5 | 3.5 | 2 | - | - | - | - | - | 4.5 | 8 | - | - | - | - | - | |
| Aurubis Hamburg | Germany | 15.5 | 6 | 1.5 | - | - | - | - | - | 2.5 | 1.5 | 4 | - | - | - | - | |
| Aurubis Luenen | Germany | 1 | 0.7 | 0.7 | - | - | - | - | - | 5 | 0.4 | 12 | - | - | - | - | |
| PT Smelting | Indonesia | 0.5 | 9.7 | 0.4 | - | - | - | - | - | 1.6 | 3.4 | 0.5 | - | - | - | - | |
| Hibi Kyodo Smelting | Japan | 32.7 | 6.8 | 1.5 | - | - | - | - | - | 4 | 0.2 | 0.4 | - | - | - | - | [19] |
| Mitsubishi | Japan | 14.5 | 5 | 2 | - | - | - | - | - | 3.4 | 2.2 | 2 | - | - | - | - | |
| Onahama | Japan | 25.5 | 9.2 | 1.4 | - | - | - | - | - | 5.3 | 4.4 | 3.7 | - | - | - | - | |
| LS-Nikko | Korea | 20.1 | 15.6 | 2.6 | - | - | - | - | - | 3.2 | 0.9 | 0.9 | - | - | - | - | |
| LS-Nikko | Korea | 16.4 | 10.9 | 2 | 2.3 | 1.4 | 1.6 | - | - | - | - | ||||||
| Mexicana de Cobre | Mexico | 0.4 | 7.8 | 2.7 | - | - | - | - | - | 1.4 | 2.5 | 9.8 | - | - | - | - | |
| Southern Peru Copper | Peru | 2 | 20 | 1.5 | - | - | - | - | - | 6 | 15 | 3 | - | - | - | - | |
| Kyshtym Copper | Russia | 11.8 | 3.5 | 0.7 | - | - | - | - | - | 1.7 | 0.2 | 4.4 | - | - | - | - | |
| Uralelectromed | Russia | 22 | 7 | 4 | - | - | - | - | - | 4 | - | 10 | - | - | - | - | |
| Atlantic Copper | Spain | 10.5 | 10.5 | 1 | - | - | - | - | - | 1 | 0.5 | 1 | - | - | - | - | |
| Boliden | Sweden | 14 | 2.9 | 2.4 | - | - | - | - | - | 2.4 | 0.81 | 4.7 | - | - | - | - | |
| Kennecott Utah Copper | USA | 25 | 7.5 | 1 | - | - | - | - | - | 5 | 5 | 0.3 | - | - | - | - | |
| Asarco | USA | 2.2 | 23.2 | 0.8 | - | - | - | - | - | 3.4 | 6.8 | 2 | - | - | - | - | |
| Freeport-McMoRan | USA | 1 | 19 | 0.4 | - | - | - | - | - | 3 | 0.7 | 5 | - | - | - | - | |
| Olympic Dam | Australia | 35 | 15 | 3 | 15 | 0.75 | - | - | - | - | - | - | - | 2 | - | - | [20] |
| - | South Africa | 26.4 | 0.53 | 0.44 | 3.39 | 0.21 | 0.01 | 0.01 | 0.81 | 0.04 | 0.01 | - | - | 0.05 | - | - | [21] |
| - | South Africa | 26.5 | 0.68 | 0.53 | 3.81 | 0.19 | - | - | 0.41 | 0.03 | - | - | - | 0.08 | - | - | |
| - | South Africa | 31.5 | 0.69 | 0.57 | 3.17 | 0.2 | - | - | 0.33 | 0.03 | - | - | - | - | - | - | |
| - | South Africa | 27 | 0.54 | 0.59 | 3.4 | 0.22 | 0.01 | 0.01 | 0.35 | 0.02 | 0.1 | - | - | 0.11 | - | - | |
| - | South Africa | 29 | 0.61 | 0.42 | 3.38 | 0.21 | 0.01 | 0.01 | 0.19 | 0.01 | 0.01 | - | - | 0.15 | - | - | |
| Hitachi (1969) | Japan | 8.9 | 2.5 | 1.8 | 20.6 | 0.26 | - | - | - | - | - | - | - | 17.8 | - | - | [22] |
| Hitachi (1970) | Japan | 9.4 | 2.9 | 1.6 | 19.7 | 0.29 | - | - | - | - | - | - | - | 17.3 | - | - | |
| Hitachi (1971) | Japan | 9.6 | 3.4 | 1.1 | 20.8 | 0.39 | - | - | - | - | - | - | - | 25.4 | - | - | |
| Hitachi (1974) | Japan | 24.2 | 10.6 | 1.2 | 13.9 | 0.8 | - | - | - | - | - | - | - | 6.4 | - | - | |
| Hitachi (1975) | Japan | 23 | 10.2 | 2 | 12.9 | 0.92 | - | - | - | - | - | - | - | 7.7 | - | - | |
| La Caridad | Mexico | 12.7 | 4.2 | 0.37 | 15.4 | 0.036 | - | - | 0 | 5 | 1.2 | 5 | - | 14.8 | 2.1 | 0.8 | [5] |
| BGMK | Kazakhstan | 3 | 6.6 | 1.2 | 20 | 1.2 | - | - | - | 5.3 | - | 19 | - | 11.9 | - | - | |
| Olympic Dam | Australia | 30 | 14.9 | 3.5 | 9.9 | 1.19 | - | - | - | 3.9 | 2.5 | - | - | 12.3 | - | - | [5] |
| Port Kembla | Australia | 29 | 8 | 0.9 | 3.1 | 1 | - | - | - | 1.6 | 2.5 | 0.5 | - | 22 | - | - | |
| Townsville | Australia | 21 | 3 | 0.5 | 6.1 | 0.63 | - | - | 0.3 | 3.9 | 0.7 | 0.6 | - | 9.8 | 0 | - | |
| Bahia | Brazil | 4.38 | 11.5 | 2.6 | 7.59 | 0.4 | - | - | 0.1 | 3.8 | 0.7 | 4.1 | - | 6.7 | - | - | |
| Med | Bulgaria | 25.3 | 9.25 | 0.83 | 4.86 | 0.49 | - | - | 0.14 | 2.96 | 0.18 | 1.16 | - | 10.3 | 0.1 | - | |
| Timmins | Canada | 17 | 8.4 | 0.7 | 23 | 0.26 | - | - | 0.9 | 2.3 | 0.8 | 0.9 | - | 23 | - | - | |
| CCR | Canada | 15 | 5 | 2 | 29 | 1.4 | - | - | 3.8 | 1.5 | 1.5 | 4.5 | - | 16 | - | - | |
| Portrerillos | Chile | 7.8 | 8.65 | 0.66 | 15.42 | 0.47 | - | - | 0.02 | 9.22 | 0.41 | 10.5 | - | 1.16 | 0.2 | - | |
| Las Ventanas | Chile | 24.3 | 7.9 | 0.8 | 14.77 | 5.4 | - | - | 0.1 | 6.2 | 0.3 | 5.5 | - | 8.1 | 0.1 | - | |
| Gresik | Indonesia | 0.6 | 11.1 | 0.2 | 4.9 | 2.1 | - | - | 0 | 1.2 | 2.8 | 0.3 | - | 51.4 | 0.1 | - | |
| Saganoseki | Japan | 25.2 | 12.1 | 3.9 | 19 | 2.1 | - | - | 0.4 | 3.3 | 0.7 | 1.8 | - | 4 | 0.2 | - | |
| Nishibara | Japan | 21.4 | 6.9 | 1.7 | 10.8 | 1.4 | - | - | 0.7 | 3.3 | 1.4 | 1.7 | - | 14 | - | - | |
| Almalyk | Uzbekistan | 29 | 9 | 11 | - | - | - | - | - | 4 | 0.2 | 2 | - | 24 | 0.1 | - | |
| Amarillo | USA | 19.6 | 14.1 | 1.45 | 18.6 | 0.12 | - | - | 0.74 | 2.91 | 0.44 | 2.96 | - | 0.15 | - | - | |
| El Paso | USA | 1 | 20 | 0.4 | 22 | 0.2 | - | - | 0.05 | 2 | 0.7 | 4 | - | 5 | 0 | - | |
| Kennecott | USA | 30 | 5 | 1 | 5 | 0.5 | - | - | 0.05 | 5 | 3 | 1 | - | 30 | 0.3 | - | |
| Kayserie | Turkey | 18 | 1 | 0.4 | 1.5 | 0.08 | - | - | 0.2 | 0.3 | 0.04 | 0.4 | - | 16.9 | - | - | |
| Lunen | Germany | 1 | 0.7 | 0.7 | 8 | 0.16 | - | - | 1 | 2.5 | 0.4 | 9 | 9.5 | 30 | - | - | |
| Beerse | Belgium | 19 | 0.5 | 0.5 | 5.5 | 0.06 | - | - | - | 2.5 | 1.5 | 16 | - | 4 | - | - | |
| Boliden | Sweden | 15.7 | 5.17 | 0.94 | 22.95 | 0.578 | - | - | 5.09 | 3.21 | 0.78 | 4.15 | - | 9.69 | - | - | |
| Montanwerke | Austria | 7 | 0.3 | 0.59 | 8.751 | 0.125 | - | - | 10.9 | - | 0.41 | 3.8 | 12.1 | 24.6 | - | - | |


References
- Dabkowski, A.; Yusheng, L. The World Copper Factbook; International Copper Study Group: Lisbon, Portugal, 2024. [Google Scholar]
- NavInfo Co., Ltd. Chinese Smelter Landscape. 2024. Available online: https://en.navinfo.com/ (accessed on 15 February 2024).
- Tripathi, N. Personal Communication; Moosavi-Khoonsari, E., Ed.; Rio Tinto: Singapore, 2024. [Google Scholar]
- Barr, G.; Grieve, W.; Jones, D.; Mayhew, K. The New CESL gold Process; ALTA: Perth, WA, Australia, 2007. [Google Scholar]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Alvear Flores, G.R.F. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Zeng, Y.L.; Liao, C.; Liu, F.; Zhou, X. Occurrence Behaviors of As/Sb/Bi in Copper Anode Slime and Their Separation by Compound Leaching Followed by Stepwise Precipitation. ACS Omega 2023, 8, 10022–10029. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, S.; Liu, B.; Li, B. Integrated process for recycling copper anode slime from electronic waste smelting. J. Clean. Prod. 2017, 165, 48–56. [Google Scholar] [CrossRef]
- Wang, S.; Cui, W.; Zhang, G.; Zhang, L.; Peng, J. Ultra fast ultrasound-assisted decopperization from copper anode slime. Ultrason. Sonochem. 2017, 36, 20–26. [Google Scholar] [CrossRef]
- Chen, A.; Peng, Z.; Hwang, J.-Y.; Ma, Y.; Liu, X.; Chen, X. Recovery of Silver and Gold from Copper Anode Slimes. JOM 2015, 67, 493–502. [Google Scholar] [CrossRef]
- Santana Barros, K.; Schaeffer Vielmo, V.; Garrido Moreno, B.; Riveros, G.; Cifuentes, G.; Moura Bernardes, A. Chemical Composition Data of the Main Stages of Copper Production from Sulfide Minerals in Chile: A Review to Assist Circular Economy Studies. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- Hait, J.; Jana, R.K.; Sanyal, S.K. Processing of copper electrorefining anode slime: A review. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. Sect. C 2009, 118, 240–252. [Google Scholar] [CrossRef]
- Xing, W.D.; Sohn, S.H.; Lee, M.S. A Review on the Recovery of Noble Metals from Anode Slimes. Miner. Process. Extr. Metall. Rev. Int. J. 2020, 41, 130–143. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y.; Tang, A.; Pan, D.; Li, B. Recovery of scattered and precious metals from copper anode slime by hydrometallurgy: A review. Hydrometallurgy 2020, 197, 105460. [Google Scholar] [CrossRef]
- Lee, J.C.; Kurniawan, K.; Chung, K.W.; Kim, S. Metallurgical Process for Total Recovery of All Constituent Metals from Copper Anode Slimes: A Review of Established Technologies and Current Progress. Met. Mater. Int. 2021, 27, 2160–2187. [Google Scholar] [CrossRef]
- Moosavi-Khoonsari, E.; Tripathi, N. Gold Recovery from Smelting Copper Sulfide Concentrate. Preprints 2024, 2024091473. [Google Scholar]
- Cooper, W.C. The Treatment of Copper Refinery Anode Slimes. JOM 1990, 42, 45–49. [Google Scholar] [CrossRef]
- Devia, M.; Luraschi, A. A Study of the Smelting and Refining of Anode Slimes to Dore Metal. In Proceedings of the Pyrometallurgy of Copper, an International Symposium, Ottawa, ON, Canada, 18–21 August 1991; pp. 209–226. [Google Scholar]
- Mahmoudi, A.; Shakibania, S.; Mokmeli, M.; Rashchi, F. Tellurium, from Copper Anode Slime to High Purity Product: A Review Paper. Metall. Mater. Trans. B 2020, 51B, 2020–2555. [Google Scholar] [CrossRef]
- Moats, M.; Alagha, L.; Awuah-Offei, K. Towards resilient and sustainable supply of critical elements from the copper supply chain: A review. J. Clean. Prod. 2021, 307, 127207. [Google Scholar] [CrossRef]
- Hall, S. Gold and Silver Recovery from Copper Anode Slimes at the Olympic Dam Joint Venture, Roxby Downs, SA. Aust. Min. Metall. Monogr. Ser. 1993, 19, 1102–1105. [Google Scholar]
- Nkuna, N.H.; Popoola, A.P.I. Effect of Chloride Electrolyte Additive on the Quality of Electrorefined Copper Cathode. Procedia Manuf. 2019, 35, 789–794. [Google Scholar] [CrossRef]
- Kurokawa, H. New precious metal refining process development at Sumitomo Metal Mining Co., Ltd. Min. Mater. Process. Inst. Jpn. 2018, 134, 74–80. [Google Scholar] [CrossRef][Green Version]
- Furuzono, T.; Fujimoto, A.; Takeuchi, T.; Takebayashi, K. Unique Hydrometallurgical Process for Copper-Anode Slime Treatment at Saganoseki Smelter and Refinery. In Extraction 2018; Springer: Cham, Switzerland, 2018; pp. 2699–2710. [Google Scholar]
- Nexhip, C.; Crossman, R.; Rockandel, M. By-Products Recovery via Integrated Copper Operations at Rio Tinto Kennecott. In Proceedings of the Exchange of Good Practices on Metal by-Products Recovery, Brussels, Belgium, 12–13 November 2015. [Google Scholar]
- Ferron, C.J. Recovery of gold as by-product from the base-metals industries. Dev. Miner. Process. 2005, 15, 861–896. [Google Scholar]
- Hoffmann, J.E.; Wesstrom, B. Hydrometallurgical processing of refinery slimes at Phelps Dodge: Theory to practice. In Hydrometallurgy; Springer: Dordrecht, The Nerthland, 1994; pp. 69–105. [Google Scholar]
- Sato, J.; Imamura, T.; Hojo, M.; Suzuki, T. New process for copper anode slime treatment at the Hitachi Smelter & Refinery. In World Mining and Metals Technology; Weiss, A., Ed.; AIME: Warrendale, PA, USA, 1976; Volume 2, pp. 585–598. [Google Scholar]
- Maeda, Y. Recent Operations at Hitachi Refinery. J. MMIJ 2007, 123, 605–607. [Google Scholar] [CrossRef]
- Ziegler, C.; Bryson, L. The Role of R&D in the Makeover of the Precious Metals Refinery at Aurubis. In Proceedings of the 63rd Annual Conference of Metallurgists (COM 2024), Halifax, NS, Canada, 19–22 August 2024; Springer: Cham, Swizerland, 2024. [Google Scholar]
- Ludvigsson, B.M.; Larsson, S.R. Anode slimes treatment: The boliden experience. JOM 2003, 55, 41–44. [Google Scholar] [CrossRef]
- Bäckström, J. Copper, Nickel and Tellurium Yields During Leaching of Anode Slime. Master’s Thesis, Lulea University of Technology, Department of Chemical Engineering and Geosciences, Lulea, Sweden, 2010. [Google Scholar]
- Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; King, S.; Liebezeit, V.; Slattery, A.D. Detailed Characterisation of Precious Metals and Critical Elements in Anode Slimes from the Olympic Dam Copper Refinery, South Australia. Miner. Eng. 2024, 206, 108539. [Google Scholar] [CrossRef]
- Knight, R.P. Further Applications of the Bottom Blown Oxygen Converter. In Internation Symposium on Injection in Process Metallurgy; Lehner, P.K.T., Ramachandran, V., Eds.; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1991. [Google Scholar]
- Navarro, L.G.; Morris, T.; Read, W.; Parameswaran, K. Metal Sustainability from a Manufacturing Perspective: Initiatives at ASARCO LLC Amarillo Copper Refinery; Izatt, R.M., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 397–423. [Google Scholar]
- Dong Xing, W.; Seung Lee, M. Leaching of Gold and Silver from Anode Slime with a Mixture of Hydrochloric Acid and Oxidizing Agents. Geosystem Eng. 2017, 20, 216–223. [Google Scholar] [CrossRef]
- Saeedi, M.; Keshavarz Alamdari, E.; Darvishi, D.; Keshavarz Alamdari, A.; Haghshenas Fatmehsari, D.; Bagheri kafash, A. Chlorine Leaching of Au from Copper Anode Slimes Using the Response Surface Method in an Agitated Reactor. Miner. Process. Extr. Metall. (Trans. Inst. Min Metall. C) 2015, 124, 9–15. [Google Scholar] [CrossRef]
- Dehghanpoor, M.H.; Zivdar, M.; Torabi, M. Extraction of Copper and Gold from Anode Slime of Sarcheshmeh Copper Complex. J. South. Afr. Institue Min. Metall. 2016, 116, 1153–1157. [Google Scholar] [CrossRef]
- Bae, M.; Lee, J.-C.; Lee, H.; Kim, S. Recovery of nitric acid and gold from gold-bearing aqua regia by tributyl phosphate. Sep. Purif. Technol. 2020, 235, 116154. [Google Scholar] [CrossRef]
- Sadeghi, N.; Keshavarz Alamdari, E. Selective Extraction of Gold (III) from Hydrochloric Acid−Chlorine Gas Leach Solutions of Copper Anode Slime by Tri-butyl Phosphate (TBP). Trans. Nonferrous Met. Soc. China 2016, 26, 3258–3265. [Google Scholar] [CrossRef]
- Becker, E. Modernisation of Precious Metals Refining at Norddeutesche Affinerie AG. World Metall.-Erzmetall 2006, 59, 87–94. [Google Scholar]
- Mahyapour, H.; Mohammadnejad, S. Optimization of the Operating Parameters in Gold Electro-Refining. Miner. Eng. 2022, 186, 107738. [Google Scholar] [CrossRef]
- Shkodin, V.G.; Malyshev, V.P.; Evtyukhova, O.V.; Malkov, V.E.; Galimova, S.A. Roasting-sintering of anode slimes with soda in shaft furnaces. Chem. Abstr. 1970, 72. [Google Scholar]
- Morrison, B.H.; Lenz, J.G.; Pageau, J.; Bard, J.G. Treatment of Anode Slimes in a Top Blown Rotary Converter. U.S. Patent 4,581,064, 8 April 1986. [Google Scholar]
- Narita, H.; Kasuya, R.; Suzuki, T.; Motokawa, R.; Tanaka, M. Precious Metal Separations. Encycl. Inorg. Bioinorg. Chem. 2020. [Google Scholar]
- Hyvärinen, O.; Lindroos, L.; Yllö, E. Recovering Slenium from Copper Refinery Slimes. JOM 1989, 41, 42–43. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Yin, W.; Jiang, T.; Li, G. Thiosulfate leaching of Au, Ag and Pd from a high Sn, Pb and Sb bearing decopperized anode slime. Hydrometallurgy 2016, 164, 278–287. [Google Scholar] [CrossRef]
- BYJUS. Reverberatory Furnaces. Available online: https://byjus.com/chemistry/reverberatory-furnace/ (accessed on 24 October 2024).
- Griffin, M. Change from top-blown to bottom-blown converter for lead bullion cupellation at Rand Refinery. In Pyrometallurgy ’95; Institution of Mining and Metallurgy: London, UK, 1995; pp. 65–87. [Google Scholar]
- Knight, R.P. Oxidation refining in the Bottom Blown Oxygen Converter. Erzmetall 1995, 48, 530–537. [Google Scholar]
- Tomine-Tech-Coperation. Technologies for Copper Anode Slime Treatment (TBRC). Available online: https://topmie.com/solution/technologies-for-copper-anode-slime-treatment-tbrc/ (accessed on 24 October 2024).
- Metalcess. Top Blown Rotary Converter (TBRC)-Pyrometallurgy. Available online: www.metalcess.com/equipment/pyrometallurgy/Top_Blown_Rotary_Converter_TBRC_19_show.html (accessed on 10 July 2024).
- Segawa, C.; Kusakabe, T. CCurrent operations in SMM’s slime treatment. In EPD Congress 1996, Warren, G.W., Ed.; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 1995; pp. 43–52. [Google Scholar]
- Swinbourne, D.; Winters, A.; Giunti, M. Theory and practice of cupellation at Port Pirie Pasminco smelter. In Proceedings of the European Metallurgical Conference EMC 2001, Friedrichshafen, Germany, 18–21 September 2001; pp. 329–345. [Google Scholar]
- Barbante, G.G.; Swinbourne, D.R.; Rankin, W.J. Pyrometallurgical treatment of tank house slimes. In Pyrometallurgy for Complex Minerals & Wastes; Nilmani, T.L.M., Rankin, W.J., Eds.; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 1994; pp. 319–337. [Google Scholar]
- Okura, T.; Takebe, H. Sulfide Smelting Development in Japan During the Past Half Century. In Extraction 2018; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 71–79. [Google Scholar]
- Ximing, L.; Jiajun, K.; Xinhui, M.; Bin, L. Chlorine Leaching of Gold-Bearing Sulphide Concentrate and its Calcine. Hydrometallurgy 1992, 29, 205–215. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching in non-cyanide lixiviant systems: Critical issues on fundamentals and applications. Miner. Eng. 2004, 17, 785–801. [Google Scholar] [CrossRef]
- Han, J.; Li, X.; Dai, S. Electrochemical influence of quartz on cyanide leaching of gold. Chem. Phys. Lett. 2020, 739, 136997. [Google Scholar] [CrossRef]
- Jing-ying, L.; Xiu-li, X.; Wen-quan, L. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Li, G.; Jiang, T. Fluidized roasting-stage leaching of a silver and gold bearing polymetallic sulfide concentrate. Hydrometallurgy 2014, 147–148, 79–82. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yang, X.; Wang, S.; Zhang, L. Gold Leaching from a Refractory Gold Concentrate by the Method of Liquid Chlorination. In Rare Metal Technology, Neelameggham, N.R., Alam, S., Oosterhof, H., Jha, A., Dreisinger, D., Wang, S., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Gui, W.; Zhu, X.; Yang, Y. Selective enrichment of low concentration Au(III) from acidic chloride media by poly ionic liquid sorbent. RSC Adv. 2016, 6, 62336–62343. [Google Scholar] [CrossRef]
- Dönmez, B.; Sevim, F.; Þolak, S. A Study on Recovery of Gold from Decopperized Anode Slime. Chem. Eng. Technol. 2001, 24, 91–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Cobley, C.M.; Zeng, J.; Wen, L.-P.; Chen, J.; Xia, Y. Dissolving Ag from Au-Ag Alloy Nanoboxes with H2O2: A Method for both Tailoring the Optical Properties and Measuring the H2O2 Concentration. J. Phys. Chem. C 2010, 114, 6396–6400. [Google Scholar] [CrossRef]
- Ghobeiti Hasab, M.; Rashchi, F.; Raygan, S. Chloride–hypochlorite leaching and hydrochloric acid washing in multi-stages for extraction of gold from a refractory concentrate. Hydrometallurgy 2014, 142, 56–59. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate Leaching of Gold—A Review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Aazami, M.; Lapidus, G.T.; Azadeh, A. The effect of solution parameters on the thiosulfate leaching of Zarshouran refractory gold ore. Int. J. Miner. Process. 2014, 131, 43–50. [Google Scholar] [CrossRef]
- Chauhan, G.; Jadhao, P.R.; Pant, K.K.; Nigam, K.D.P. Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: Challenges & opportunities—A review. J. Environ. Chem. Eng. 2018, 6, 1288–1304. [Google Scholar]
- Ding, Y.; Zhang, S.; Liua, B.; Zheng, H.; Chang, C.-c.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Ranjbar, R.; Naderi, M.; Omidvar, H.; Amoabediny, G. Gold recovery from copper anode slime by means of magnetite nanoparticles (MNPs). Hydrometallurgy 2014, 143, 54–59. [Google Scholar] [CrossRef]
- Schulze, R.G. New Aspects in Thiourea Leaching of Precious Metals. JOM 1984, 36, 62–65. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Khaleghi, A.; Ghader, S.; Afzali, D. Ag recovery from copper anode slime by acid leaching at atmospheric pressure to synthesize silver nanoparticles. Int. J. Min. Sci. Technol. 2014, 24, 251–257. [Google Scholar] [CrossRef]
- Hoh, Y.C.; Chang, C.C.; Chuang, W.S.; Lee, B.D.; Tai, J.J.; Feng, W.L.; Ma, T.; Ko, C.S.; Chen, W.L. INER process for recovering precious metals from copper refinery anode slime. In Hydrometallurgy Research, Development and Plant Practice; Osseo-Asare, K., Miller, J.D., Eds.; TMS/AIME (The Minerals, Metals & Materials Society/American Institute of Mining, Metallurgical, and Petroleum Engineers): Warrendale, PA, USA, 1983; pp. 151–163. [Google Scholar]
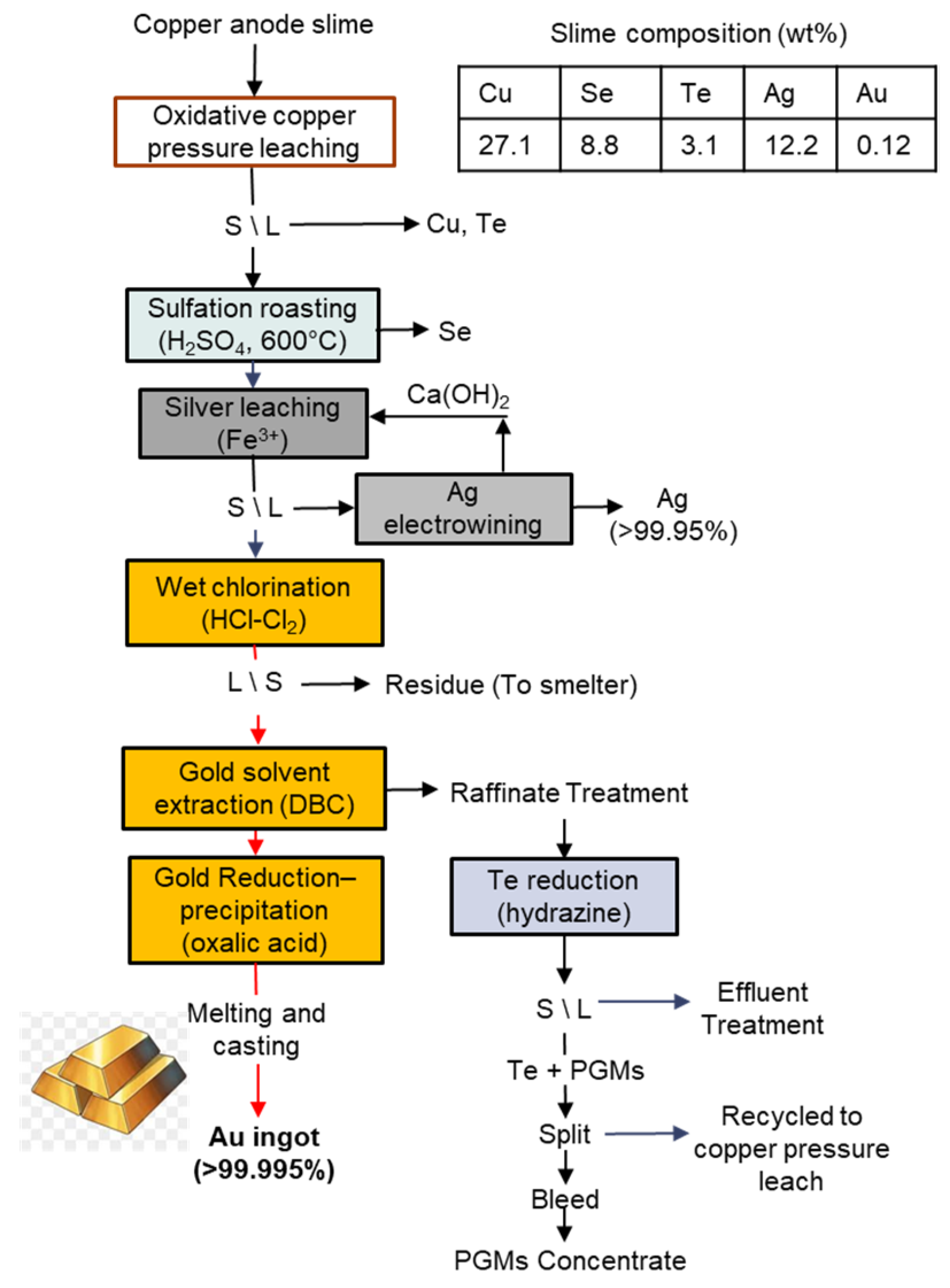

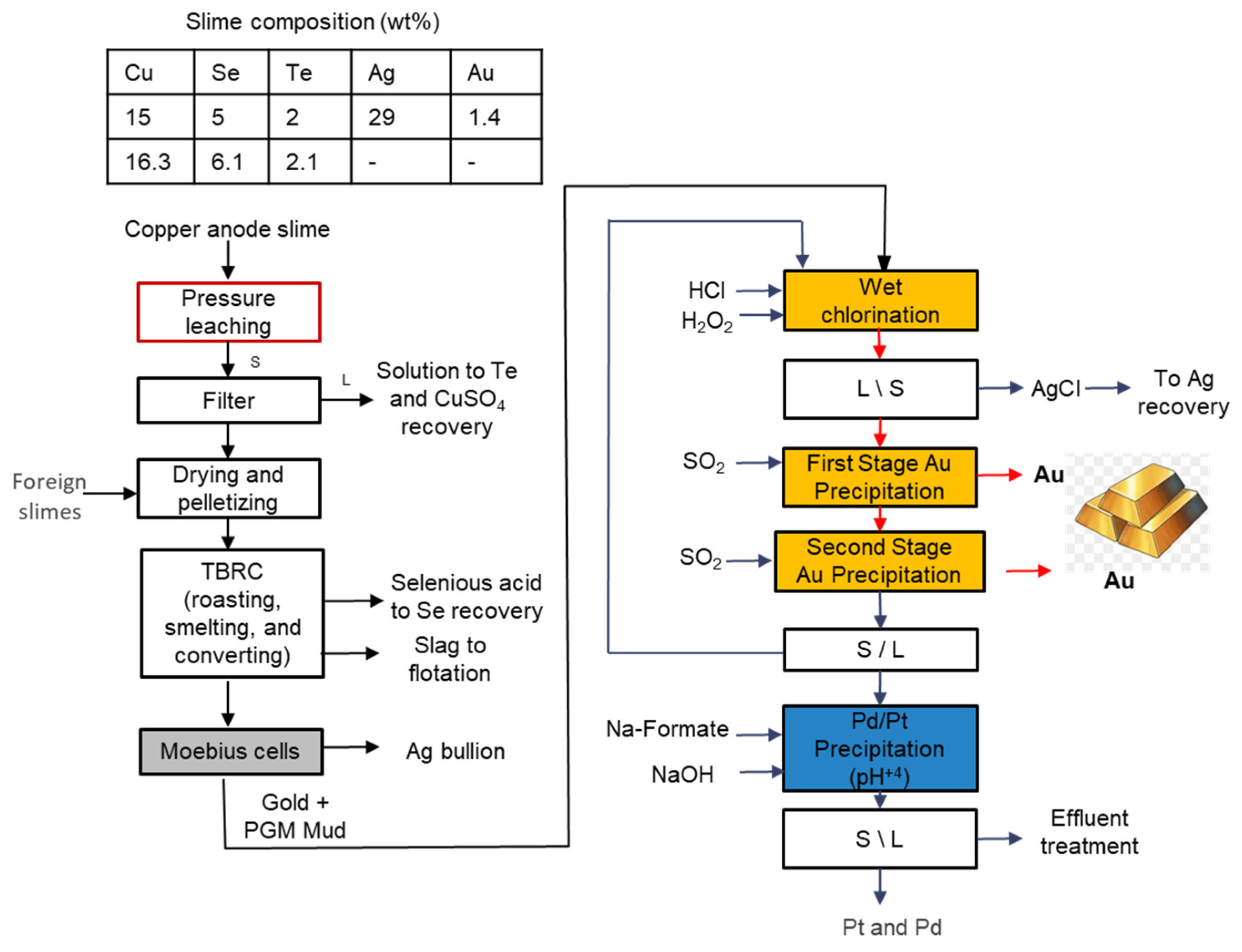
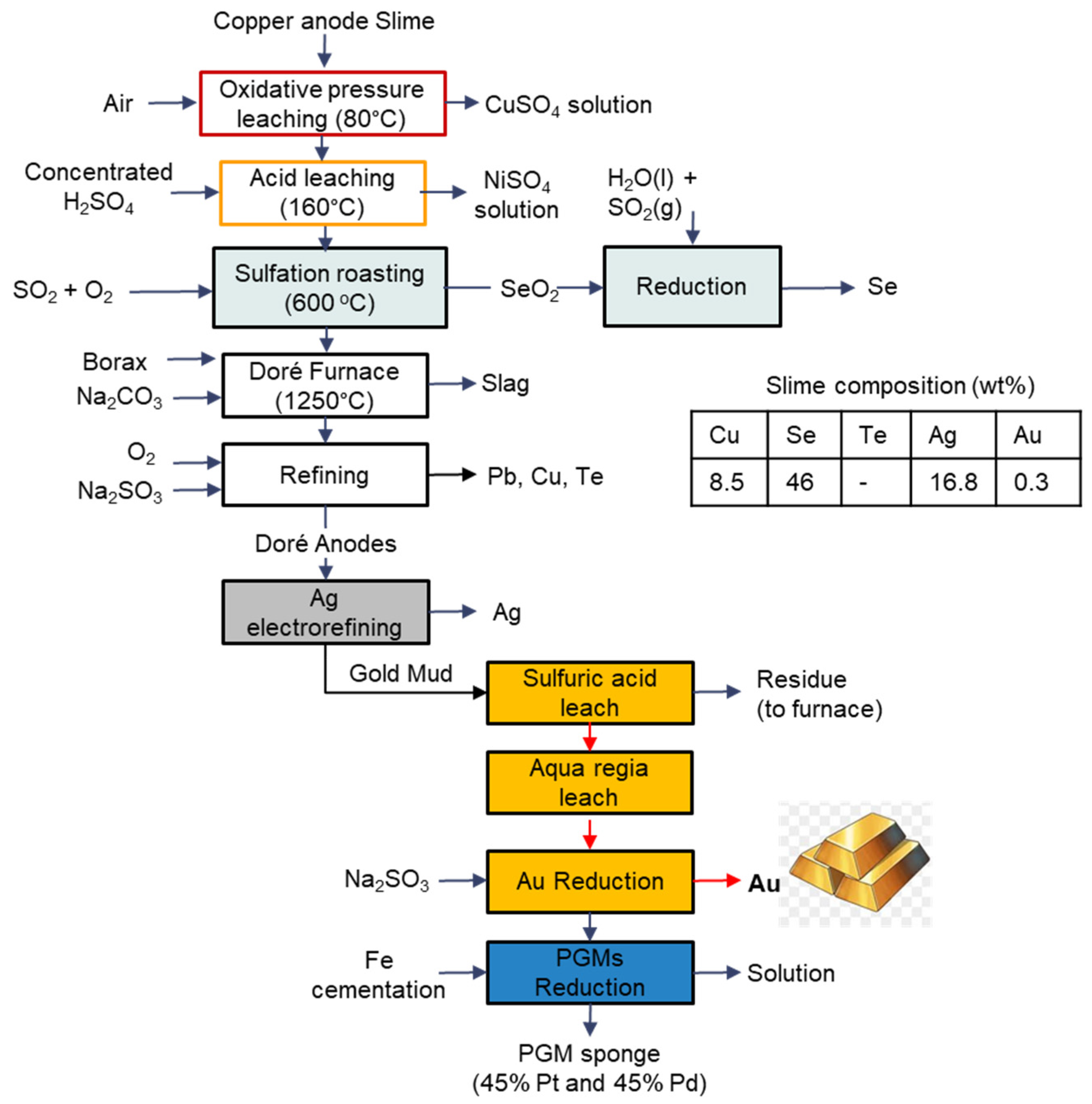
| Feature/Technology | Reverberatory Doré Furnace | Short Rotary Furnace | TBRC * | BBOC ** |
|---|---|---|---|---|
| Production cycle time | 7–20 days | Medium (multiple vessels) | Shorter than doré furnaces (30 h) | Short (18 h) |
| Gold in-process hold-ups and losses | High (large inventory) | High (large inventory) | Moderate (better design) | Lower than TBRC |
| PM first-pass recovery rate | 70%, 92.5% | - | - | 95%, 97.5% |
| Capacity | High | Medium | High (over 2 kt/year) | Medium |
| Operational costs | Low | Higher than reverberatory | Moderate (efficiency gains) | Lower due to efficiency |
| Initial investment | Moderate–High | Moderate | High | Moderate |
| Oxygen utilization | Low | Moderate | Moderate (60%) | High (up to 100%) |
| Energy efficiency | Low | Moderate | Higher than reverberatory but lower than BBOC (<60%) | High |
| Feature/Aspect | Hybrid Routes | Hydrometallurgy Routes |
|---|---|---|
| Gold separation step | End of flow sheet | Initial stage of flow sheet [22,23,24,25,26] |
| Total processing time | Long: up to 45 days [22,34] | Faster (7–8 days) [22,23,46] |
| Hold-up in process | High: PM recirculation in slag and high inventory in electrorefining [5,26,29,33,41] | Low |
| First-pass recovery rate | Not reported but could be low due to internal recirculation [26] | 99% (Au) and 98% (Ag) [11] |
| Pollution | High: flue gases, dust [5,22,26,30,46] | Low: minimal workplace emissions, recyclable organics and acids [11,46] |
| Energy consumption | High: significant energy use [11,75] | Lower: approximately 11% more efficient than pyro processes [11,75] |
| Cost considerations | High operating and environmental costs [22,30,46] | Lower treatment and investment costs [30,46,50] |
| Capacity | Large-scale operations [34] | Suitable for smaller capacities (<2 kt/year) [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosavi-Khoonsari, E.; Tripathi, N. Copper Anode Slime Processing with a Focus on Gold Recovery: A Review of Traditional and Recent Technologies. Processes 2024, 12, 2686. https://doi.org/10.3390/pr12122686
Moosavi-Khoonsari E, Tripathi N. Copper Anode Slime Processing with a Focus on Gold Recovery: A Review of Traditional and Recent Technologies. Processes. 2024; 12(12):2686. https://doi.org/10.3390/pr12122686
Chicago/Turabian StyleMoosavi-Khoonsari, Elmira, and Nagendra Tripathi. 2024. "Copper Anode Slime Processing with a Focus on Gold Recovery: A Review of Traditional and Recent Technologies" Processes 12, no. 12: 2686. https://doi.org/10.3390/pr12122686
APA StyleMoosavi-Khoonsari, E., & Tripathi, N. (2024). Copper Anode Slime Processing with a Focus on Gold Recovery: A Review of Traditional and Recent Technologies. Processes, 12(12), 2686. https://doi.org/10.3390/pr12122686