Development of a Niobium-Based Coordination Compound with Catalytic Applications for Green Hydrogen Evolution
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
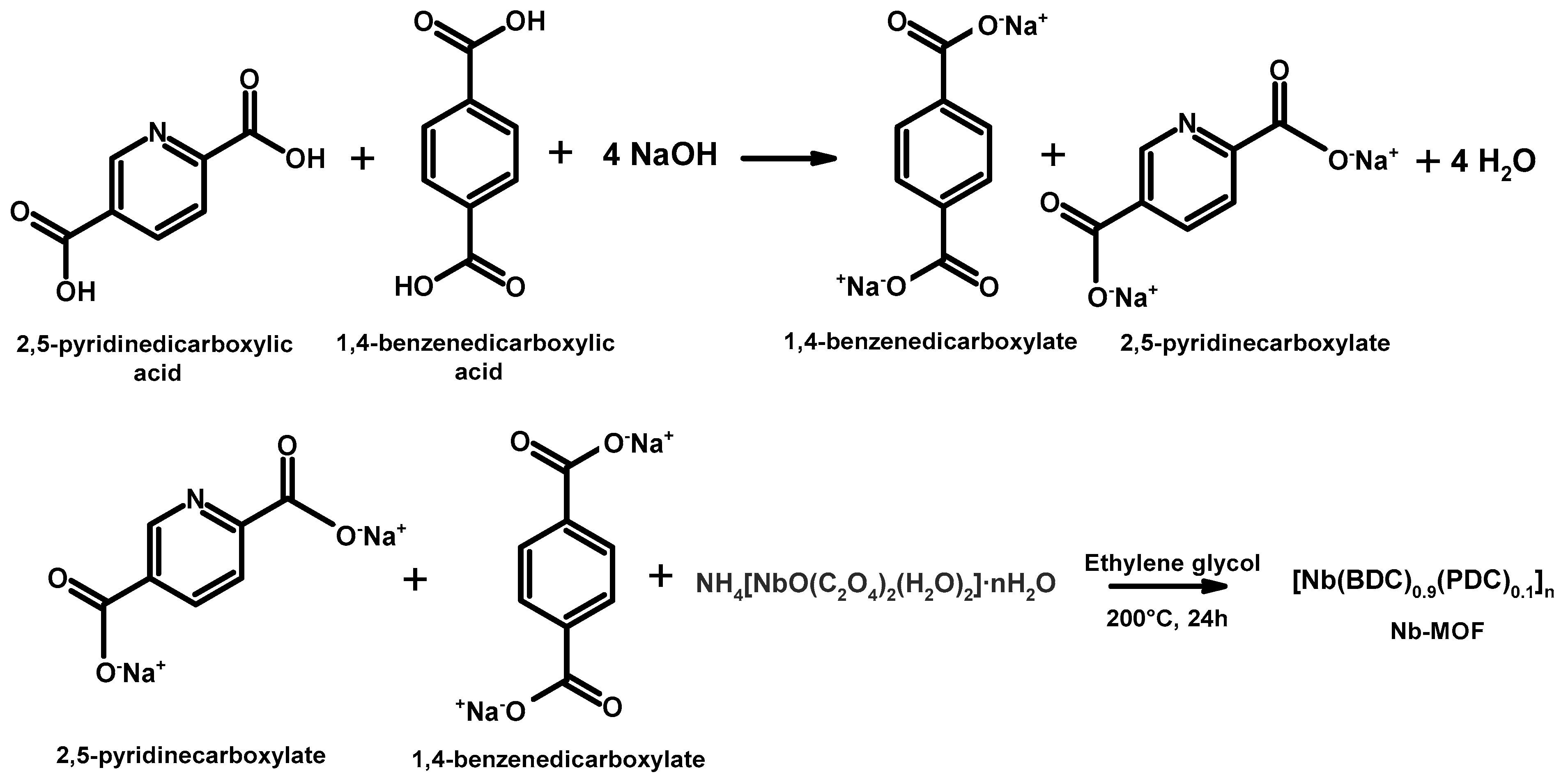
2.2. Synthesis of the Coordination Compound ([Nb(BDC)0.9(PDC)0.1]n)
2.3. MOF Chemical Characterization
2.4. Hydrogen Evolution from NaBH4
2.5. Parameters Optimization
2.5.1. Composition of Monometallic Catalysts
2.5.2. Effect of Catalyst Dose
2.5.3. Effect of NaBH4 Concentration
2.5.4. Effect of Temperature
2.5.5. Effect of NaOH Concentration
2.6. Catalyst Reuse
2.7. Effect of Deuterated Water (D2O)
3. Results and Discussion
3.1. Characterization of [Nb(BDC)0.9(PDC)0.1]ₙ
3.2. Characterization of [Nb(BDC)0.9(PDC)0.1]ₙ/Pt-NP
3.3. Application of [Nb(BDC)0.9(PDC)0.1]ₙ in H2 Evolution from NaBH4
3.3.1. Evaluation of the Effect of NaBH4 Concentration
3.3.2. Evaluation of Catalyst Dosage
3.3.3. Evaluation of Effect of NaOH Concentration
3.3.4. Evaluation of the Temperature Effect
3.4. Evaluation of Catalyst Reuse
3.5. Effect of Deuterated Water (D2O)
3.6. Mechanistic Proposal for H2 Generation from NaBH4 Catalyzed by [Nb(BDC)0.9(PDC)0.1]n/Pt-NPs
3.7. Performance of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kılkış, Ş. Urban emissions and land use efficiency scenarios for avoiding increments of global warming. Energy 2024, 307, 132174. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tareq, S.M.; Chowdhury, A.H. Environmental impacts of industrial waste: The case of Bangladesh. Environ. Chall. 2021, 4, 100–107. [Google Scholar] [CrossRef]
- Bruland, K.; Smith, K. Assessing the role of steam power in the first industrial revolution: The early work of Nick von Tunzelmann. Res. Policy 2013, 42, 1716–1723. [Google Scholar] [CrossRef]
- Howell, J.P. Finding Oil: The Nature of Petroleum Geology. J. Hist. Geogr. 2013, 41, 1859–1920. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Delbeke, J.; Vis, P. Towards a Climate-Neutral Europe; Delbeke, J., Vis, P., Eds.; Routledge: London, UK, 2019. [Google Scholar] [CrossRef]
- Li, S.-C.; Wang, F.-C. The development of a sodium borohydride hydrogen generation system for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2016, 41, 3038–3051. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Y.; Li, B.; Liu, Y. Novel Ni–Co–B hollow nanospheres promote hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2018, 43, 15245–15254. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.E.; Wijayantha, K.G.U. Heterogeneous and homogenous catalysts for hydrogen generation by hydrolysis of aqueous sodium borohydride (NaBH4) solutions. Energy Sci. Eng. 2015, 3, 174–188. [Google Scholar] [CrossRef]
- Ding, X.-L.; Yuan, X.; Jia, C.; Ma, Z.-F. Hydrogen generation from catalytic hydrolysis of sodium borohydride solution using Cobalt–Copper–Boride (Co–Cu–B) catalysts. Int. J. Hydrogen Energy 2010, 35, 11077–11084. [Google Scholar] [CrossRef]
- Bozkurt, G.; Özer, A.; Yurtcan, A.B. Development of effective catalysts for hydrogen generation from sodium borohydride: Ru, Pt, Pd nanoparticles supported on CO3O4. Energy 2019, 180, 702–713. [Google Scholar] [CrossRef]
- Amer, M.S.; Ghanem, M.A.; Al-Mayouf, A.M.; Arunachalam, P.; Khdary, N.H. Low-loading of oxidized platinum nanoparticles into mesoporous titanium dioxide for effective and durable hydrogen evolution in acidic media. Arab. J. Chem. 2020, 13, 2257–2270. [Google Scholar] [CrossRef]
- Coelho, L.O.; Sperandio, G.H.; da Silva, R.C.; Moreira, R.P.L.; de Jesus, J.R. Niobium Metal–Organic Framework Is an Efficient Catalytic Support for the Green Hydrogen Evolution Process from Metal Hydride. Processes 2024, 12, 2342. [Google Scholar] [CrossRef]
- Hayat, A.; Rauf, S.; Al Alwan, B.; El Jery, A.; Almuqati, N.; Melhi, S.; Amin, M.A.; Al-Hadeethi, Y.; Sohail, M.; Orooji, Y.; et al. Recent advance in MOFs and MOF-based composites: Synthesis, properties, and applications. Mater. Today Energy 2024, 41, 101542. [Google Scholar] [CrossRef]
- Udourioh, G.A.; Solomon, M.M.; Matthews-Amune, C.O.; Epelle, E.I.; Okolie, J.A.; Agbazue, V.E.; Onyenze, U. Current trends in the synthesis, characterization and application of metal-organic frameworks. React. Chem. Eng. 2022, 8, 278–310. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, R.; Xu, Q. Metal–Organic Framework Based Catalysts for Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801193. [Google Scholar] [CrossRef]
- Mandegarzad, S.; Raoof, J.B.; Hosseini, S.R.; Ojani, R. MOF-derived Cu-Pd/nanoporous carbon composite as an efficient catalyst for hydrogen evolution reaction: A comparison between hydrothermal and electrochemical synthesis. Appl. Surf. Sci. 2018, 436, 451–459. [Google Scholar] [CrossRef]
- de Jesus, J.R.; Ribeiro, I.S.; de Carvalho, J.P.; de Oliveira, K.L.A.; Moreira, R.P.L.; da Silva, R.C. Successful synthesis of eco-friendly Metal-Organic framework ([Ni(BDC)]n) allows efficient extraction of multiresidues pesticides and dyes from fish samples. Microchem. J. 2024, 201, 110592. [Google Scholar] [CrossRef]
- Li, J.; Fouda, M.M.; Sharaby, C.M.; Zhang, X.; Ma, N.; Agathos, S.N.; Abd-El-Aziz, A.S. Advances in nanoarchitectonics of metal-organic frameworks and metal-/metalloid-containing nanomaterials for antibacterial and antifungal applications. Appl. Mater. Today 2024, 40, 102335. [Google Scholar] [CrossRef]
- Biondi, J.C.; Braga, J.M. Geology and mineralization of Nb, P, Fe and light rare earth elements of the Araxá alkaline-carbonatite complex, Minas Gerais state, Brazil. J. South Am. Earth Sci. 2023, 131, 104623. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Zhang, Y. The application of MOFs for hydrogen storage. Inorganica Chim. Acta 2023, 557, 121683. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Jia, Q.; Ball, R.J.; Zhu, Y.; Xia, Y. Emerging applications of metal-organic frameworks and derivatives in solar cells: Recent advances and challenges. Mater. Sci. Eng. R Rep. 2022, 152, 100714. [Google Scholar] [CrossRef]
- Kanchanakanho, P.; Jansoda, I.; Kenyotha, K.; Krachuamram, S.; Siriwong, K.; Poo-Arporn, Y.; Chanapattharapol, K.C. Catalytic activity of two-dimensional cobalt-ZIF catalyst for NaBH4 hydrolysis. Microporous Mesoporous Mater. 2023, 363, 112805. [Google Scholar] [CrossRef]
- Mirshafiee, F.; Rezaei, M. Co/Fe3O4@GO catalyst for one-step hydrogen generation from hydrolysis of NaBH4: Optimization and kinetic study. Int. J. Hydrogen Energy 2023, 48, 32356–32370. [Google Scholar] [CrossRef]
- Junior, I.M.; Sperandio, G.H.; Lopes, R.P. Efficient hydrogen evolution from NaBH4 using bimetallic nanoparticles (Ni–Co) supported on recycled Zn–C battery electrolyte paste. Int. J. Hydrogen Energy 2023, 53, 1323–1331. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Nafady, A.; Alanazi, N.B.; Abdulhameed, M.M.; Shaikh, S.F. Waste polyethylene terephthalate plastic derived Zn-MOF for high performance supercapacitor application. J. King Saud Univ. Sci. 2024, 36, 103179. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Hassan, H.; Shoaib, M.; Iqbal, M.W.; Alqorashi, A.K.; Sunny, M.A.; Yaseen, T.; Alrobei, H.; Afzal, A.M. Synergistic performance of Ag-MOF@V2CTx composite for asymmetric supercapacitors and hydrogen evolution reaction. J. Phys. Chem. Solids 2024, 193, 112151. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, C.; Liu, X.; El-Seedi, H.R.; Gómez, P.L.; Alzamora, S.M.; Zou, X.; Guo, Z. Enhanced composite Co-MOF-derived sodium carboxymethyl cellulose visual films for real-time and in situ monitoring fresh-cut apple freshness. Food Hydrocoll. 2024, 157, 110475. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, J.; Hu, Z.; Zhang, J.; Yan, J.; Sheng, J. Novel fabrication of rod-like CoAl2O4/halloysite hybrid pigment derived from Co-MOF/nano-clay and mechanism exploration. Dye. Pigment. 2022, 201, 110216. [Google Scholar] [CrossRef]
- Griemsmann, T.; Abel, A.; Hoff, C.; Hermsdorf, J.; Weinmann, M.; Kaierle, S. Laser-based powder bed fusion of niobium with different build-up rates. Int. J. Adv. Manuf. Technol. 2021, 114, 305–317. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhou, C.; Sun, P.; Liu, Y.; Fang, Z.Z. Hydrogen storage properties of Ti-Fe-Zr-Mn-Nb alloys. J. Alloy. Compd. 2022, 938, 168466. [Google Scholar] [CrossRef]
- Tella, A.C.; Olayemi, V.T.; Adekola, F.A.; Oladipo, A.C.; Adimula, V.O.; Ogar, J.O.; Hosten, E.C.; Ogunlaja, A.S.; Argent, S.P.; Mokaya, R. Synthesis, characterization and density functional theory of copper(II) complex and cobalt(II) coordination polymer for detection of nitroaromatic explosives. Inorg. Chim. Acta 2020, 515, 120048. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.-H.; Wang, H.; Zhan, C.-L. Two Zn(II) coordination polymers for highly selective detection of phenol based nitroaromatics and removal of water soluble organic dyes. J. Solid State Chem. 2020, 289, 121481. [Google Scholar] [CrossRef]
- Qi, H.-X.; Wang, Q.; Wang, S.; Jo, H.; Ning, X. Synthesis, structure, and thermal stability of new one-dimensional rubidium coordination supramolecular compound. J. Mol. Struct. 2023, 1300, 137284. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Li, Y.; Murayama, T.; Ishida, T.; Lin, M.; Xiu, G. In-situ Raman unveiled Nb-O-bond-dependency selectivity for methanol electro-oxidation at high current density. Appl. Catal. A Gen. 2023, 664, 119341. [Google Scholar] [CrossRef]
- Su, T.; Peng, R.; Hood, Z.D.; Naguib, M.; Ivanov, I.N.; Keum, J.K.; Qin, Z.; Guo, Z.; Wu, Z. One-Step Synthesis of Nb2O5/C/Nb2C (MXene) Composites and Their Use as Photocatalysts for Hydrogen Evolution. ChemSusChem 2017, 11, 688–699. [Google Scholar] [CrossRef]
- Choudhury, A.; Dey, B.; Ahmad, W.; Sarkhel, G.; Lee, G.H. Fabrication of Niobium Metal Organic Frameworks Anchored Carbon Nanofiber Hybrid Mat for Simultaneous Detection of Xanthine, Hypoxanthine and Uric Acid. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Abuzalat, O.; Tantawy, H.; Mokhtar, M.; Baraka, A. Nano-porous bimetallic organic frameworks (Fe/Co)-BDC, a breathing MOF for rapid and capacitive removal of Cr-oxyanions from water. J. Water Process. Eng. 2022, 46, 102537. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Ren, P.; Wang, D.; Lu, X.; Xu, R.; Li, Y.; Xue, J.; Zhang, J.; et al. Synergistic interface engineering and structural optimization of non-noble metal telluride-nitride electrocatalysts for sustainably overall seawater electrolysis. Appl. Catal. B Environ. 2022, 318, 121834. [Google Scholar] [CrossRef]
- Gusev, A.I. Anisotropy of elastic properties of cubic Nb3O3 niobium monoxide. Solid State Commun. 2023, 372, 115310. [Google Scholar] [CrossRef]
- Daniel, N.K.; Varghese, A.; Devi, K.S.; Chundattu, S.J.; Sreekanth, A. Evaluating the effect of different ligands on the supercapacitance and hydrogen evolution reaction studies of Zn-Co MOF. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 703, 135177. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Qiu, Y.; Song, Y.; Zhou, X.; Ding, Y. Colorimetric aptasensor based on Fe3O4@MOF@Pt nanozymes for ultrasensitive detection of histamine in fish. Food Control 2024, 166, 110702. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, F.; Liu, W.; He, R.; Zhang, J.; Tan, Y.; Sun, W.; Bao, S.-J. Water-regulated 2D Ni-MOF-derived heat-sheared Nano-Ni@TC for efficient hydrogen evolution. Int. J. Hydrogen Energy 2024, 64, 389–397. [Google Scholar] [CrossRef]
- Larichev, Y.V. Dynamic Light Scattering for Studying Supported Metal Catalysts. Kinet. Catal. 2021, 62, 528–535. [Google Scholar] [CrossRef]
- Sun, B.; Panferov, V.; Guo, X.; Xiong, J.; Zhang, S.; Qin, L.; Yin, C.; Wang, X.; Liu, C.; Han, K.; et al. A novel triple-signal biosensor based on ZrFe-MOF@PtNPs for ultrasensitive aflatoxins detection. Biosens. Bioelectron. 2024, 267, 116797. [Google Scholar] [CrossRef]
- Larichev, Y.V. Experience of Using DLS to Study the Particle Sizes of Active Component in the Catalysts Based on the Oxide and Non-Oxide Supports. Inorganics 2022, 10, 248. [Google Scholar] [CrossRef]
- Luo, X.; Sun, L.; Xu, F.; Cao, Z.; Zeng, J.; Bu, Y.; Zhang, C.; Xia, Y.; Zou, Y.; Zhang, K.; et al. Metal boride-decorated CoNi layered double hydroxides supported on muti-walled carbon nanotubes as efficient hydrolysis catalysts for sodium borohydride. J. Alloy. Compd. 2022, 930, 167339. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Guo, J.; Sun, L.; Ming, J.; Dong, H.; Zhao, Y.; Tian, J.; Yang, X. Ceria-Induced Strategy to Tailor Pt Atomic Clusters on Cobalt–Nickel Oxide and the Synergetic Effect for Superior Hydrogen Generation. ACS Sustain. Chem. Eng. 2018, 6, 7451–7457. [Google Scholar] [CrossRef]
- Ababaii, M.A.; Gilani, N.; Pasikhani, J.V. Hydrogen evolution from NaBH4 solution using Cr-doped Ni–B metallic catalyst deposited on rice husk via electroless plating. Int. J. Hydrogen Energy 2023, 51, 648–662. [Google Scholar] [CrossRef]
- Al-Shaikh, H.; Lasri, J.; Knight, J.G.; Al-Goul, S.T. Palladium mesoporous nanoparticles Pd NPs@[KIT-6] and Pd NPs@[KIT-6]-PEG-imid as efficient heterogeneous catalysts for H2 production from NaBH4 hydrolysis. Fuel 2022, 325, 124962. [Google Scholar] [CrossRef]
- Churikov, A.; Gamayunova, I.; Zapsis, K.; Churikov, M.; Ivanishchev, A. Influence of temperature and alkalinity on the hydrolysis rate of borohydride ions in aqueous solution. Int. J. Hydrogen Energy 2012, 37, 335–344. [Google Scholar] [CrossRef]
- Sun, L.; Liu, M.; Zhang, T.; Huang, Y.; Song, H.; Yang, J.; Dou, J.; Li, D.; Gao, X.; Zhang, Q.; et al. Co@SiO2/C catalyst shielded by hierarchical shell for robust hydrogen production. Appl. Catal. B Environ. 2023, 343, 123537. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, N. Superior reusability of metal catalysts prepared within poly(ethylene imine) microgels for H2 production from NaBH4 hydrolysis. Fuel Process. Technol. 2014, 127, 88–96. [Google Scholar] [CrossRef]
- Sermiagin, A.; Meyerstein, D.; Rolly, G.S.; Mondal, T.; Kornweitz, H.; Zidki, T. Mechanistic implications of the solvent kinetic isotope effect in the hydrolysis of NaBH4. Int. J. Hydrogen Energy 2021, 47, 3972–3979. [Google Scholar] [CrossRef]
- Paterson, R.; Alharbi, A.A.; Wills, C.; Dixon, C.; Šiller, L.; Chamberlain, T.W.; Griffiths, A.; Collins, S.M.; Wu, K.; Simmons, M.D.; et al. Heteroatom modified polymer immobilized ionic liquid stabilized ruthenium nanoparticles: Efficient catalysts for the hydrolytic evolution of hydrogen from sodium borohydride. Mol. Catal. 2022, 528, 112476. [Google Scholar] [CrossRef]
- Abraham, A.; Silviya, R.; Patel, R.; Patel, N.; Fernandes, R. MOF derived cobalt-phospho-boride for rapid hydrogen generation via NaBH4 hydrolysis. Int. J. Hydrogen Energy 2024, 77, 1245–1253. [Google Scholar] [CrossRef]
- Hashem, Z.H.; Abdel-Rahman, L.H.; Gómez-Ruiz, S.; Abdelhamid, H.N. Cerium-Organic Framework (CeOF) for hydrogen generation via the hydrolysis of NaBH4. Results Chem. 2024, 7, 101412. [Google Scholar] [CrossRef]
- Mengesha, D.N.; Baye, A.F.; Kim, H. Modulating effect of urea/melamine on Co2+/Co3+ ratio of CO3O4 microplates for rapid hydrogen generation via NaBH4 hydrolysis. Int. J. Hydrogen Energy 2024, 57, 856–868. [Google Scholar] [CrossRef]
- Bu, Y.; Liu, J.; Cai, D.; Huang, P.; Wei, S.; Luo, X.; Liu, Z.; Xu, F.; Sun, L.; Wei, X. Magnetic recyclable catalysts with dual protection of hollow Co/N/C framework and surface carbon film for hydrogen production from NaBH4 hydrolysis. J. Alloy. Compd. 2022, 938, 168495. [Google Scholar] [CrossRef]
- Sperandio, G.H.; de Carvalho, J.P.; de Jesus, C.B.R.; Junior, I.M.; de Oliveira, K.L.A.; Puiatti, G.A.; de Jesus, J.R.; Moreira, R.P.L. Hydrogen evolution from NaBH4 using novel Ni/Pt nanoparticles decorated on a niobium-based composite. Int. J. Hydrogen Energy 2024, 83, 774–783. [Google Scholar] [CrossRef]



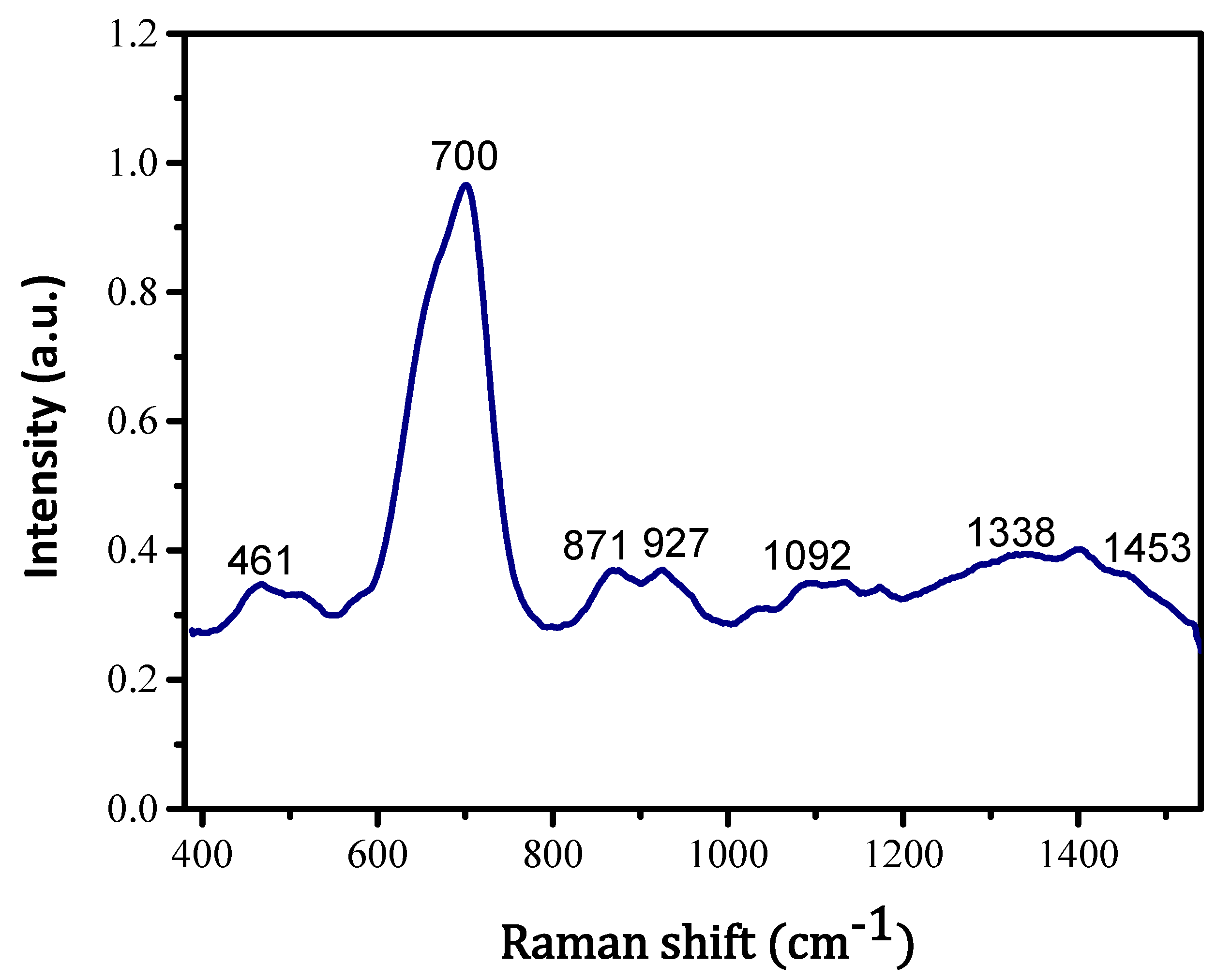










| Temperature (Kelvin) | Kinetic Constants (s−1) |
|---|---|
| 303.15 | 2.9755 |
| 313.15 | 5.2268 |
| 323.15 | 4.7531 |
| 333.15 | 5.8433 |
| Catalyst | EA (kJ mol−1) | HGR (mL min−1 gcat−1) | Reuse | Ref. |
|---|---|---|---|---|
| MOF-derived cobalt-phospho-boride for rapid hydrogen generation via NaBH4 hydrolysis | 20.7 | 1800 | ~98% efficiency in the 5th cycle | [58] |
| Cerium-Organic Framework (CeOF) for hydrogen generation via the hydrolysis of NaBH4 | 58.8 | 1800 | ~100%efficiency in the 4th cycle | [59] |
| Modulating effect of urea/melamine on Co2+/Co3+ ratio of Co3O4 microplates for rapid hydrogen generation via NaBH4 hydrolysis | 46.9 | 2042 | ~100% efficiency in the 5th cycle | [60] |
| Magnetic recyclable catalysts with dual protection of hollow Co/N/C framework and surface carbon film for hydrogen production from NaBH4 hydrolysis | 26.9 | 9815.82 | 81.3% efficiency in the 25th cycle | [61] |
| Hydrogen evolution from NaBH4 using novel Ni/Pt nanoparticles decorated on a niobium-based composite | 23.1 | 1782 | ~100% efficiency in the 16th cycle | [62] |
| Niobium coordination compound with catalytic application for green hydrogen evolution is developed | 16.38 | 119,020 | 92.82% efficiency in the 8th cycle | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squizzatto, E.P.; Andrade, T.d.A.; Lopes Moreira, R.P.; Guimarães, L.d.M.; da Silva, M.J.; Novaes, F.J.M.; de Jesus, J.R. Development of a Niobium-Based Coordination Compound with Catalytic Applications for Green Hydrogen Evolution. Processes 2024, 12, 2677. https://doi.org/10.3390/pr12122677
Squizzatto EP, Andrade TdA, Lopes Moreira RP, Guimarães LdM, da Silva MJ, Novaes FJM, de Jesus JR. Development of a Niobium-Based Coordination Compound with Catalytic Applications for Green Hydrogen Evolution. Processes. 2024; 12(12):2677. https://doi.org/10.3390/pr12122677
Chicago/Turabian StyleSquizzatto, Emily Pacheco, Tatianny de Araujo Andrade, Renata Pereira Lopes Moreira, Luciano de Moura Guimarães, Márcio José da Silva, Fábio Junior Moreira Novaes, and Jemmyson Romário de Jesus. 2024. "Development of a Niobium-Based Coordination Compound with Catalytic Applications for Green Hydrogen Evolution" Processes 12, no. 12: 2677. https://doi.org/10.3390/pr12122677
APA StyleSquizzatto, E. P., Andrade, T. d. A., Lopes Moreira, R. P., Guimarães, L. d. M., da Silva, M. J., Novaes, F. J. M., & de Jesus, J. R. (2024). Development of a Niobium-Based Coordination Compound with Catalytic Applications for Green Hydrogen Evolution. Processes, 12(12), 2677. https://doi.org/10.3390/pr12122677









