Research on the Chloramphenicol Removal Performance of Co-Doped Porous Carbon Materials Derived from Co-Zn Bimetallic ZIFs
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
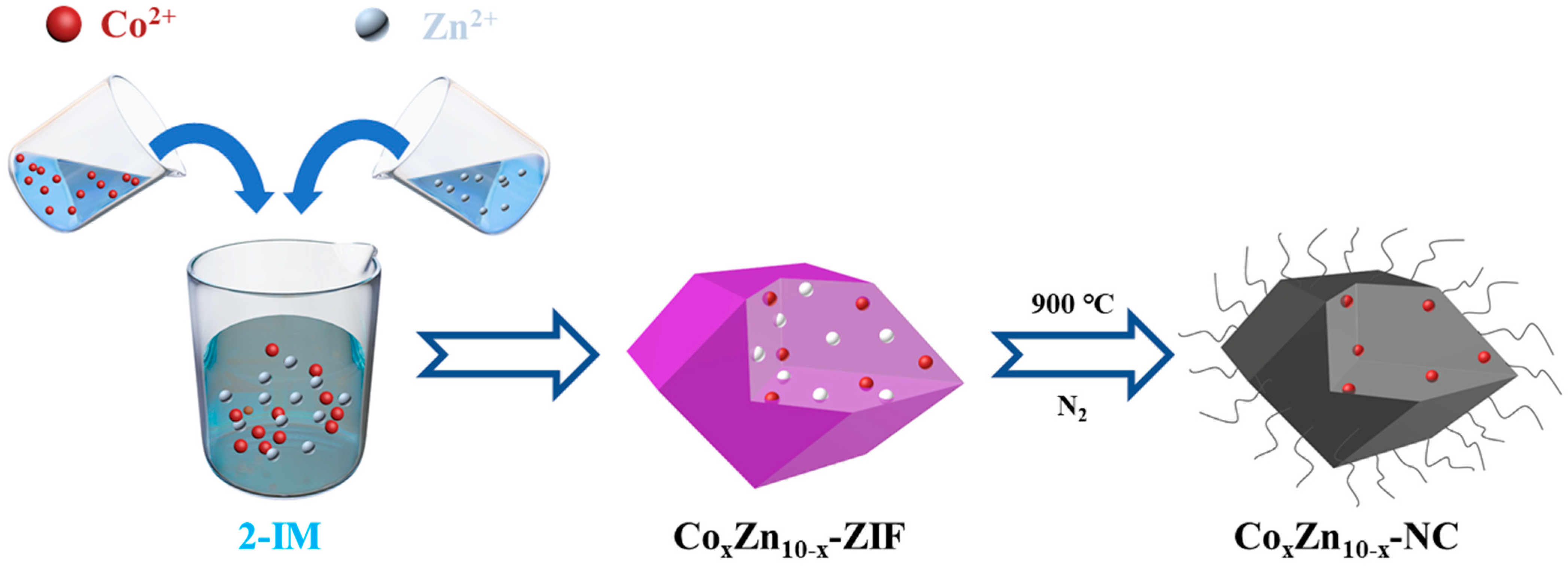
2.2. Preparation of Materials
2.2.1. Preparation of CoxZn10−x-ZIF
2.2.2. Preparation of CoxZn10−x-NC
2.3. Experimental Process
3. Discussion
3.1. The Characterization Results of CoxZn10−x-NC
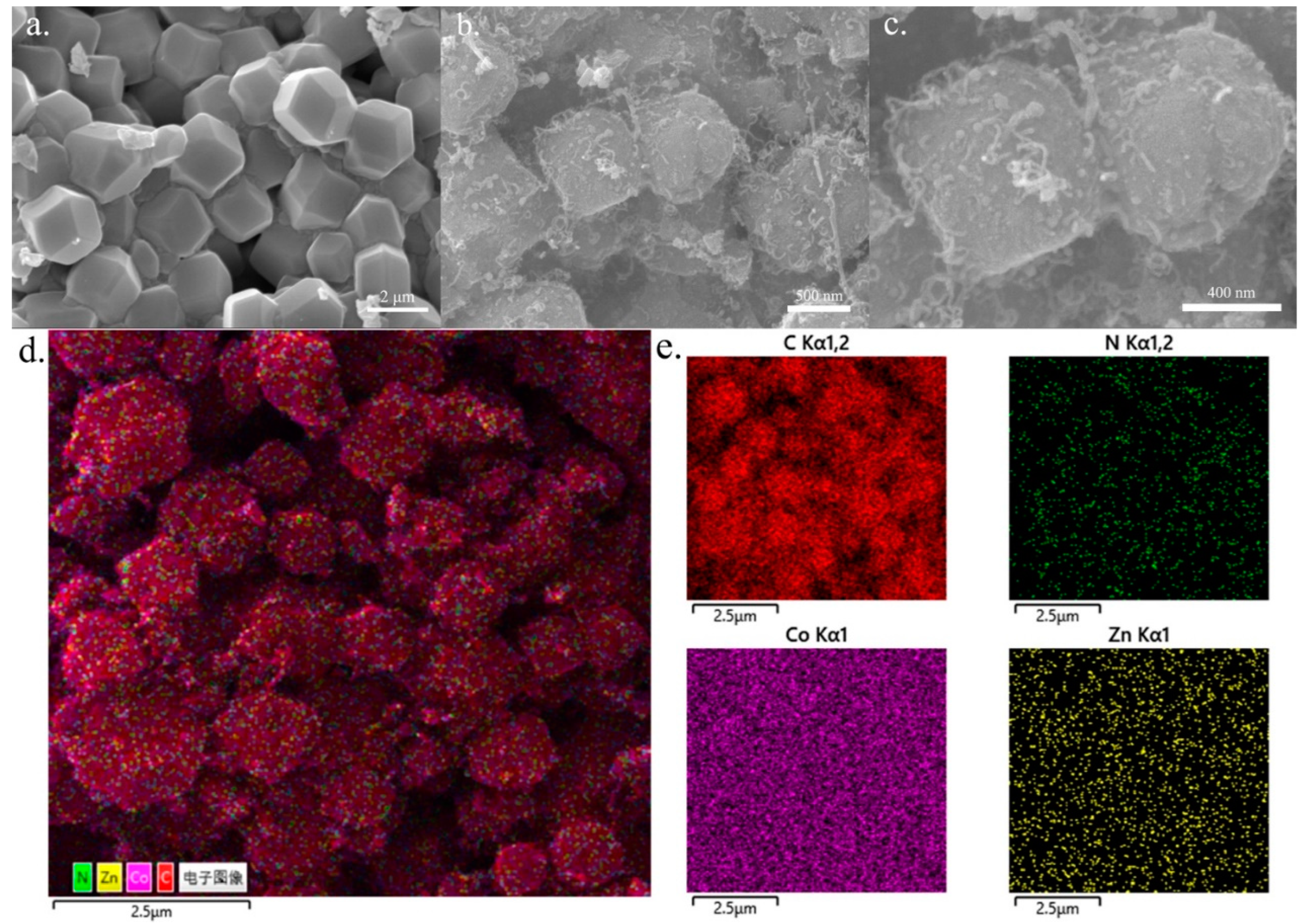
3.1.1. SEM Characterization Results
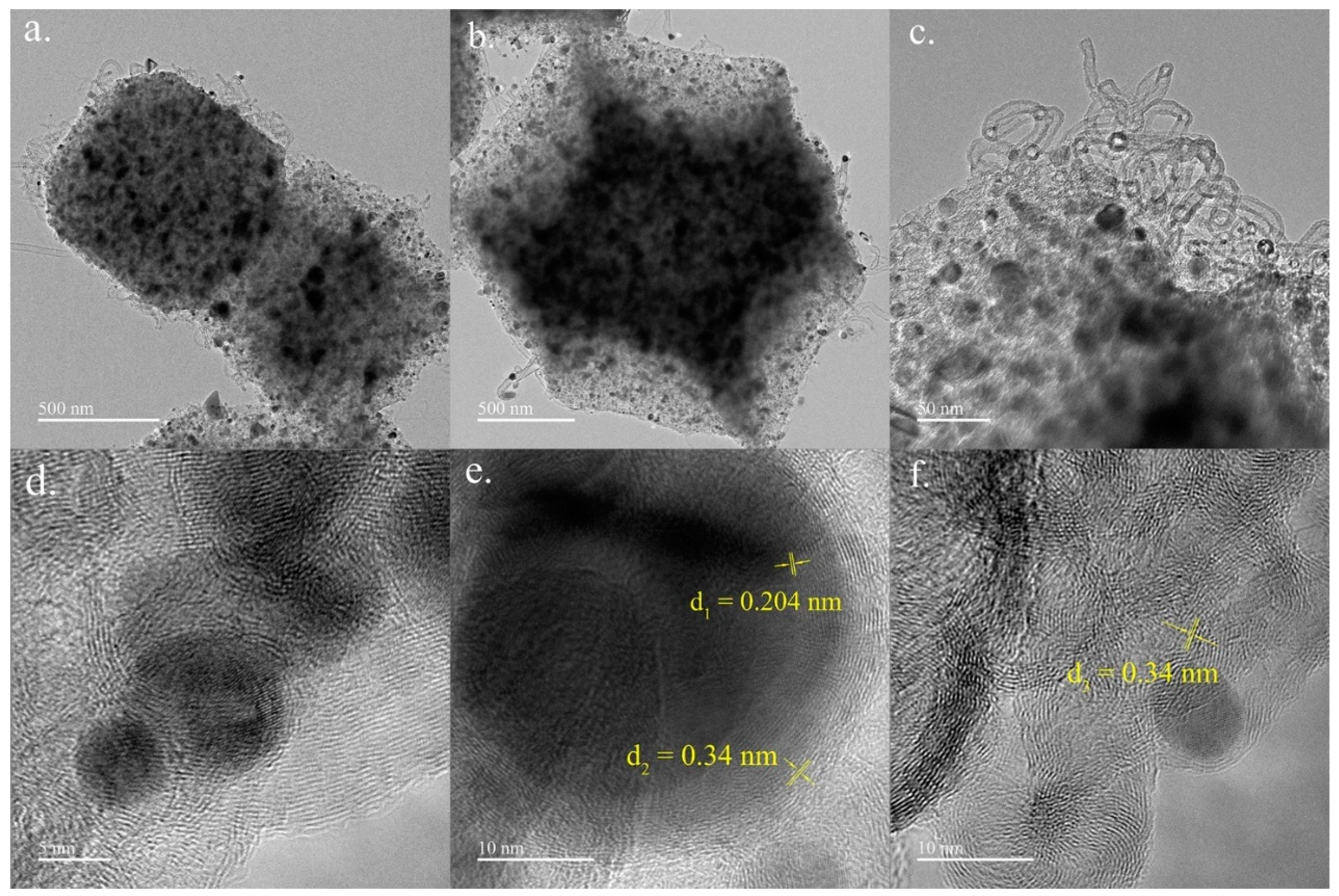
3.1.2. TEM Characterization Results
3.1.3. XRD and Raman Characterization Results
3.1.4. BET Characterization Results
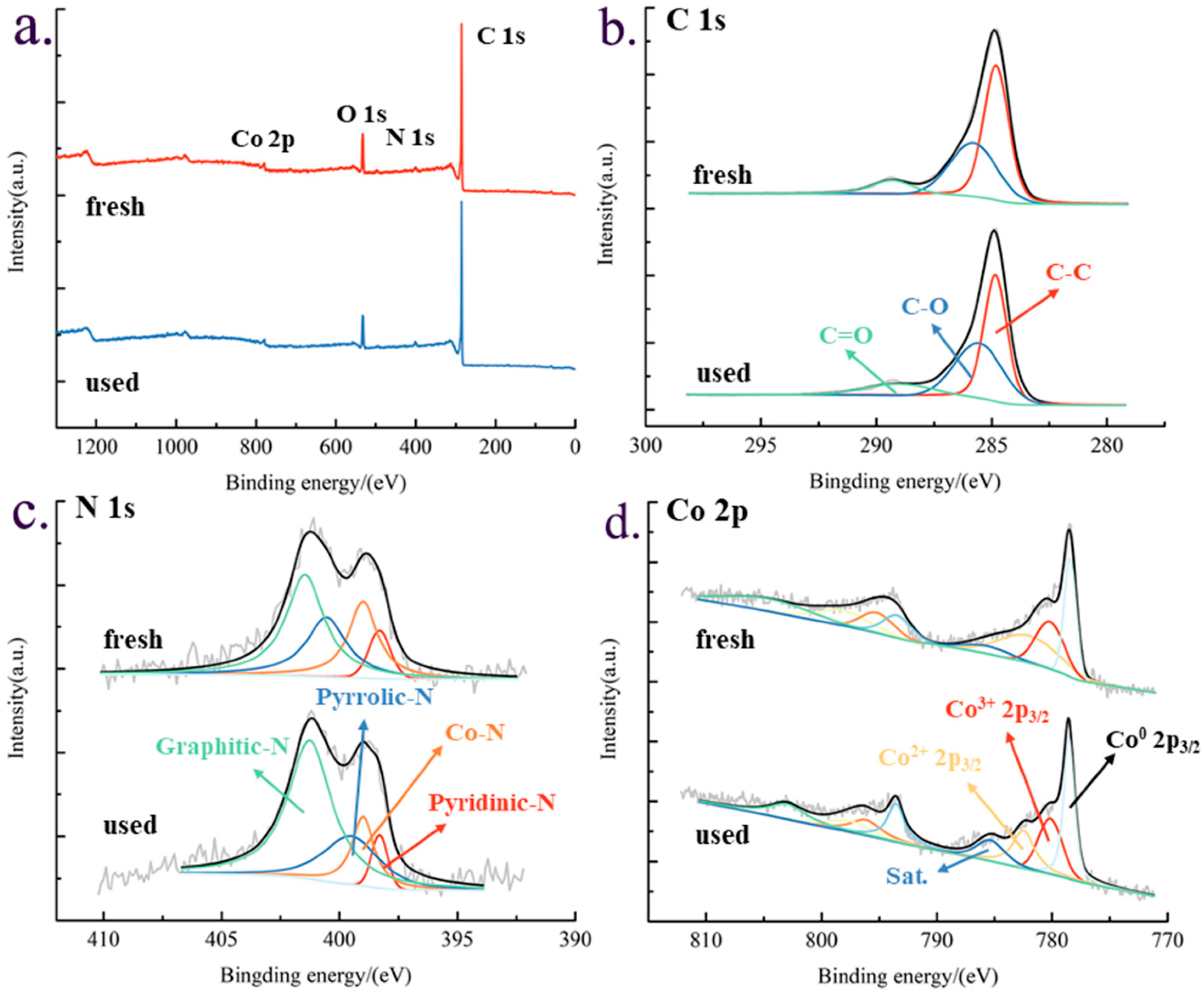
3.1.5. XPS Characterization Results
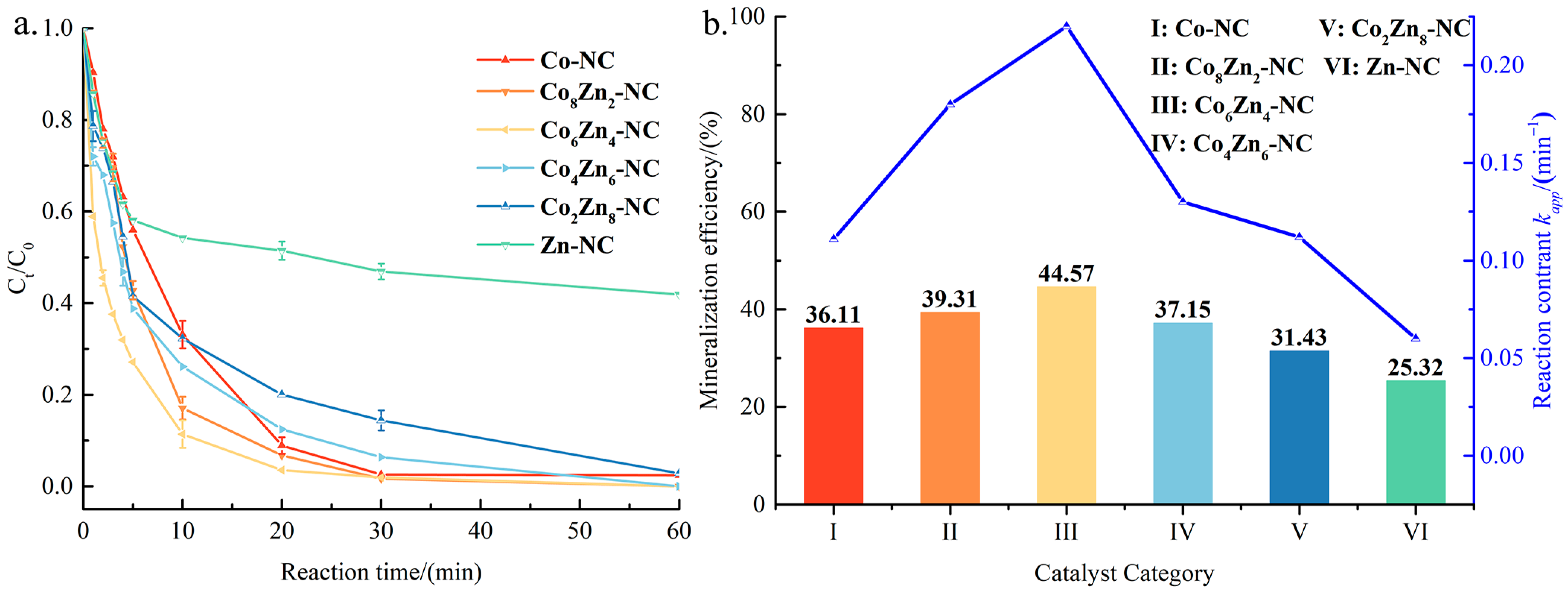
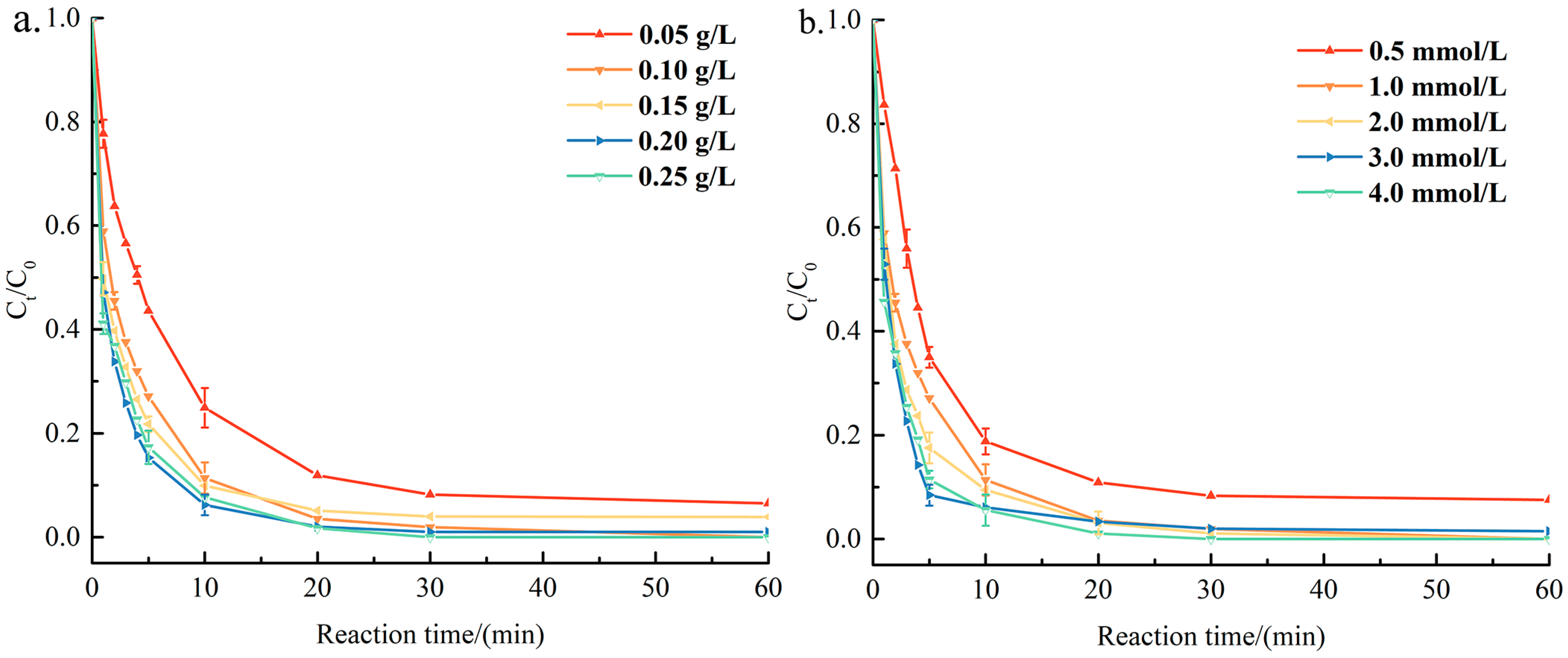
3.2. Catalytic Performance of CoxZn10−x-NC
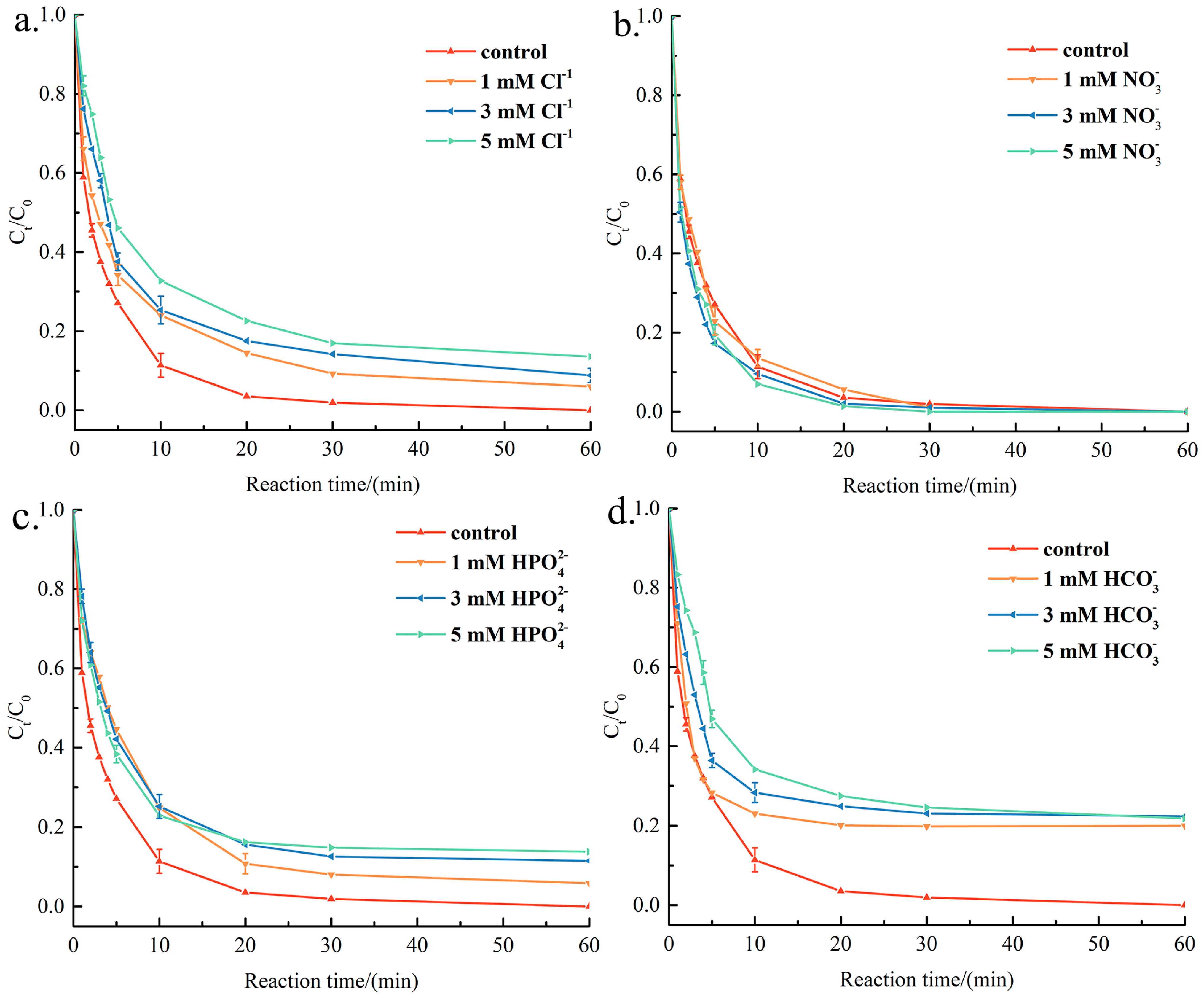
3.3. Potential Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Cheng, S.; Chen, G. Bioelectrochemical systems for efficient recalcitrant wastes treatment. J. Chem. Technol. Biotechnol. 2011, 86, 481–491. [Google Scholar] [CrossRef]
- Alexievaa, Z.; Gerginova, M.; Zlateva, P.; Peneva, N. Comparison of growth kinetics and phenol metabolizing enzymes of Trichosporon cutaneum R57 and mutants with modified degradation abilities. Enzym. Microb. Technol. 2004, 34, 242–247. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Lu, Y.; Hu, Z.; Chen, S.; Zeng, R.J. The performance and microbial communities of an anaerobic membrane bioreactor for treating fluctuating 2-chlorophenol wastewater. Bioresour. Technol. 2020, 317, 124001. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Anisuzzaman, S.M.; Bono, A.; Sarbatly, R. Adsorption of 2,4,6-trichlorophenol (TCP) onto activated carbon. J. King Saud Univ.-Sci. 2013, 25, 251–255. [Google Scholar] [CrossRef]
- Lai, L.; Yan, J.; Li, J.; Lai, B. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism. Chem. Eng. J. 2018, 343, 676–688. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: Performance and mechanism for organic pollutant degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Jin, L.; Li, Y.; Geng, P.; Xue, H.; Pang, H.; Xu, Q. Metal-Organic Framework-Derived Carbons for Battery Applications. Adv. Energy Mater. 2018, 8, 1800716. [Google Scholar] [CrossRef]
- Chen, C.; Tuo, Y.; Lu, Q.; Lu, H.; Zhang, S.; Zhou, Y.; Zhang, J.; Liu, Z.; Kang, Z.; Feng, X.; et al. Hierarchical trimetallic Co-Ni-Fe oxides derived from core-shell structured metal-organic frameworks for highly efficient oxygen evolution reaction. Appl. Catal. B Environ. 2021, 287, 119953. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal–Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Accounts Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, C.; Yan, X.; Chen, S.; Wang, C.; Luo, R.; Qi, J.; Sun, X.; Wang, L.; Li, J. Efficient Removal of Organic Pollutants by Metal–organic Framework Derived Co/C Yolk–Shell Nanoreactors: Size-Exclusion and Confinement Effect. Environ. Sci. Technol. 2020, 54, 10289–10300. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.D.; Rafiq, K.; Yoo, H. Nano Metal-Organic Framework-Derived Inorganic Hybrid Nanomaterials: Synthetic Strategies and Applications. Chemistry 2017, 23, 5631–5651. [Google Scholar] [CrossRef]
- Geng, S.; Wang, H.; Liang, F.; Wang, L.; Wei, D.; Yan, T.; Yan, L. Cobalt-manganese co-doped metal-organic framework compound activates peroxymonosulfate for tetracycline degradation. China Powder Sci. Technol. 2024, 30, 113–122. [Google Scholar]
- Yao, S.; Qian, W.; Wang, Y.; Hu, X.; Zhang, W.; Song, J.; Dong, X. Catalytic performance of CuO-kaolinite composite for norfloxacin degradation by activating peroxymonosulfate. China Powder Sci. Technol. 2024, 30, 1–12. [Google Scholar]
- Yang, L.-X.; Yang, J.-C.E.; Fu, M.-L. Magnetic CoFe2O4 nanocrystals derived from MIL-101 (Fe/Co) for peroxymonosulfate activation toward degradation of chloramphenicol. Chemosphere 2021, 272, 129567. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, J.; Huang, J.; Ma, W.; Li, K.; Li, J.; Zhang, S.; Li, Z. Accelerated electron transfer process via MOF-derived FeCo/C for enhanced degradation of antibiotic contaminants towards heterogeneous electro-Fenton system. Chemosphere 2023, 335, 138994. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Chen, J.; Gao, Q.; Khan, M.M.; Wang, P.; Jiao, Y.; Zhang, Z.; Liu, Y.; Yang, Y. The preparation of Ag/ZIF-8@ZIF-67 core-shell composites as excellent catalyst for degradation of the nitroaromatic compounds. Appl. Surf. Sci. 2020, 516, 146160. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Yan, X.; Zhang, H.; Xiao, C.; Qi, J.; Zhu, Z.; Zhou, Y.; Sun, X.; Duan, X.; et al. Rational Regulation of Co–N–C Coordination for High-Efficiency Generation of 1O2 toward Nearly 100% Selective Degradation of Organic Pollutants. Environ. Sci. Technol. 2022, 56, 8833–8843. [Google Scholar] [CrossRef]
- Gu, A.; Gong, C.; He, M.; Chen, K.; Zhou, X.; Wang, P.; Chen, K.; Jiao, Y.; Yang, Y. MOFs-derived Co-Fe sulfides as highly efficient and stable catalysts to activate peroxymonosulfate for the degradation of trichlorophenol. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132462. [Google Scholar] [CrossRef]
- Mi, X.; Wang, P.; Xu, S.; Su, L.; Zhong, H.; Wang, H.; Li, Y.; Zhan, S. Almost 100% Peroxymonosulfate Conversion to Singlet Oxygen on Single-Atom CoN2+2 Sites. Angew. Chem. Int. Ed. 2021, 60, 4588–4593. [Google Scholar] [CrossRef]
- Xiong, X.; Sun, B.; Zhang, J.; Gao, N.; Shen, J.; Li, J.; Guan, X. Activating persulfate by Fe0 coupling with weak magnetic field: Performance and mechanism. Water Res. 2014, 62, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liang, X.; Zhong, Y.; Zhu, J.; Zhu, S.; Yuan, P.; He, H.; Zhang, J. Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products. Water Res. 2012, 46, 4633–4644. [Google Scholar] [CrossRef]
- Li, M.; Luo, R.; Wang, C.; Zhang, M.; Zhang, W.; Klu, P.K.; Yan, Y.; Qi, J.; Sun, X.; Wang, L.; et al. Iron-tannic modified cotton derived Fe0/graphitized carbon with enhanced catalytic activity for bisphenol A degradation. Chem. Eng. J. 2019, 372, 774–784. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.; Sun, Y.; Jin, P.; Bai, X.; Jin, X.; Shi, X.; Wang, Y.; Nie, S. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.-S.; Tu, Y.-J.; Huang, Y.-H.; Zhang, H. Heterogeneous Degradation of Organic Pollutants by Persulfate Activated by CuO-Fe3O4: Mechanism, Stability, and Effects of pH and Bicarbonate Ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, H.; Li, Y.; Yu, J.; Ding, L.; Kong, Y.; Ma, J. Nitrogen-doped biochar encapsulated Fe/Mn nanoparticles as cost-effective catalysts for heterogeneous activation of peroxymonosulfate towards the degradation of bisphenol-A: Mechanism insight and performance assessment. Sep. Purif. Technol. 2022, 283, 120136. [Google Scholar] [CrossRef]
- Gu, A.; Wang, P.; Chen, K.; Miensah, E.D.; Gong, C.; Jiao, Y.; Mao, P.; Chen, K.; Jiang, J.; Liu, Y.; et al. Core-shell bimetallic Fe-Co MOFs to activated peroxymonosulfate for efficient degradation of 2-chlorophenol. Sep. Purif. Technol. 2022, 298, 121461. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Luo, H.; Ni, C.; Zhang, C.; Wang, W.; Yang, Y.; Xiong, W.; Cheng, M.; Zhou, C.; Zhou, Y.; Tian, S.; et al. Lignocellulosic biomass derived N-doped and CoO-loaded carbocatalyst used as highly efficient peroxymonosulfate activator for ciprofloxacin degradation. J. Colloid Interface Sci. 2022, 610, 221–233. [Google Scholar] [CrossRef]
- Cen, Z.; Ni, Z.; Zhou, X.; Liu, Y.; Fang, Y.; Qiu, R.; Zhang, S. Enhancing mediated electron transfer in for efficient chloramphenicol degradation through Cu-modified Co@C catalyst. J. Environ. Chem. Eng. 2023, 11, 110079. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ. Pollut. 2020, 257, 113610. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, L.; Guo, J.; Zhou, L.; Lan, Y. Sulfur-doped copper-cobalt bimetallic oxides with abundant Cu(I): A novel peroxymonosulfate activator for chloramphenicol degradation. Chem. Eng. J. 2019, 361, 1304–1316. [Google Scholar] [CrossRef]
- Tan, C.; Dong, Y.; Fu, D.; Gao, N.; Ma, J.; Liu, X. Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: Kinetics and mechanism of radical generation. Chem. Eng. J. 2018, 334, 1006–1015. [Google Scholar] [CrossRef]

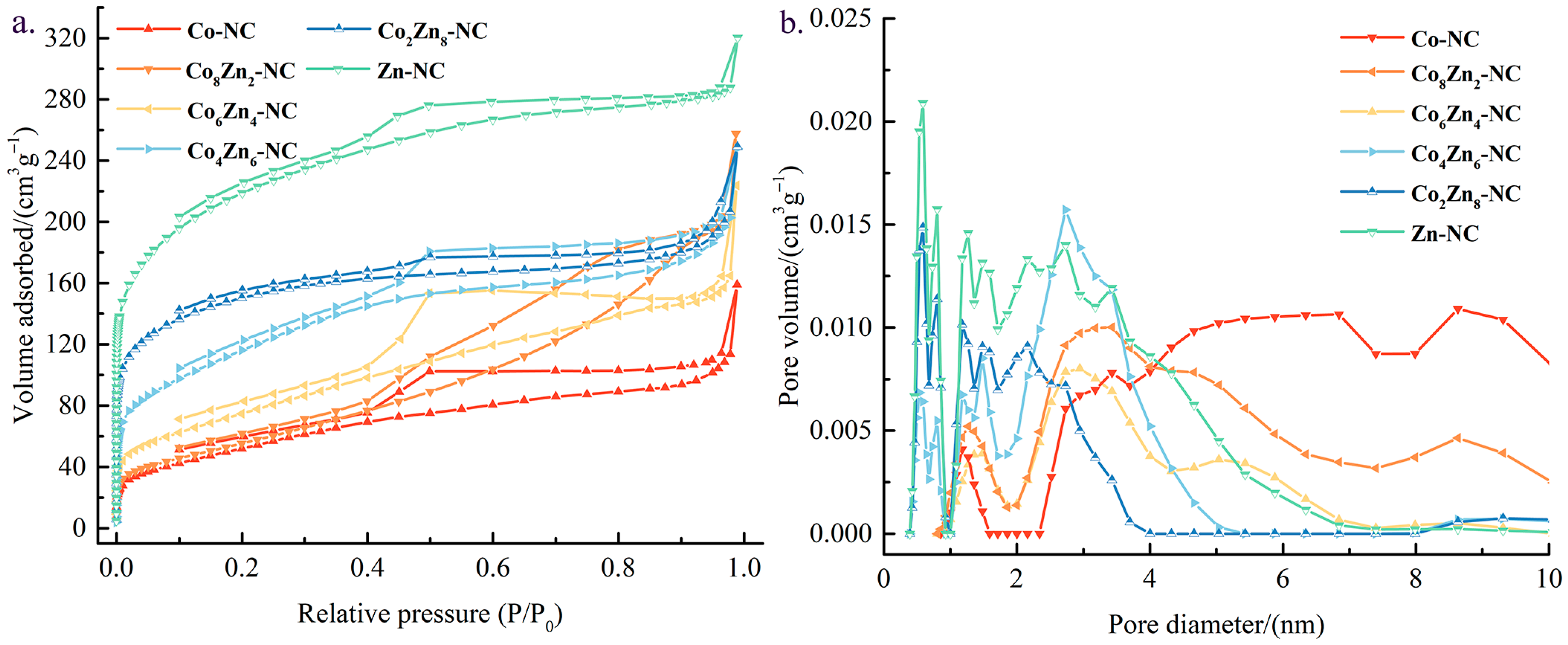

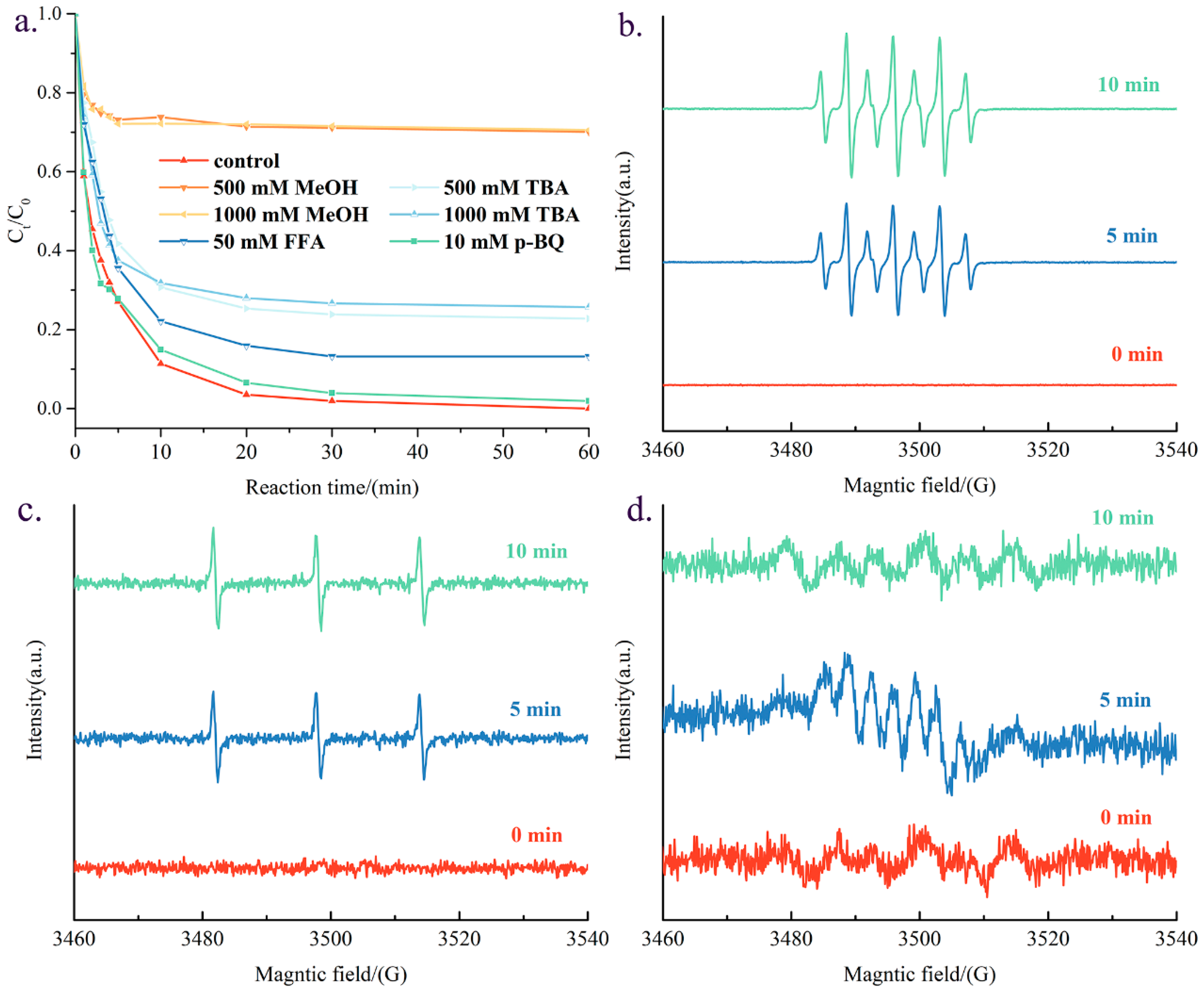
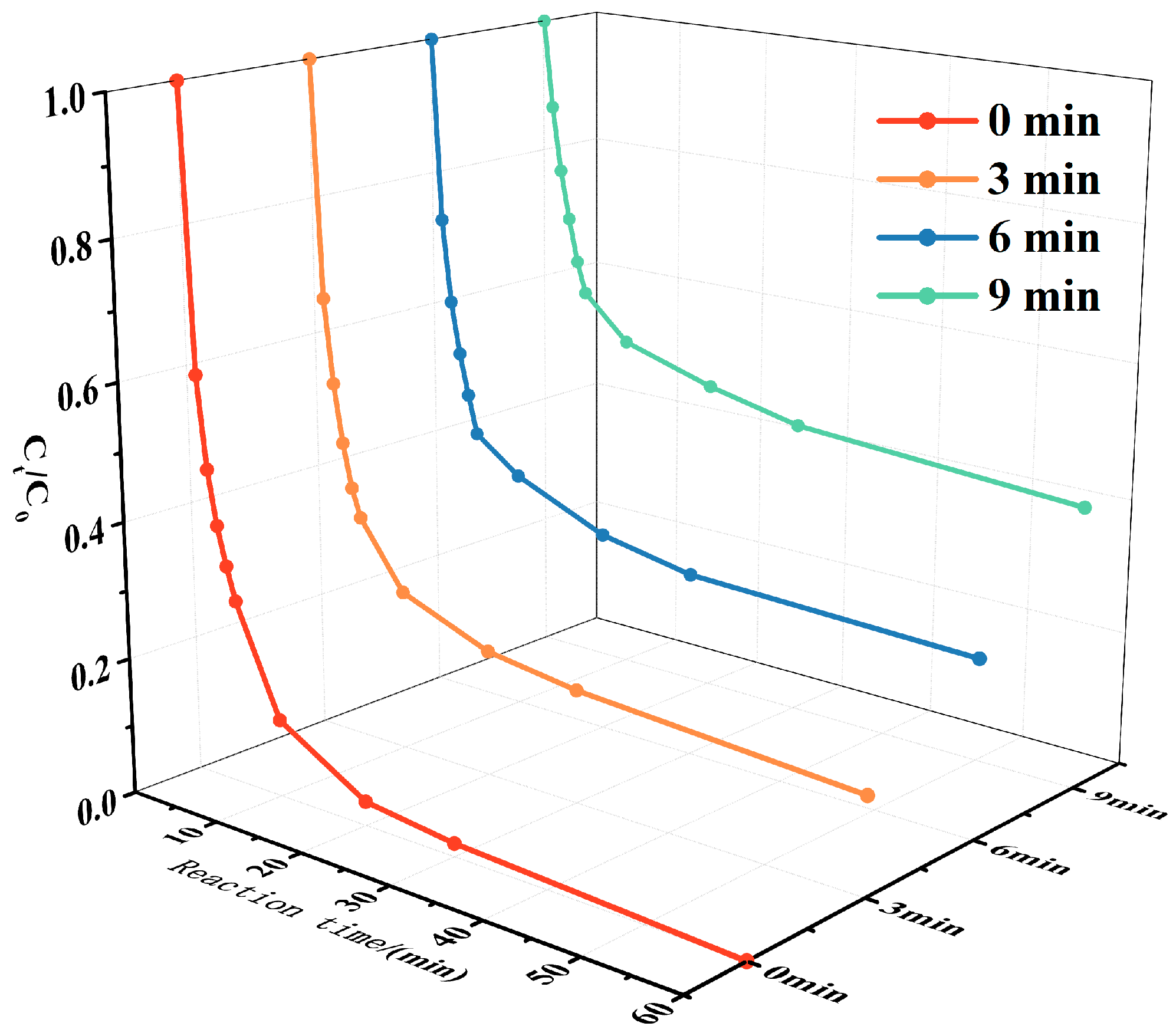

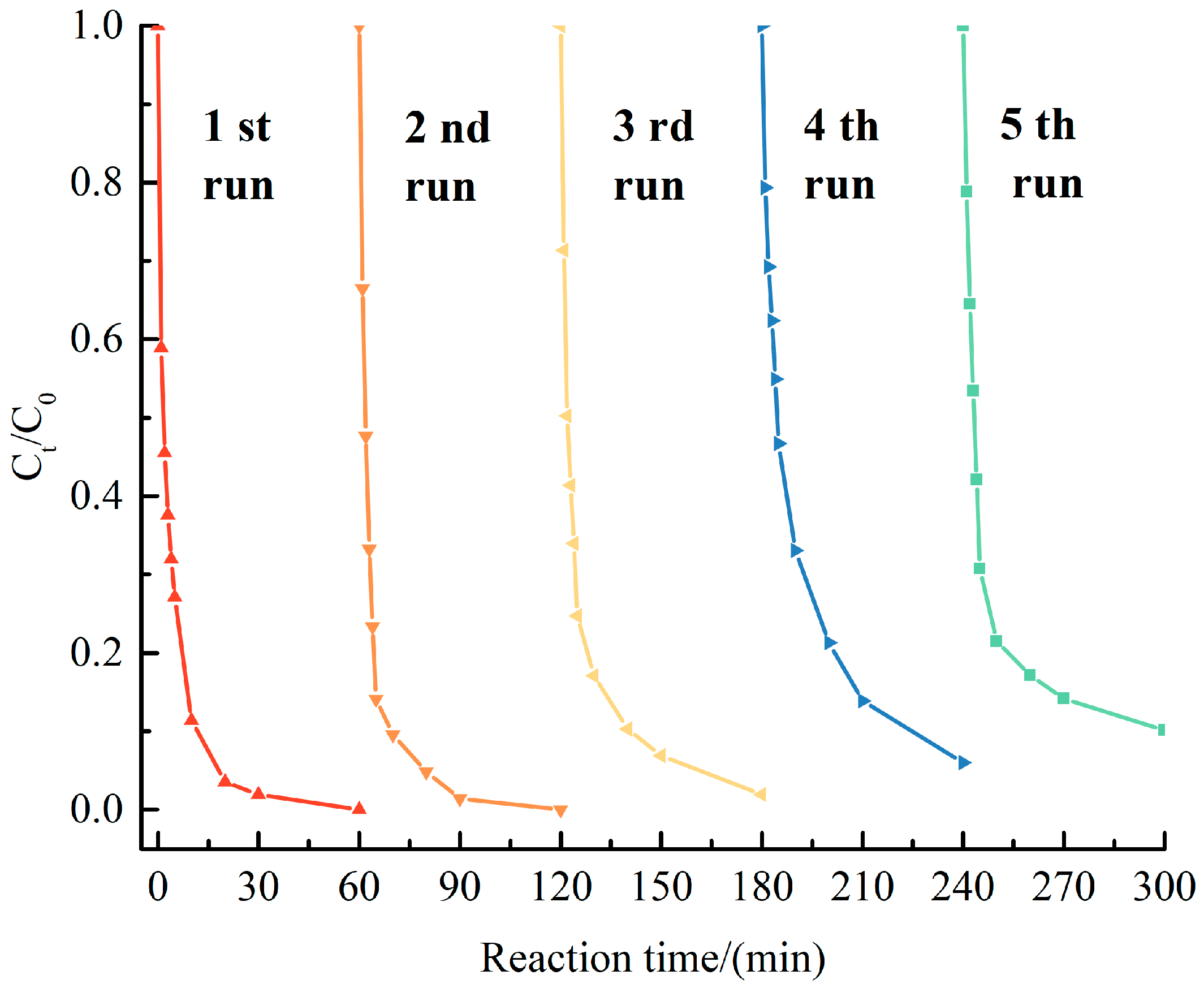
| Catalyst | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Aperture (nm) |
|---|---|---|---|
| Co-NC | 198.49 | 2.46 × 10−1 | 7.62 |
| Co8Zn2-NC | 209.56 | 3.47 × 10−1 | 5.05 |
| Co6Zn4-NC | 274.94 | 3.84 × 10−1 | 4.97 |
| Co4Zn6-NC | 421.58 | 3.86 × 10−1 | 3.65 |
| Co2Zn8-NC | 554.22 | 3.99 × 10−1 | 2.79 |
| Zn-NC | 797.24 | 4.97 × 10−1 | 2.49 |
| Catalyst | Pollutant (mg/L) | Catalyst Dose (g/L) | Oxone (mmol/L) | Removal Efficiency Kapp (min−1) | Ref. |
|---|---|---|---|---|---|
| CoFe2O4 NCs | CAP(10) | 0.1 | 2 | 0.08 | [16] |
| CuCo@C | CAP(20) | 0.1 | 2 | 0.12 | [31] |
| Co3O4-BC | CAP(30) | 0.3 | 10 | 0.34 | [32] |
| CuCoO−1 | CAP(20) | 0.1 | 0.5 | 98%, 15 min | [33] |
| Fe0 | CAP | 0.5 | 0.2 | 0.03 | [34] |
| Co-NC | CAP(20) | 0.1 | 1 | 0.07 | This work |
| Co6Zn4-NC | CAP(20) | 0.1 | 1 | 0.22 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-H.; Gu, A.-T.; Zhang, L.; Tang, H.; Zhang, Z.-M.; Zhou, Y.-M.; Cheng, Y.-Q.; Yang, Y. Research on the Chloramphenicol Removal Performance of Co-Doped Porous Carbon Materials Derived from Co-Zn Bimetallic ZIFs. Processes 2024, 12, 2670. https://doi.org/10.3390/pr12122670
Chen K-H, Gu A-T, Zhang L, Tang H, Zhang Z-M, Zhou Y-M, Cheng Y-Q, Yang Y. Research on the Chloramphenicol Removal Performance of Co-Doped Porous Carbon Materials Derived from Co-Zn Bimetallic ZIFs. Processes. 2024; 12(12):2670. https://doi.org/10.3390/pr12122670
Chicago/Turabian StyleChen, Ke-Hong, Ao-Tian Gu, Liang Zhang, Hao Tang, Zhi-Ming Zhang, Yi-Ming Zhou, Yu-Qi Cheng, and Yi Yang. 2024. "Research on the Chloramphenicol Removal Performance of Co-Doped Porous Carbon Materials Derived from Co-Zn Bimetallic ZIFs" Processes 12, no. 12: 2670. https://doi.org/10.3390/pr12122670
APA StyleChen, K.-H., Gu, A.-T., Zhang, L., Tang, H., Zhang, Z.-M., Zhou, Y.-M., Cheng, Y.-Q., & Yang, Y. (2024). Research on the Chloramphenicol Removal Performance of Co-Doped Porous Carbon Materials Derived from Co-Zn Bimetallic ZIFs. Processes, 12(12), 2670. https://doi.org/10.3390/pr12122670






