The Use of a Trichoderma reesei Culture for the Hydrolysis of Wheat Straw to Obtain Bioethanol
Abstract
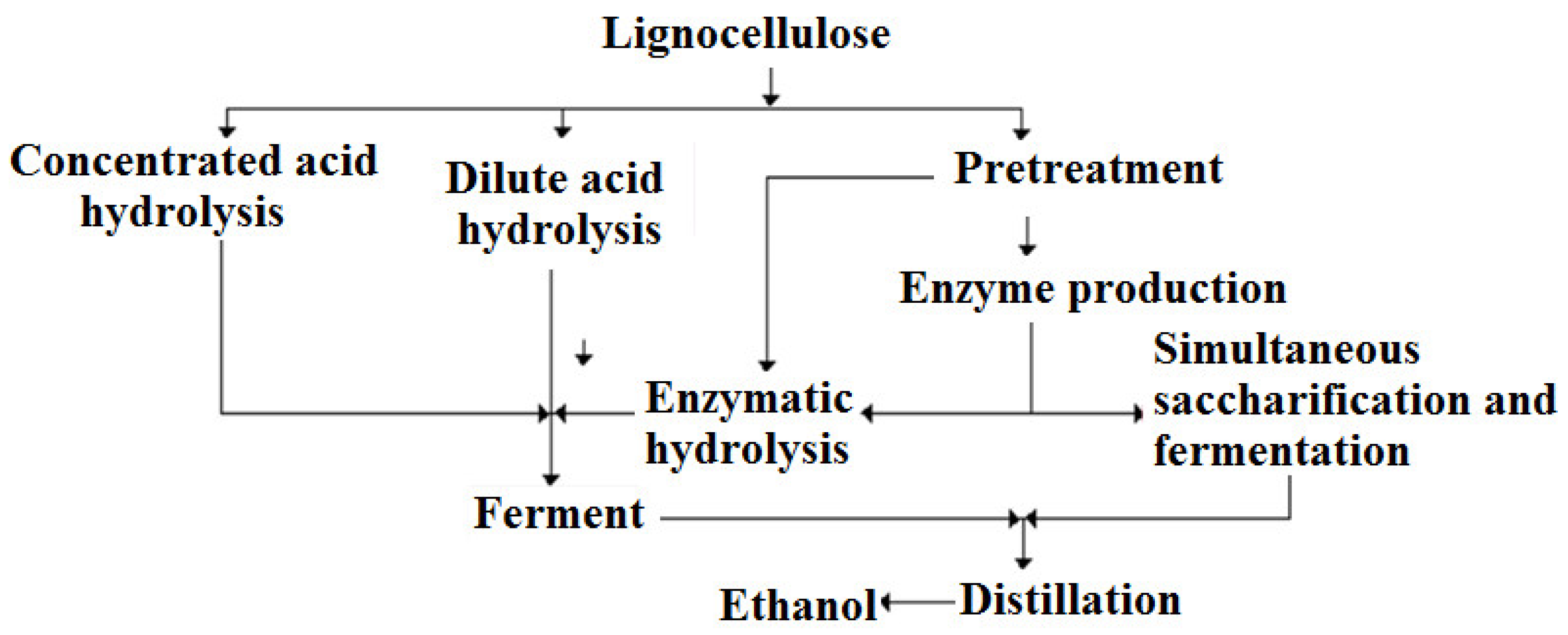
:1. Introduction
2. Materials and Methods
3. Results
3.1. Thermal Pretreatment of Wheat Straw
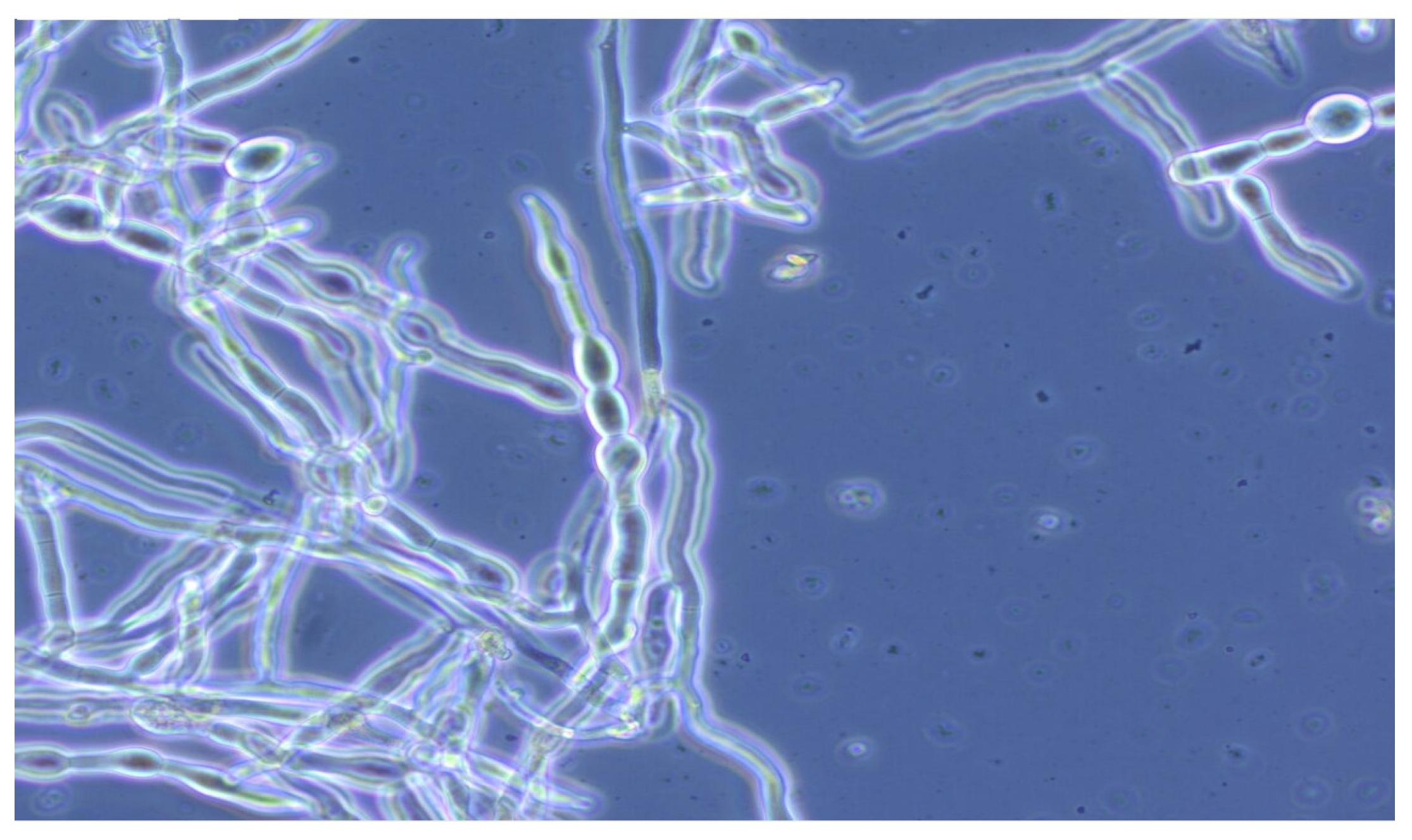
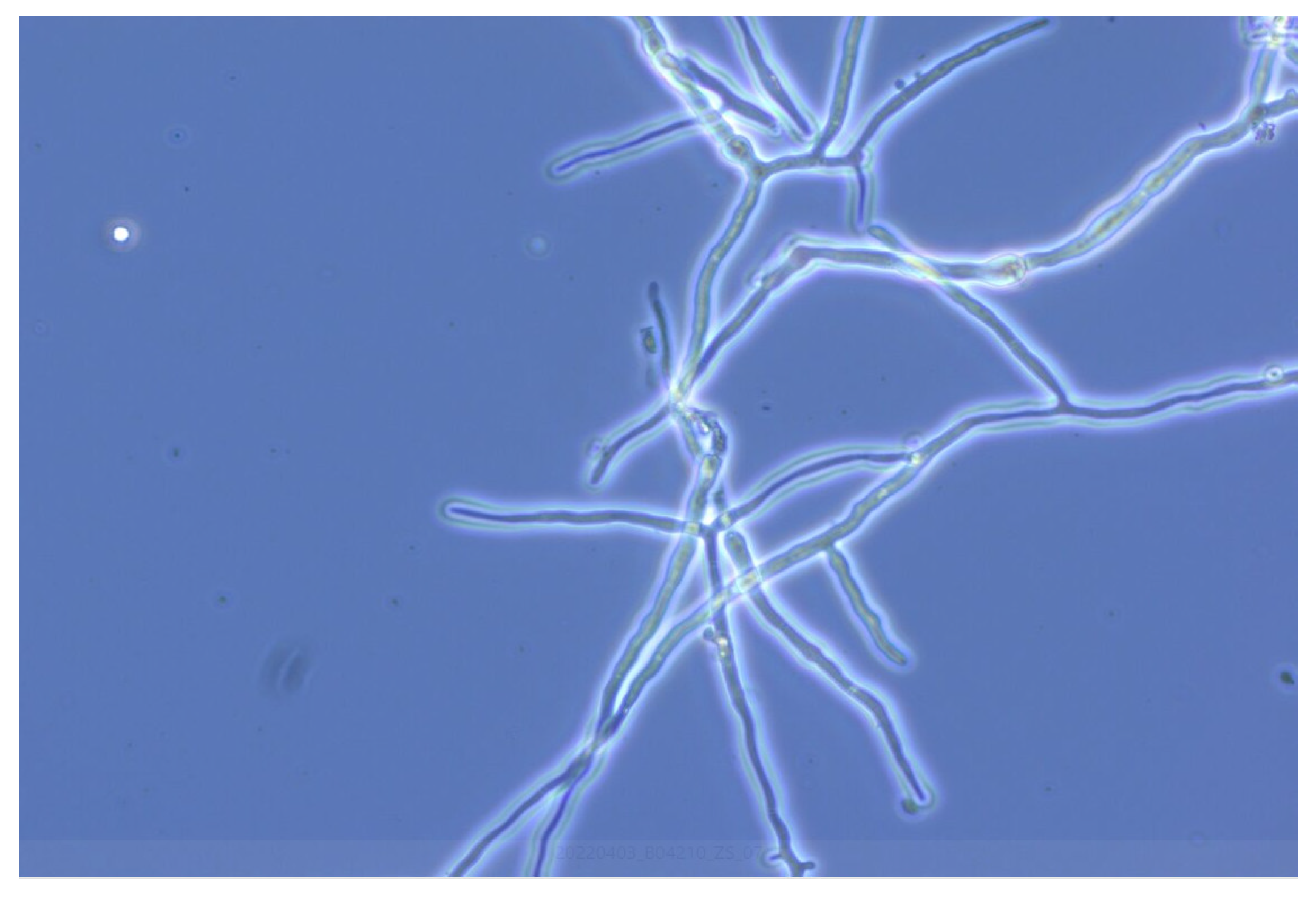
3.2. Enzymatic Hydrolysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taghizadeh-Alisaraei, A.; Tatari, A.; Khanali, M.; Keshavarzi, M. Potential of biofuels production from wheat straw biomass, current achievements and perspectives: A review. Biofuels 2022, 14, 79–92. [Google Scholar] [CrossRef]
- Fatma, S.; Saleem, A.; Tabassum, R. Wheat straw hydrolysis by using co-cultures of Trichoderma reesei and Monascus purpureus toward enhanced biodegradation of the lignocellulosic biomass in bioethanol biorefinery. Biomass Convers. Biorefinery 2021, 11, 743–754. [Google Scholar] [CrossRef]
- Karim, R.A.; Hussain, A.S.; Zain, A.M. Production of bioethanol from empty fruit bunches cellulosic biomass and Avicel PH-101 cellulose. Biomass Convers. Biorefinery 2014, 4, 333–340. [Google Scholar] [CrossRef]
- Kolasa, M.; Ahring, B.K.; Lübeck, P.S.; Lübeck, M. Cocultivation of Trichoderma reesei RutC30 with three black Aspergillus strains facilitates efficient hydrolysis of pretreated wheat straw and shows promises for on-site enzyme production. Bioresour. Technol. 2014, 169, 143–148. [Google Scholar] [CrossRef]
- Meehnian, H.; Jana, A.K.; Jana, M.M. Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification. Int. Biodeterior. Biodegrad. 2017, 117, 68–77. [Google Scholar] [CrossRef]
- Bhattacharya, A.S.; Bhattacharya, A.; Pletschke, B.I. Synergism of fungal and bacterial cellulases and hemicellulases: A novel. perspective for enhanced bio-ethanol production. Biotechnol. Lett. 2015, 37, 1117–1129. [Google Scholar] [CrossRef]
- Energia din Surse Regenerabile. Available online: https://www.europarl.europa.eu/factsheets/ro/sheet/70/energia-din-surse-regenerabile (accessed on 19 July 2024).
- Renewable Energy Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Renewable_energy_statistics (accessed on 20 July 2024).
- Cererea UE de Biocombustibili Tradiționali se va Reduce la Jumătate Până în 2050. Available online: https://www.economistul.ro/opinii-si-analize/cererea-ue-de-biocombustibili-traditionali-se-va-reduce-la-jumatate-pana-in-2050-64822/ (accessed on 28 July 2024).
- Rusănescu, C.O.; Ciobanu, M.; Rusănescu, M.; Dinculoiu, R.L. Pretreatments Applied to Wheat Straw to Obtain Bioethanol. Appl. Sci. 2024, 14, 1612. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Becze, A.; Vincze, E.-B.; Lányi, S.; Mara, G. Evaluation of structural carbohydrate degrading capacity of PGP bacterial strains using different methods. U.P.B. Sci. Bull. Ser. B 2022, 84, 1. [Google Scholar]
- Palonen, H. Role of Lignin in Enzymatic Hydrolysis of Lignocellulose; VTT Technical Research: Espoo, Finland, 2004; pp. 11–22+26–32. [Google Scholar]
- Hamelinck, C.N.; van Hooijdonk, G.A.; Faaij, P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Talluri, G.; Scarlat, N.; Prussi, M. The challenge of forecasting the role of biofuel in EU transport decarbonisation at 2050: A meta-analysis review of published scenarios. Renew. Sustain. Energy Rev. 2021, 139, 110715. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Sivakumar, N.; Verma, J.P. Deconstruction of lignocellulosic biomass for bioethanol production: Recent advances and future prospects. Fuel 2022, 327, 125109. [Google Scholar] [CrossRef]
- Valin, H.; Havlík, P.; Mosnier, A.; Herrero, M.; Schmid, E.; Obersteiner, M. Agricultural productivity and greenhouse gas emissions: Trade-offs or synergies between mitigation and food security? Environ. Res. Lett. 2013, 8, 035019. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- European Commission. COM. 640 Final—The European Green New Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar] [CrossRef]
- Danielis, R.; Scorrano, M.; Giansoldati, M. Decarbonising transport in Europe: Trends, goals, policies and passenger car scenarios. Res. Transp. Econ. 2022, 91, 101068. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Donoso-Bravo, A.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresour. Technol. 2013, 143, 251–257. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Balsari, P. The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 2012, 43, 273–282. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, X.; Dai, R.; Wang, Y.; Ma, P. Effect of low temperature of thermal pretreatment on anaerobic digestion of textile dyeing sludge. Bioresour. Technol. 2017, 243, 426–432. [Google Scholar] [CrossRef]
- Kontogianni, N.; Barampouti, E.M.; Mai, S.; Malamis, D.; Loizidou, M. Effect of alkaline pretreatments on the enzymatic hydrolysis of wheat straw. Environ. Sci. Pollut. Res. 2019, 26, 35648–35656. [Google Scholar] [CrossRef]
- Asghar, U.; Irfan, M.; Iram, M.; Huma, Z.; Nelofer, R.; Nadeem, M.; Syed, Q. Effect of alkaline pretreatment on delignification of wheat straw. Nat. Prod. Res. 2015, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jaisamut, K.; Paulová, L.; Patáková, P.; Kotúčová, S.; Rychtera, M. Effect of sodium sulfite on acid pretreatment of wheat straw with respect to its final conversion to ethanol. Biomass Bioenergy 2016, 95, 1–7. [Google Scholar] [CrossRef]
- Rahikainen, J.L.; Martin-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on nonproductive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Guan, X. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Yu, G.; Afzal, W.; Yang, F.; Padmanabhan, S.; Liu, Z.; Xie, H.; Shafy, M.; Bell, A.; Prausnitz, J. Pretreatment of Miscanthus × giganteus using aqueous ammonia with hydrogen peroxide to increase enzymatic hydrolysis to sugars. J. Chem. Technol. Biotechnol. 2014, 89, 698–706. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Shahryari, Z.; Fazaelipoor, M.H.; Setoodeh, P.; Nair, R.B.; Taherzadeh, M.J.; Ghasemi, Y. Utilization of wheat straw for fungal phytase production. Int. J. Recycl. Org. Waste Agric. 2018, 7, 345–355. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016, 215, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, Z.; Syed, Q.; Nadeem, M.; Irfan, M.; Wu, J.; Samra, Z.; Gul, I.; Athar, A. Enhanced production of cellulase by Trichoderma reesei using wheat straw as a carbon source. World Appl. Sci. J. 2014, 30, 1095–1104. [Google Scholar]
- Arnthong, J.; Chuaseeharonnachai, C.; Boonyuen, N.; Tachaapaikun, C.; Chimchana, D.; Eurwilaichitr, L.; Champreda, V.; Chantasingh, D. Cooperative decomposition of rice straw by co-cultivation of cellulolytic fungi. Chiangmai J. Sci. 2018, 2, 645–652. [Google Scholar]
- Anasontzis, G.E.; Thuy, N.T.; Hang, D.T.M.; Huong, H.T.; Thanh, D.T.; Hien, D.D.; Thanh, V.N.; Olsson, L. Rice straw hydrolysis using secretomes from novel fungal isolates from Vietnam. Biomass Bioenergy 2017, 99, 11–20. [Google Scholar] [CrossRef]





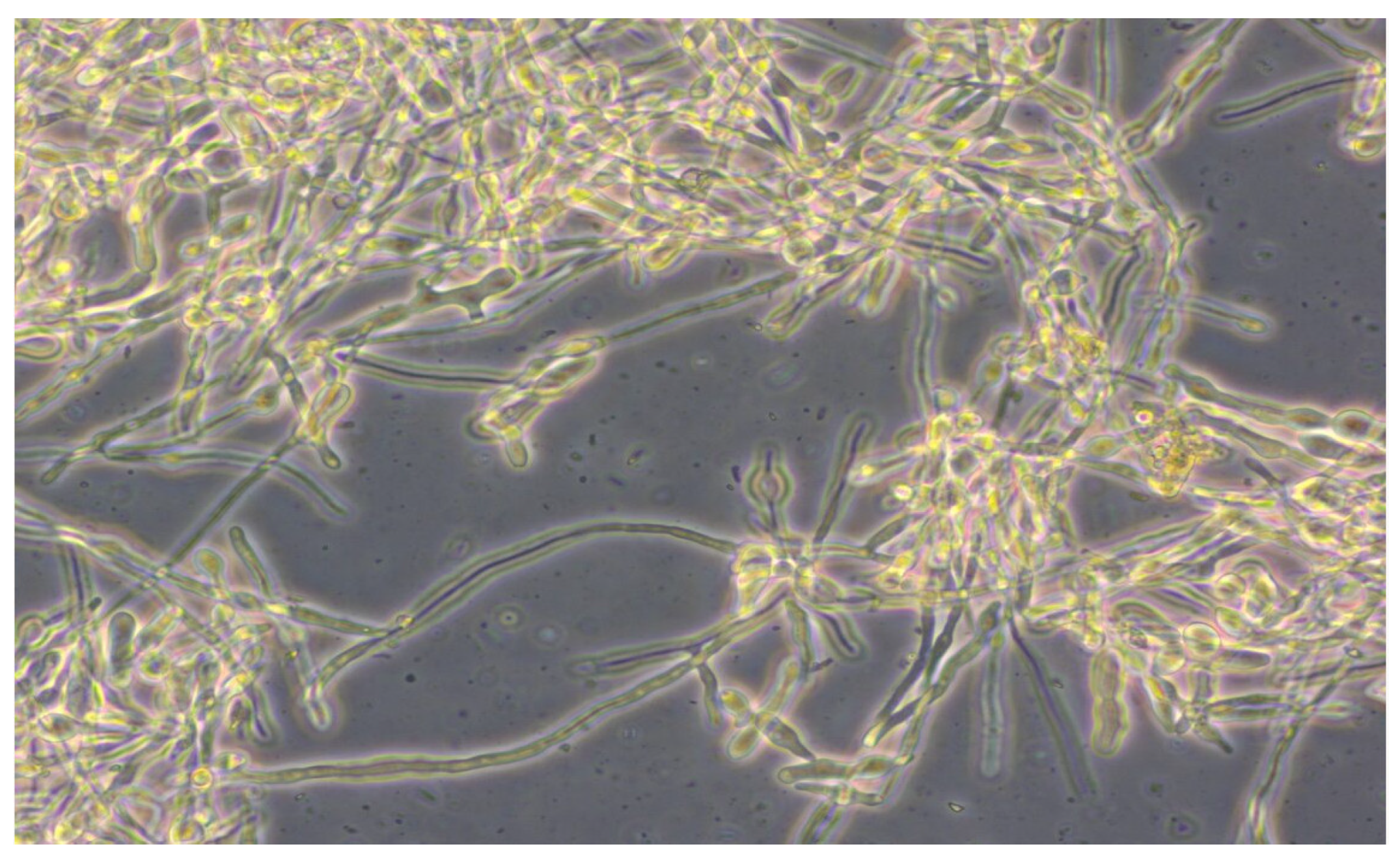

| Lot of Straw | Raw Material | Steam Pressure [bar] | Thermal Treatment Time [min] | Water Added [lt H2O] | Process Time [s] | pH Before Thermal Treatment | pH After Thermal Treatment |
|---|---|---|---|---|---|---|---|
| 1 | Wheat straw/1 t | 11.5 | 10 | 175 | 72 | 4.19 | 4.91 |
| 2 | Wheat straw/1 t | 11.5 | 10 | 175 | 72 | 4.19 | 4.88 |
| 3 | Wheat straw/1 t | 11.5 | 10 | 175 | 72 | 4.19 | 4.85 |
| Batch Straw Substrate | Glucose [mg/g] | Xylose [mg/g] | Sodium Lactate (NaDL) [mg/g] | Acetic Acid [mg/g] |
|---|---|---|---|---|
| 1 | 55.96 | 27.02 | 0.75 | 5.50 |
| 56.04 | 26.52 | 0.59 | 5.34 | |
| 59.67 | 29.07 | 0.72 | 5.65 | |
| Average value [mg/g] | 57.22 | 27.54 | 0.69 | 5.49 |
| Standard deviation (SD) | 2.12 | 1.35 | 0.85 | 0.15 |
| 2 | 54.73 | 26.76 | 0.26 | 5.04 |
| 54.61 | 26.57 | 0.25 | 5.03 | |
| 54.47 | 26.71 | 0.26 | 5.04 | |
| Average value [mg/g] | 54.60 | 26.68 | 0.26 | 5.04 |
| Standard deviation (SD) | 0.13 | 0.098 | 0 | 0 |
| 3 | 55.54 | 26.17 | 0.19 | 5.31 |
| 56.78 | 27.33 | 0.21 | 5.35 | |
| 55.43 | 26.22 | 0.30 | 6.28 | |
| Average value [mg/g] | 55.92 | 26.57 | 0.23 | 5.65 |
| Standard deviation (SD) | 0.75 | 0.65 | 0.06 | 0.55 |
| Lot of Straw | Raw Material | Dry Component [%] | Glucose [g/L] | Xylose [g/L] | Furfural [g/L] |
|---|---|---|---|---|---|
| 1 | Average wheat straw/1 t | 48.74 | 62.77 | 28.94 | 0.23 |
| 2 | Average wheat straw/1 t | 48.74 | 64.52 | 29.25 | 0.22 |
| 3 | Average wheat straw/1 t | 48.74 | 67.38 | 30.48 | 0.22 |
| Wheat Straw Size [mm] | Glucose [g/L] | Xylose [g/L] | Lactate (NaDL) [g/L] | Acetic Acid [g/L] | Hydroxymethyl Furfural (HMF) [g/L] | Furfural [g/L] |
|---|---|---|---|---|---|---|
| 20–30 | 55.67 | 18.85 | 1.25 | 8.75 | 1.20 | 1.15 |
| 54.76 | 18.76 | 1.23 | 8.67 | 1.3 | 1.1 | |
| 55.87 | 18.67 | 1.22 | 8.6 | 1.2 | 1.3 | |
| Average [g/L] | 55.43 | 18.76 | 1.23 | 8.67 | 1.23 | 1.18 |
| Standard deviation | 0.59 | 0.09 | 0.015 | 0.075 | 0.06 | 0.10 |
| 70–90 | 54.52 | 19.25 | 0.65 | 6.44 | 0.19 | 0.84 |
| 54.23 | 19.56 | 0.45 | 6.34 | 0.18 | 0.77 | |
| 53.87 | 18.78 | 0.55 | 6.21 | 0.17 | 0.85 | |
| Average [g/L] | 54.21 | 19.19 | 0.55 | 6.33 | 0.18 | 0.82 |
| Standard deviation | 0.32 | 0.39 | 0.1 | 0.11 | 0.01 | 0.04 |
| 50 | 64.89 | 29.56 | 0.67 | 4.58 | 0.17 | 0.22 |
| 63.78 | 28.987 | 0.56 | 4.87 | 0.15 | 0.18 | |
| 64.78 | 27.98 | 0.62 | 4.54 | 0.16 | 0.23 | |
| Average [g/L] | 64.48 | 28.84 | 0.62 | 4.66 | 0.16 | 0.21 |
| Standard deviation | 0.61 | 0.80 | 0.05 | 0.18 | 0.01 | 0.02 |
| Treatment | Moisture [%] | Lignin [%] | Hemicellulose [%] | Cellulose [%] |
|---|---|---|---|---|
| Untreated WS | 9.7 | 17.8 | 24.6 | 39.3 |
| Trichoderma reesei-treated WS | 9.9 | 11.06 | 20.9 | 33.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, M.; Rusănescu, C.O.; Dinculoiu, R.L. The Use of a Trichoderma reesei Culture for the Hydrolysis of Wheat Straw to Obtain Bioethanol. Processes 2024, 12, 2625. https://doi.org/10.3390/pr12122625
Ciobanu M, Rusănescu CO, Dinculoiu RL. The Use of a Trichoderma reesei Culture for the Hydrolysis of Wheat Straw to Obtain Bioethanol. Processes. 2024; 12(12):2625. https://doi.org/10.3390/pr12122625
Chicago/Turabian StyleCiobanu, Maria, Carmen Otilia Rusănescu, and Raluca Lucia Dinculoiu. 2024. "The Use of a Trichoderma reesei Culture for the Hydrolysis of Wheat Straw to Obtain Bioethanol" Processes 12, no. 12: 2625. https://doi.org/10.3390/pr12122625
APA StyleCiobanu, M., Rusănescu, C. O., & Dinculoiu, R. L. (2024). The Use of a Trichoderma reesei Culture for the Hydrolysis of Wheat Straw to Obtain Bioethanol. Processes, 12(12), 2625. https://doi.org/10.3390/pr12122625







