Pyrolysis of Hydrothermal Sewage Sludge and Food Waste Digestate for Heavy Metals Stabilization and Ecological Risk Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
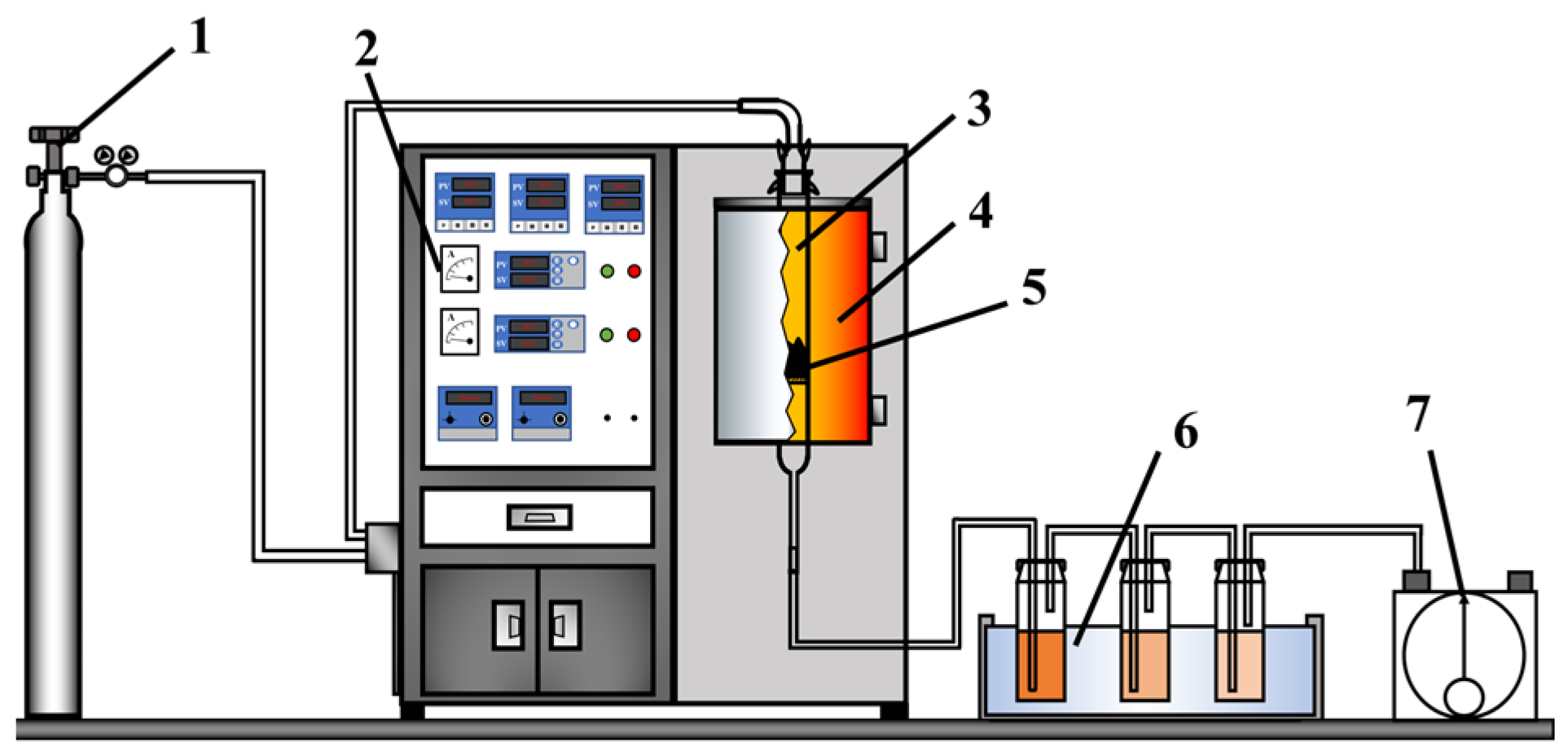
2.2. Co-Pyrolysis for Biochar Generation
2.3. Heavy Metals Analysis
3. Results
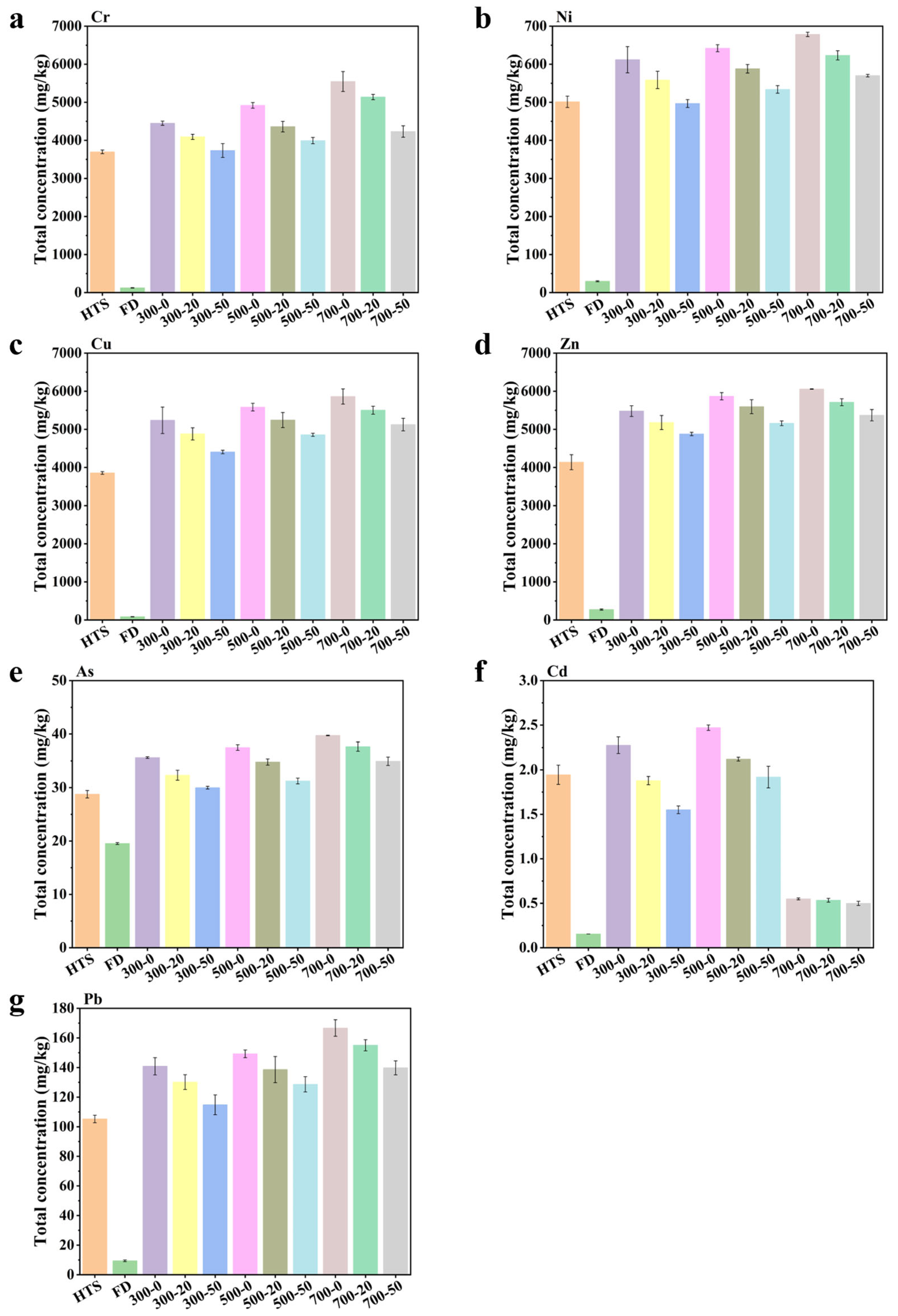
3.1. Heavy Metals Concentration
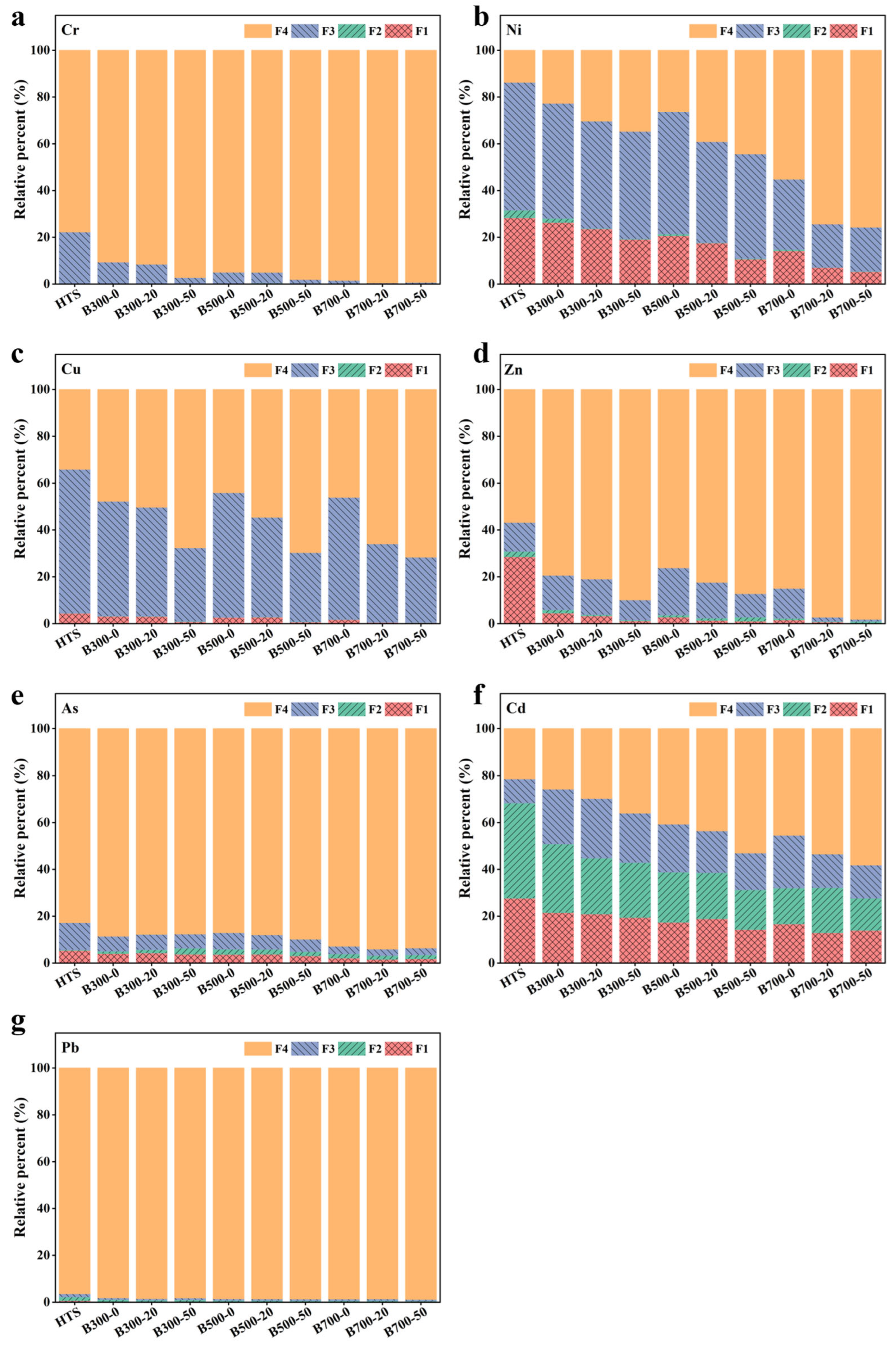
3.2. Heavy Metals Speciation Distribution
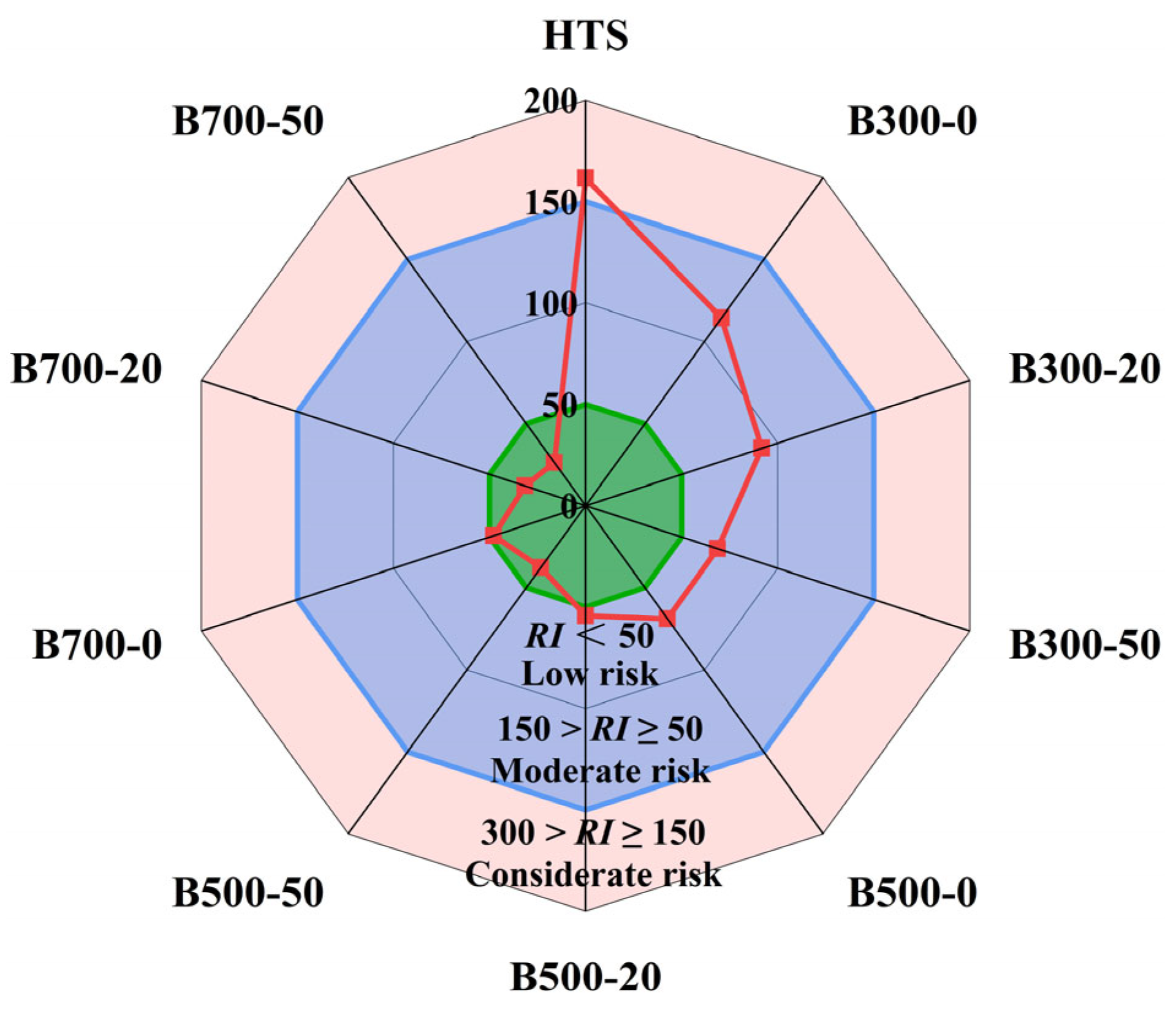
3.3. Risk Analysis of Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, G.; Zhang, G.M.; Wang, H.C. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Zhang, G.; Xu, Y.; Gao, N. Recent advances on the speciation distribution of heavy metals in sludge pyrolysis residue. CIESC J. 2022, 73, 134–143. [Google Scholar] [CrossRef]
- Abubakar, I.R.; Maniruzzaman, K.M.; Dano, U.L.; AlShihri, F.S.; AlShammari, M.S.; Ahmed, S.M.S.; Al-Gehlani, W.A.G.; Alrawaf, T.I. Environmental sustainability impacts of solid waste management practices in the global South. Int. J. Environ. Res. Public Health 2022, 19, 12717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wang, X.B.; Yu, S.L.; Deng, S.; Tan, H.Z.; Mikulčić, H. Process design and optimization on self-sustaining pyrolysis and carbonization of municipal sewage sludge. Waste Manag. 2023, 159, 125–133. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.Y.; Li, C.X. Hydrothermal Coupled Pyrolytic Treatment of Sewage Sludge and Food Waste Digestate for Heavy Metal Immobilization and Biochar Properties Enhancement. Energy Fuels 2024, 38, 3148–3158. [Google Scholar] [CrossRef]
- Li, C.X.; Wang, R.M.; Yuan, Z.W.; Xie, S.Y.; Wang, Y.; Zhang, Y.F. Novel strategy for efficient energy recovery and pollutant control from sewage sludge and food waste treatment. Water Res. 2024, 261, 122050. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Z.; Zhu, K.L.; Fan, J.J.; Clark, J.H.; Luo, G.; Zhang, S.C. Speciation evolution and transformation mechanism of P during microwave hydrothermal process of sewage sludge. Sci. Total Environ. 2022, 815, 152801. [Google Scholar] [CrossRef]
- Ling, M.X.; Ma, D.C.; Hu, X.; Liu, Z.; Wang, D.B.; Feng, Q.G. Hydrothermal treatment of polyvinyl chloride: Reactors, dechlorination chemistry, application, and challenges. Chemosphere 2023, 316, 137718. [Google Scholar] [CrossRef]
- Wang, Y.D.; Li, W.; Wang, Y.K.; Turap, Y.S.; Wang, Z.T.; Zhang, Z.; Xia, Z.; Wang, W. Anaerobic co-digestion of food waste and sewage sludge in anaerobic sequencing batch reactors with application of co-hydrothermal pretreatment of sewage sludge and biogas residue. Bioresour. Technol. 2022, 364, 128006. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.W.; Lin, J.J.; Li, C.J.; Jiang, R.Q.; Xing, Z.J.; Yu, C. Preparation of building ceramsite from food waste digestate residues, incineration fly ash and sludge biochar. Chem. Ind. Eng. Prog. 2022, 42, 1039–1050. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.W.; Xie, S.Y.; Jiang, R.Q.; Li, C.J.; Xing, Z.J. Pyrolysis of food waste digestate residues for biochar: Pyrolytic properties, biochar characterization, and heavy metal behaviours. Fuel 2023, 353, 129185. [Google Scholar] [CrossRef]
- Alves, C.T.; Onwudili, J.A.; Ghorbannezhad, P.; Kumagai, S. A review of the thermochemistries of biomass gasification and utilisation of gas products. Sustain. Energy Fuels 2023, 7, 3505–3540. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Zhou, X.; Peng, Z.L.; Wan, S.; Gao, Z.F.; Deng, S.S.; Tong, L.L.; Han, W.; Chen, X. Co-pyrolysis technology for enhancing the functionality of sewage sludge biochar and immobilizing heavy metals. Chemosphere 2023, 317, 137929. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.-C.; Cho, J.-S.; Lee, S.-E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Cui, M.H.; Ren, Y.G.; Guo, J.C.; Zheng, Z.Y.; Liu, H. Towards Understanding the Mechanism of Heavy Metals Immobilization in Biochar Derived from Co-pyrolysis of Sawdust and Sewage Sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 489–496. [Google Scholar] [CrossRef]
- Liu, J.Y.; Huang, L.M.; Zou, H.H.; Xie, W.M.; Evrendilek, D.E.; Luo, G.; Ninomiya, Y. Do FeCl3 and FeCl3/CaO conditioners change pyrolysis and incineration performances, emissions, and elemental fates of textile dyeing sludge? J. Hazard. Mater. 2021, 413, 125334. [Google Scholar] [CrossRef]
- Sun, S.C.; Huang, X.F.; Lin, J.H.; Ma, R.; Fang, L.; Zhang, P.X.; Qu, J.L.; Zhang, X.H.; Liu, Y.L. Study on the effects of catalysts on the immobilization efficiency and mechanism of heavy metals during the microwave pyrolysis of sludge. Waste Manag. 2018, 77, 131–139. [Google Scholar] [CrossRef]
- Shemwell, B.; Atal, A.; Levendis, Y.A.; Simons, G.A. A Laboratory Investigation on Combined In-Furnace Sorbent Injection and Hot Flue-Gas Filtration to Simultaneously Capture SO2, NOx, HCl, and Particulate Emissions. Environ. Sci. Technol. 2000, 34, 4855–4866. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, T.; Yao, P.; Xiang, W.; Wu, Y.; Li, J. Co-pyrolysis of sewage sludge and phosphate tailings: Synergistically enhancing heavy metal immobilization and phosphorus availability. Waste Manag. 2024, 181, 44–56. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, W.; Wang, B.Y.; Zhang, X.Y.; Li, J. Effect of different calcium salts on phosphorus availability and immobilization of heavy metals in municipal sludge derived biochar. J. Water Process Eng. 2024, 57, 104708. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.W.; Lin, J.J.; Jiang, R.Q.; Xu, X.H.; Xie, S.Y.; Wang, Y. Synergistic hydrothermal treatment of food waste digestate residues and incineration fly ash: Dehydration performance and heavy metals safety. React. Chem. Eng. 2022, 7, 1797–1806. [Google Scholar] [CrossRef]
- Gotore, O.; Itayama, T.; Dang, B.-T.; Nguyen, T.-D.; Ramaraj, R.; Osamu, N.; Shuji, T.; Maseda, H. Adsorption analysis of ciprofloxacin and delafloxacin onto the corn cob derived-biochar under different pyrolysis conditions. Biomass Convers. Biorefinery 2024, 14, 10373–10388. [Google Scholar] [CrossRef]
- Barrow, C. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- O’Laughlin, J.; McElligott, K. Biochar for environmental management: Science and technology, Johannes Lehmann, Stephen, M. Joseph (Eds.), Earthscan, London UK (2009), 448 p. For. Policy Econ. 2009, 11, 535–536. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Thomas Klasson, K.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.X.; Wang, H.T.; Lu, W.J.; Zhou, Z.Y.; Zhang, Y.C.; Ren, L.L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Li, C.X.; Li, J.; Pan, L.J.; Zhu, X.Y.; Xie, S.Y.; Yu, G.W.; Wang, Y.; Pan, X.F.; Zhu, G.F.; Angelidaki, I. Treatment of digestate residues for energy recovery and biochar production: From lab to pilot-scale verification. J. Clean. Prod. 2020, 265, 121852. [Google Scholar] [CrossRef]
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. Standardization Administration of the People’s Republic of China (SAC), 2012.
- Udayanga, W.D.C.; Veksha, A.; Giannis, A.; Liang, Y.N.; Lisak, G.; Hu, X.; Lim, T.-T. Insights into the speciation of heavy metals during pyrolysis of industrial sludge. Sci. Total Environ. 2019, 691, 232–242. [Google Scholar] [CrossRef]
- Wang, X.D.; Chi, Q.Q.; Liu, X.J.; Wang, Y. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 2019, 216, 698–706. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Guo, S.; Yu, S.X.; Che, D.Y.; Liu, H.P.; Sun, B.Z. Migration characteristics of heavy metals during co-combustion of dehydrated sludge with straw. J. Fuel Chem. Technol. 2022, 50, 283–294. [Google Scholar] [CrossRef]
- Xie, S.Y.; Yu, G.W.; Li, C.X.; Li, J.; Wang, G.; Dai, S.Q.; Wang, Y. Treatment of high-ash industrial sludge for producing improved char with low heavy metal toxicity. J. Anal. Appl. Pyrolysis 2020, 150, 104866. [Google Scholar] [CrossRef]
- Yu, F.; Lv, H.B.; Fan, L.A.; Chen, L.S.; Hu, Y.J.; Wang, X.; Guo, Q.Q.; Cui, X.Q.; Zhou, N.; Jiao, L. Co-pyrolysis of sewage sludge and poplar sawdust under controlled low-oxygen conditions: Biochar properties and heavy metals behavior. J. Anal. Appl. Pyrolysis 2023, 169, 105868. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol. 2014, 162, 308–315. [Google Scholar] [CrossRef]
- Kistler, R.C.; Widmer, F.; Brunner, P.H. Behavior of chromium, nickel, copper, zinc, cadmium, mercury, and lead during the pyrolysis of sewage sludge. Environ. Sci. Technol. 1987, 21, 704–708. [Google Scholar] [CrossRef]
- Trinkel, V.; Mallow, O.; Thaler, C.; Schenk, J.; Rechberger, H.; Fellner, J. Behavior of Chromium, Nickel, Lead, Zinc, Cadmium, and Mercury in the Blast Furnace—A Critical Review of Literature Data and Plant Investigations. Ind. Eng. Chem. Res. 2015, 54, 11759–11771. [Google Scholar] [CrossRef]
- Huang, H.J.; Yuan, X.Z. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour. Technol. 2016, 200, 991–998. [Google Scholar] [CrossRef]
- Li, C.X.; Xie, S.Y.; You, F.T.; Zhu, X.Y.; Li, J.; Xu, X.H.; Yu, G.W.; Wang, Y.; Angelidaki, I. Heavy metal stabilization and improved biochar generation via pyrolysis of hydrothermally treated sewage sludge with antibiotic mycelial residue. Waste Manag. 2021, 119, 152–161. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Guo, R.X.; Yang, J.L.; Liu, D.Y.; Liu, Z.Y. The fate of As, Pb, Cd, Cr and Mn in a coal during pyrolysis. J. Anal. Appl. Pyrolysis 2003, 70, 555–562. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Ju, R.; Zhou, H.T.; Chen, H.W. Migration characteristics of heavy metals during sludge pyrolysis. Waste Manag. 2021, 120, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Gu, W.H.; Geng, Z.X.; Bai, J.F.; Dong, B.; Zhao, J.; Zhuang, X.N.; Shih, K.M. Immobilization of heavy metals in biochar by co-pyrolysis of sludge and CaSiO3. J. Environ. Manag. 2023, 326, 116635. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liang, L.; Wang, L.H.; Ni, Q.L.; Yang, K.; Zhang, J.; Chen, D.L.; Yang, J.J.; Shen, X.D. Roles of pyrolysis on availability, species and distribution of Cu and Zn in the swine manure: Chemical extractions and high-energy synchrotron analyses. Chemosphere 2013, 93, 2094–2100. [Google Scholar] [CrossRef]
- Yuan, H.R.; Lu, T.; Huang, H.Y.; Zhao, D.D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, L.; Lv, J.T.; Li, G.; Wen, B.; Ma, Y.B.; Huang, R.X. Copper Speciation Evolution in Swine Manure Induced by Pyrolysis. Environ. Sci. Technol. 2020, 54, 9008–9014. [Google Scholar] [CrossRef]
- Jin, J.W.; Wang, M.Y.; Cao, Y.C.; Wu, S.C.; Liang, P.; Li, Y.N.; Zhang, J.Y.; Zhang, J.; Wong, M.H.; Shan, S.D.; et al. Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: Biochar properties and environmental risk from metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef]
- Huang, R.X.; Zhang, B.; Saad, E.M.; Ingall, E.D.; Tang, Y. Speciation evolution of zinc and copper during pyrolysis and hydrothermal carbonization treatments of sewage sludges. Water Res. 2018, 132, 260–269. [Google Scholar] [CrossRef]
- Udayanga, W.D.C.; Veksha, A.; Giannis, A.; Lisak, G.; Lim, T.-T. Effects of sewage sludge organic and inorganic constituents on the properties of pyrolysis products. Energy Convers. Manag. 2019, 196, 1410–1419. [Google Scholar] [CrossRef]
- Han, H.D.; Hu, S.; Lu, C.F.; Wang, Y.; Jiang, L.; Xiang, J.; Su, S. Inhibitory effects of CaO/Fe2O3 on arsenic emission during sewage sludge pyrolysis. Bioresour. Technol. 2016, 218, 134–139. [Google Scholar] [CrossRef]
- Wang, X.D.; Chang, V.W.-C.; Li, Z.W.; Song, Y.; Li, C.X.; Wang, Y. Co-pyrolysis of sewage sludge and food waste digestate to synergistically improve biochar characteristics and heavy metals immobilization. Waste Manag. 2022, 141, 231–239. [Google Scholar] [CrossRef]
| Sample | Proximate Analysis (wt.%) | Elemental Analysis (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ash | VM a | FC b | N | C | H | S | O | H/C | N/C | |
| HTS | 57.54 | 40.13 | 2.33 | 2.68 | 22.21 | 7.75 | 1.91 | 7.91 | 4.19 | 0.10 |
| FD | 48.12 | 46.53 | 5.35 | 2.06 | 19.86 | 4.81 | 1.13 | 24.02 | 2.91 | 0.09 |
| Temperature | Feedstocks | Naming of Biochar Samples |
|---|---|---|
| 300 °C | HTS + 0% FD | B300-0, B500-0, B700-0 |
| 500 °C | HTS + 20% FD | B300-20, B500-20, B700-20 |
| 700 °C | HTS + 50% FD | B300-50, B500-50, B700-50 |
| Samples | Cr | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|
| HTS | 0.11/NR | 28.38/MR | 4.47/LR | 28.62/MR | 5.37/LR | 27.70/MR | 0.64/NR |
| B300-0 | 0.02/NR | 26.33/MR | 3.19/LR | 4.53/LR | 4.14/LR | 21.55/MR | 0.23/NR |
| B300-20 | 0.01/NR | 23.58/MR | 3.12/LR | 3.34/LR | 4.28/LR | 20.99/MR | 0.13/NR |
| B300-50 | 0.01/NR | 19.1/MR | 0.86/NR | 1.20/LR | 3.79/LR | 19.44/MR | 0.13/NR |
| B500-0 | 0.01/NR | 20.68/MR | 2.69/LR | 2.80/LR | 3.74/LR | 17.40/MR | 0.14/NR |
| B500-20 | 0.01/NR | 17.57/MR | 2.81/LR | 1.40/LR | 3.77/LR | 18.92/MR | 0.12/NR |
| B500-50 | 0.01/NR | 10.67/MR | 0.72/NR | 1.09/LR | 3.13/LR | 14.36/MR | 0.10/NR |
| B700-0 | 0.01/NR | 14.22/MR | 1.73/LR | 1.61/LR | 2.12/LR | 16.73/MR | 0.12/NR |
| B700-20 | 0.00/NR | 7.08/LR | 0.55/NR | 0.78/NR | 1.59/LR | 12.98/MR | 0.09/NR |
| B700-50 | 0.00/NR | 5.31/LR | 0.43/NR | 0.22/NR | 1.84/LR | 13.99/MR | 0.10/NR |
| Sample | Cf | Er | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Cu | Zn | As | Cd | Pb | Cr | Ni | Cu | Zn | As | Cd | Pb | |
| HTS | 0.29 | 6.30 | 1.94 | 0.76 | 0.21 | 3.68 | 0.04 | 0.57 | 37.80 | 9.69 | 0.76 | 2.09 | 110.5 | 0.19 |
| B300-0 | 0.10 | 3.42 | 1.10 | 0.26 | 0.13 | 2.89 | 0.02 | 0.21 | 20.49 | 5.48 | 0.26 | 1.29 | 86.60 | 0.09 |
| B300-20 | 0.09 | 2.30 | 0.99 | 0.24 | 0.14 | 2.37 | 0.02 | 0.19 | 13.79 | 4.94 | 0.24 | 1.39 | 71.09 | 0.08 |
| B300-50 | 0.03 | 1.88 | 0.48 | 0.11 | 0.14 | 1.78 | 0.02 | 0.06 | 11.29 | 2.39 | 0.11 | 1.42 | 53.34 | 0.09 |
| B500-0 | 0.05 | 2.81 | 1.27 | 0.31 | 0.15 | 1.46 | 0.01 | 0.11 | 16.87 | 6.35 | 0.31 | 1.49 | 43.82 | 0.07 |
| B500-20 | 0.05 | 1.56 | 0.83 | 0.21 | 0.14 | 1.30 | 0.01 | 0.10 | 9.37 | 4.16 | 0.21 | 1.37 | 38.96 | 0.07 |
| B500-50 | 0.02 | 1.26 | 0.44 | 0.15 | 0.11 | 0.89 | 0.01 | 0.04 | 7.54 | 2.18 | 0.15 | 1.13 | 26.59 | 0.07 |
| B700-0 | 0.02 | 0.81 | 1.17 | 0.18 | 0.08 | 1.20 | 0.01 | 0.03 | 4.88 | 5.86 | 0.18 | 0.78 | 36.06 | 0.07 |
| B700-20 | 0.01 | 0.35 | 0.52 | 0.03 | 0.06 | 0.87 | 0.01 | 0.01 | 2.08 | 2.59 | 0.03 | 0.64 | 26.16 | 0.07 |
| B700-50 | 0.01 | 0.32 | 0.40 | 0.02 | 0.07 | 0.72 | 0.01 | 0.02 | 1.93 | 1.98 | 0.02 | 0.69 | 21.60 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, R.; Zhang, G. Pyrolysis of Hydrothermal Sewage Sludge and Food Waste Digestate for Heavy Metals Stabilization and Ecological Risk Reduction. Processes 2024, 12, 2614. https://doi.org/10.3390/pr12122614
Wang Y, Wang R, Zhang G. Pyrolysis of Hydrothermal Sewage Sludge and Food Waste Digestate for Heavy Metals Stabilization and Ecological Risk Reduction. Processes. 2024; 12(12):2614. https://doi.org/10.3390/pr12122614
Chicago/Turabian StyleWang, Yu, Ruming Wang, and Guangyi Zhang. 2024. "Pyrolysis of Hydrothermal Sewage Sludge and Food Waste Digestate for Heavy Metals Stabilization and Ecological Risk Reduction" Processes 12, no. 12: 2614. https://doi.org/10.3390/pr12122614
APA StyleWang, Y., Wang, R., & Zhang, G. (2024). Pyrolysis of Hydrothermal Sewage Sludge and Food Waste Digestate for Heavy Metals Stabilization and Ecological Risk Reduction. Processes, 12(12), 2614. https://doi.org/10.3390/pr12122614







