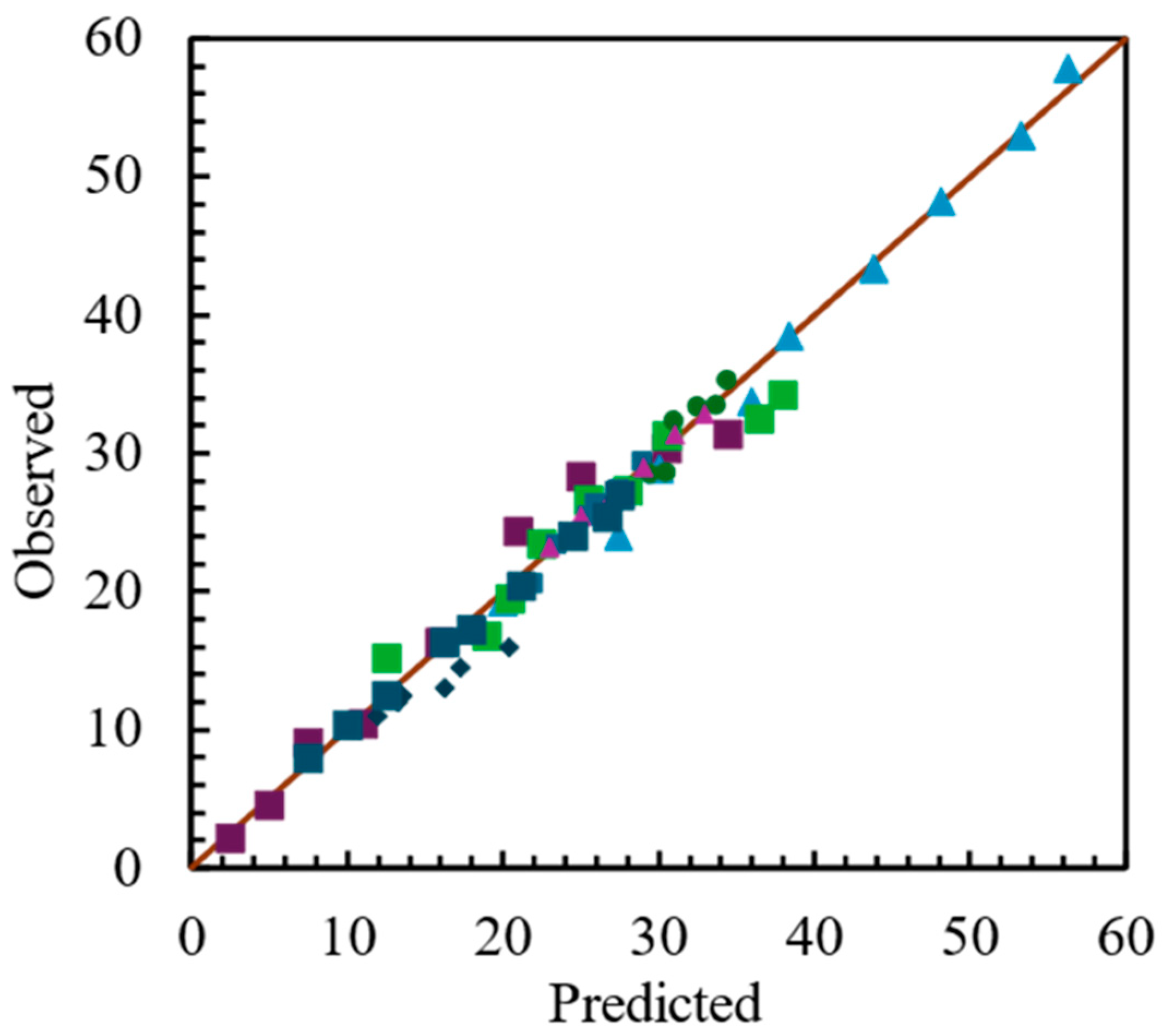
The compositions of the reactor effluent with different dual mixtures of one mole of total substrate at three different proportions were simulated. Because each mixture is different to any other, and there are an infinite number of possible dual mixtures, there are no experimental results available for the mixtures below. Consequently, the parameters adjusted for individual substrates were used in the prediction of the composition of the reactor effluent.
Cane bagasse was used as the ‘pivot’ waste, because of its high content of water. Two more substrates, pine sawdust and wheat straw, were mixed with the cane bagasse in order to assess the effect of using a dry substrate on the product distribution at the gasification reactor outlet. The results can be used to predict the behavior of the gasification reactor for each mixture of substrates, based on the individual (and validated) results for the gasification of each one individually.
3.1. Sugar Cane Bagasse and Pine Sawdust
The first mixture to be analyzed was sugar cane bagasse and pine sawdust, in three different proportions—8:2, 7:3, and 6:4. These proportions were selected because the incorporation of more pine sawdust prevents the gasification reactions. An atomic account of the first mixture, with the proportion 8:2, is given in
Table 2.
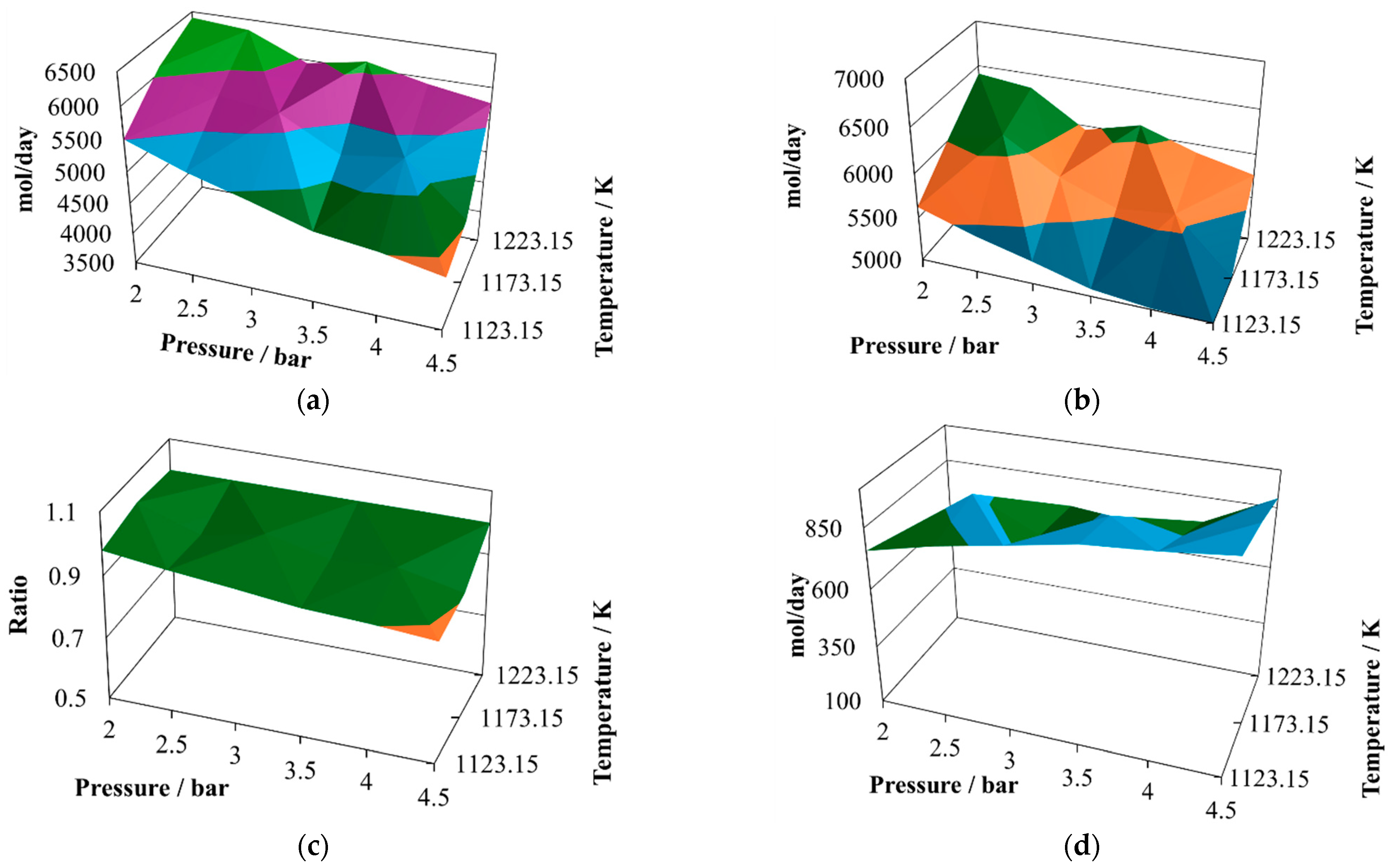
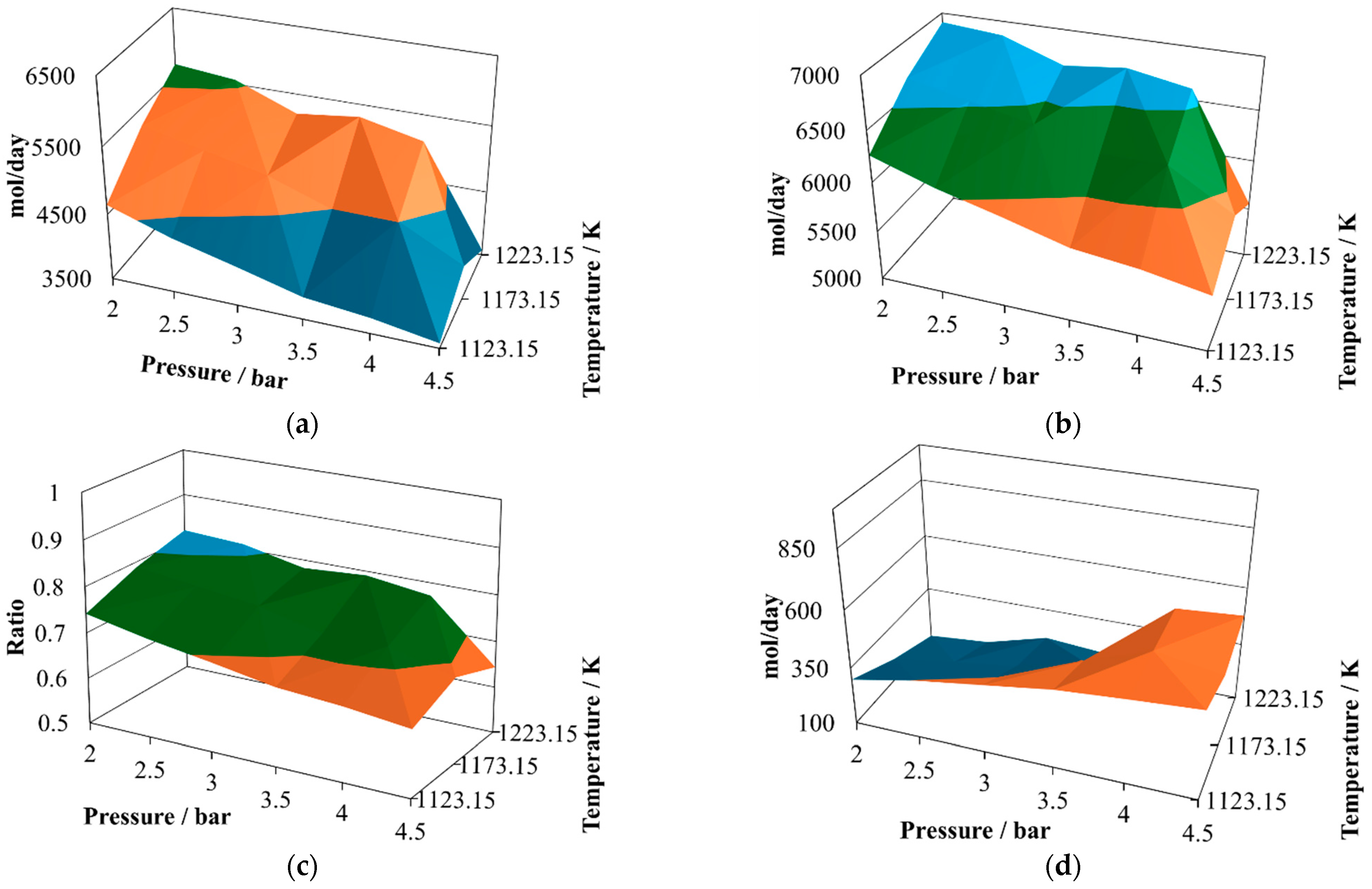
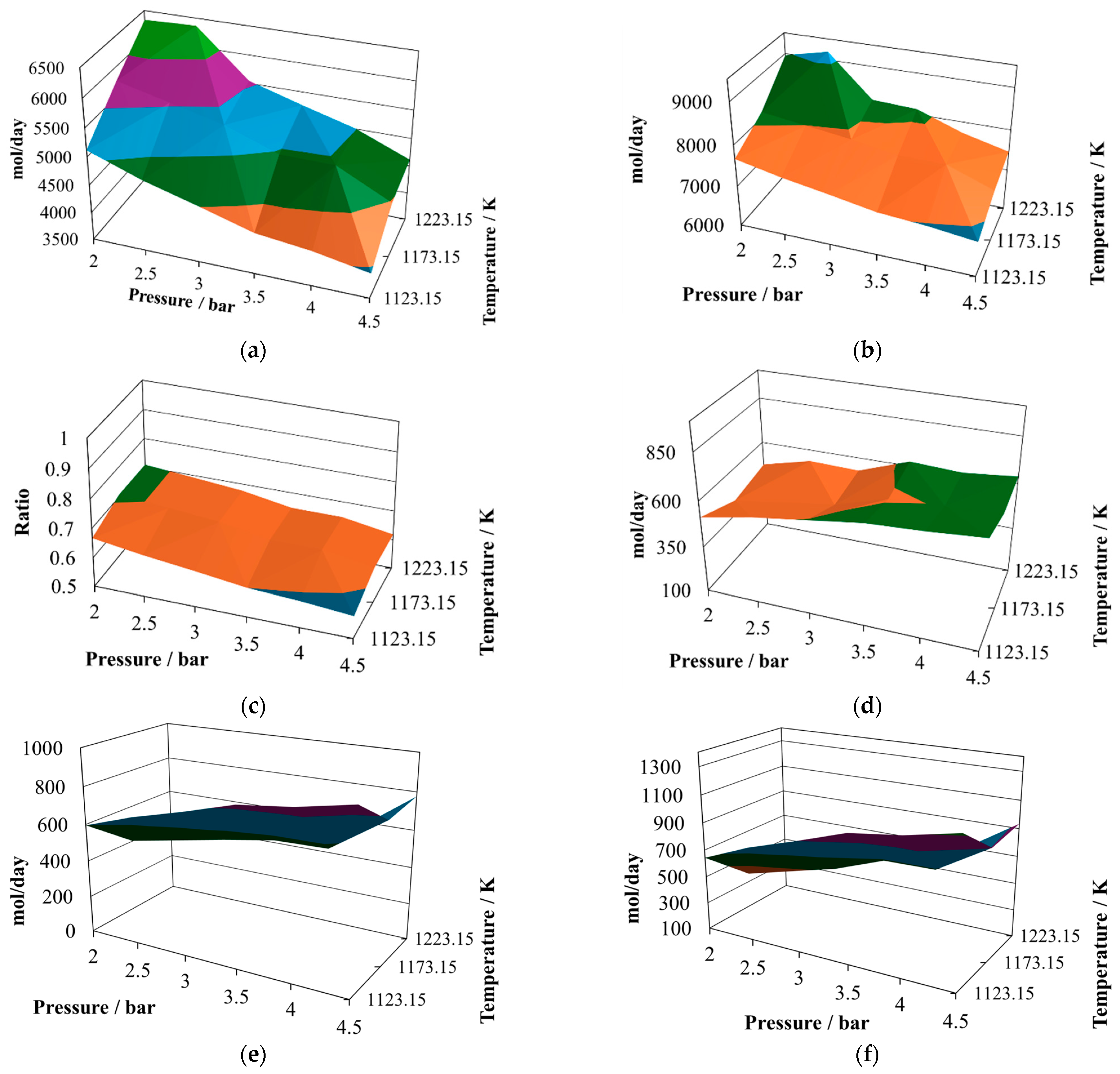
The production of molecular hydrogen at the proportion 8:2 (
Figure 2a) exhibits two noticeable maxima, one at 2.5 bar and the other at 3.8 bar, both at 1223.15 K. Therefore, it is possible to infer that there should be points close to the optimum yield of hydrogen. On the other hand, the minimum values are reached at the maximum operating pressure, which means that the pressure inversely affects the hydrogen production.
The production of CO at the proportion 8:2 (
Figure 2b) follows a similar trend to that exhibited by molecular hydrogen, showing two possible relative maximum points of production, at 2.0 bar and 1223.15 K and 3.5 bar and 1223.15 K. Both gasses are the principal components of the syngas, and therefore it is possible to infer that syngas production is favored at low pressures and the maximum operating temperature.
The H
2/CO ratio at the proportion 8:2 (
Figure 2c) follows a different pattern, which is important given the effect this ratio has on the potential uses of this syngas. In the simulated cases, this ratio remains mostly unchanged, oscillating between values of 0.85 and unity. This result agrees with experimental observations made previously [
6,
13].
The water available in the reactor effluent at the proportion 8:2 exhibits slight changes (
Figure 2d), exhibiting its relative maximum at 4.5 bar and 1223.15 K. Although the water profile oscillates, it is possible to infer that its content in the reactor effluent is improved by increasing the pressure, and the same behaviors were observed previously in the context of CLC production, taking water–gas shift as one of the main reactions; further, in gasification, water shift was proposed as one of the main schematic reactions [
16].
The production of carbon dioxide at the proportion 8:2 (
Figure 2e) exhibits an oscillating behavior, with its relative maximum value achieved at 4.5 bar and 1173.15 K, and two relative minima reached at 2.0 bar and 1123.15 K and 4.5 bar and 1223.15 K. Therefore, its production is favored at high pressure and intermediate temperature and prevented at high pressure and either low or elevated temperature.
Finally, the production of methane at the proportion 8:2 (
Figure 2f) exhibits medium oscillations, reaching its relative maximum at 4.5 bar, 1173.15 K, and its relative minimum at 4.5 bar, 1223.15 K. It also shows an incline with the relative maximum at the intermediate value.
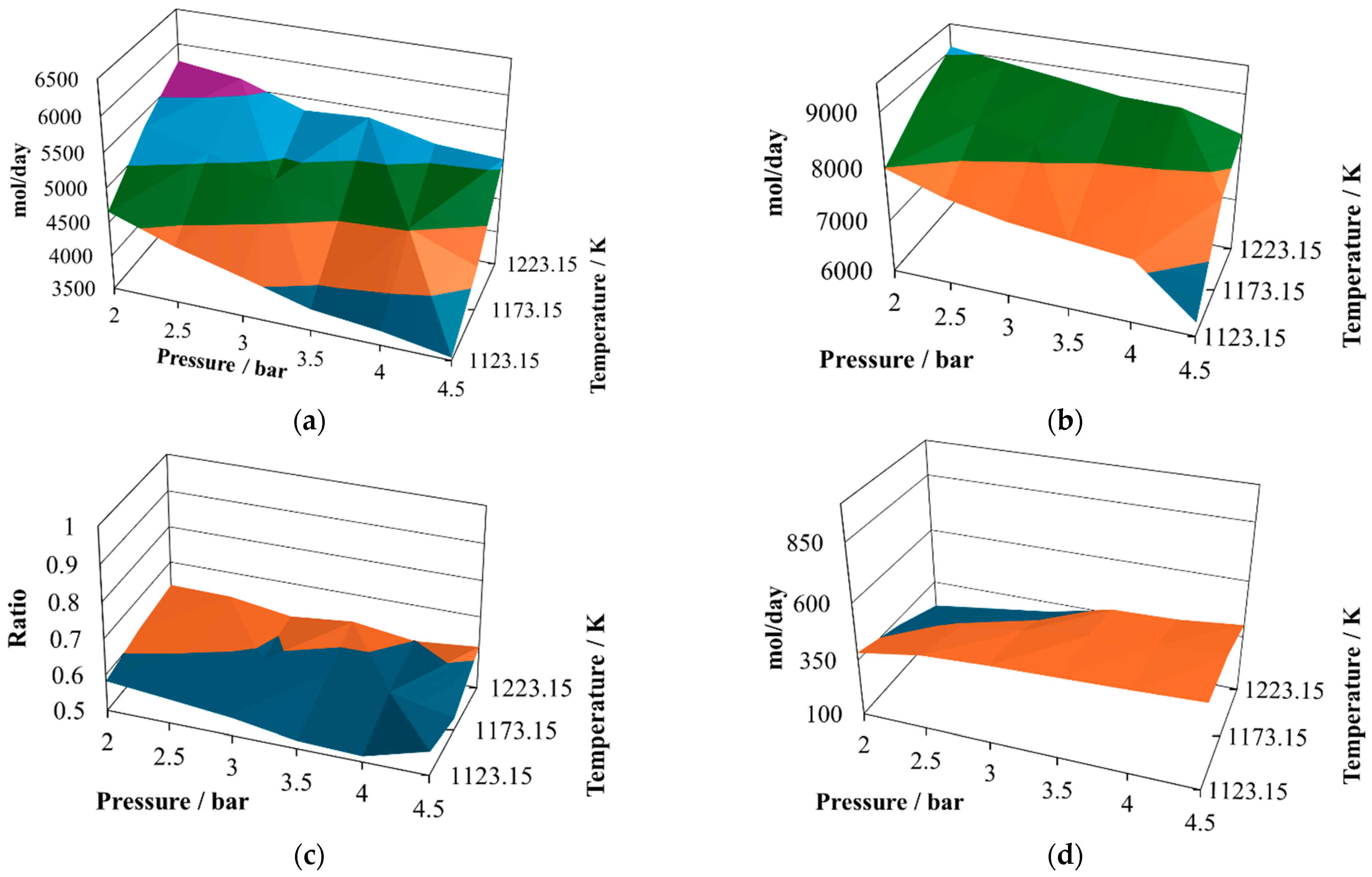
When the proportion is changed to 7:3, the global behavior will be similar, with changes in some variables. The atomic account of the second mixture, with a proportion of 7:3, is given in
Table 3.
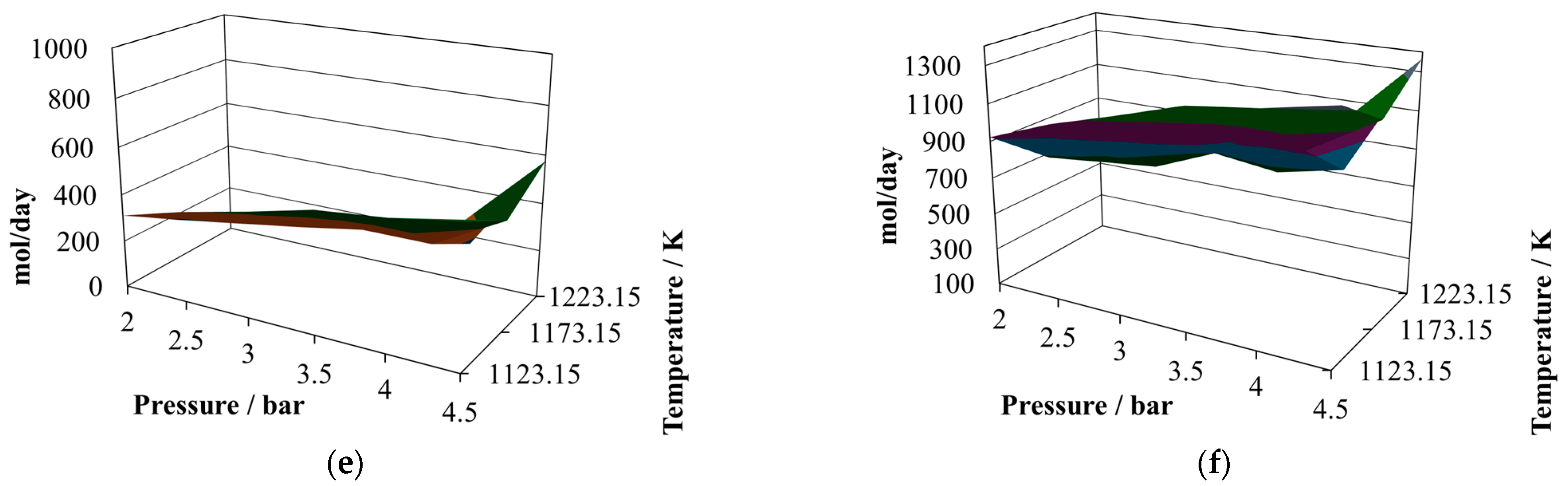
The first change is that the H
2/CO ratio exhibits some decrease, reaching a relative maximum close to one at 2.0 bar and 1223.15 K, but with a smaller minimum value than in the case of the proportion 7:3. However, the productivities of hydrogen and carbon monoxide at the proportion 8:2 are similar to those reached at the proportion 7:3. Hence, the H
2/CO ratio is not a good quality criteria, as has been mentioned in the literature [
17].
As regards the water in the reactor effluent at the proportion 7:3 (
Figure 3d), there is a decrease in all its values, and its relative minimum is observed at intermediate temperatures. This situation changes the shapes of the profiles to hill-like ones.
The level of carbon dioxide produced remains at similar values for the proportion 7:3 (
Figure 3e) in comparison with 8:2 (
Figure 2e); however, the shape of the profile changes, reaching its relative maximum at 2.5 bar and 1123.15 K and its relative minimum at 4.5 bar and 1223.15 K.
The production of methane changes shape at the proportion 7:3, increasing its content in the reactor effluent and reaching two relative maximum values at 2.0 bar and 1173.15 K and 4.5 bar and 1173.15 K. Further, in the case of the relative minima, it is possible to observe oscillatory behavior, with the minima reached at 2.5 bar and 1123.15 K and 4.5 bar and 1223.15 K.
The atomic account of the third mixture, with the proportion 6:4, is given in
Table 4.
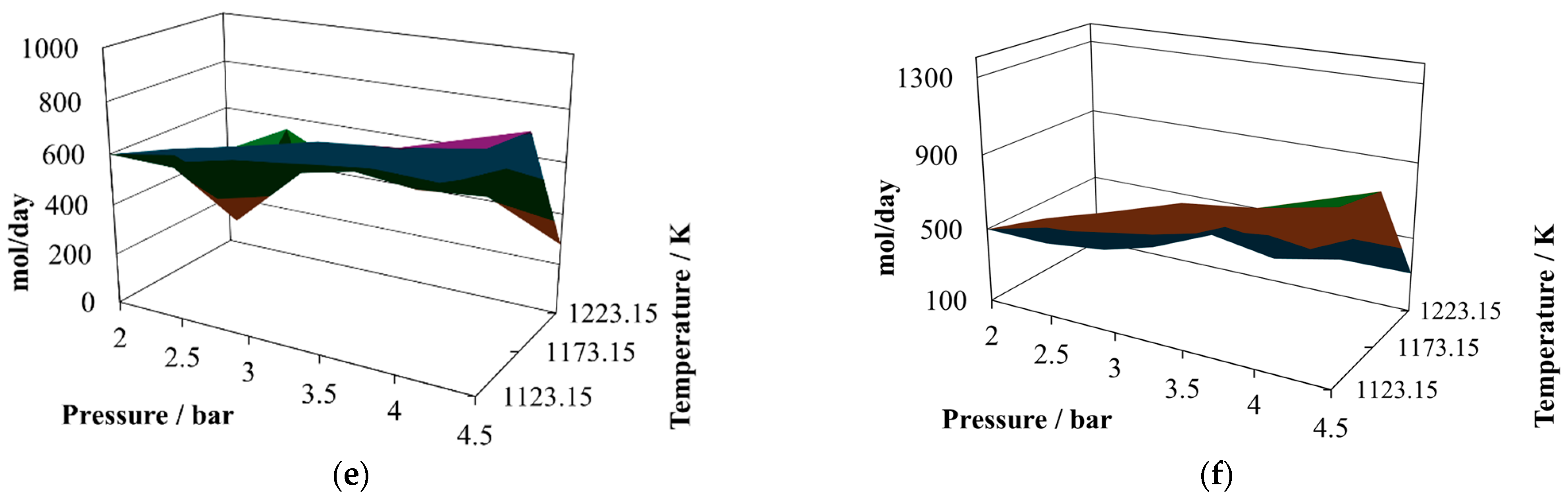
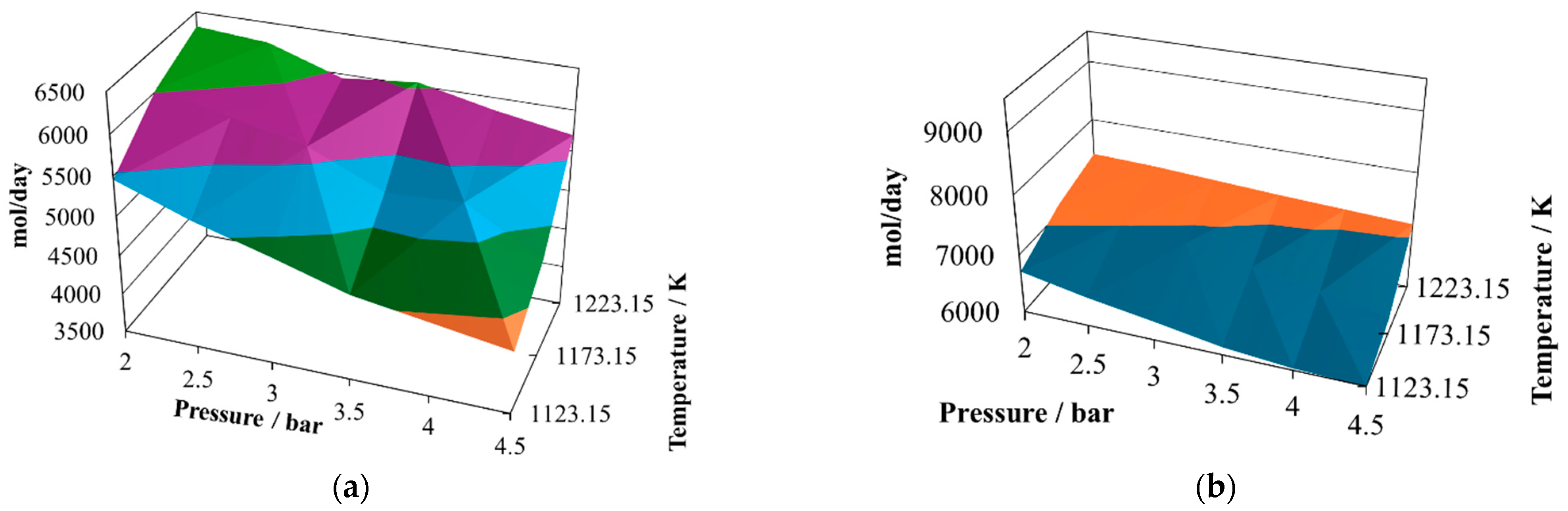
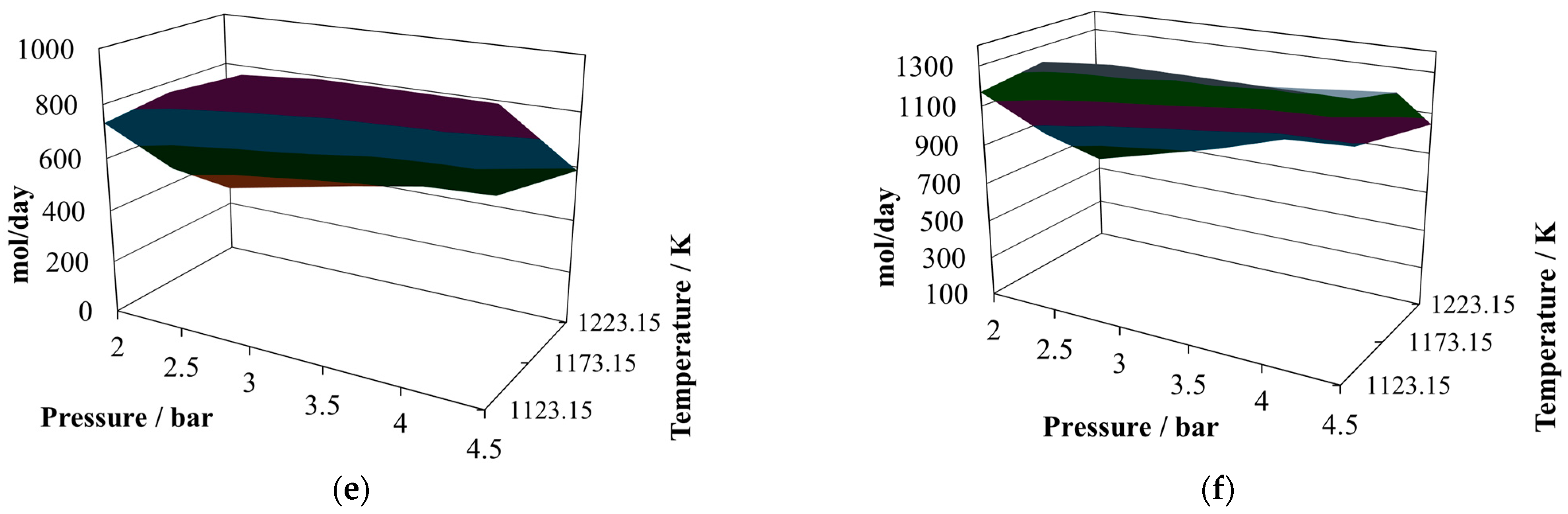
The production of hydrogen (
Figure 4a) decreases with respect to the previous proportions and exhibits an oscillating behavior. Its relative maximum is reached at 2.0 bar and 1223.15 K, like in the previous two cases, but now there are two relative minima at 4.5 bar and 1123.15 K and 4.5 bar and 1223.15 K. Therefore, the conclusion is that lower pressure favors hydrogen production. The production of CO (
Figure 4b) remains similar to that in the other two cases, as does its relative maximum and relative minimum. Due to the changes in hydrogen production, the profiles of the H
2/CO ratio (
Figure 4c) change to a hill-like shape, reaching the relative maximum at 2.0 bar and 1223.15 K and showing two relative minima, both close to 0.65, at 4.5 bar and 1123.15 K and 4.5 bar and 1223.15 K. Again, although the levels of production of hydrogen and CO are similar to those in the previous cases, this ratio changes significantly, which is not a particularly good quality parameter to use to classify the syngas. In comparing the mixtures containing the three proportions of sugar cane bagasse and pine sawdust, it can be noted that the levels of production of hydrogen and carbon monoxide remained similar in the three cases; however, the H
2/CO ratio changed its behavior noticeably. The amounts of water (
Figure 4d), carbon dioxide (
Figure 4e) and methane (
Figure 4f) in the reactor effluents decreased in the sequence 8:2, 7:3, and 6:4; this situation is a consequence of the equilibrium conditions and difficult to associate with any single influence.
3.2. Sugar Cane Bagasse and Wheat Straw
The second mixture to be analyzed comprised sugar cane bagasse and wheat straw, in the same three proportions (8:2, 7:3, and 6:4). The operating conditions for the gasification reactor here were the same as in the last case, so it is possible to compare the reactor effluents directly. An atomic account of the fourth mixture, with the proportion 8:2, is given in
Table 5.
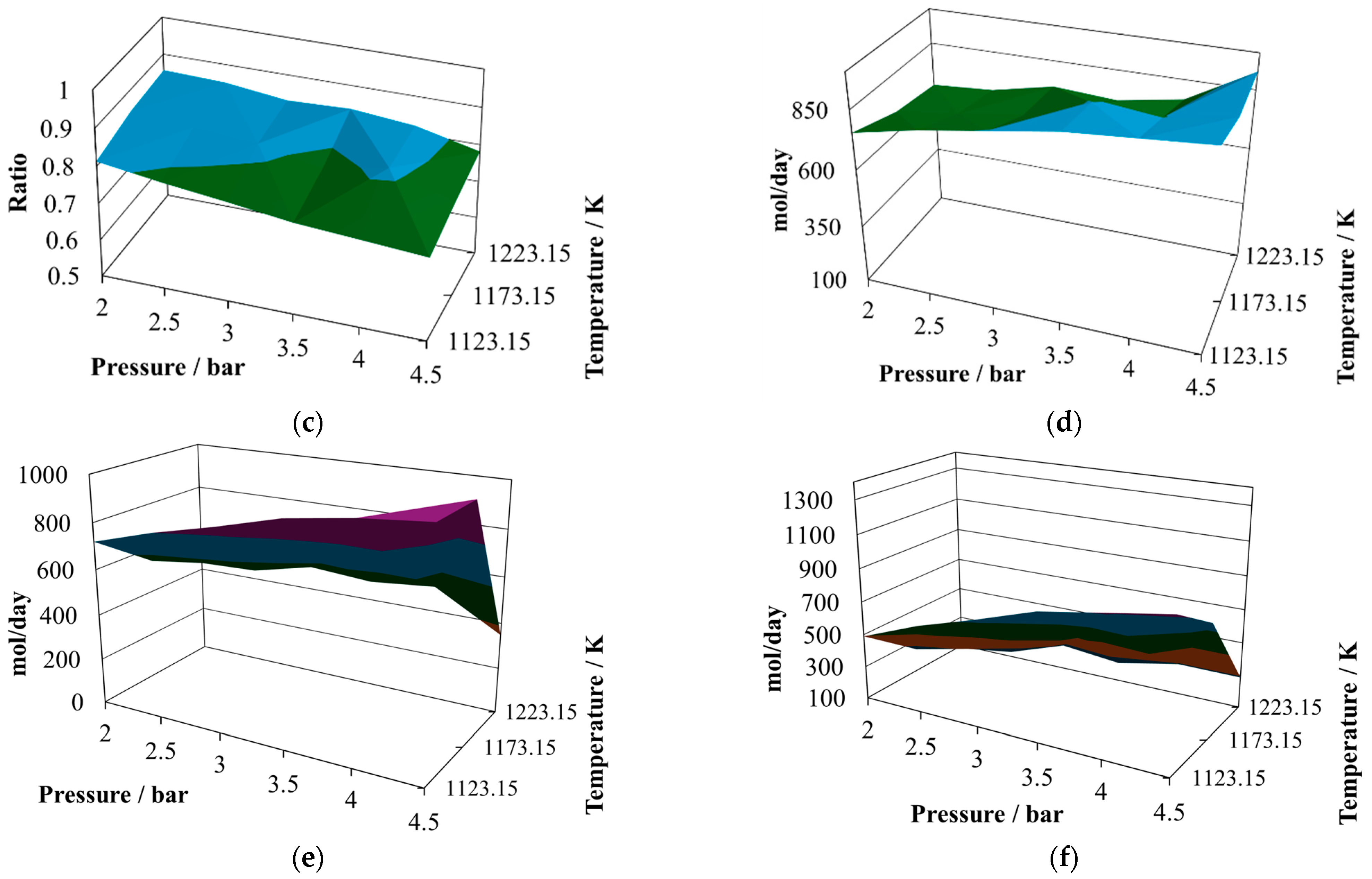
The production of molecular hydrogen at the proportion 8:2 (
Figure 5a) exhibits two noticeable maxima, one at 2.5 bar and the other at 3.8 bar, both at 1223.15 K. Again, it is possible to infer that there are points that should be close to the optimum yield of hydrogen. The relative minimum values are reached at the maximum operating pressure, which means that the pressure inversely affects the hydrogen production. The production of carbon monoxide at the proportion 8:2 (
Figure 5b) still shows slight changes in all operating regions, reaching two relative maxima at 2.0 bar and 1123 K and 4.5 bar and 1223 K. This level of production reaches its relative minimum at 4.5 bar and 1123 K. Although these values are stable, they are larger than in the case of the other mixture.
The H
2/CO ratio values exhibited are small at the proportion 8:2 (
Figure 5c), reaching two relative maxima of about 0.84 at 2.0 bar and 1223 K and 4.5 bar and 1173 K. However, for this mixture, the level of hydrogen production is similar to that yielded by the mixture of sugar cane bagasse and pine sawdust, but the level of production of carbon monoxide is higher.
The amount of water in the gasification reactor outlet at the proportion 8:2 (
Figure 5d) reaches its relative maximum at 4.5 bar and 1223 K and its relative minimum at 2.0 bar and 1223 K. Its shape is hill-like and oscillating, while the relative changes are sensitive.
The production of carbon dioxide at the proportion 8:2 (
Figure 5e) exhibits an oscillating behavior, with its relative maximum value reached at 4.5 bar and 1173.15 K and its relative minimum reached at 4.5 bar and 1223.15 K. Therefore, its production is favored at high pressures and intermediate temperatures and prevented at high pressures and elevated temperatures.
The production of methane at the proportion 8:2 (
Figure 5f) exhibits medium oscillations, reaching its relative maximum at 4.5 bar and 1173.15 K and its relative minimum at 4.5 bar and 1223.15 K. Therefore, high pressures and intermediate temperatures favor the production of methane.
The fifth mixture, with the proportion 7:3, exhibits a somewhat different and oscillating behavior, and the atomic account of this mixture is given in
Table 6.
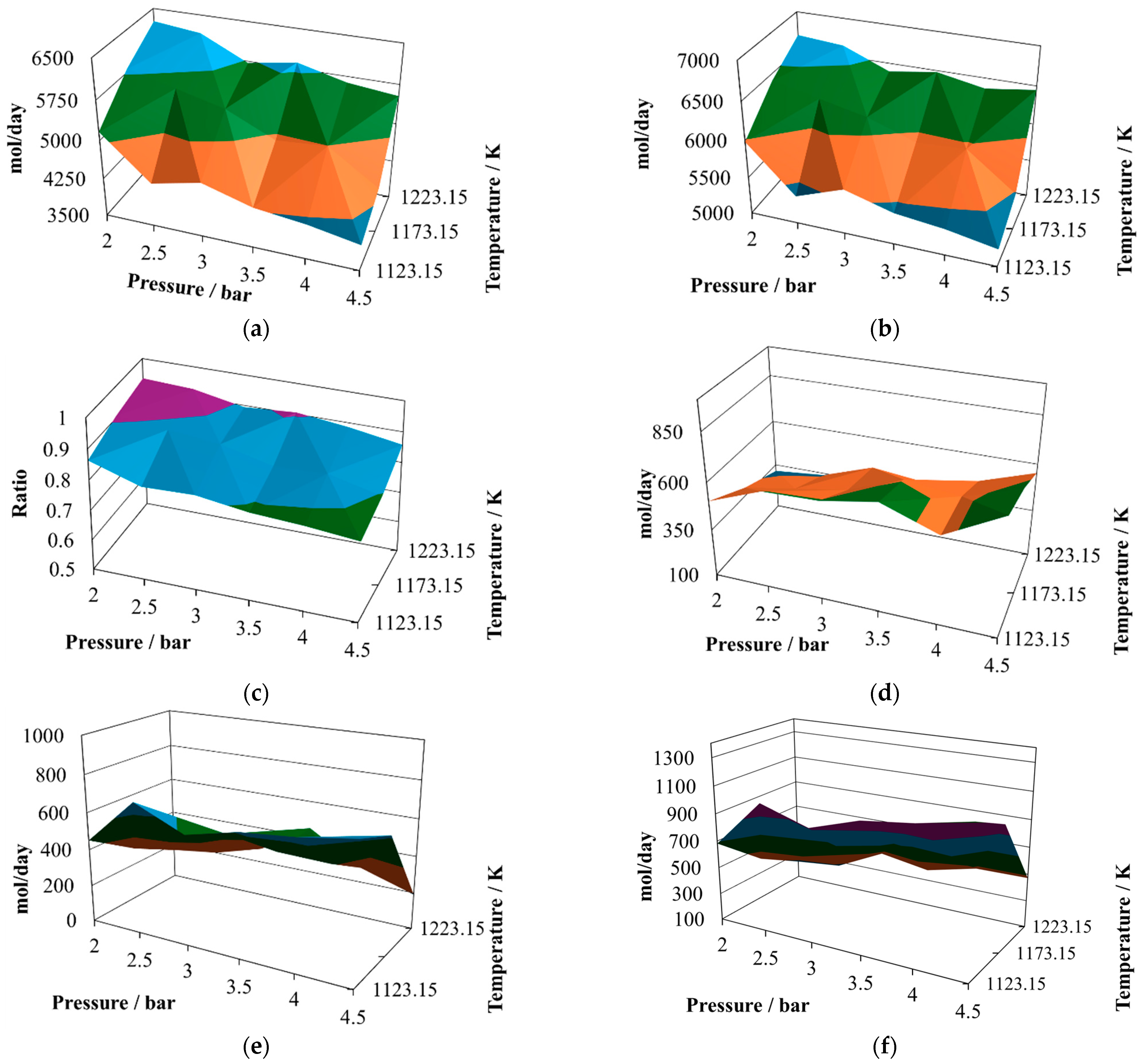
The productivities of hydrogen (
Figure 6a) and carbon monoxide (
Figure 6b) exhibit hill-like shapes, with their relative maximum showing a plateau between 2.0 bar and 2.5 bar and at a temperature of 1223.15 K. Both exhibit two relative minima at 4.5 bar and 1123.15 K and 4.5 bar and 1223.15 K. The amounts produced are the highest for both gasses analyzed in this work. In fact, the profiles of both production curves are remarkably similar.
As a consequence of the similarity mentioned above, the H
2/CO ratio (
Figure 6c) continues to show slight changes in all the operating regions, exhibiting values as low as 0.73 at 2.0 bar and 1223.15 K and 0.54 at 4.5 bar and 1123.15 K. Again, this ratio is not a suitable quality index to indicate the availability of hydrogen.
The amount of water in the reactor effluent (
Figure 6d) exhibits its relative maximum at 4.5 bar and 1223.15 K and its relative minimum at 2.0 bar and 1173 K. Its behavior is consistent, without profound changes in the operating zone, as a consequence of the equilibrium reached by the reactions.
The production of carbon dioxide (
Figure 6e) remains consistent without profound changes in the operating zone, reaching its relative maximum at 4.5 bar and 1123.15 K and its relative minimum at 2.0 bar and 1173.15 K.
Finally, the production of methane (
Figure 6f) exhibits slight changes in the operating region, reaching two relative maxima at 4.5 bar and 1123.15 K and 4.5 bar and 1223 K, and reaching its relative minimum at 2.0 bar and 1173.15 K.
For the last simulation, the atomic account of the sixth mixture, the proportion 6:4, is given in
Table 7.
The levels of hydrogen production for this mixture oscillate (
Figure 7a), reaching three relative maxima at 2.0 bar and 1223.15 K, 2.5 bar and 1223.15 K, and 3.5 bar and 1223.15 K. The CO production (
Figure 7b) exhibits slight changes in the operating region, reaching relative maxima at 2.0 bar and 1223.15 K and 4.0 bar and 1223.15 K. It also exhibits sensitive changes at elevated temperatures, reaching its minimum at 4.5 bar and 1123.15 K.
The H
2/CO ratio (
Figure 7c) exhibits very low values, reaching its maximum of 0.6 at 4.0 bar, 1173.15 K, and its minimum of 0.5 at 4.0 bar, 1173.15 K. Again, these values do not reflect the amount of hydrogen in the reactor effluent.
The water in the reactor effluent (
Figure 7d) exhibits an oscillating behavior, reaching two relative maxima at 4.5 bar and 1123.15 K and 4.5 bar and 1173.15 K. The production of carbon dioxide (
Figure 7e) is very flat, reaching its maximum at 4.0 bar and 1226.15 K and its minimum at 2.0 bar and 1223.15 K. Finally, the production of methane (
Figure 7f) exhibits very low changes, reaching its relative maximum as a plateau between 2.0 bar and 1223.15 K and 2.5 bar and 1223 K; its minimum is reached at 2.0 bar and 1223.15 K.
The amounts of gasses produced changed according to the proportions of the sugar cane bagasse–wheat straw mixture. The productions of hydrogen and carbon monoxide changed minutely for the mixture with a proportion of 7:3, exhibiting their maximum values. As regards the other mixtures, the production of hydrogen was slower for the mixture with a proportion of 6:4, and the lowest level of carbon monoxide production was obtained for the mixture 8:2. As regards the H2/CO ratio, the observed values decreased from mixture to mixture, even when the levels of production of the gasses were very different, indicating again that this ratio does not closely reflect the characteristics of the syngas. The amount of water in the reactor effluent decreased from simulation to simulation, as a consequence of the equilibrium reached by the reactions. The production of carbon dioxide exhibited its maximum level for the mixture with the proportion 8:2, its minimum in the mixture with the proportion 7:3, and an intermediate value in the mixture with a proportion of 6:4: again, all as consequences of the equilibrium. The production of methane increased slowly from the first to the second mixture but increased very notably for the third mixture.
Comparing the production profiles between the mixtures of pine sawdust with sugar cane bagasse and wheat straw with sugar cane bagasse, we found that all the values were higher for the mixtures with wheat straw. This was expected, given the greater amounts of carbon and hydrogen atoms supplied by each mixture; however, it is not easy to predict the final distribution of these atoms in the products of the gasification reactions. Therefore, this methodology of using empiric stoichiometry to reflect the mass balances in the gasification reactor, constrained by the equilibrium conditions for the chemical reactions, contributes to the prediction of production profiles during the gasification of mixtures of lignocellulosic waste, giving results similar to the experimental results. Simultaneously, substrates typically considered as waste are here valued as useful, contributing to the circular economy in Mexico, as suggested by Nizami et al. (2018) [
17].