Investigation of a Hydrophobic Sputtered Cu Electrode to Electrocatalyze CO2 Towards a C2+ Product: The Effect of Substrate and Catalyst Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Cathode Electrode Preparation
2.2. Anode Electrode Preparation
2.3. Characterization
2.4. Electrochemical CO2RR and Product Analysis
3. Results
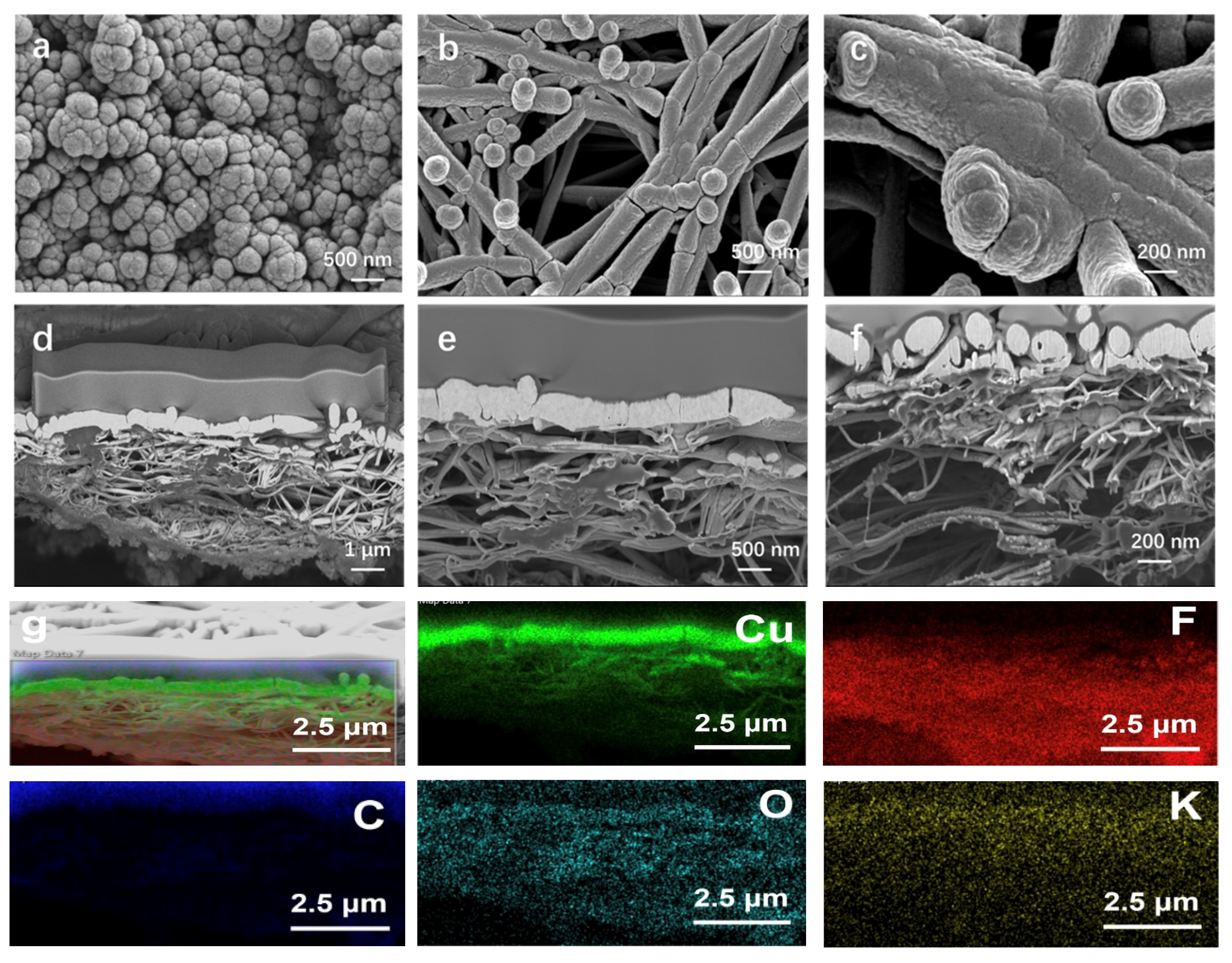
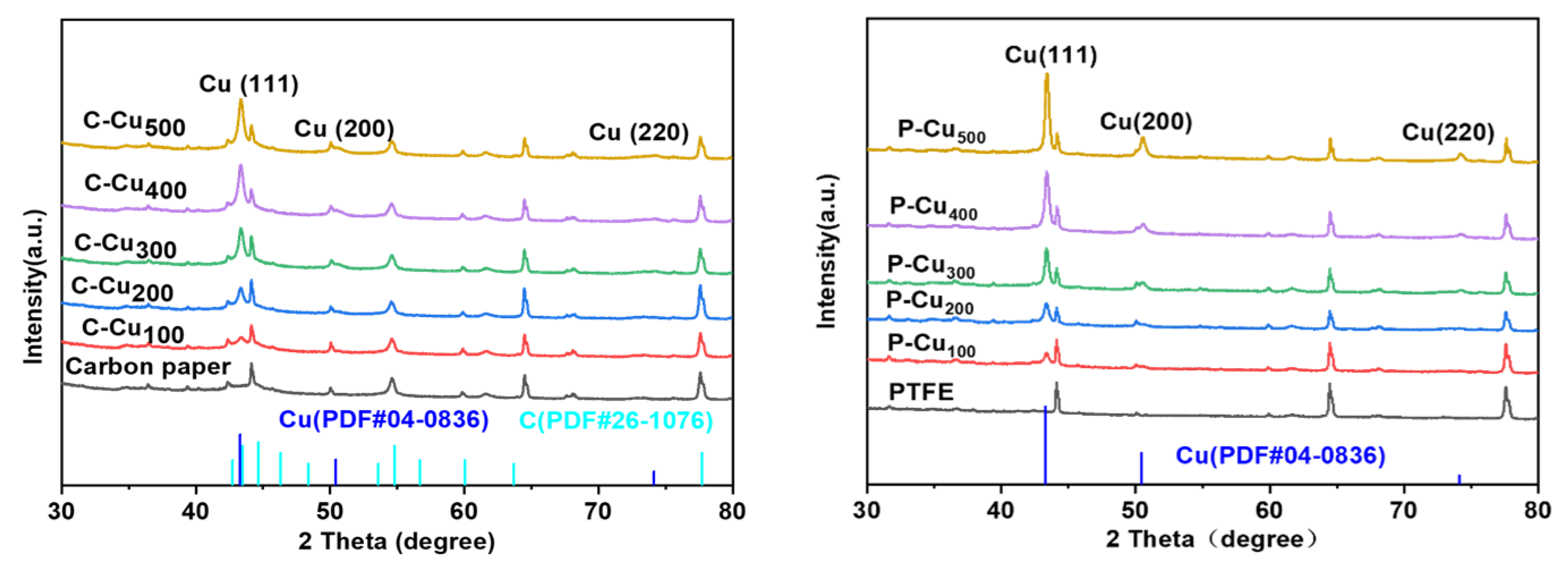
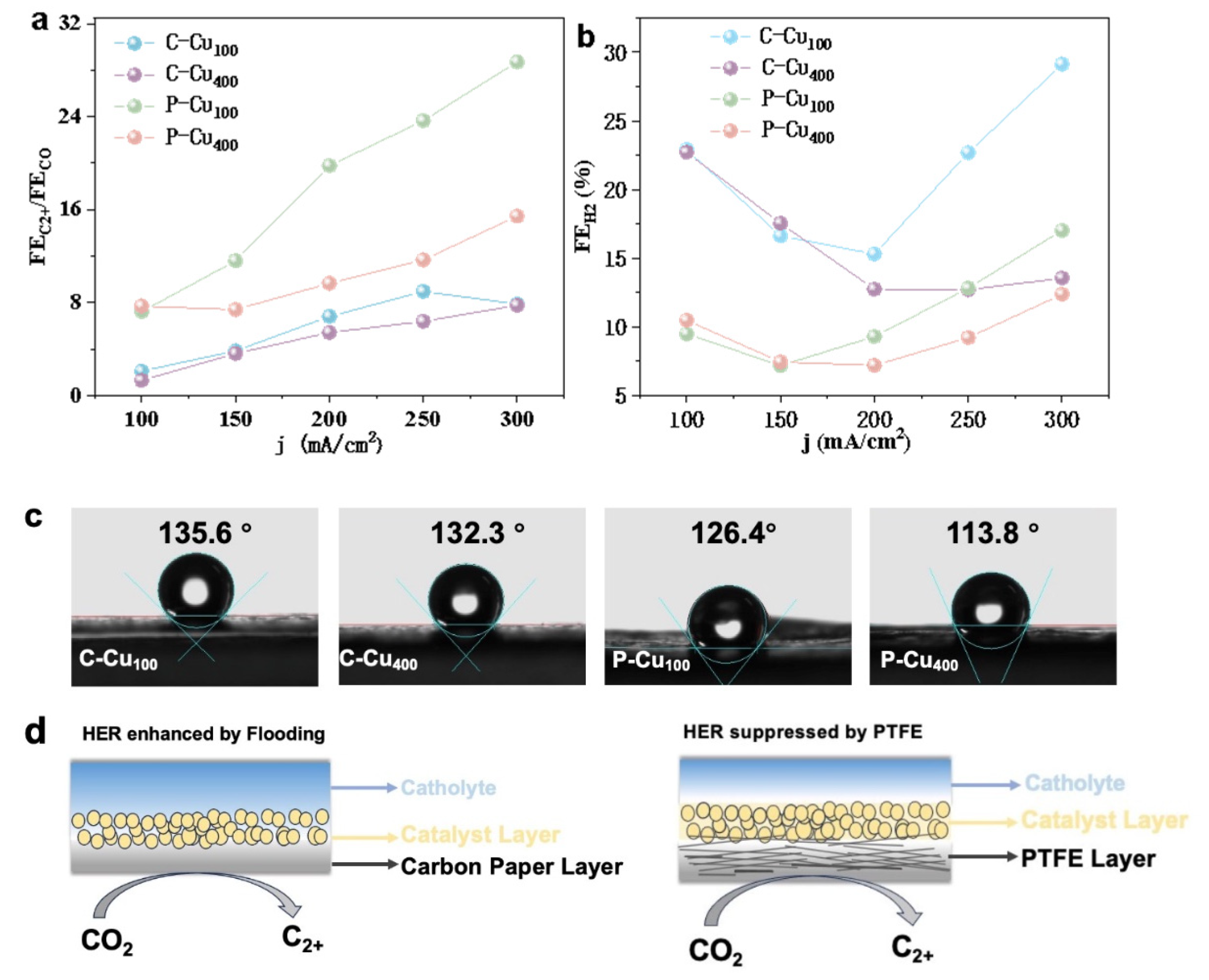
3.1. Characterization of the Sputtered Cu Electrocatalyst on Carbon and PTFE
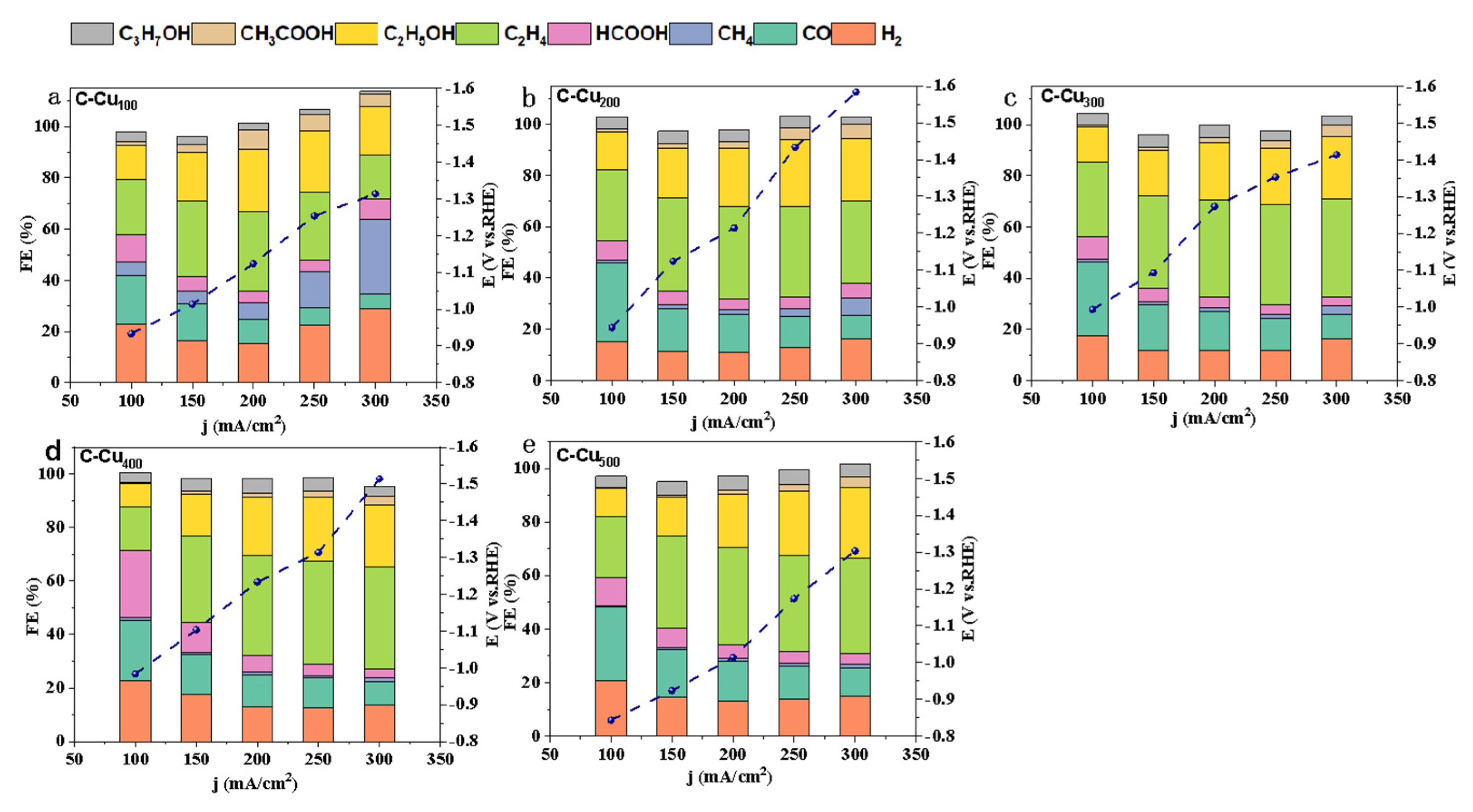
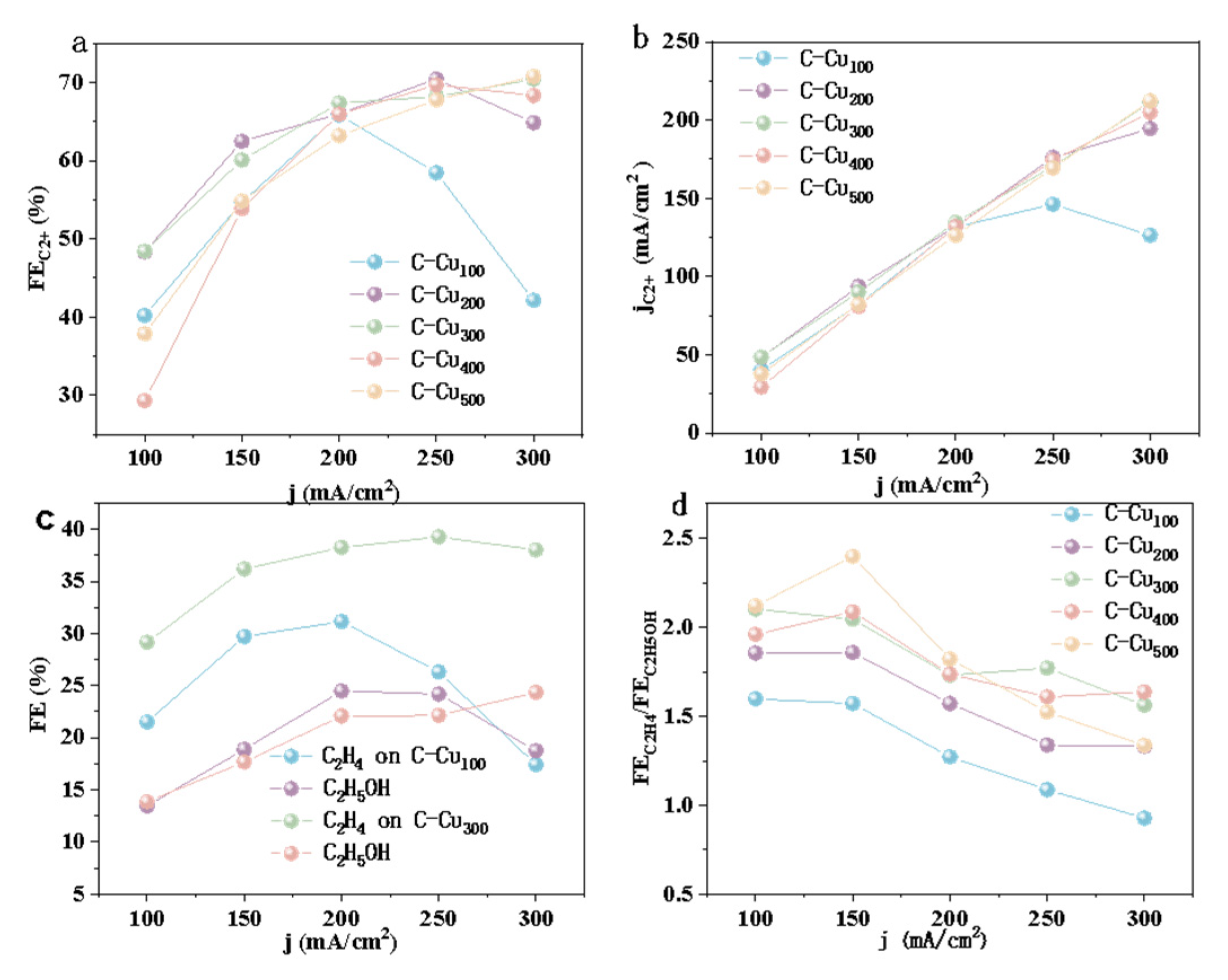
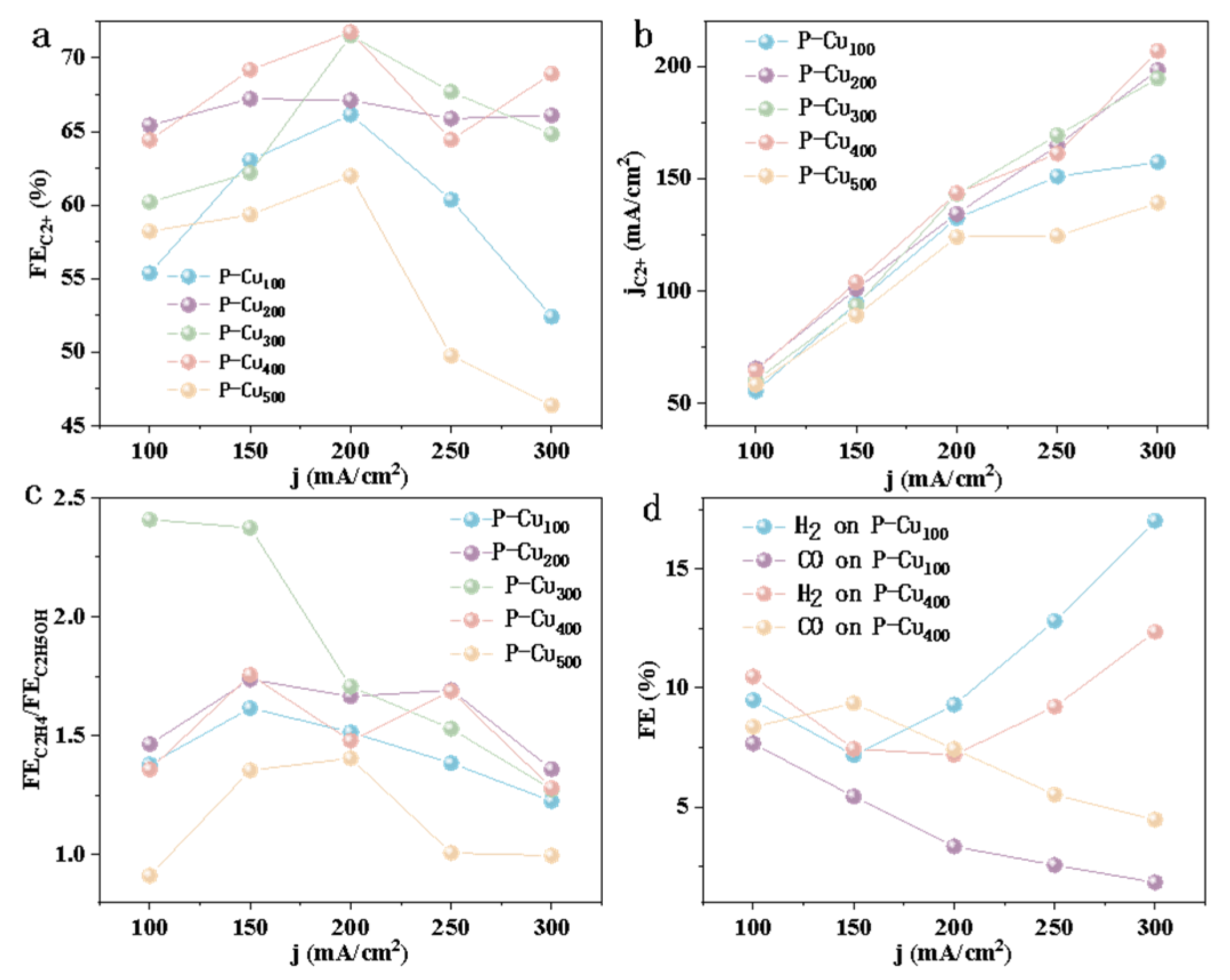
3.2. Electrocatalytic Activity of CO2RR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, C.; Stringer, L.C.; Lv, T. The spatial spillover effect of fossil fuel energy trade on CO2 emissions. Energy 2021, 223, 120038. [Google Scholar] [CrossRef]
- Shanmugam, G. 200 Years of Fossil Fuels and Climate Change (1900–2100). J. Geol. Soc. India 2023, 99, 1043–1062. [Google Scholar] [CrossRef]
- Stougie, L.; Giustozzi, N.; van der Kooi, H.; Stoppato, A. Environmental, economic and exergetic sustainability assessment of power generation from fossil and renewable energy sources. Int. J. Energy Res. 2018, 42, 2916–2926. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. Optimizing the Electrocatalytic Selectivity of Carbon Dioxide Reduction Reaction by Regulating the Electronic Structure of Single-Atom M-N-C Materials. Adv. Funct. Mater. 2022, 32, 2111504. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Yu, Q. Research progress on electrochemical CO2 reduction for Cu-based single-atom catalysts. Sci. China Mater. 2023, 66, 3765–3781. [Google Scholar] [CrossRef]
- Zhao, X.; Du, L.; You, B.; Sun, Y. Integrated design for electrocatalytic carbon dioxide reduction. Catal. Sci. Technol. 2020, 10, 2711–2720. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, S.; Zhou, P.; Ren, X.; Wang, Z.; Zheng, Z.; Wang, P.; Cheng, H.; Liu, Y.; Wei, W.; et al. Promoting Electrocatalytic Reduction of CO2 to C2H4 Production by Inhibiting C2H5OH Desorption from Cu2O/C Composite. Small 2022, 18, 2105212. [Google Scholar] [CrossRef]
- Li, M.; Song, N.; Luo, W.; Chen, J.; Jiang, W.; Yang, J. Engineering Surface Oxophilicity of Copper for Electrochemical CO2 Reduction to Ethanol. Adv. Sci. 2023, 10, 2204579. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Guo, J.-Y.; Zhang, W.; Jie, Y.; Song, H.T.; Lu, H.; Zhang, Y.-F.; Zhao, J.; Hua, C.-X.; Yan, H. Theoretical study on electrocatalytic carbon dioxide reduction over copper with copper-based layered double hydroxides. Phys. Chem. Chem. Phys. 2024, 26, 4480–4491. [Google Scholar] [CrossRef]
- Morrison, A.R.T.; Ramdin, M.; van der Broeke, L.J.P.; De Jong, W.; Vlugt, T.J.; Kortlever, R. Surface Coverage as an Important Parameter for Predicting Selectivity Trends in Electrochemical CO2 Reduction. J. Phys. Chem. C 2022, 126, 11927–11936. [Google Scholar] [CrossRef]
- da Silva AH, M.; Raaijman, S.J.; Santana, C.S.; Assaf, J.M.; Gomes, J.F.; Koper, M.T. Reprint of “Electrocatalytic CO2 reduction to C2+ products on Cu and CuxZny electrodes: Effects of chemical composition and surface morphology”. J. Electroanal. Chem. 2021, 896, 115609. [Google Scholar] [CrossRef]
- Kang, M.; Kolb, M.; Calle-Vallejo, F.; Yeo, B.S.J. Tandem Electrochemical Conversion of CO2 to Liquid Fuels and Chemical Feedstocks. ECS Meet. Abstr. 2022, MA2022-01, 1615. [Google Scholar] [CrossRef]
- Zhong, X.; Zhong, Z.; Liang, S.; Zeng, G.; Cheng, S.; Deng, H.; Lin, Z. Towards a broad-operation window for stable CO2 electroreduction to HCOOH by a design involving upcycling electroplating sludge-derived Sn@N/P-doped carbon. Environ. Sci. Nano 2022, 9, 511–522. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, Y.; Li, C.; Campos dos Santos, E.; Wang, T.; Li, H.; Xu, J.; Liu, C.; Li, H. Deciphering Structure-Activity Relationship Towards CO2 Electroreduction over SnO2 by A Standard Research Paradigm. Angew. Chem. Int. Ed. 2024, 63, e202319913. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Lin, S.-F.; Lee, D.-W.; Chou, J.; Lee, S.-W.; Wang, K.-W. Selective Formate Production from the Electrochemical CO2 Reduction Reaction of Surface Oxide-Modified InSn4 Binary Catalysts. ACS Appl. Energy Mater. 2022, 5, 9895–9901. [Google Scholar] [CrossRef]
- Shi, H.; Luo, L.; Li, C.; Li, Y.; Zhang, T.; Liu, Z.; Cui, J.; Gu, L.; Zhang, L.; Hu, Y.; et al. Stabilizing Cu+ Species in Cu2O/CuO Catalyst via Carbon Intermediate Confinement for Selective CO2RR. Adv. Funct. Mater. 2024, 34, 2310913. [Google Scholar] [CrossRef]
- Woldu, A.R.; Huang, Z.; Zhao, P.; Hu, L.; Astruc, D. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts. Coord. Chem. Rev. 2022, 454, 214340. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, B.; Cai, W.-B.; Shao, M. Direct Observation on Reaction Intermediates and the Role of Bicarbonate Anions in CO2 Electrochemical Reduction Reaction on Cu Surfaces. J. Am. Chem. Soc. 2017, 139, 15664–15667. [Google Scholar] [CrossRef]
- Jang, J.; Zhu, S.; Delmo, E.P.; Li, T.; Zhao, Q.; Wang, Y.; Zhang, L.; Huang, H.; Ge, J.; Shao, M. Facile design of oxide-derived Cu nanosheet electrocatalyst for CO2 reduction reaction. EcoMat 2023, 5, e12334. [Google Scholar] [CrossRef]
- Mu, S.; Lu, H.; Wu, Q.; Li, L.; Zhao, R.; Long, C.; Cui, C. Hydroxyl radicals dominate reoxidation of oxide-derived Cu in electrochemical CO2 reduction. Nat. Commun. 2022, 13, 3694. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Qiu, Z.-F.; Zhang, X.Y.; Chen, J.Q.; Zhao, Y.; Gong, F.; Sun, W.-Y. Tailoring Coordination Microenvironment of Cu(I) in Metal–Organic Frameworks for Enhancing Electroreduction of CO2 to CH4. Funct. Mater. 2022, 32, 2203677. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Bai, S.; Zhang, L.; Lai, F.; Chen, Y.; Zhu, X.; Liu, T. Strain-Regulated Pd/Cu Core/Shell Icosahedra for Tunable Syngas Electrosynthesis from CO2. Chem. Mater. 2022, 34, 7995–8003. [Google Scholar] [CrossRef]
- Ting LR, L.; Piqué, O.; Lim, S.Y.; Tanhaei, M.; Calle-Vallejo, F.; Yeo, B.S. Enhancing CO2 Electroreduction to Ethanol on Copper–Silver Composites by Opening an Alternative Catalytic Pathway. ACS Catal. 2020, 10, 4059–4069. [Google Scholar] [CrossRef]
- Huo, S.; Lu, J.; Wang, X. Electrodeposition of Ni on MWNTs as a promising catalyst for CO2RR. Energy Sci. Eng. 2021, 9, 1042–1047. [Google Scholar] [CrossRef]
- Huo, S.; Lu, J.; Wang, X. Recent progress in electrochemical reduction of carbon dioxide on metal single-atom catalysts. Energy Sci. Eng. 2022, 10, 1584–1600. [Google Scholar] [CrossRef]
- Li, X.; Qin, M.; Wu, X.; Lv, X.; Wang, J.; Wang, Y.; Bin Wu, H. Enhanced CO Affinity on Cu Facilitates CO2 Electroreduction toward Multi-Carbon Products. Small 2023, 19, 2302530. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qin, Y.; Gao, B.; Wang, K.; Li, D.; Li, Y.; Ding, S.; Song, Z.; Su, Y.; Xiao, C. Plasma-assisted and oxygen vacancy-engineered In2O3 films for enhanced electrochemical reduction of CO2. Appl. Surf. Sci. 2021, 563, 150405. [Google Scholar] [CrossRef]
- Wang, X.; Ou, P.; Wicks, J.; Xie, Y.; Wang, Y.; Li, J.; Tam, J.; Ren, D.; Howe, J.Y.; Wang, Z.; et al. Gold-in-copper at low *CO coverage enables efficient electromethanation of CO2. Nat. Commun. 2021, 12, 3387. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Li, Y.C.; Li, J.; Li, F.; Lum, Y.; Nam, D.-H.; Chen, B.; Wicks, J.; Xu, A.; et al. Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen. Nat. Commun. 2019, 10, 5814. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Magnetron sputtering. J. Vac. Sci. Technol. A 2020, 38, 060805. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Zhang, Z.; Li, S.; Liu, H.; Kang, X.; Liu, J.; Ge, S.; Wang, J.; Lv, W.; et al. Ampere-Level Current Density CO2 Reduction with High C2+ Selectivity on La(OH)3-Modified Cu Catalysts. Small 2024, 20, 2308226. [Google Scholar] [CrossRef] [PubMed]
- Pellessier, J.; Gong, X.; Li, B.; Zhang, J.; Gang, Y.; Hambleton, K.; Podder, C.; Gao, Z.; Zhou, H.-C.; Wang, G.; et al. PTFE nanocoating on Cu nanoparticles through dry processing to enhance electrochemical conversion of CO2 towards multi-carbon products. J. Mater. Chem. A 2023, 11, 26252–26264. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Wang, M.; Cheng, Q.; Qian, T.; Yan, C. Deciphering engineering principle of three-phase interface for advanced gas-involved electrochemical reactions. J. Energy Chem. 2023, 80, 302–323. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Liu, H.; Hashimoto, K.; Jiang, L. Highly Boosted Oxygen Reduction Reaction Activity by Tuning the Underwater Wetting State of the Superhydrophobic Electrode. Small 2016, 13, 1601250. [Google Scholar] [CrossRef]
- Shi, R.; Shang, L.; Zhang, T. Three Phase Interface Engineering for Advanced Catalytic Applications. ACS Appl. Energy Mater. 2021, 4, 1045–1052. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Mao, X.; Peng, Y.; Zhang, W.; Ye, Q.; Yang, Y.; Yu, F.; Ma, Y.; Wu, A.; Qi, Z. Investigation of a Hydrophobic Sputtered Cu Electrode to Electrocatalyze CO2 Towards a C2+ Product: The Effect of Substrate and Catalyst Thickness. Processes 2024, 12, 2374. https://doi.org/10.3390/pr12112374
Wang D, Mao X, Peng Y, Zhang W, Ye Q, Yang Y, Yu F, Ma Y, Wu A, Qi Z. Investigation of a Hydrophobic Sputtered Cu Electrode to Electrocatalyze CO2 Towards a C2+ Product: The Effect of Substrate and Catalyst Thickness. Processes. 2024; 12(11):2374. https://doi.org/10.3390/pr12112374
Chicago/Turabian StyleWang, Dongdong, Xiaoyu Mao, Yaqi Peng, Wei Zhang, Qiulin Ye, Yan Yang, Fengping Yu, Yan Ma, Angjian Wu, and Zhifu Qi. 2024. "Investigation of a Hydrophobic Sputtered Cu Electrode to Electrocatalyze CO2 Towards a C2+ Product: The Effect of Substrate and Catalyst Thickness" Processes 12, no. 11: 2374. https://doi.org/10.3390/pr12112374
APA StyleWang, D., Mao, X., Peng, Y., Zhang, W., Ye, Q., Yang, Y., Yu, F., Ma, Y., Wu, A., & Qi, Z. (2024). Investigation of a Hydrophobic Sputtered Cu Electrode to Electrocatalyze CO2 Towards a C2+ Product: The Effect of Substrate and Catalyst Thickness. Processes, 12(11), 2374. https://doi.org/10.3390/pr12112374





