Determination of Hafnium in Zirconium by Spectrophotometry
Abstract
1. Introduction
2. Experimental
2.1. Materials
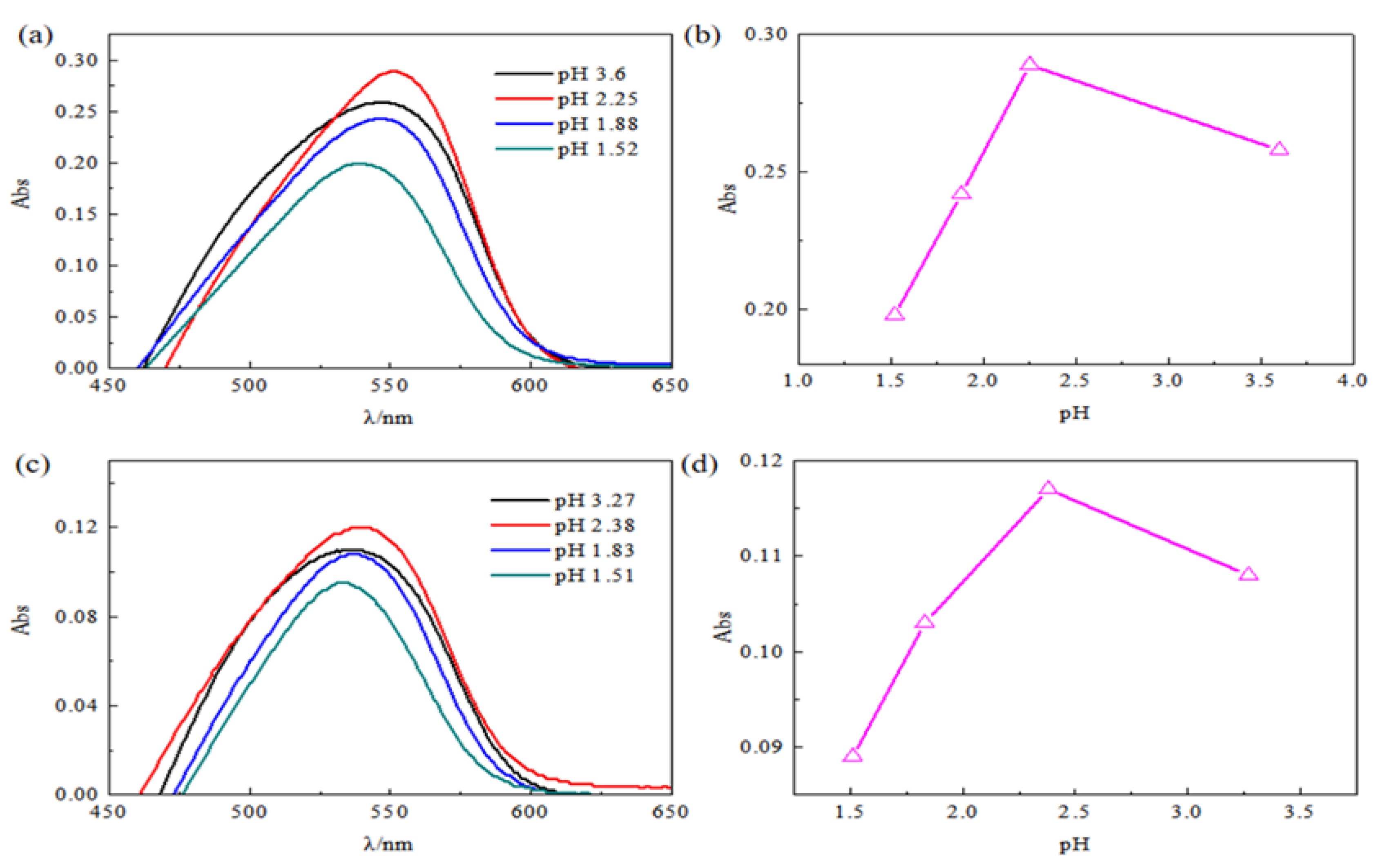
2.2. The Influence of pH on Absorbance
2.3. The Influence of Indicator Content on Absorbance
2.4. Work Curves of Zirconium and Hafnium Ions
2.5. Zirconium and Hafnium Ion Component Determination
3. Results and Discussion
3.1. Effect of pH
3.2. The Influence of Indicator Content
3.3. Working Curve Drawing and Determination of Hafnium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, J.; Duan, L.; Wang, Y.; Zhai, X.; Chen, G.; Yu, G. Preparation of DMHMP Levextrel resin and separation of zirconium and hafnium by extraction chromatography in HNO3-HCl mixture. Colloids Surf. Physicochem. Eng. Asp. 2022, 638, 128216. [Google Scholar] [CrossRef]
- Qin, W.; Han, X.; Wang, J.; Wang, X.; Zhang, J. Selectivity of novel PAMAM dendrimers modified with salicylaldehyde and their extraction/separation performance on Zr(Ⅳ) and Hf(Ⅳ). J. Mol. Struct. 2023, 1276, 134817. [Google Scholar] [CrossRef]
- Yadollahi, A.; Saberyan, K.; Torab-Mostaedi, M.; Charkhi, A.; Pourjavid, M.R. Solvent extraction separation of zirconium and hafnium from nitric acid solutions using mixture of Cyanex-272 and TBP. Radiochim. Acta 2018, 106, 675–684. [Google Scholar] [CrossRef]
- Kozma, K.; Zakharov, L.N.; Nyman, M. Countercation-controlled nuclearity of Zr/Hf peroxo oxalates. Cryst. Growth Des. 2020, 20, 6519–6527. [Google Scholar] [CrossRef]
- Luther, L.C.; Kometani, T.Y. Chemical Analysis of Bi-containing magnetic bubble garnet films. J. Am. Ceram. Soc. 1983, 66, 619–622. [Google Scholar] [CrossRef]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using total reflection X-Ray fluorescence spectrometry. Food Chem. Toxicol. 2018, 116, 233–237. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Frankowski, M.; Simeonov, V.; Polkowska, Ż.; Namieśnik, J. Determination of metals content in wine samples by inductively coupled plasma-mass spectrometry. Molecules 2018, 23, 2886. [Google Scholar] [CrossRef]
- Majeed, A.; Khan, M.S.; Ballantyne, E.K. Spectrophotometric Determination of Zirconium in Steels with Xylenol Orange. Anal. Chim. Acta 1987, 192, 125–128. [Google Scholar] [CrossRef]
- Liu, Y.X.; Li, Q.X.; Ma, N.; Sun, X.L.; Bai, J.F.; Zhang, Q. Application of the Zr/Hf ratio in the determination of hafnium in geochemical samples by high-resolution inductively coupled plasma mass spectrometry. Anal. Chem. 2014, 86, 11570–11577. [Google Scholar] [CrossRef]
- Lu, L.; Shao, X.; Lin, X.; Ding, L.; Song, B.; Sun, J. Tb3+-xylenol orange complex-based colorimetric and luminometric dual-readout sensing platform for dipicolinic acid and metal ions. Chin. Chem. Lett. 2023, 34, 107203. [Google Scholar] [CrossRef]
- Dayan Elshan, N.G.R.; Patek, R.; Vagner, J.; Mash, E.A. Spectrophotometric determination and removal of unchelated europium ions from solutions containing Eu-Diethylenetriaminepentaacetic acid chelate-peptide conjugates. Anal. Biochem. 2014, 464, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Averill, D.J.; Garcia, J.; Siriwardena-Mahanama, B.N.; Vithanarachchi, S.M.; Allen, M.J. Preparation, purification, and characterization of lanthanide complexes for use as contrast agents for magnetic resonance imaging. J. Vis. Exp. JoVE 2011, 53, 2844. [Google Scholar]
- Larsson, K.; Cullen, T.D.; Mezyk, S.P.; McDowell, R.G.; Martin, L.R. Complications in complexation kinetics for lanthanides with DTPA using dye probe molecules in aqueous solution. RSC Adv. 2017, 7, 26507–26512. [Google Scholar] [CrossRef]
- Hafez, M.A.H. Study of the chromogenic reactions of semi-Xylenol Orange with rare earths and thorium. Anal. Lett. 1992, 25, 1779–1796. [Google Scholar] [CrossRef]
- Munshi, K.N.; Dey, A.K. Absorptiometric study of the chelates formed between the lanthanoids and xylenol orange. Microchim. Acta 1968, 56, 1059–1065. [Google Scholar] [CrossRef]
- Havel, J. Spectrophotometric determination of rare earth elements by flow injection analysis based on their reaction with xylenol orange and cetylpyridinium bromide. Talanta 1994, 41, 1251–1254. [Google Scholar] [CrossRef]
- Liosi, G.M.; Dondi, D.; Vander Griend, D.A.; Lazzaroni, S.; D’Agostino, G.; Mariani, M. Fricke-gel dosimeter: Overview of xylenol orange chemical behavior. Radiat. Phys. Chem. 2017, 140, 74–77. [Google Scholar] [CrossRef]
- Sato, H.; Yokoyama, Y.; Momoki, K. Chelate formation of zirconium with xylenol orange and semi-xylenol orange. Anal. Chim. Acta 1978, 99, 167–175. [Google Scholar] [CrossRef]
- Hurst, F.J. Separation of hafnium from zirconium in sulfuric acid solutions using pressurized ion exchange. Hydrometallurgy 1983, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Kuang, S.; Li, Y.; Huang, X.; Liao, W. Separation of zirconium from hafnium in sulfate medium using solvent extraction with a new reagent BEAP. Hydrometallurgy 2017, 169, 607–611. [Google Scholar] [CrossRef]
- Challis, H.J.G. Separate and simultaneous determination of zirconium and hafnium in nickel-base alloys with xylenol orange. Analyst 1969, 94, 94. [Google Scholar] [CrossRef]
- Mori, I.; Fujita, Y.; Fujita, K.; Nakahashi, Y.; Kishida, Y. Xylenol orange, zirconium(IV) and fluoride color reaction in mixed micelles of N-hexadecylpyridinium chloride and Brij 35, and its analytical application. Anal. Lett. 1988, 21, 563–581. [Google Scholar] [CrossRef]
- Miyachi, K.; Kurokawa, H.; Moriyama, K.; Morimoto, S.; Tabuse, K.; Asano, M.; Yamaguchi, T.; Fujita, Y. Spectrophotometric determination of micro amounts of albumin using zirconium(IV) and xylenol orange. Chem. Lett. 2012, 41, 58–59. [Google Scholar] [CrossRef]
- Amaral, J.C.B.S.; Morais, C.A. Equilibrium of zirconium and hafnium in the process of extraction with TBP in nitric medium–influence in the Zr/Hf separation. Miner. Eng. 2020, 146, 106138. [Google Scholar] [CrossRef]
- Das, A.; Chandrakumar, K.R.S.; Paul, B.; Chopade, S.M.; Majumdar, S.; Singh, A.K.; Kain, V. Enhanced adsorption and separation of zirconium and hafnium under mild conditions by phosphoric acid based ligand functionalized silica gels: Insights from experimental and theoretical investigations. Sep. Purif. Technol. 2020, 239, 116518. [Google Scholar] [CrossRef]
- Cheng, K.L. Determination of zirconium and hafnium with xylenol orange and methylthymol blue. Anal. Chim. Acta 1963, 28, 41–53. [Google Scholar] [CrossRef]



| Concentration Zr/Hf (mg/L) | Absorbance Abs | Calculated Concentration Zr/Hf (mg/L) | Hafnium Concentration Error |
|---|---|---|---|
| 0.6/0.2 | 0.231 | 0.59/0.21 | 5% |
| 0.4/0.4 | 0.194 | 0.3999/0.4001 | 0.025% |
| 0.2/0.6 | 0.152 | 0.184/0.616 | 2.67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, X.; Lv, X.; Li, S.; Lv, Z.; Song, J. Determination of Hafnium in Zirconium by Spectrophotometry. Processes 2024, 12, 2286. https://doi.org/10.3390/pr12102286
Jiao X, Lv X, Li S, Lv Z, Song J. Determination of Hafnium in Zirconium by Spectrophotometry. Processes. 2024; 12(10):2286. https://doi.org/10.3390/pr12102286
Chicago/Turabian StyleJiao, Xiuhao, Xiaotao Lv, Shaolong Li, Zepeng Lv, and Jianxun Song. 2024. "Determination of Hafnium in Zirconium by Spectrophotometry" Processes 12, no. 10: 2286. https://doi.org/10.3390/pr12102286
APA StyleJiao, X., Lv, X., Li, S., Lv, Z., & Song, J. (2024). Determination of Hafnium in Zirconium by Spectrophotometry. Processes, 12(10), 2286. https://doi.org/10.3390/pr12102286





