Molecular Insights into CO2 Diffusion Behavior in Crude Oil
Abstract
1. Introduction
2. Methodology
2.1. Molecular Model
2.2. Simulation Details
2.3. Diffusion Coefficient
2.4. Model Validation
3. Results and Discussion
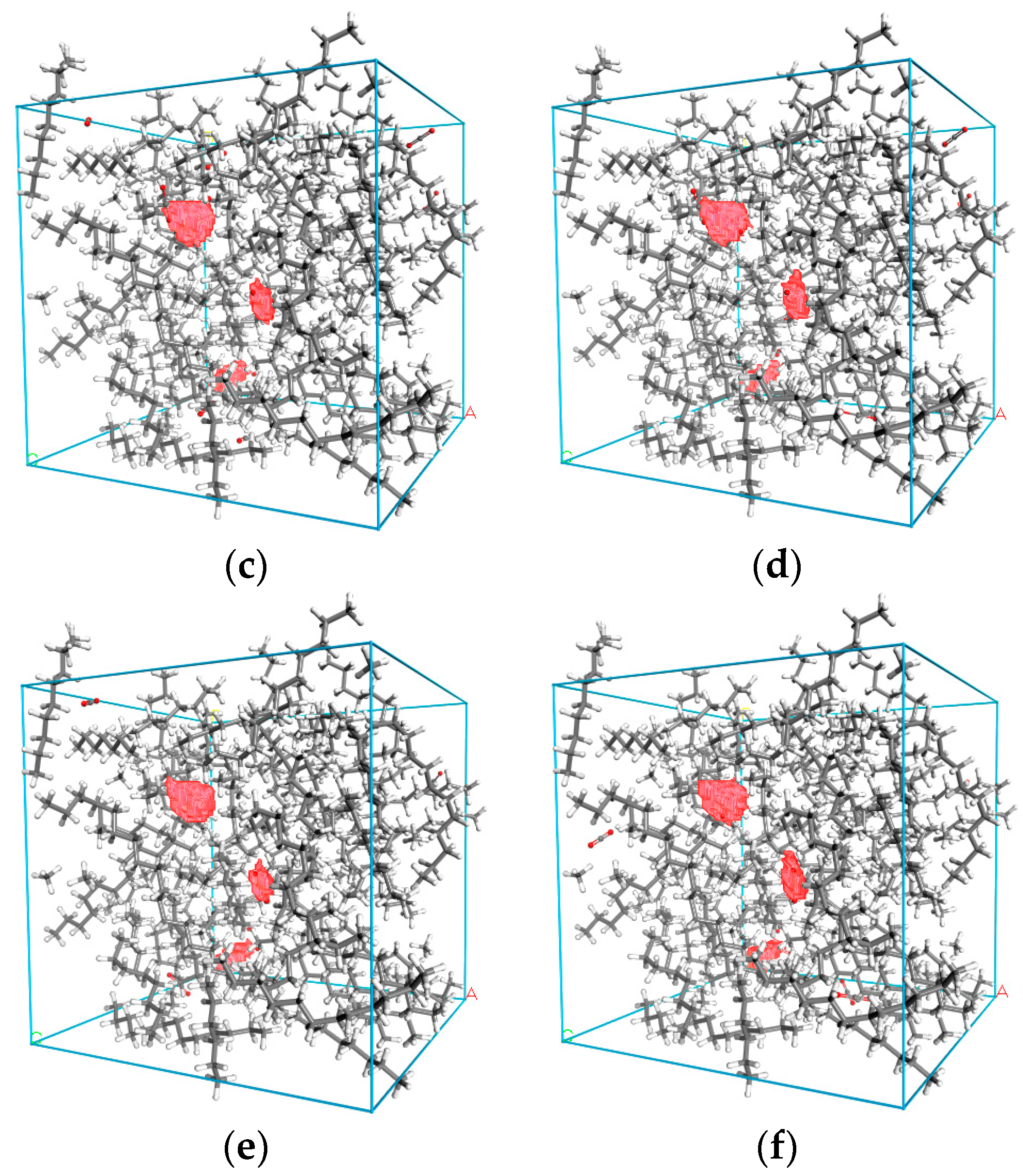
3.1. Density Distribution of CO2
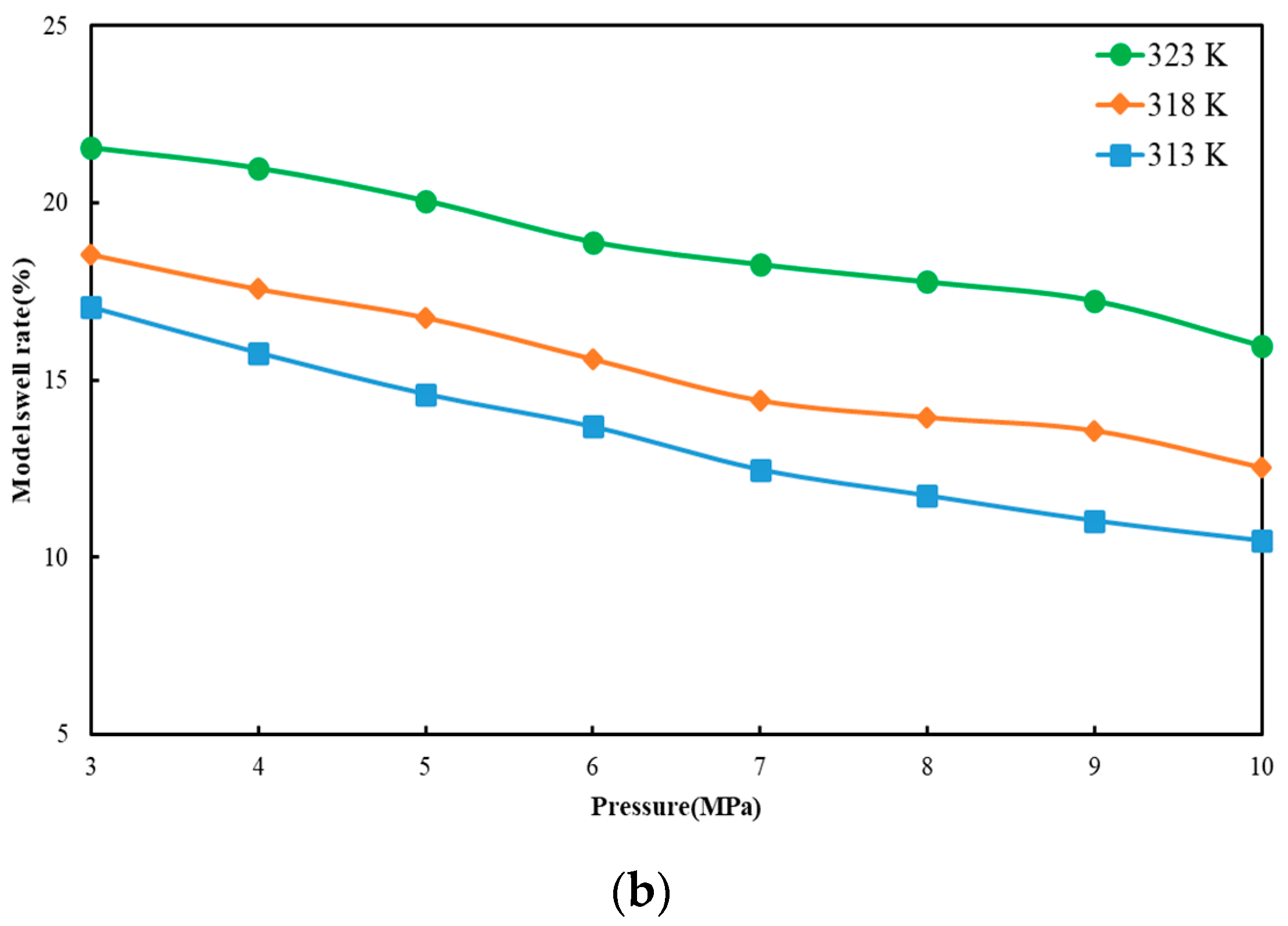
3.2. Model Swelling Rate
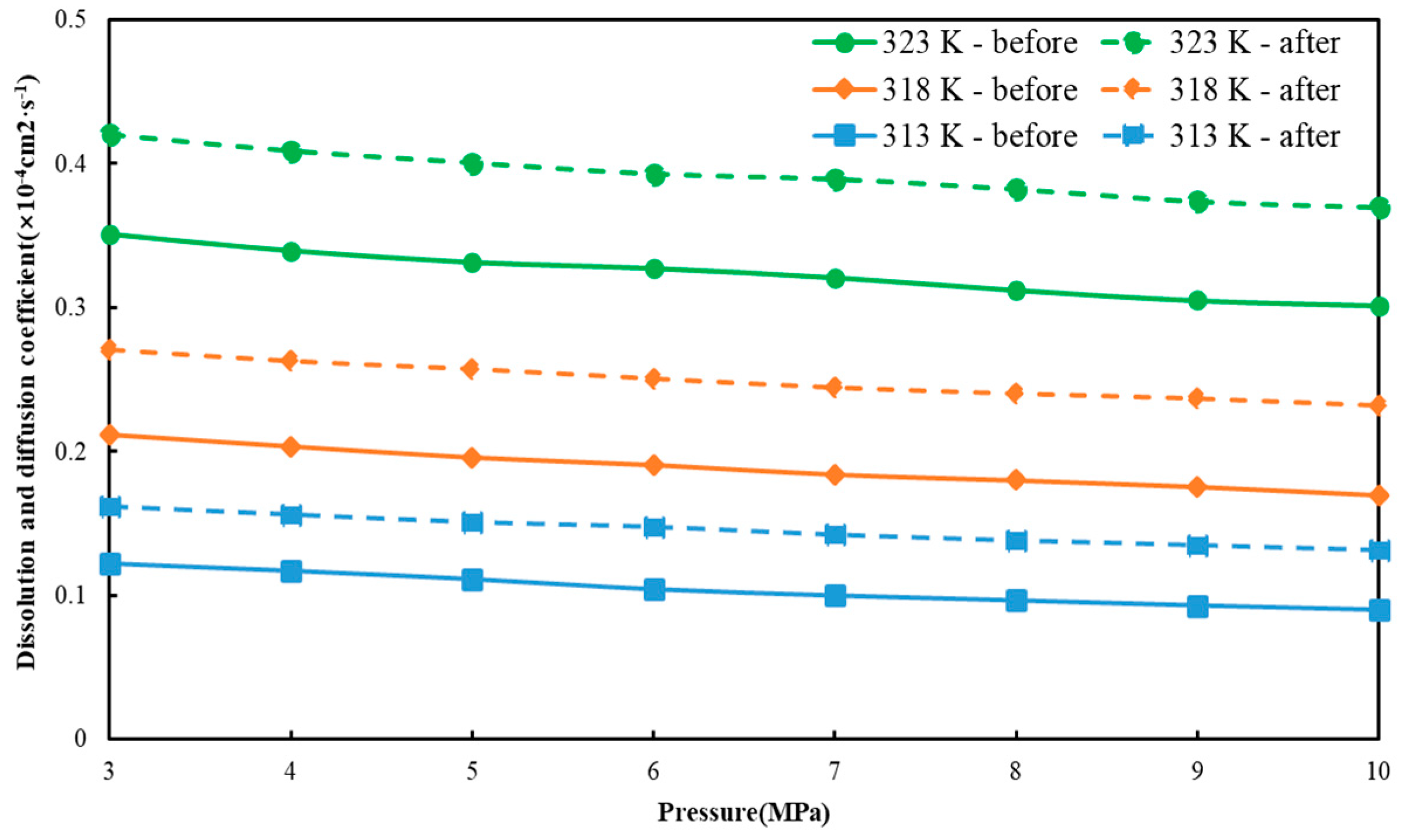
3.3. Dissolution and Diffusion
3.4. Prediction of Diffusion Coefficient
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Description | Unit |
| Eij | the potential well depth | |
| rij0 | the zero potential distance for the atom pair | |
| r | the distance between the two atoms | |
| Nα | the amount of diffused atoms in the system | |
| ri(t) | the displacement vector of molecule i from time 0 to time t | |
| T | temperature | K |
| α | CO2 molecular radius | 1.65 × 10−8 cm |
| k | Boltzmann’s constant | 1.38 × 10−23 J/K |
| D | the diffusion coefficient | cm2/s |
| μ | the oil viscosity | Pa·s |
| Vfin | the final model’s volume | Å3 |
| Vini | the initial model’s volume | Å3 |
| ε | the model swelling rate | % |
| P | pressure | MPa |
References
- Kaya, Y.; Gale, J. Greenhouse Gas Control Technologies. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Kyoto, Japan, 1–4 October 2002; Available online: https://www.sciencedirect.com/book/9780080442761/greenhouse-gas-control-technologies-6th-international-conference#book-description (accessed on 14 July 2024).
- Ye, H.; Liu, Q.; Peng, B. Research progress in evaluation of carbon storage potential based on CO2 flooding technology. Clean Coal Technol. 2021, 27, 107–116. [Google Scholar] [CrossRef]
- Song, X.; Wang, F.; Ma, D.; Gao, M.; Zhang, Y. Progress and prospect of carbon dioxide capture, utilization and storage in CNPC oilfields. Pet. Explor. Dev. 2023, 50, 229–244. [Google Scholar] [CrossRef]
- Ricky, E.X.; Mwakipunda, G.C.; Nyakilla, E.E.; Kasimu, N.A.; Wang, C.; Xu, X. A comprehensive review on CO2 thickeners for CO2 mobility control in enhanced oil recovery: Recent advances and future outlook. J. Ind. Eng. Chem. 2023, 126, 69–91. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Wang, S.; Guo, Y.; Han, X.; Li, Q.; Cheng, Y.; Dong, Z.; Li, X.; Zhang, X. Numerical insights into factors affecting collapse behavior of horizontal wellbore in clayey silt hydrate-bearing sediments and the accompanying control strategy. Ocean Eng. 2024, 297, 117029. [Google Scholar] [CrossRef]
- Qin, J.; Han, H.; Liu, X. Application and enlightenment of carbon dioxide flooding in the United States of America. Pet. Explor. Dev. 2015, 42, 232–240. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Gamage, R.P.; Rathnaweera, T.D.; Ranathunga, A.S.; Koay, A.; Choi, X. A Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, 9, 481. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Miao, J.; Yang, G. Status and Prospect of CO2 Capture and Oil Displacement in China. Pet. Petrochem. Today 2020, 28, 32–37. [Google Scholar]
- Orr, F.M.; Heller, J.P.; Taber, J.J. Carbon dioxide flooding for enhanced oil recovery: Promise and problems. J. Am. Oil Chem. Soc. 1982, 59, 810A–817A. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Hyndman, C.L.; Maini, B.B. Measurement of gas diffusivity in heavy oils. J. Pet. Sci. Eng. 2000, 25, 37–47. [Google Scholar] [CrossRef]
- Yang, C.; Gu, Y. Diffusion coefficients and oil swelling factors of carbon dioxide, methane, ethane, propane, and their mixtures in heavy oil. Fluid Phase Equilibria 2006, 243, 64–73. [Google Scholar] [CrossRef]
- Abedini, A.; Torabi, F. On the CO2 storage potential of cyclic CO2 injection process for enhanced oil recovery. Fuel 2014, 124, 14–27. [Google Scholar] [CrossRef]
- Song, Z.; Song, Y.; Li, Y.; Bai, B.; Song, K.; Hou, J. A critical review of CO2 enhanced oil recovery in tight oil reservoirs of North America and China. Fuel 2020, 276, 118006. [Google Scholar] [CrossRef]
- Ren, D.; Wang, X.; Kou, Z.; Wang, S.; Wang, H.; Wang, X.; Tang, Y.; Jiao, Z.; Zhou, D.; Zhang, R. Feasibility evaluation of CO2 EOR and storage in tight oil reservoirs: A demonstration project in the Ordos Basin. Fuel 2023, 331, 125652. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Sheng, J.J. Effect of the Injection Pressure on Enhancing Oil Recovery in Shale Cores during the CO2 Huff-n-Puff Process When It Is above and below the Minimum Miscibility Pressure. Energy Fuels 2017, 31, 3856–3867. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J. A comparison of crude oil hydrocarbon mobilization by vaporization gas drive into methane, ethane, and carbon dioxide at 15.6 MPa and 42 °C. Fuel 2019, 249, 392–399. [Google Scholar] [CrossRef]
- Assef, Y.; Almao, P.P. Evaluation of Cyclic Gas Injection in Enhanced Recovery from Unconventional Light Oil Reservoirs: Effect of Gas Type and Fracture Spacing. Energies 2019, 12, 1370. [Google Scholar] [CrossRef]
- Li, L.; Sheng, J.J. Experimental study of core size effect on CH4 huff-n-puff enhanced oil recovery in liquid-rich shale reservoirs. J. Nat. Gas Sci. Eng. 2016, 34, 1392–1402. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Sorensen, J.A.; Miller, D.J.; Gorecki, C.D.; Harju, J.A.; Pospisil, G. Laboratory Studies of Rich Gas Interactions with Bakken Crude Oil to Support Enhanced Oil Recovery. In Proceedings of the 7th Unconventional Resources Technology Conference, Denver, CO, USA, 22–24 July 2019. [Google Scholar]
- Hill, E.S.; Lacey, W.N. Rate of solution of propane in quiescent liquid hydrocarbons. Am. Chem. Soc. 1934, 26, 1327–1331. [Google Scholar] [CrossRef]
- Reamer, H.H.; Opfell, J.B.; Sage, B.H. Diffusion coefficients in hydrocarbon systems methane-decane-methane in liquid phase-methane-decane-methane in liquid phase. Am. Chem. Soc. 1956, 48, 275–282. [Google Scholar] [CrossRef]
- Riazi, M.R. A new method for experimental measurement of diffusion coefficients in reservoir fluids. J. Pet. Sci. Eng. 1996, 14, 235–250. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, Y.; Lu, G.; Jiang, L.; Zhao, J.; Zhang, Y.; Song, Y. Experimental study on CO2 diffusion in bulk n-decane and n-decane saturated porous media using micro-CT. Fluid Phase Equilibria 2016, 417, 212–219. [Google Scholar] [CrossRef]
- Fayazi, A.; Kantzas, A. Determining Diffusivity, Solubility, and Swelling in Gaseous Solvent–Heavy Oil Systems. Ind. Eng. Chem. Res. 2019, 58, 10031–10043. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, X.; Huang, M.; Song, Y. Experimental method for in-situ measurement of CO2-oil diffusion coefficient in porous media using NMR. Exp. Technol. Manag. 2022, 39, 19–23. [Google Scholar] [CrossRef]
- Li, Z.; Dong, M. Experimental Study of Carbon Dioxide Diffusion in Oil-Saturated Porous Media under reservoir conditions. Ind. Eng. Chem. Res. 2009, 48, 9307–9317. [Google Scholar] [CrossRef]
- Song, L.; Kantzas, A.; Bryan, J. Experimental Measurement of Diffusion Coefficient of CO2 in Heavy Oil Using X-ray Computed-Assisted Tomography under Reservoir Conditions. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar] [CrossRef]
- Afari, S.; Ling, K.; Sennaoui, B.; Maxey, D.; Oguntade, T.; Porlles, J. Optimization of CO2 huff-n-puff EOR in the Bakken Formation using numerical simulation and response surface methodology. J. Pet. Sci. Eng. 2022, 215, 110552. [Google Scholar] [CrossRef]
- Amarasinghe, W.; Fjelde, I.; Guo, Y. CO2 dissolution and convection in oil at realistic reservoir conditions: A visualization study. J. Nat. Gas Sci. Eng. 2021, 95, 104113. [Google Scholar] [CrossRef]
- Li, C.; Pu, H.; Zhong, X.; Li, Y.; Zhao, J.X. Interfacial interactions between Bakken crude oil and injected gases at reservoir temperature: A molecular dynamics simulation study. Fuel 2020, 276, 118058. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Y.; Guo, L.; Su, X. Molecular dynamic investigation on hydrogen production by polycyclic aromatic hydrocarbon gasification in supercritical water. Int. J. Hydrogen Energy 2016, 41, 3837–3843. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; He, M. Investigation on the condensation process of HFO refrigerants by molecular dynamics simulation. J. Mol. Liq. 2019, 288, 111034. [Google Scholar] [CrossRef]
- Ding, W.; Shi, J.; Wei, W.; Cao, C.; Jin, H. A molecular dynamics simulation study on solubility behaviors of polycyclic aromatic hydrocarbons in supercritical water/hydrogen environment. Int. J. Hydrogen Energy 2021, 46, 2899–2904. [Google Scholar] [CrossRef]
- Li, B.; Liu, G.; Xing, X.; Chen, L.; Lu, X.; Teng, H.; Wang, J. Molecular dynamics simulation of CO2 dissolution in heavy oil resin-asphaltene. J. CO2 Util. 2019, 33, 303–310. [Google Scholar] [CrossRef]
- Seyyedattar, M.; Ghamartale, A.; Zendehboudi, S.; Butt, S. Assessment of CO2-Oil swelling behavior using molecular dynamics simulation: CO2 utilization and storage implication. J. Mol. Liq. 2023, 379, 121582. [Google Scholar] [CrossRef]
- Moh, D.Y.; Zhang, H.; Sun, S.; Qiao, R. Molecular anatomy and macroscopic behavior of oil extraction from nanopores by CO2 and CH4. Fuel 2022, 324, 124662. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Liu, S.; Zhang, J.; Song, Y. Molecular dynamics simulation of CO2-oil miscible fluid distribution and flow within nanopores. J. Mol. Liq. 2023, 380, 121769. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Liu, K.; Burke, N. Molecular Simulation of CO2 Solubility and Its Effect on Octane Swelling. Energy Fuels 2013, 27, 2741–2747. [Google Scholar] [CrossRef]
- Li, C.; Pu, H.; Zhao, J.X. Molecular Simulation Study on the Volume Swelling and the Viscosity Reduction of n-Alkane/CO2 Systems. Ind. Eng. Chem. Res. 2019, 58, 8871–8877. [Google Scholar] [CrossRef]
- Zhang, J.; Seyyedi, M.; Clennell, M.B. Molecular Dynamics Simulation of Transport and Structural Properties of CO2–Alkanes. Energy Fuels 2021, 35, 6700–6710. [Google Scholar] [CrossRef]
- Song, Y.-L.; Gu, S.-H.; Song, Z.-J.; Zhang, Z.-Y.; Chang, X.-Y.; Guo, J. Effect of confinement on the three-phase equilibrium of water-oil-CO2 mixtures in nanopores. Pet. Sci. 2022, 19, 203–210. [Google Scholar] [CrossRef]
- Loya, A.; Stair, J.L.; Jafri, A.R.; Yang, K.; Ren, G. A molecular dynamic investigation of viscosity and diffusion coefficient of nanoclusters in hydrocarbon fluids. Comput. Mater. Sci. 2015, 99, 242–246. [Google Scholar] [CrossRef]
- Materials Studio. A Material Modeling Software of Accelrys Company; United States [EB/OL]. Available online: https://www.3ds.com/products-services/biovia/ (accessed on 14 July 2024).
- Gingrich, T.R.; Wilson, M. On the Ewald summation of Gaussian charges for the simulation of metallic surfaces. Chem. Phys. Lett. 2010, 500, 178–183. [Google Scholar] [CrossRef]
- Higgoda, U.A.; Hellmann, R.; Koller, T.M.; Fröba, A.P. Enhancement of the predictive power of molecular dynamics simulations for the determination of self-diffusion coefficient and viscosity demonstrated for propane. Fluid Phase Equilibria 2019, 496, 69–79. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Jian, Z. Molecular dynamics simulation of diffusion coefficient of Al-Cu alloy. J. Xi’an Technol. Univ. 2018, 38, 559–564. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, C.; Qiu, Q.; Yang, L. Effect of water on the diffusion of small molecular weight acids in nano-SiO2 modified insulating oil. J. Mol. Liq. 2020, 314, 113670. [Google Scholar] [CrossRef]
- Zeebe, R.E. On the molecular diffusion coefficients of dissolved, and and their dependence on isotopic mass. Geochim. Cosmochim. Acta 2011, 75, 2483–2498. [Google Scholar] [CrossRef]
- Costigliola, L.; Heyes, D.M.; Schrøder, T.B.; Dyre, J.C. Revisiting the Stokes-Einstein relation without a hydrodynamic diameter. J. Chem. Phys. 2019, 150, 021101. [Google Scholar] [CrossRef]
- Qi, H.; Han, J.; Xu, N.; Bouwmeester, H.J.M. Hybrid organic-inorganic microporous membranes with high hydrothermal stability for the separation of carbon dioxide. ChemSusChem 2010, 3, 1375–1378. [Google Scholar] [CrossRef]
- Thermophysical Properties of Fluid Systems. Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 14 July 2024).









| Component | Mole % | Plus Fraction Analysis | Mole % |
|---|---|---|---|
| C1 | 14.446 | ||
| C2 | 6.487 | C1–C4 | 37.793 |
| C3 | 10.674 | C5+ | 62.207 |
| C4 | 6.186 | ||
| C5 | 3.958 | ||
| C6 | 3.223 | C1–C8 | 57.605 |
| C7 | 4.893 | C9+ | 42.395 |
| C8 | 7.738 | ||
| C9 | 5.632 | ||
| C10 | 5.162 | C1–C16 | 84.856 |
| C11 | 3.577 | C17+ | 15.144 |
| C12 | 3.109 | ||
| C13 | 2.969 | ||
| C14 | 2.527 | ||
| C15 | 2.368 | ||
| C16 | 1.907 | ||
| C17+ | 15.144 | ||
| Total | 100 |
| Pressure MPa | Final Model Volume Å3 | Volumetric Strain Å3 | Model Swelling Rate % |
|---|---|---|---|
| 3 | 20,672.023 | 3356.741 | 19.39 |
| 4 | 20,329.764 | 3014.482 | 17.41 |
| 5 | 20,325.377 | 3010.095 | 17.38 |
| 6 | 20,218.541 | 2903.259 | 16.77 |
| 7 | 20,202.760 | 2887.478 | 16.68 |
| 8 | 20,070.536 | 2755.254 | 15.91 |
| 9 | 20,043.743 | 2728.461 | 15.76 |
| 10 | 19,804.000 | 2488.718 | 14.37 |
| Pressure MPa | Final Model Volume Å3 | Volumetric Strain Å3 | Model Swelling Rate % |
|---|---|---|---|
| 3 | 21,047.038 | 3731.756 | 21.55 |
| 4 | 20,948.019 | 3632.737 | 20.98 |
| 5 | 20,789.597 | 3474.315 | 20.07 |
| 6 | 20,588.867 | 3273.585 | 18.91 |
| 7 | 20,478.810 | 3163.528 | 18.27 |
| 8 | 20,393.628 | 3078.346 | 17.78 |
| 9 | 20,301.949 | 2986.667 | 17.25 |
| 10 | 20,082.561 | 2767.279 | 15.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Zhang, Y.; Fan, W.; Chen, D.; Wu, K.; Pan, S.; Guo, Y.; Wang, H.; Wu, K. Molecular Insights into CO2 Diffusion Behavior in Crude Oil. Processes 2024, 12, 2248. https://doi.org/10.3390/pr12102248
Gao C, Zhang Y, Fan W, Chen D, Wu K, Pan S, Guo Y, Wang H, Wu K. Molecular Insights into CO2 Diffusion Behavior in Crude Oil. Processes. 2024; 12(10):2248. https://doi.org/10.3390/pr12102248
Chicago/Turabian StyleGao, Chunning, Yongqiang Zhang, Wei Fan, Dezhao Chen, Keqin Wu, Shuai Pan, Yuchuan Guo, Haizhu Wang, and Keliu Wu. 2024. "Molecular Insights into CO2 Diffusion Behavior in Crude Oil" Processes 12, no. 10: 2248. https://doi.org/10.3390/pr12102248
APA StyleGao, C., Zhang, Y., Fan, W., Chen, D., Wu, K., Pan, S., Guo, Y., Wang, H., & Wu, K. (2024). Molecular Insights into CO2 Diffusion Behavior in Crude Oil. Processes, 12(10), 2248. https://doi.org/10.3390/pr12102248







