A Study on Acid Dissolution Characteristics and the Permeability Enhancement of Deep Coal Rock
Abstract
1. Introduction
2. Experiment
2.1. Experimental Material
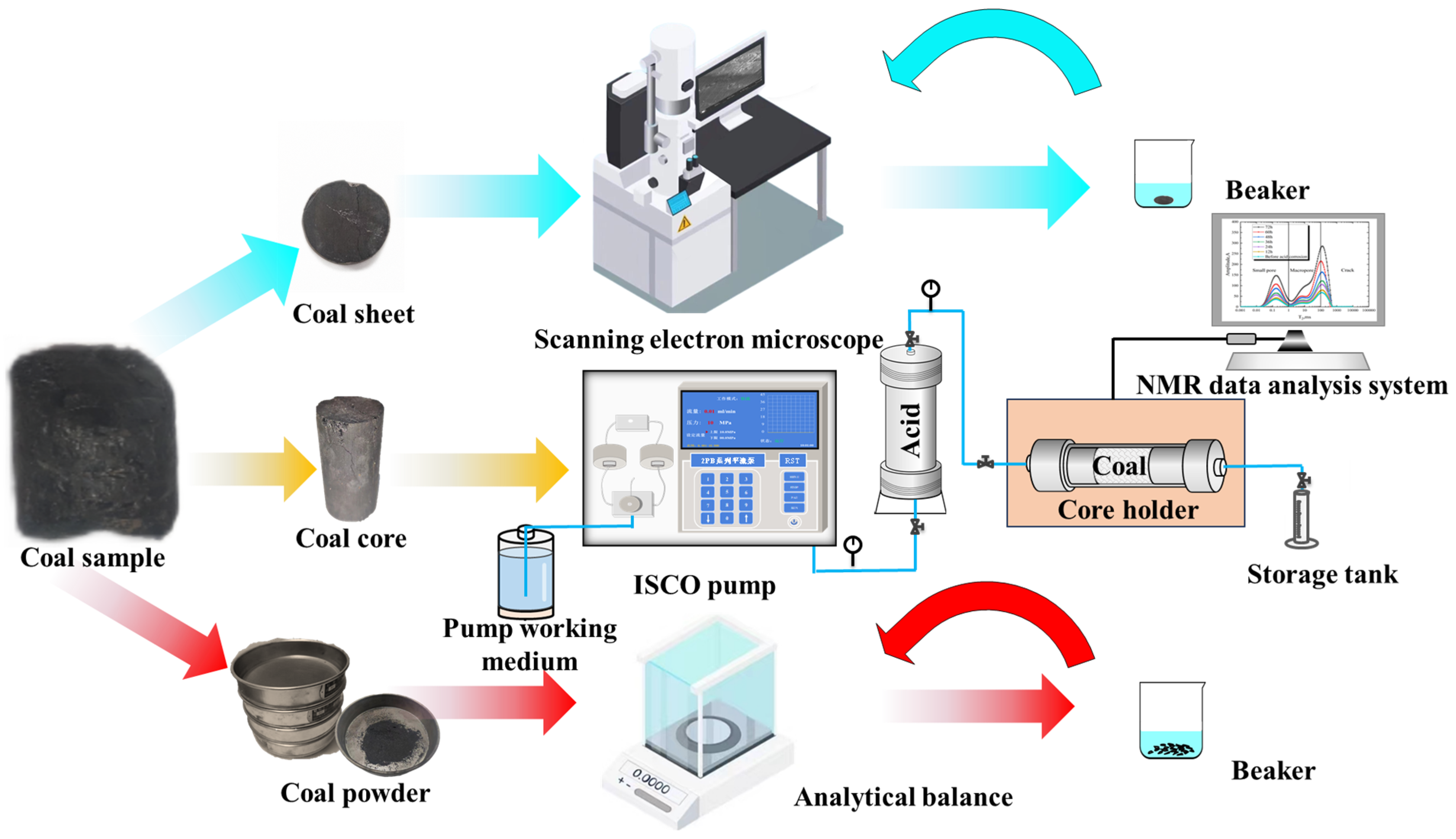
2.2. Experimental Steps and Methods
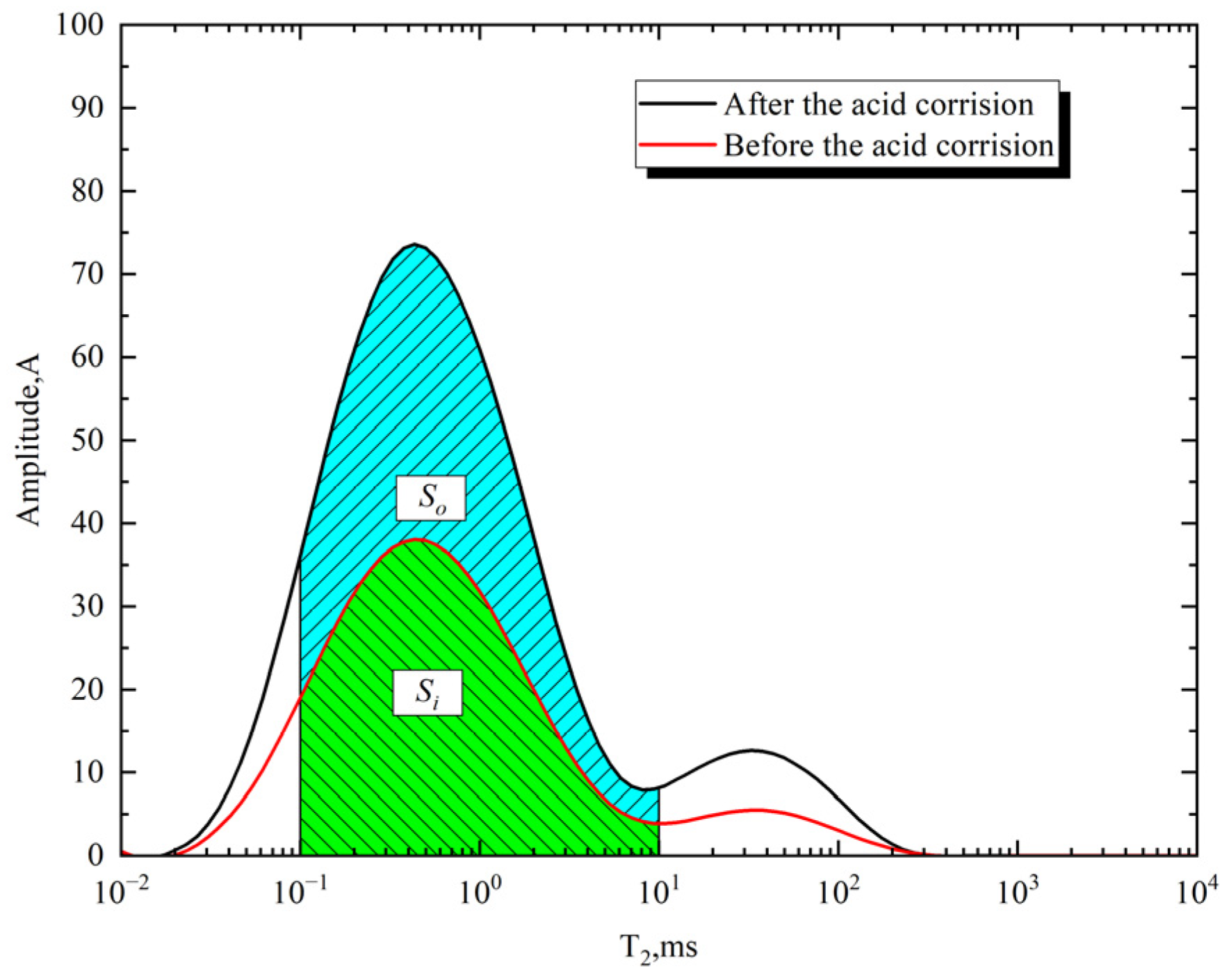
2.3. Calculation Method of Pore Volume Growth Rate
3. Results and Discussion
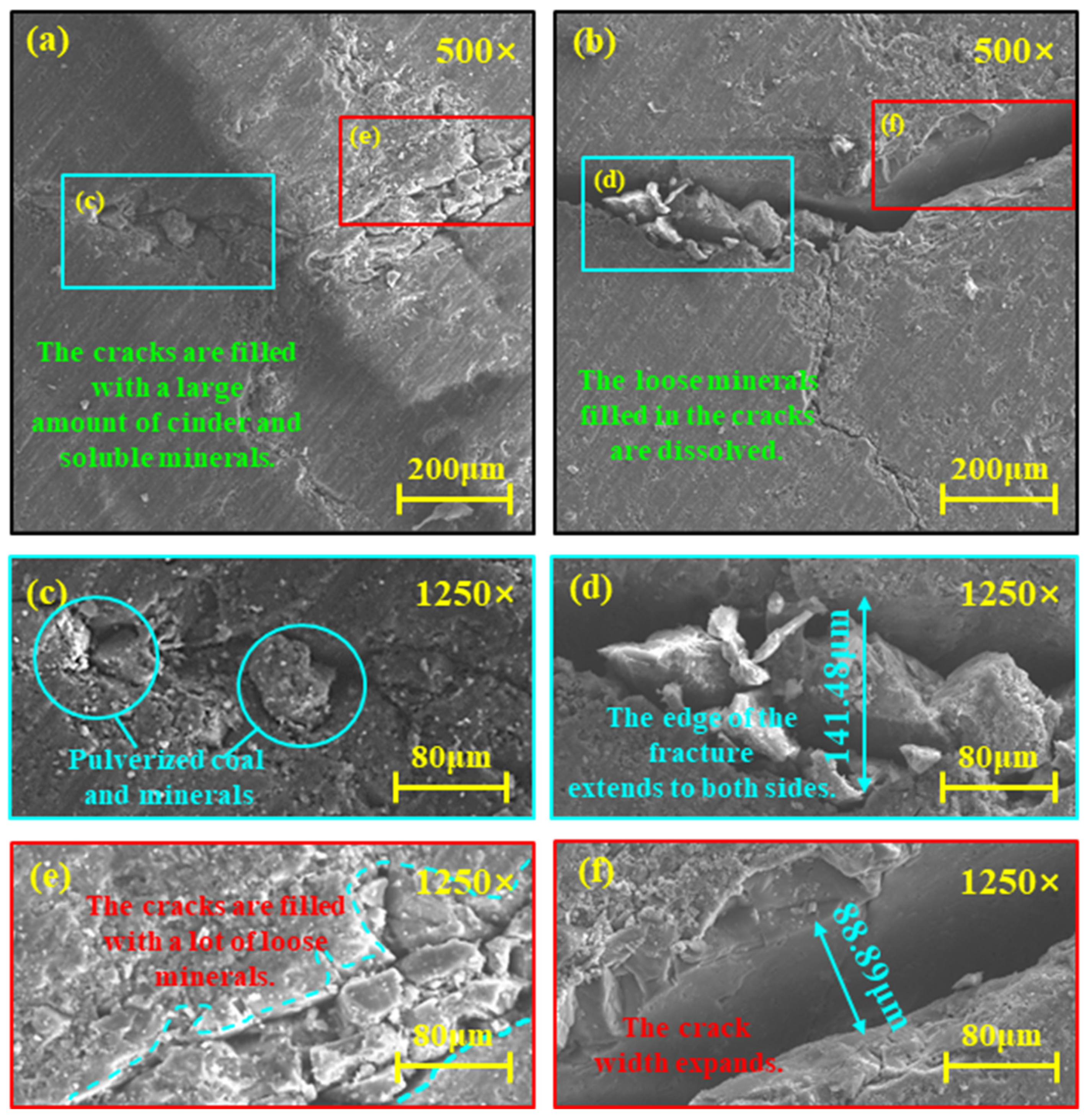
3.1. Pore Dissolution Characteristics
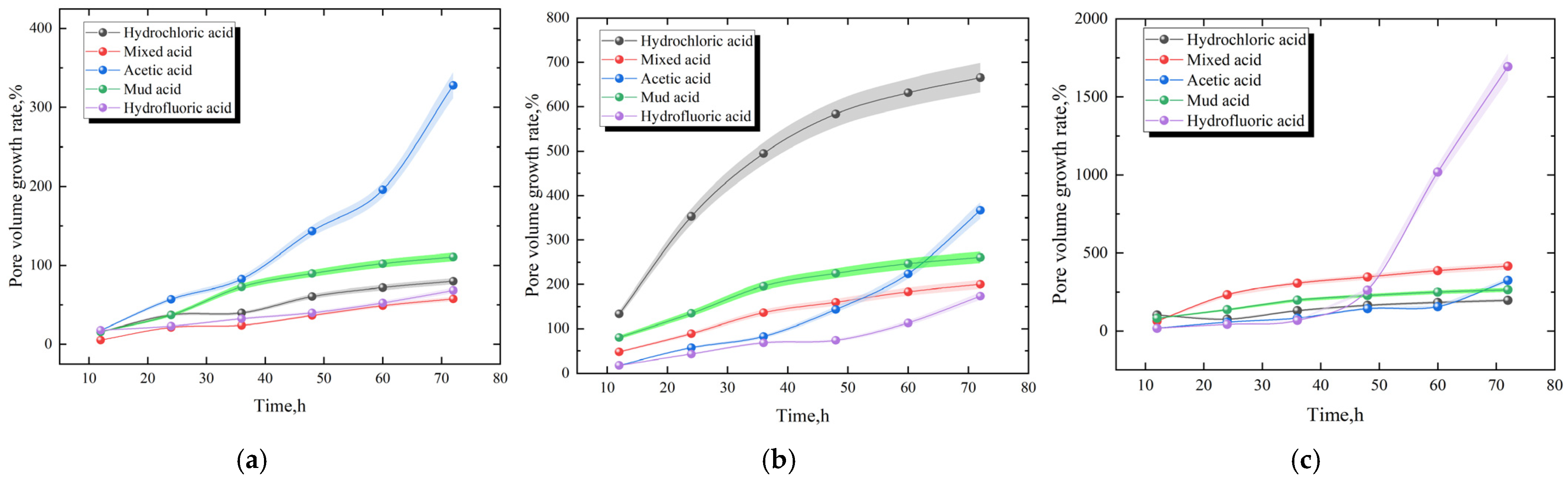
3.2. Cumulative Pore Volume Growth Rate
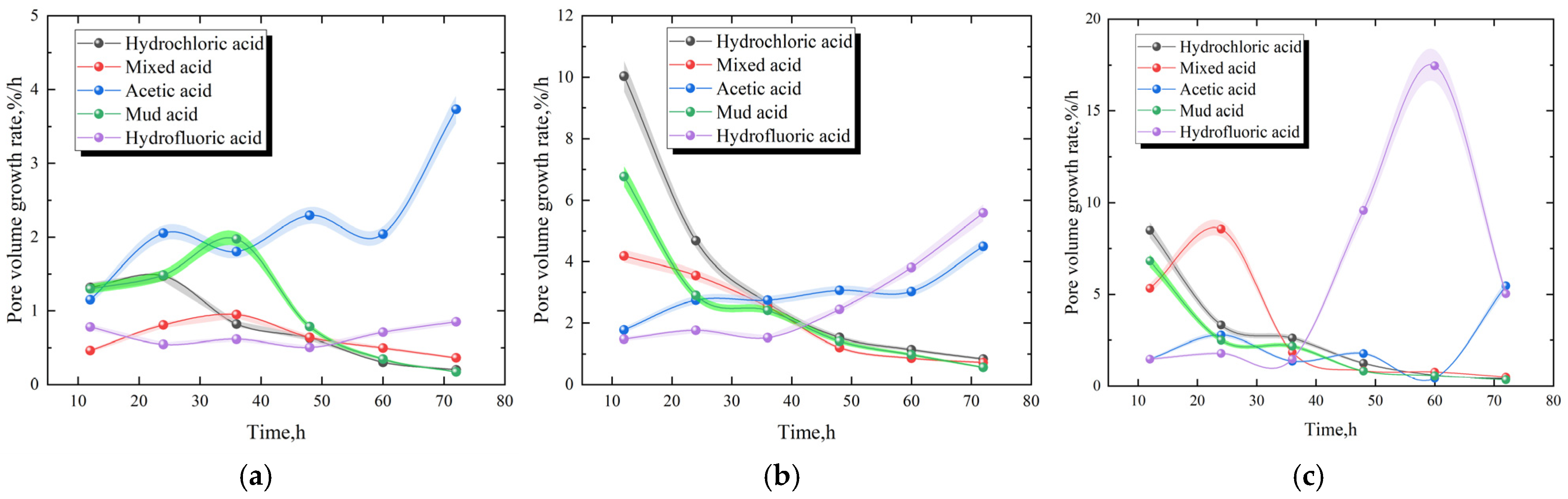
3.3. Change Rate of Pore Volume Growth
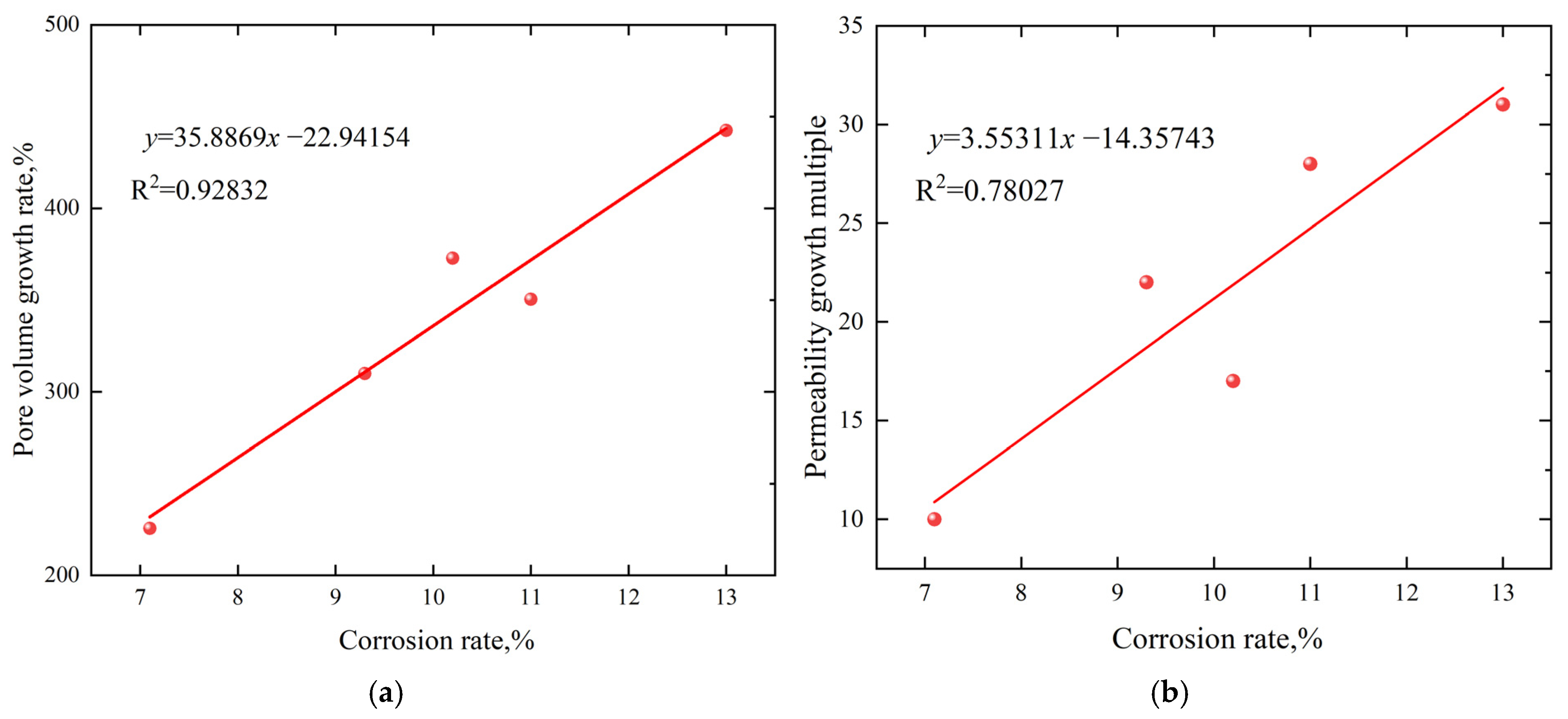
3.4. Analysis of Permeability Increasing Law
3.5. Main Controlling Factors of Coal Rock Dissolution
4. Conclusions
- (1)
- Among different acid solutions, 15% acetic acid has the best dissolution effect on coal rock, with a pore volume growth rate of 442.49% and a 32-time permeability increase. Considering 36 h as the node, 0–36 h can be defined as the high-speed dissolution stage of mud acid, hydrochloric acid, and mixed acid. Further, 36–72 h can be divided into the high-speed dissolution stages of acetic acid and hydrofluoric acid. In this paper, the maximum concentration of acetic acid is 15%, the concentration can be increased to 75% or higher later, and the dissolution characteristics of different acid types can also be observed.
- (2)
- After the dissolution of core samples using mud acid, mixed acid, and hydrochloric acid, the volume growth rate of larger pores and fractures showed a trend of initial rapid increase, followed by a slower increase, and then a gradual decrease. The volume growth rates of macropores and fractures after the dissolution of core samples by hydrofluoric acid and acetic acid were slower before 36 h; however, the pore volume growth rate gradually increased after 36 h.
- (3)
- There is a strong positive correlation between porosity, permeability, and dissolution rate. The greater the degree of dissolution, the better the seepage performance. Acetic acid has the greatest impact on the degree of acid corrosion, followed by hydrochloric acid and hydrofluoric acid. The greater the concentration of acetic acid, the greater the degree of coal rock dissolution.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, F.; Yan, X.; Lin, C.; Li, S.; Xiong, X.; Yan, D.; Wang, H.; Zhang, S.; Xu, B.; Ma, X.; et al. Research progress and development direction of key technologies for efficient coalbed methane development in China. Coal Geol. Explor. 2022, 50, 1–14. [Google Scholar]
- Yang, C.; Yang, Z.; Li, X.; He, Y.; Yi, L. Status and prospect of reservoir stimulation technologies for CBM surface well production in China. Nat. Gas Ind. 2022, 42, 154–162. [Google Scholar]
- Xu, F.; Hou, W.; Xiong, X.; Xu, B.; Wu, P.; Wang, H.; Feng, K.; Yun, J.; Li, S.; Zhang, L.; et al. The status and development strategy of coalbed methane industry in China. Pet. Explor. Dev. 2023, 50, 669–682. [Google Scholar] [CrossRef]
- Liu, S.; Tang, S.; Yin, S. Coalbed methane recovery from multilateral horizontal wells in Southern Qinshui Basin. Adv. Geo-Energy Res. 2018, 2, 34–42. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Lei, Y.; Cao, J. Current situation and development trend of coalbed methane development and utilization technology in coal mine area. J. China Coal Soc. 2020, 45, 258–267. [Google Scholar]
- Xu, C.; Lai, W.; Zhang, X.; Zhang, J. New progress and future exploration thinking of CNOOC oil and gas exploration. China Offshore Oil Gas 2023, 35, 1–12. [Google Scholar]
- Gao, L.; Pang, J.; Xie, Y.; Zhou, L. Analysis on potential geological mining model of deep coalbed methane in Linxing Block. Coal Sci. Technol. 2019, 47, 89–96. [Google Scholar]
- Wang, C.; Gao, J.; Yang, M.; Zhang, X.; Ren, J.; Li, X.; Yang, J.; Chen, S. Study on influencing factors and mechanism of acidizing antireflection effect of high-rank coal. J. Saf. Environ. 2023, 23, 3070–3080. [Google Scholar]
- Yang, H.; Xu, Z.; Fan, M.; Rajender, G.; Rachid, B.S.; Alan, E.B.; Ian, W. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Sun, Y.; Song, Z.; Zhang, L. Experimental Study on the Influence Mechanism of Coal–Rock Fracture Differential Deformation on Composite Permeability. Nat. Resour. Res. 2022, 31, 2853–2868. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; He, G.; Zhou, Z.; Wang, D.; Zhang, D. Research on a permeability model of coal damaged under triaxial loading and unloading. Fuel 2023, 354, 129375. [Google Scholar] [CrossRef]
- Ren, X.; Yu, H. Interaction between sequestered supercritical CO2 and minerals in deep coal seams. Int. J. Coal Geol. 2019, 202, 1–13. [Google Scholar]
- Liu, Y.; Rajendar, G.; Terry, W. Ash formation from excluded minerals including consideration of mineral–mineral associations. Energy Fuels 2007, 21, 461–467. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Robeck, E.; Huo, D. A more accurate method for estimating in situ coal density and mineral matter from ash and specific energy determinations. Int. J. Coal Geol. 2016, 168, 237–252. [Google Scholar] [CrossRef]
- Zhao, W.; Li, R.; Wu, X.; Tang, J.; Wang, K.; Wang, Y. Preliminary indoor experiments on enhancing permeability rate of coal reservoir by using acidification technology. China Coalbed Methane 2012, 9, 10–13. [Google Scholar]
- Yang, H.; Yu, Y.; Cheng, W.; Jun, R.; Xu, Q. Influence of acetic acid dissolution time on evolution of coal phase and surface morphology. Fuel 2021, 286, 119464. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, C.; Yang, H.; Cheng, W.; Xin, Q.; Zhang, X. Effect of acetic acid concentration and dissolution time on the evolution of coal phases: A case report of bituminous coal. J. Mol. Liq. 2021, 340, 117298. [Google Scholar] [CrossRef]
- Lu, H.; Wei, K.; Ma, W.; Xie, K.; Wu, J.; Lei, Y.; Dai, Y. Effect of acetic acid on the leaching behavior of impurities in metallurgical grade silicon. Sep. Sci. Technol. 2017, 52, 1257–1264. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, R.; Seeram, R. Comparative experimental study on the effects of organic and inorganic acids on coal dissolution. J. Mol. Liq. 2021, 339, 116730. [Google Scholar] [CrossRef]
- Wei, Z.; Lin, B.; Tong, L.; Ting, L.; Wei, Y. Effect of acidification on microscopic properties and pore structure of coal. Fuel 2023, 343, 127834. [Google Scholar] [CrossRef]
- Wei, W.; Yu, H.; Guo, C.; Zhang, X.; Liu, G.; Cheng, S.; Zhao, B. Effect of Fouling Layer (Acid–Ash Reaction) on Low-Temperature Corrosion Covering Heating Surface in Coal-Fired Flue Gas. Processes 2022, 10, 241. [Google Scholar] [CrossRef]
- Li, S.; Fan, Y.; Guo, Y.; Wang, Y.; He, T.; Zhang, H.; Ye, J.; Chen, W.; Zhang, X. Simulation and Control Strategies for Longitudinal Propagation of Acid Fracture in a Low-Permeability Reservoir Containing Bottom Water. Processes 2024, 12, 792. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Wu, J.; Wang, Y. The Carrying Behavior of Water-Based Fracturing Fluid in Shale Reservoir Fractures and Molecular Dynamics of Sand-Carrying Mechanism. Processes 2024, 12, 2051. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Han, Y. A Numerical Investigation on Kick Control with the Displacement Kill Method during a Well Test in a Deep-Water Gas Reservoir: A Case Study. Processes 2024, 12, 2090. [Google Scholar] [CrossRef]
- Yang, C.; Tang, Y.; Kang, H.; Gou, M.; Yu, X. Occurrence of minerals in coal and its geological controlling factors-significance in evaluation of coal washability. ACS Omega 2021, 6, 24362–24376. [Google Scholar] [CrossRef]
- Li, X.; Kang, J.; Wang, P.; Liu, Y.; Fu, Q. Geochemistry and mineralogy of Late Carboniferous coal (No. 8) from the Jialequan Mine, Xishan Coalfield, Shanxi Province, China: Evidences for the geologic controls of trace elements. Arab. J. Geosci. 2020, 13, 1171. [Google Scholar] [CrossRef]
- Magni, M.; Postiglione, E.; Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Agri-Food Wastes: The Case of Punica granatum Extract and Its Constituent Ellagic Acid. A Validation Study. Processes 2020, 8, 272. [Google Scholar] [CrossRef]
- Hiei, Y.; Shirai, H. Basic Study on Mineral Removal from Coal-The Influence of Mineral Distribution and Size of Pulverized Coal on Characteristics of Mineral Removal. Coal Prep. 2006, 26, 137–148. [Google Scholar] [CrossRef]
- Dou, H.; Xie, J.; Xie, J.; Sun, G.; Li, Z.; Wang, Z.; Miao, Y. Study on the mechanism of the influence of HNO3 and HF acid treatment on the CO2 adsorption and desorption characteristics of coal. Fuel 2022, 309, 122187. [Google Scholar] [CrossRef]
- Li, R.; Wang, K.; Wang, Y. Indoor study on acidification for enhancing the permeability of coal. J. China Coal Soc. 2014, 39, 913–917. [Google Scholar]
- Fang, H.; Sang, S.; Liu, S. Three-dimensional spatial structure of the macro-pores and flow simulation in anthracite coal based on X-ray μ-CT scanning data. Pet. Sci. 2020, 17, 1221–1236. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Zhang, R.; Zhang, L.; Xu, W. Optimization and Performance Evaluation of an Atomized Acid System for the Expansion of Carbonate Gas Injection. Processes 2023, 11, 3080. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Li, Y.; Liu, F.; Martyushev, D.A.; Yang, Y. Effects of microfractures on permeability in carbonate rocks based on digital core technology. Adv. Geo-Energy Res. 2022, 6, 86–90. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, M.; Zhang, Q.; Tang, J.; Lu, S. Dynamic capillary pressure analysis of tight sandstone based on digital rock model. Capillarity 2020, 3, 28–35. [Google Scholar] [CrossRef]
- Quanle, Z.; Baiquan, L.; Ting, L.; Yan, Z.; Zhen, Z.; Fazhi, Y. Variation of methane adsorption property of coal after the treatment of hydraulic slotting and methane pre-drainage: A case study. J. Nat. Gas Sci. Eng. 2014, 20, 396–406. [Google Scholar]
- Quanle, Z.; Tiancheng, Z.; Tengfei, M.; Shixiang, T.; Xueqi, J.; Zebiao, J. Effect of water-based SiO2 nanofluid on surface wettability of raw coal. Energy 2022, 254, 124228. [Google Scholar]
- Quanle, Z.; Baiquan, L.; Jinyan, L.; Ting, L.; Yan, Z.; Fazhi, Y.; Chuanjie, Z. Variation in the Pore Structure of Coal after Hydraulic Slotting and Gas Drainage. Adsorpt. Sci. Technol. Vol. 2014, 32, 623–705. [Google Scholar]
- GB/T 23561.1-2009; Methods for Determining the Physical and Mechanical Properties of Coal and Rock—Part 1: General Requirements for Sampling. Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Wei, J.; Su, E.; Xu, G.; Yang, Y.; Han, S.; Chen, X.; Chen, H.; An, F. Comparative analysis of permeability rebound and recovery of tectonic and intact coal: Implications for coalbed methane recovery in tectonic coal reservoirs. Energy 2024, 301, 131702. [Google Scholar] [CrossRef]
- Nie, L.; Zhou, D.; Guo, X.; Hui, J.; Bian, J.; Gao, L. Analysis on influencing factors of post fracture response in low permeability gas reservoir with gray correlation method. Fault-Block Oil Gas Field 2013, 20, 133–136. [Google Scholar]



| Core Number | Depth (m) | Length (cm) | Diameter (cm) | Porosity (%) | Permeability (10−3 μm) |
|---|---|---|---|---|---|
| 1 | 2087.80 | 5.02 | 2.50 | 5.16 | 1.38 |
| 2 | 2088.26 | 4.98 | 2.50 | 4.98 | 1.41 |
| 3 | 2088.45 | 4.97 | 2.51 | 5.17 | 1.39 |
| 4 | 2088.65 | 5.01 | 2.51 | 5.21 | 1.40 |
| 5 | 2088.72 | 5.03 | 2.50 | 5.09 | 1.37 |
| Acid | Acid Concentration (%) | Purity | Manufacturer |
|---|---|---|---|
| HF | 40 | Analytically pure | Xilong Scientific Co., Ltd., Shantou, China |
| CH3COOH | 36 | Analytically pure | Damao Chemical Reagent Factory, Tianjin, China |
| HCl | 36 | Analytically pure | Tianjin Chemical Reagent Factory No.1, Tianjin, China |
| A | B | C | D | E | F | G | H | I | J | |
|---|---|---|---|---|---|---|---|---|---|---|
| HF | 3% | / | 15% | / | 5% | 3% | 3% | 5% | 7% | 7% |
| CH3COOH | / | / | / | 15% | 3% | 5% | 7% | 7% | 5% | 3% |
| HCl | 12% | 15% | / | / | 7% | 7% | 5% | 3% | 3% | 5% |
| Time | HF | Mud Acid | CH3COOH | Mixed Acid | HCl | |
|---|---|---|---|---|---|---|
| Total pore volume growth rate | 0–36 h | 53.31% | 154.26% | 82.56% | 77.59% | 189.56% |
| 36–72 h | 128.55% | 20.95% | 142.39% | 27.08% | 28.74% | |
| Total | 350.39% | 310.07% | 442.49% | 225.54% | 372.77% | |
| Macropore volume growth rate | 0–36 h | 68.07% | 195.81% | 82.56% | 136.06% | 494.69% |
| 36–72 h | 62.76% | 21.95% | 155.45% | 28.03% | 28.68% | |
| Total | 273.55% | 360.74% | 466.33% | 299.80% | 765.57% | |
| Small pore volume growth rate | 0–36 h | 32.19% | 72.63% | 84.32% | 23.92% | 39.57% |
| 36–72 h | 27.17% | 21.95% | 134.50% | 27.00% | 28.74% | |
| Total | 168.10% | 210.53% | 428.09% | 157.37% | 179.67% | |
| Fracture volume growth rate | 0–36 h | 68.07% | 198.18% | 82.56% | 305.67% | 130.40% |
| 36–72 h | 966.88% | 21.95% | 132.31% | 26.99% | 28.74% | |
| Total | 1793.08% | 363.64% | 424.09% | 515.21% | 296.60% |
| HF | Mud Acid | CH3COOH | Mixed Acid | HCl | |
|---|---|---|---|---|---|
| Pre-dissolution mass (g) | 5 | 5 | 5 | 5 | 5 |
| Mass after dissolution (g) | 4.45 | 4.54 | 4.35 | 4.65 | 4.49 |
| Dissolution rate (%) | 11 | 9.3 | 13 | 7.1 | 10.2 |
| Initial permeability (10−3 μm) | 0.65 | 0.59 | 0.63 | 0.58 | 0.61 |
| Permeability after dissolution (10−3 μm) | 18.85 | 13.57 | 20.16 | 6.38 | 10.98 |
| Permeability growth multiple | 28.00 | 22.00 | 31.00 | 10.00 | 17.00 |
| HF | CH3COOH | HCl | Corrosion Rate | |
|---|---|---|---|---|
| A | 3% | / | 12% | 9.3% |
| B | / | / | 15% | 10.2% |
| C | 15% | / | / | 11% |
| D | / | 15% | / | 13% |
| E | 5% | 3% | 7% | 7.1% |
| F | 3% | 5% | 7% | 8.1% |
| G | 3% | 7% | 5% | 9.3% |
| H | 5% | 7% | 3% | 5.5% |
| I | 7% | 5% | 3% | 5.3% |
| J | 7% | 3% | 5% | 4.2% |
| HF | CH3COOH | HCl | ||
|---|---|---|---|---|
| Correlation coefficient | A | 3.54 | 4.90 | 17.73 |
| B | 2.93 | 5.44 | 4.69 | |
| C | 5.42 | 3.80 | 27.09 | |
| D | 12.93 | 244.48 | 7.90 | |
| E | 72.31 | 14.80 | 8.56 | |
| F | 13.84 | 11.38 | 23.39 | |
| G | 4.86 | 1.26 | 1.26 | |
| H | 0.72 | 0.72 | 0.72 | |
| I | 0.72 | 1.26 | 0.72 | |
| J | 0.54 | 0.51 | 0.72 | |
| Correlation degree | 11.78 | 28.86 | 14.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Luo, W.; Dai, X.; Wu, J.; Zhou, X.; Huang, K.; Zhang, N. A Study on Acid Dissolution Characteristics and the Permeability Enhancement of Deep Coal Rock. Processes 2024, 12, 2209. https://doi.org/10.3390/pr12102209
Wang C, Luo W, Dai X, Wu J, Zhou X, Huang K, Zhang N. A Study on Acid Dissolution Characteristics and the Permeability Enhancement of Deep Coal Rock. Processes. 2024; 12(10):2209. https://doi.org/10.3390/pr12102209
Chicago/Turabian StyleWang, Chen, Weijiang Luo, Xiancai Dai, Jian Wu, Xing Zhou, Kai Huang, and Nan Zhang. 2024. "A Study on Acid Dissolution Characteristics and the Permeability Enhancement of Deep Coal Rock" Processes 12, no. 10: 2209. https://doi.org/10.3390/pr12102209
APA StyleWang, C., Luo, W., Dai, X., Wu, J., Zhou, X., Huang, K., & Zhang, N. (2024). A Study on Acid Dissolution Characteristics and the Permeability Enhancement of Deep Coal Rock. Processes, 12(10), 2209. https://doi.org/10.3390/pr12102209






