Abstract
In the last decade, with the rise in customer awareness about the quality of the food they consume and its health benefits, new methods for producing food fat replacers have been developed. Since then, significant progress has been made in enhancing these techniques. Methods such as emulsion template, foam template, and solvent exchange are frequently employed for creating fat replacers known as oleo- or emulsion gels, commonly used in food products. As the interest in developing fat replacers continues to grow, it has become essential to explore and pursue new materials suitable for producing protein-based fat replacers. Given the increasing food consumption, food waste is on the rise. The goal is to maximize food utilization and create high-protein, nutritionally rich foods with minimal waste. This involves using new materials, such as alternative proteins or food by-products, and finding effective methods for their utilization. This review aims to provide insights into the variety of materials and methods employed to prepare protein-based fat replacers as documented in the available literature.
1. Introduction
In the past two decades, high-protein diets have become increasingly popular. Due to the rise in customer awareness regarding eating habits, the quality of foods, and most importantly their impact on human health, consumers are being cautious about the ingredients that are part of their diet [1]. In the literature, increasing numbers of studies show the negative effect of overconsumption of saturated or trans-fatty acids on human welfare [2,3,4]. The aforementioned fatty acids are commonly found in food products containing hydrogenated fats, which are originally extracted from plants in the form of oil [5].
To create more solid fats, liquid vegetable oils undergo a common process called hydrogenation. During hydrogenation, the double bonds in unsaturated fatty acids are eliminated, and hydrogen is added to saturate their tri-, di-, or mono-acyl glycerol. This process can be partial, where some bonds remain and undergo a cis/trans conversion and positional shift in the fatty acid chain. Complete hydrogenation results in all unsaturated fatty acids becoming saturated, yielding solid fat as a result. As a result of the chemical changes brought about by hydrogenation, two important quality aspects are affected: the melting range is shifted to higher temperatures, and the stability against oxidation and flavor deterioration is improved. However, the hydrogenation process also brings about the formation of trans-fatty acids, which can have negative impacts on individual health. Industrial processing at elevated temperatures, such as physical refining, deodorization, and partial hydrogenation of oils, can lead to the production of trans-isomers in oils and fats from their cis forms [5,6,7]. Many studies [8,9,10,11] have examined the connection between the consumption of saturated and trans-fatty acids and the level of cholesterol, mainly LDL in the blood. Based on the facts obtained from the previously mentioned studies, saturated fatty acids (SFAs) can increase low-density lipoprotein (LDL) cholesterol concentration, thus increasing the risk of cardiovascular disease [9,11]. SFAs, as well as trans-fatty acids, are recognized as the dietary factor that has the greatest negative effect on LDL cholesterol [12,13]. In a study conducted by Mensink and Katan [14], where the influence of saturated and trans-fatty acids in the diet on human health was measured, it was found that diets that contained trans-fatty acids at 10% of energy increased LDL and decreased levels of high-density lipoprotein (HDL).
On the other hand, diets with saturated fats increased LDL but did not affect HDL. This can be explained by the result of the measurement of apolipoproteins A and B, which are the main proteins in the composition of HDL and LDL cholesterol, respectively. The results indicated that the values of apolipoprotein A were significantly lower in consumers with a trans-fatty acid diet, resulting in the lowering of HDL cholesterol. Additionally, higher values were observed for apolipoprotein B regarding both diets, saturated- and trans-fatty-acid-based, thus resulting in higher HDL [14]. In other studies, these results were confirmed by conducting trials in which the diets consisted of 3% [15], 6% [15], and 7.8% [16] trans fats [10]. In contrast, monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) have decreased the levels of cholesterol in clinical and animal trials [11,17,18,19]. The results of the study [20] showed that the replacement of 1% of total daily energy from SFAs with MUFAs or PUFAs lowers the LDL cholesterol concentration by approximately 1.3, 1.6, and 2.1 mg/dL, respectively [19,20,21].
Although MUFAs and PUFAs positively impact human welfare, they are prone to oxidation and do not have the desired physical and chemical properties, which makes them inadequate for the replacement of saturated and trans-fatty acids in most food products [22]. Because of that, there was a need for alternatives that could replace unwanted fats and include other materials with minimal influence on the properties of a final food product. After comprehensive research, in 2012, the first oleogel system was developed [23].
Oleogels are fat replacers, defined as liquid oils that have been converted into semi-solid systems with viscoelastic properties as well as a hydrophobic nature. Additionally, emulsion gels are also recognized as potential fat substitutes in food matrices, because of their gel-like network structure and textural properties similar to solid fats [24,25]. Proteins and polysaccharides are gelling agents and can be used in the preparation of the above-mentioned fat replacers [26,27,28]. The formation of the structure can be explained by the presence of weak intermolecular forces—electrostatic and Van der Waals forces, hydrogen bonds, and hydrophobic interactions—which hold the molecules together. Additionally, cross-linkages are present between two or more polymers, forming structures known as junction zones [28].
Protein is a nutrient needed to maintain body processes because of its structural and functional role in the human organism. Proteins are made of amino acids, and in human organisms, 20 of them are used as a base for protein synthesis. Only nine of them are regarded as essential; in other words, the human organism cannot produce those amino acids [29]. To formulate oleogels based on these biopolymers and hydrophilic components, indirect methods such as the emulsion template method, the foam template method, and the solvent exchange method are used [30].
This review seeks to offer an understanding of the utilization of conventional and, recently, unconventional proteins, such as plant proteins or proteins derived from food waste, as well as the techniques used to create protein-based fat substitutes, as evidenced in the existing literature.
2. Techniques and Mechanisms for Manufacturing Protein-Based Oleogels
Techniques used for the preparation of oleogels can be direct and indirect. The direct method is known to be used for the preparation of oleogels consisting of ethyl cellulose (ES). Since proteins have a hydrophilic nature, it was necessary to develop a new method for oleogelation. The indirect methods are nonconventional, innovative methods designed for the production of protein- and polysaccharide-based oleogels [31]. The next sections will provide more information about the previously mentioned indirect methods such as the emulsion template, foam template, and solvent exchange methods.
2.1. Emulsion Template Method
The emulsion template method has been recognized as an adequate method for preparing oleogel/emulsion gel. This method was first utilized by Romoscanu and Mazzenga in 2006 [32]. The structures prepared using this technique have been used in various food products, as a replacement for shortening in cakes and fats in sausages [28,33,34], because of their properties, such as improving the stability and controlled release of selected bioactive compounds [23]. Using oleogels as bakery fats instead of liquid oils significantly improved the resulting batter and cake properties. The structure of the oleogel batter was similar to that of commercial shortening batter, but it had a runny nature similar to oil batter. While runny batter allows better homogenization and easier processing in large-scale manufacturing, it can lead to denser cake products with low volume. Moreover, the appearance and sensory properties of cakes prepared using oleogel were much better than those prepared with oil and more similar to those prepared with shortening [33]. However, properties like storage conditions and long-term effects on human health are yet to be examined. The mechanism of this method can be explained in a few steps (Figure 1). First, it is necessary to prepare concentrated O/W emulsion. At this point, it is crucial to obtain stabilization and stiffness of the layer between the phases via cross-linking or particle adsorption. After the successful stabilization, the next step includes drying, by evaporating the water phase. Lastly, mild shearing of the dried product is needed to obtain oleogel. Mild shearing is performed using an Ultraturrax at a low rotor speed [33]. In other words, this method involves the addition of a polymer into an aqueous solvent or a continuous emulsion to structure the oil into an emulsion gel, and then after the water is removed by freeze-drying or heating-drying, to maintain the tightness of the produced gel. This structure is formed to entrap high amounts of liquid oil without any oil leakage even after a long storage time [35]. This method provides the opportunity to produce oleogels with dissimilar characteristics by selecting the portion of crucial compounds and differentiating processing conditions.
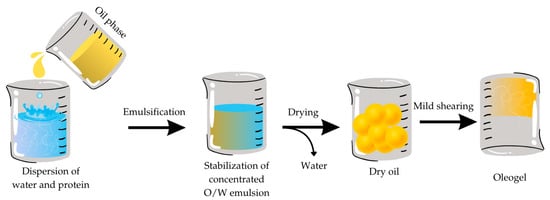
Figure 1.
Schematic view of the production of the oleogel step by step using the emulsion template approach.
Ensuring the stability of the interface layer is crucial when producing oleogels using this method. This stability can be increased by combining proteins and different polysaccharides [35]. Patel et al. [36] used the emulsion template method to formulate oleogels manufactured utilizing gelatin and xanthan gum. Their investigation resulted in stable oleogels, with an interesting microstructure. The oil phase, in the form of droplets, was tightly packed with a protein layer to protect it from the coalescence of oil droplets. Since gelatin and xanthan gum have “GRAS” status (generally recognized as safe), oleogels produced using this combination have the potential to be utilized in the food industry. In addition, Qui et al. [37] produced oleogels using a combination of protein–gelatin and polysaccharide–flaxseed gum and tannic acid. This combination (0.075% wt) with protein increased the strength of oleogels and insinuated that the addition of tannic acid and flaxseed gum positively impacted the stability of the produced oleogels. Another study [38] also indicated that the addition of polysaccharides like HPMC and pectin increased the mechanical strength of the assembled oleogel.
2.2. Foam Template Method
Another common method for producing oleogels is the foam template method (Figure 2). Oleogels obtained by this method are commonly used for cakes as a fat replacement because they have mechanical properties similar to shortening used for the production of this type of product. This method is similar to the previously mentioned method, but the main difference is in the first step, where air is incorporated into a dispersion of water and protein. After foaming and drying, the aerogel is formed. The next step includes the absorption of edible oil into the aerogel and then mild shearing to obtain the oleogel. In this method, oil is added after the drying step, or the template is placed into the oil to let the structure fill the void in the formed structure [39,40,41]. The amount of absorbed oil depends on the properties of the produced aerogel.
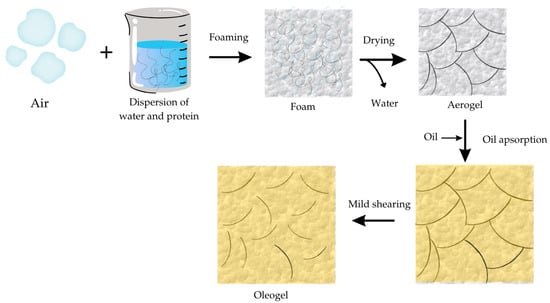
Figure 2.
Schematic diagram of the production of the oleogel step by step using the foam template method.
This method was used in research by Abdollahi, Goli, and Soltanizadeh [40]. Their study aimed to develop gelatin and gelatin–xanthan gum oleogels using the foam template technique and determine the physicochemical properties of the produced samples. Preparation of the samples included aeration with Ultraturrax at 13,000 rpm for 5 min and, afterward, drying using the freeze-drying technique for 24 h. Lastly, canola oil was added to produce cryogels, which were shredded after the oil absorption to obtain oleogels. The obtained oleogels showed high gel strength, high values for oil binding capacity (OBC) (more than 90%), and thixotropic behavior. Adding the xanthan gum increased the firmness and network density but did not significantly impact the oil binding capacity. In another study, by Chen and Zhang [41], the alginate/soy protein conjugates were used to manufacture oleogels loaded with 5% thymol using this method. This research gave excellent results regarding antimicrobial activity and OBC and agrees with the previously mentioned research.
2.3. Solvent Exchange Template
Another innovative indirect method for achieving oleogelation is the solvent exchange template. The first step includes the formation of a heat-set protein hydrogel in water as a continual phase. Afterward, through the intermediate solvent, the exchange of the water phase with oil, as a continual phase, occurs. In the second step, the solvent’s polarity was gradually adjusted to displace the surrounding water from the protein aggregates and substitute it as the continuous phase for oil. This process is common in the production of aerogels, where the water is replaced with alcohol or acetone and then removed by supercritical CO2 to produce aerogels. These aerogels are then mixed with oil to obtain oleogel [42,43]. The properties of the obtained oleogels can be changed by adjusting the protein concentration or by modifying the strength of interaction among the aggregates. This can be achieved by adding the water phase to increase capillary interaction or by heating to enhance van der Waals interactions [44]. The results of the study [43] showed that the oil binding capacity and the structure of the obtained oleogels produced using this method were highly dependent on the composition of the intermediate solvent and the number of steps used for the solvent exchange. In another study [45], a solvent exchange template was utilized to produce oleogels using various protein sources. This study suggested that the oleogels with smaller particle size distribution and higher hydrophilicity exhibited greater strength and suitability for use. This method does not have any food application so far in the literature, which can be explained by the use of organic solvents that are not favorable in food production. Additionally, this method is more complicated than the previously mentioned methods.
3. Common and Innovative Solutions for Protein Sources of Organogelators
Proteins, as macronutrients, are vital in the human diet due to their structural and functional role in human organisms. Proteins from animals are generally present in the human diet, but in the last decade, proteins derived from plant sources have been attracting growing attention. The increase in the need for protein production and consumption has led to extensive research on new sources of proteins that could be utilized as organogelators. The properties of plant-based proteins, such as gelling or foaming ability and solubility, are often considered inadequate compared to animal-based proteins. Due to disparities in their functional properties, it is generally challenging to directly substitute animal proteins with plant proteins [46]. The difference among proteins of different origins lies in the different structures which result from their native environment. Proteins like milk and egg proteins are found in aqueous habitats and are therefore mostly hydrophilic. In contrast, plant-derived proteins are generally considered storage proteins, possessing more compact structures and exhibiting higher hydrophobicity [47].
3.1. Animal-Based Proteins
The increase in consumer awareness about protein intake and modern trends in protein-based nutrition have led to an increase in the variety of products available over the counter. The traditional proteins found in human diets come from animals, including eggs, meat, milk, cheese, and yogurt. However, the protein market is changing due to consumer demand for healthier, more sustainable, and ethically acceptable sources. Factors like limited availability, economic concerns, and environmental considerations are directing growing interest in nonconventional protein sources such as plants, food processing by-products, insects, and microalgae [48]. Some of these are examined as potential oleogelators (Table 1).
The protein content in meat ranges from 16 to 40%, varying according to the type of tissue and the age of the animal. Meat also provides essential minerals such as zinc, magnesium, copper, and iron [48]. This animal-based protein can be potentially replaced with plant-based proteins because of the similar properties such as water and oil binding, needed for products like sausages and burgers [49].
Milk, like meat, has varying protein content based on the type, age of the animal, and environmental conditions on the farm. It is recognized for its high digestibility, at approximately 95%, with a total protein utilization value of 74% [46,50]. Different milk protein fractions, such as whey protein and casein (80:20 percent, respectively), are available on the market, with varying levels of purity. Whey protein, obtained as a by-product during cheese production, is an economical source of milk protein. Its nutritional composition makes it a useful functional ingredient in the food industry [51]. In studies [42,45,52], whey protein isolates (WPIs) were used to produce oleogels using the solvent exchange method. Small particle sizes of WPIs (~200 nm) were responsible for obtaining the gel network in oil. Additionally, low protein concentrations (3–7%) were enough to achieve gel-like behavior. Furthermore, the protein network structure was examined through the values of fractal dimension. Crucial parameters for understanding this analysis are G′ (storage modulus) and γ0 (limit of linearity), related to the volume fraction, φ. Comparing the relation between these parameters, we can understand the strength of the formed gel network (weak or strong). A strong link is present when the value of γ0 lowers with an increase in the protein concentration and fractal flocs interaction [53]. Using WPIs as an oleogelator resulted in a strong gel network. In another study [54] WPIs were used as an organogelator by applying a foam template. Oleogels were prepared from aerogels, using two different approaches, freeze-drying of hydrogels or drying with supercritical CO2. After drying, the aerogels were dispersed into the oil phase to obtain protein-based oleogels. The starting point, for aerogels produced either way, showed oil structuring properties, which resulted from their capability to entrap the oil in pores through capillary forces. Furthermore, it was demonstrated that the particle–particle interaction via hydrogen bonds affects the gel strength [42]. The different physical appearances of oleogels were observed, with a higher appeal for the products produced with the dispersion of CO2-dried particles in oil. This research suggests that whey protein aerogel particles can potentially be used as innovative food organogelators to prepare oleogels with tailored properties. Recent trends use proteins combined with polysaccharides to formulate stable emulsions first, and lastly oleogels. Pectin, combined with WPIs, was used in research [55] for producing an emulsion with a high oil fraction. This combination is based on forming a complex between biopolymers, which resulted in higher stability compared to the systems where only one kind of biopolymer was used [55,56].
Eggs are a source of protein, containing about 13% protein, in addition to other nutrients. They can be applied in solid (powdered), liquid, and frozen form. For the formulation of various food products, eggs can be added by using only egg whites, only yolks, or by adding them whole. Due to its amino acid composition (including essential amino acids), as well as good functional properties such as gelling and emulsifying, and excellent digestibility, egg white is considered a very valuable food ingredient [48,57]. Egg protein has been used as a possible protein oleogelator in the production of oleogels [45]. The study showed that low protein concentrations were enough for gel formation (~4%), but the particle size distribution analysis indicated that the presence of larger protein aggregates (around 10–100 µm) weakened the formed gel network and the ability of oil binding (7.3 g/g). Other studies involved a combination of egg protein and polysaccharides and the exploration of properties of oleogels obtained from a foam template [58,59].
Collagen is a protein obtained from the connective tissue of mammals, such as bones, cartilage, tendons, teeth, and skin. Gelatin, a hydrolyzed form of collagen, is widely used as a gelling agent. In comparison to collagen, gelatin has better digestibility and is more easily absorbed [48,60,61]. A recent study [34] mentioned collagen as a promising structuring agent for oleogels and their utilization as fat replacers in meat products. According to the results, authors indicate that the replacement of fat with oleogels positively affected textural, sensory (with replacement no further than 50%), and oxidative stability properties. A few studies [62] investigated gelatin, a form of collagen, as a potential protein source for oleogelation using different methods like foam or emulsion templates.
3.2. Plant-Based Proteins
Recent developments in nutrition and evolving consumer preferences have led to the emergence of numerous new alternative proteins. The first category of alternative proteins comprises those derived from processing and refining plant-based raw materials. Plant proteins are known for their high nutritional value, which is determined by the amino acid composition and the presence of essential amino acids in the protein. Plant proteins can influence the texture, consistency, and sensory properties of food products due to their physico-chemical characteristics. Furthermore, certain plant proteins exhibit biological activity, making them suitable for use in functional foods, as well as in cosmetic and pharmaceutical products [63,64,65,66]. Upon review of the literature, it was found plant material obtained from pulses (peas, lentils, faba beans, and chickpeas) and cereals (rice), which have high nutritional value, a positive effect on human health, and a low cost of production, can be considered as suitable substitutes for animal-based protein oleogels (refer to Table 1).
Soy protein is the most popular plan protein present in the human diet, and a major component of animal feed. Soybeans contain 35–40% total protein with a well-balanced amino acid composition. This property makes soy protein an important alternative to meat and dairy proteins in human diets. Different soy protein derivatives are commercially available, including flour, protein concentrate, and protein isolate. Soy protein concentrate contains over 65% protein, while soy protein isolate is the most refined form, with over 90% protein content [46,67]. There are many studies [45,68,69,70] involving soy protein isolate (SPI) in the formulation of these structured networks, mostly combined with polysaccharides for obtaining lasting stability. Oleogels were mostly prepared using the emulsion template approach with only protein or protein–polysaccharide complex. The two compound systems reacted and formed a complex that provided a layer that is more resistant to destabilization, thus simultaneously increasing the viscoelasticity of the samples by approximately one amplitude, and the authors concluded that the addition of the polysaccharides was justified. This can be explained by the adsorption of polysaccharides on the surface of a single layer, thus increasing the negative charge. This resulted in forming a more compact oleogel [68]. Additionally, the addition of polysaccharides increased the OBC (more than 97%) and oxidation stability, hence reducing the peroxide value. The results from other studies were in agreement with the enhanced properties of oleogels formulated using complex biopolymers.
Pea protein, with a content of around 25%, has gained interest because of its low allergenicity and low cost but high nutritional value and availability [71,72]. It is the second most important legume crop and is used in human diets worldwide because of its emulsification and water- and fat-binding abilities. Peas and faba beans have been mentioned as possible sources of plant protein in manufacturing protein-based fat replacers [73,74]. This study aimed to compare the functionality of concentrates and isolates from both sources of protein. Foams for aero- and then oleogels were prepared using different values of pH, and the influence was observed. The OBC of pea and faba concentrate at pH 9 was significantly (p < 0.05) higher (25.1 and 27.8 g/g) than the values for corresponding foams of protein isolates (17.2 and 14.4 g/g). The maximum oil binding capacity (OBC) occurred at pH 9 for protein concentrates and at pH 7 for protein isolates. This can be explained by the significantly higher values of pores present in protein isolate foam microstructure. Additionally, the number and connection between the pores play a significant role in oil binding capacity.
Bamboo shoots are the newly expanded buds or meristems of bamboo, known for their rapid growth and high nutritional value [75]. Based on the results of the study [76] regarding the nutritional value of bamboo shoots, it was concluded that bamboo shoots can be a potentially great source of protein. Because of its great nutritional value, [77] aimed to determine the possibility of utilization of bamboo shoots for oleogelation. The template used for oleogel production was emulsion, and it was based on obtaining the oleogels by using bamboo shot/soy protein complex. The results indicated that the higher amounts of protein increased the dominance of elastic solid-like behavior. The complex successfully formed a gel network, which was weak, but notably had an OBC of more than 50% and also had good thermal properties, which are desirable for food products. The conclusion of this study indicated that bamboo shoots can be considered innovative organogelators for obtaining new formulations of oleogels.
3.3. Proteins Obtained from Food Waste as Potential Oleogelators
Increased demand for food production and hence, food consumption, have led to generating enormous amounts of food waste, since the rejection of food waste follows the entire food supply chain. Food waste comprises material suitable for a human diet that is in due course degraded, polluted, or rejected. Additionally, industries that include food processing generate food waste for many reasons such as damage as a consequence of inadequate transportation, storage conditions, and losses during production [78]. Those losses can be rich in highly valuable nutrients such as proteins, carbohydrates, and dietary fiber.
Proteins can be extracted from by-products of various food production processes, which helps to enhance the sustainability of the entire food supply chain and positively impact the environmental problem caused by the disposal of food waste [79]. Increasing efforts are being focused on finding and improving techniques that could help extract valuable macro- and micro-nutrients from generated waste. Novel techniques such as electrostatic separation, enzyme-assisted extraction, and subcritical water extraction can improve the yield of extracted protein as well as their functional and nutritional characteristics. Furthermore, those techniques are considered effective, economical, and environmentally friendly, providing the clean label status of the extracted protein [79,80]. Extracted protein, after the convenient degree of purification, can be added to the human diet as a highly valuable ingredient. Additionally, food waste can be used as a starting material for obtaining the proteins and examining their abilities as potential oleogelators (Table 1).
In the meat industry, many by-products are discharged without any additional utilization of the produced waste. Some of the animal by-products include blood, blood plasma, large intestines, skin, lungs, testicles, and udders [81]. Blood consists of plasma and serum. Liquid plasma is the largest fraction, made of albumin, globulin, and fibrinogen. Because of its properties, it is utilized as an emulsifier, color additive, and stabilizer [82]. In a study [83] dexydrated bovine blood plasma has been utilized to encapture flaxseed oil into a form of oleogel. The advantages of using this material lie in its availability; gelation is observed at low protein concentration (2%), and this oleogel is self-stabilizing. Additionally, good oxidation stability for samples prepared both ways (at 60 and 80 °C) was presented even after 18 days of storage. Gel strength was stronger for samples prepared at higher temperatures, and that can be explained by stronger protein–protein interactions. Another study [84] investigated the potential of plasma blood oleogels as a carrier for hem iron and their possible application in food products as a prevention for anemia. The second study [34] utilized another by-product, pig skin, as a source of collagen.
Besides animal by-products, plants can also be a source of exceedingly valuable proteins. The agro-food industry produces a significant amount of plant by-products each year as a result of cultivating and processing agricultural products [85]. One of the by-products derived from plants was used as a potential organogelator combined with sodium alginate to secure the formation of oleogels. Protein extracted from rice bran, a significant by-product of rice processing, is known as rice bran protein (RBP). RBP is considered to be a high-quality plant protein, containing a well-balanced composition of amino acids that closely aligns with the pattern recommended by FAO/WHO. Notably, the lysine content of RBP exceeds that of rice protein and most other grain proteins. The authors of [37] provided a new approach for the development of oleogels by the Maillard reaction and physical gelation. Ultrasound treatment can be used as a mild process to break up protein aggregates and alter the tertiary structure of proteins while causing minimal changes to the secondary structures. Additionally, ultrasound treatment can speed up the Maillard reaction, leading to the formation of protein–polysaccharide conjugates in a shorter time frame [37]. The first step enhanced properties like the structure and density of the pores, and thus the OBC. The second step decreased the yield stress and compressive strength of the gel but increased the OBC. This approach presents an improved foam template method by double cross-linking and securing the stability of the formed levels. In another study [86], the authors used rice bran protein as an organogelator to form oleogel using the foam template method. They formed a stable oleogel but concluded that the pH value significantly impacted the foaming capacity and stability, which is explained by the change in the secondary structure and surface hydrophobicity of the used protein. Higher values of pH were more adequate for the production of more stable foams, later used for oleogelation. The oleogels obtained from RBP foam templates exhibited robust mechanical properties similar to shortening. This offers a potential alternative for replacing hard-stock fat in the future, with lower levels of saturated fatty acids and without trans-fatty acids.

Table 1.
Overview of protein sources and methods used for oleogelation.
Table 1.
Overview of protein sources and methods used for oleogelation.
| Protein Source | Oleogelation Method | Results of the Study | Reference |
|---|---|---|---|
| Animal Origin | |||
| Whey protein isolates | Solvent exchange | Gel properties observed at low concentrations (3–5%). High oil binding capacity is connected to the presence of small particle size. Similar fractal dimension (2.2–2.47) in oil, compared to the value in aqueous solution. High gel strength. | [42,45] |
| Whey protein isolate | Foam template | Gelation obtained at a concentration of 0.2% wt. Oleogel produced by freeze-drying had rheological values similar to commercial fat. | [52] |
| Egg protein isolate | Solvent exchange | Gelation at 4% wt. Larger particles (10–100 µm) weakened the oleogel, OBC moderate (7.3%). | [45] |
| Bovine blood plasma | Emulsion template | Oleogels prepared at higher temperatures were stronger and more stable, but with lower oxidative stability. Retained high value of OBC after 30 days of storage. | [83,84] |
| Pork skin protein—collagen | Emulsion template | High protein content. Strong and stable oleogels. | [34] |
| Plant Origin | |||
| Soy protein isolate | Emulsion template | Firm oleogels without oil leakage (G′ > 40,000 Pa). Higher concentration of protein leads to a stronger network. | [68] |
| Pea protein stabilized with xanthan gum | Foam template | Low protein concentration—5% wt. OBC was higher for concentrate (27.8 ± 1.2) compared to protein isolate (14.4 ± 1.6) at pH 9. Highest OBC for isolates was obtained at pH 4. | [73,74] |
| Pea | Solvent exchange | Gel properties observed at concentrations of 8%. High oil binding capacity is connected to the presence of small particle size. | [45] |
| Potato | Solvent exchange | Oleogel obtained at low concentrations (4%). Homogenous protein network. Strong oleogel. | [45] |
| Rice bran | Foam template | Low-porosity structure obtained, which resulted in low oil absorption. Formed oleogels had high gel strength. | [86] |
| Bamboo shoot/soy protein complex | Emulsion templated | OBC higher than 50%. High viscosity recovered (more than 70%) for oleogels obtained by 4:1 ratio. Good thermal stability. Oleogels exhibited elastic-dominated solid-like behavior. | [77] |
| Faba bean protein stabilized with xanthan gum | Foam template | Low protein concentration—5% wt. Highest OBC was obtained at pH 9 in the case of protein concentrates and pH 7 in the case of protein isolates. | [73,74] |
4. Applications of Protein Oleogels in Food Products
Recent trends in nutrition, eating habits, and the influence of food products on human health have expanded the almost non-existent research regarding the possible implementation of protein-based oleogels in food. Table 2 presents the list of food products containing oleogels. Fats can be linked to the onset of different health issues, including obesity, cardiovascular diseases (CVDs), metabolic syndrome, and diabetes [87]. By implementing oleogels into food systems, the aim is to reduce the content of saturated and trans-fats, hence improving human well-being.

Table 2.
Application of protein-based oleogels in food products.
The addition of oleogels impacted the properties of produced food products, mainly by empowering them, nutritionally and physically. Even though there is a small number of products enriched with protein oleogels as fat replacers, there is a growing interest in utilizing and improving proteins and corresponding oleogels derived from different sources, mainly originating from food waste.
5. Conclusions and Future Prospects
In recent years, a small number of studies have been focused on exploiting the proteins extracted from different resources and investigating their functional and structural properties for oleogelation. This process, either through direct or indirect methods, has advantages in formulating a gel-like network to entrap the oil phase. This technique provides the opportunity to replace solid fats, comprising mostly saturated and/or trans-fatty acids, which are known to have a negative effect on levels of cholesterol in the blood. Additionally, it is proven that the overconsumption of these fats increases the risk of cardiovascular disease. Oleogelation can be a possible solution in replacing the saturated and trans-fatty acids with structures comprising valuable compounds, such as proteins and polyunsaturated fatty acids (PUFAs) famous for their positive impact on human health. Another advantage of protein levels lies in their broad availability and low concentration required for gelation and profitability. Additionally, future research can focus on finding different solvents for solvent exchange methods that are safer and more adequate for the food industry. Moreover, based on the obtained information, it is necessary to investigate and find more resources for obtaining protein, i.e., by exploiting food waste, and different alternative proteins such as plant, algae, or insect protein and to examine the possibility of using them in the form of oleogel. Some of the proteins can be suitable for the production of oleogels, but alone, they are not stable enough. Given this, it is necessary to find ways to enhance their stability by improving the existing methods or by blending the protein with other biopolymers. Furthermore, oleogels can be food structurants, and it is imperative to conduct more studies such as clinical trials to examine their properties, such as sensory and digestive characteristics including the influence on cholesterol levels and overall effect on human health during regular diet comprising food products containing oleogels. Finally, it is necessary to assess the shelf life of the produced oleogels and food matrices upon implementation.
Author Contributions
Conceptualization: I.N. and D.Z.; investigation: M.S.; writing—original draft preparation and writing—review and editing: M.S. and J.P.; visualization: I.L.; supervision: B.P. and B.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was supported by the Ministry of Science, Technological Development and Innovations, Serbia, program (451-03-47/2023-01/200134).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the collection, analysis, or interpretation of the collected data, or in the writing of the manuscript.
References
- Berryman, C.E.; Lieberman, H.R.; Fulgoni, V.L., III; Pasiakos, S.M. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: Analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am. J. Clin. Nutr. 2018, 108, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated fatty acids and cardiovascular disease: Replacements for saturated fat to reduce cardiovascular risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 8, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Chiu, S.; Bergeron, N.; Krauss, R.M. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu. Rev. Nutr. 2015, 35, 517–543. [Google Scholar] [CrossRef] [PubMed]
- Farr, W.E.; Ghazani, S.M.; Marangoni, A.G. Hydrogenation: Processing technologies. Ind. Oil Fat Prod. 2020. [Google Scholar] [CrossRef]
- Tarrago-Trani, M.T.; Phillips, K.M.; Lemar, L.E.; Holden, J.M. New and existing oils and fats used in products with reduced trans-fatty acid content. J. Am. Diet. Assoc. 2006, 106, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Coenen, J.W. Hydrogenation of edible oils. J. Am. Oil Chem. Soc. 1976, 53, 382–389. [Google Scholar] [CrossRef]
- Parodi, P.W. Has the association between saturated fatty acids, serum cholesterol and coronary heart disease been over emphasized? Int. Dairy J. 2009, 19, 345–361. [Google Scholar] [CrossRef]
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664. [Google Scholar] [CrossRef]
- Ascherio, A.; Willett, W.C. Health effects of trans fatty acids. Am. J. Clin. Nutr. 1997, 66, 1006S–1010S. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Effects of dietary fats on blood lipids: A review of direct comparison trials. Open Heart 2018, 5, e000871. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; West, K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef]
- Judd, J.T.; Clevidence, B.A.; Muesing, R.A.; Wittes, J.; Sunkin, M.E.; Podczasy, J.J. Dietarytrans fatty acids: Effects on plasma lipids and lipoproteins of healthy men and women. Am. J. Clin. Nutr. 1994, 59, 861–868. [Google Scholar] [CrossRef]
- Zock, P.L.; Katan, M.B. Hydrogenation alternatives: Effects of trans fatty acids and stearic acid versus linoleic acid on serum lipids and lipoproteins in humans. J. Lipid Res. 1992, 33, 399–410. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Manku, M.S. How do polyunsaturated fatty acids lower plasma cholesterol levels? Lipids 1983, 18, 558–562. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Maki, K.C.; Eren, F.; Cassens, M.E.; Dicklin, M.R.; Davidson, M.H. ω-6 polyunsaturated fatty acids and cardiometabolic health: Current evidence, controversies, and research gaps. Adv. Nutr. 2018, 9, 688–700. [Google Scholar] [CrossRef]
- Mensink, R.P.; World Health Organization. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kirkpatrick, C.F.; Maki, K.C. Dietary influences on atherosclerotic cardiovascular disease risk. Curr. Atheroscler. Rep. 2021, 23, 62. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Goldstein, A.; Seetharaman, K. Effect of a novel monoglyceride stabilized oil in water emulsion shortening on cookie properties. Food Res. Int. 2011, 44, 1476–1481. [Google Scholar] [CrossRef]
- Tarancón, P.; Salvador, A.; Sanz, T. Sunflower oil–water–cellulose ether emulsions as trans-fatty acid-free fat replacers in biscuits: Texture and acceptability study. Food Bioprocess Technol. 2013, 6, 2389–2398. [Google Scholar] [CrossRef]
- Oakenfull, D.; Glicksman, M. Gelling agents. Crit. Rev. Food Sci. Nutr. 1987, 26, 1–25. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1631. [Google Scholar] [CrossRef]
- Guo, J.; Cui, L.; Meng, Z. Oleogels/emulsion gels as novel saturated fat replacers in meat products: A review. Food Hydrocoll. 2023, 137, 108313. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef]
- Romoscanu, A.I.; Mezzenga, R. Emulsion-templated fully reversible protein-in-oil gels. Langmuir 2006, 22, 7812–7818. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R. Formation and properties of biopolymer-based oleogels. In Crystallization of Lipids: Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals; Wiley: Hoboken, NJ, USA, 2018; pp. 385–404. [Google Scholar] [CrossRef]
- da Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastião, E.E.; Vargas, C.; de Lima Franzen, F.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J.; et al. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef]
- Xu, H.J.; Li, T.; Zhang, H.X.; Shi, C.H.; Cao, J.Q.; Zhang, X.R. The application of oleogels in food products: Classification, preparation, and characterisation. Acta Aliment. 2022, 51, 462–478. [Google Scholar] [CrossRef]
- Feichtinger, A.; Scholten, E. Preparation of protein oleogels: Effect on structure and functionality. Foods 2020, 9, 1745. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Cludts, N.; Lewille, B.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Biopolymer-based structuring of liquid oil into soft solids and oleogels using water-continuous emulsions as templates. Langmuir 2015, 31, 2065–2073. [Google Scholar] [CrossRef]
- Qiu, C.; Huang, Y.; Li, A.; Ma, D.; Wang, Y. Fabrication and characterization of oleogel stabilized by gelatin-polyphenol-polysaccharides nanocomplexes. J. Agric. Food Chem. 2018, 66, 13243–13252. [Google Scholar] [CrossRef]
- Jiang, Z.; Bai, X. Effects of Polysaccharide Concentrations on the Formation and Physical Properties of Emulsion-Templated Oleogels. Molecules 2022, 27, 5391. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M. Oil structuring using polysaccharides. Curr. Opin. Food Sci. 2019, 27, 29–35. [Google Scholar] [CrossRef]
- Abdollahi, M.; Goli, S.A.H.; Soltanizadeh, N. Physicochemical properties of foam-templated oleogel based on gelatin and xanthan gum. Eur. J. Lipid Sci. Technol. 2020, 122, 1900196. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Fabrication of oleogels via a facile method by oil absorption in the aerogel templates of protein–polysaccharide conjugates. ACS Appl. Mater. Interfaces 2020, 12, 7795–7804. [Google Scholar] [CrossRef]
- de Vries, A.; Wesseling, A.; van der Linden, E.; Scholten, E. Protein oleogels from heat-set whey protein aggregates. J. Colloid Interface Sci. 2017, 486, 75–83. [Google Scholar] [CrossRef]
- de Vries, A.; Hendriks, J.; Van Der Linden, E.; Scholten, E. Protein oleogels from protein hydrogels via a stepwise solvent exchange route. Langmuir 2015, 31, 13850–13859. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.; Jansen, D.; van der Linden, E.; Scholten, E. Tuning the rheological properties of protein-based oleogels by water addition and heat treatment. Food Hydrocoll. 2018, 79, 100–109. [Google Scholar] [CrossRef]
- Feichtinger, A.; Nibbelink, D.G.; Poppe, S.; Bozzo, L.; Landman, J.; Scholten, E. Protein oleogels prepared by solvent transfer method with varying protein sources. Food Hydrocoll. 2022, 132, 107821. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Popović, L. Proteins and Biochemical Transformations; Faculty of Technology: Novi Sad, Serbia, 2022; pp. 6–8. [Google Scholar]
- Wyness, L.; Weichselbaum, E.; O'’Connor, A.; Williams, E.B.; Benelam, B.; Riley, H.; Stanner, S. Red meat in the diet: An update. Nutr. Bull. 2011, 36, 34–77. [Google Scholar] [CrossRef]
- Bos, C.; Morens, C.; Luengo, C.; Tomé, D.; Gaudichon, C.; Pueyo, M.E.; Benamouzig, R.; Gausserès, N. Increasing habitual protein intake accentuates differences in postprandial dietary nitrogen utilization between protein sources in humans. J. Nutr. 2003, 133, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef]
- Park, C.; Campanella, O.; Maleky, F. The effects of whey protein and oleogel interactions on mechanical properties of oleocolloids and hydro-oleocolloids matrices. Food Hydrocoll. 2022, 124, 107285. [Google Scholar] [CrossRef]
- Shih, W.H.; Shih, W.Y.; Kim, S.I.; Liu, J.; Aksay, I.A. Scaling behavior of the elastic properties of colloidal gels. Phys. Rev. A 1990, 42, 4772. [Google Scholar] [CrossRef] [PubMed]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structural characterization of oleogels from whey protein aerogel particles. Food Res. Int. 2020, 132, 109099. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Wijaya, C.H.; Patel, A.R. High internal phase emulsions stabilized solely by whey protein isolate-low methoxyl pectin complexes: Effect of pH and polymer concentration. Food Funct. 2017, 8, 584–594. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Patel, A.R. Oleogels from emulsion (HIPE) templates stabilized by protein–polysaccharide complexes. In Edible Oil Structuring: Concepts, Methods and Applications; Royal Society of Chemistry: London, UK, 2017. [Google Scholar] [CrossRef]
- Layman, D.K.; Rodriguez, N.R. Egg protein as a source of power, strength, and energy. Nutr. Today 2009, 44, 43–48. [Google Scholar] [CrossRef]
- Li, J.; Shi, W.; Sun, Y.; Qin, Z.; Zheng, S.; Liang, S.; Li, Y.; Ritzoulis, C.; Zhang, H. Fabrication, characterization, and oxidation resistance of gelatin/egg white protein cryogel-templated oleogels through apple polyphenol crosslinking. Int. J. Biol. Macromol. 2024, 277, 134077. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, R.; Pedram Nia, A.; Naji-Tabasi, S.; Elhamirad, A.H.; Shafafi Zenoozian, M. Rheological and structural properties of oleogel base on soluble complex of egg white protein and xanthan gum. J. Texture Stud. 2020, 51, 925–936. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Pan, H.; Xu, X.; Qian, Z.; Cheng, H.; Shen, X.; Chen, S.; Ye, X. Xanthan gum-assisted fabrication of stable emulsion-based oleogel structured with gelatin and proanthocyanidins. Food Hydrocoll. 2021, 115, 106596. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wada, Y.; Wäsche, A. Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem. 2008, 107, 32–39. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Yu, D.; Chen, Y.; Chen, X.; Huang, Y.; Wang, L.; Pan, M.; Elfalleh, W. Electrolysis soy protein isolate-based oleogels prepared with an emulsion-templated approach. Int. J. Food Eng. 2021, 17, 583–594. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, W.; Jiang, H.; Zhang, T.; Zhang, L.; Yang, C.; Guo, X.; Chen, F. Characterization of soybean protein isolate-chitosan-based emulsion template-oleogel as fast-frozen special fat substitute and its mechanism on the quality improvement of fast-frozen food. LWT 2024, 205, 116485. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Toews, R.; Wang, N. Physicochemical and functional properties of protein concentrates from pulses. Food Res. Int. 2013, 52, 445–451. [Google Scholar] [CrossRef]
- Mohanan, A.; Tang, Y.R.; Nickerson, M.T.; Ghosh, S. Oleogelation using pulse protein-stabilized foams and their potential as a baking ingredient. RSC Adv. 2020, 10, 14892–14905. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Utilization of pulse protein-xanthan gum complexes for foam stabilization: The effect of protein concentrate and isolate at various pH. Food Chem. 2020, 316, 126282. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Zhang, J.; Brooks, M.S.L. Potential for value-added utilization of bamboo shoot processing waste—Recommendations for a biorefinery approach. Food Bioprocess Technol. 2018, 11, 901–912. [Google Scholar] [CrossRef]
- Sayanika, D.W.; Louis, B.; Pranab, R.; Narayan, C.T. Insights on predominant edible bamboo shoot proteins. Afr. J. Biotechnol. 2015, 14, 1511–1518. [Google Scholar] [CrossRef]
- Li, J.; Xi, Y.; Wu, L.; Zhang, H. Preparation, characterization and in vitro digestion of bamboo shoot protein/soybean protein isolate based-oleogels by emulsion-templated approach. Food Hydrocoll. 2023, 136, 108310. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Silva, V.D.; Silvestre, M.P. Functional properties of bovine blood plasma intended for use as a functional ingredient in human food. LWT-Food Sci. Technol. 2003, 36, 709–718. [Google Scholar] [CrossRef]
- Fernández, C.L.; Romero, M.C.; Rolhaiser, F.; Fogar, R.A.; Doval, M.M. Fat substitutes based on bovine blood plasma and flaxseed oil as functional ingredients. Int. J. Gastron. Food Sci. 2021, 25, 100365. [Google Scholar] [CrossRef]
- Fernández, C.L.; Fogar, R.A.; Rolhaiser, F.A.; Romero, M.C. Functional gels from bovine blood proteins as fat substitutes and potential carriers of heme iron. Innov. Food Sci. Emerg. Technol. 2023, 87, 103389. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Wei, F.; Lu, M.; Li, J.; Xiao, J.; Rogers, M.A.; Cao, Y.; Lan, Y. Construction of foam-templated oleogels based on rice bran protein. Food Hydrocoll. 2022, 124, 107245. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.; Harrison, K.; Cooper, D.M.L.; Nickerson, M.T.; Ghosh, S. Conversion of Pulse Protein Foam-Templated Oleogels into Oleofoams for Improved Baking Application. Foods 2022, 11, 2887. [Google Scholar] [CrossRef]
- Tang, Y.R.; Ghosh, S. Canola protein thermal denaturation improved emulsion-templated oleogelation and its cake-baking application. RSC Adv 2021, 11, 25141–25157. [Google Scholar] [CrossRef]
- Salama, H.H.; Hashim, A.F. A functional spreadable canola and milk proteins oleogels as a healthy system for candy gummies. Sci. Rep. 2022, 12, 12619. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Abd El-Aty, A.M.; Su, W.; Tan, M. Preparation of bilayer nano-oleogel by whey protein isolate and soy lecithin for fish oil encapsulation and its application in cookies. Food Hydrocoll. 2024, 146, 109280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).