The Impact of Farm and Industrial Feed Waste on the Safety Parameters of Tenebrio molitor Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Research Material
Insect Rearing
2.2. Lyophilization of Larvae and Preparation of Samples
2.3. Biogenic Amines
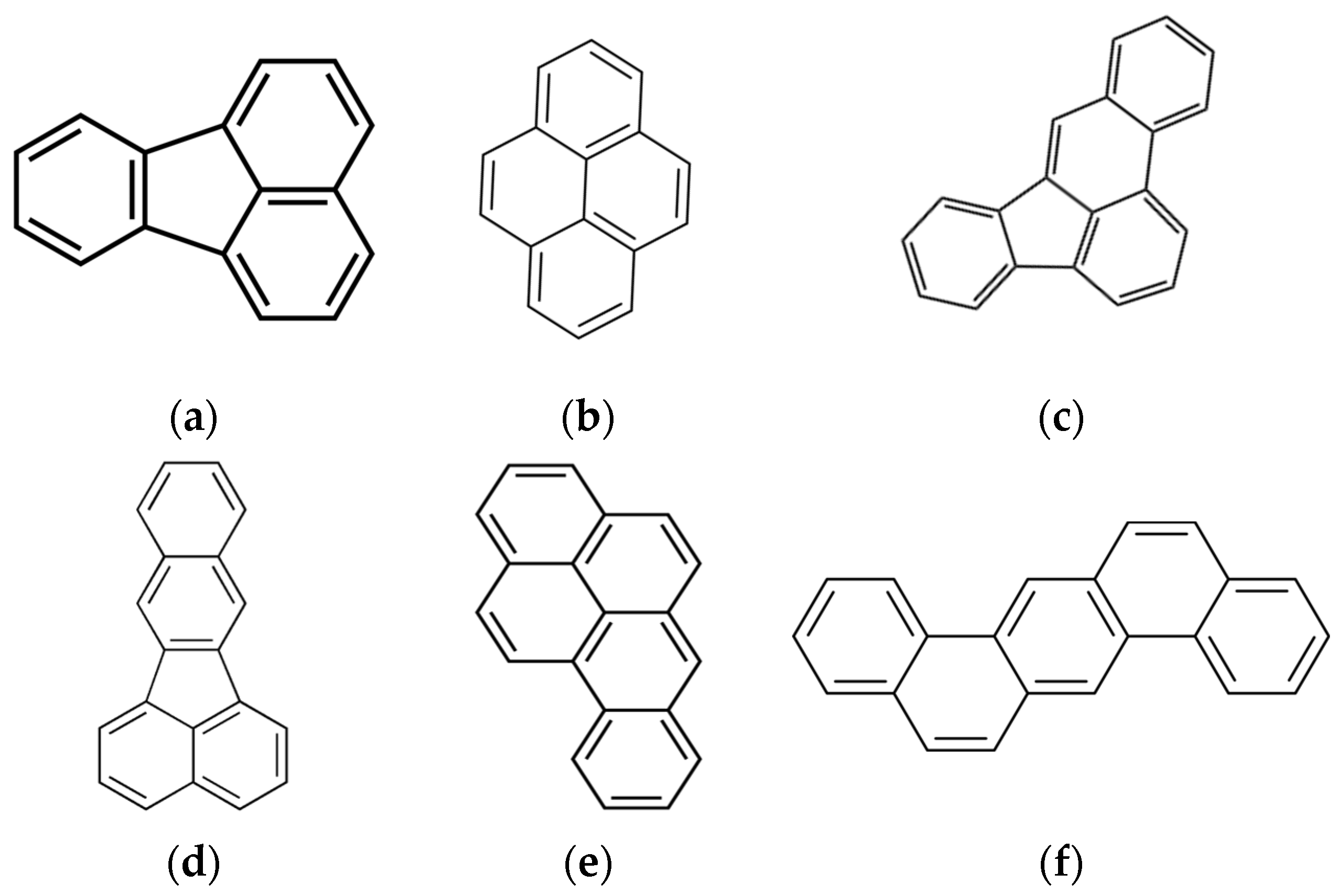
2.4. Polycyclic Aromatic Hydrocarbons
2.5. Heavy Metals
2.5.1. Determination of Copper, Manganese, Zinc
2.5.2. Determination of Copper, Manganese, Zinc
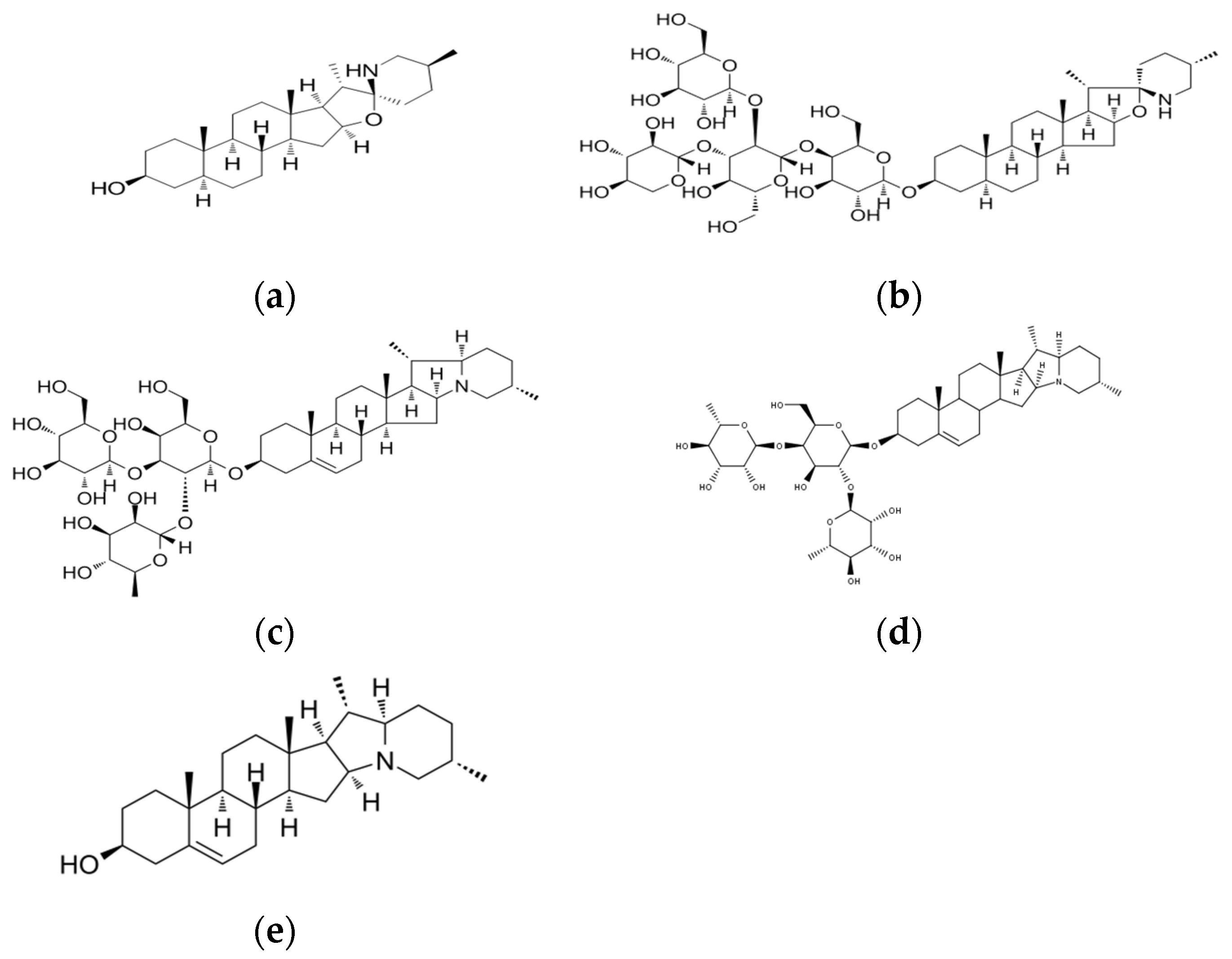
2.6. Glycoalkaloids
2.6.1. Determination of α-Solanine, α-Chaconine, and Solanidin
2.6.2. Determination of Tomatidin and Tomatin
2.7. Pesticides
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biogenic Amines
3.2. Polycyclic Aromatic Hydrocarbons
3.3. Heavy Metals
3.4. Glycoalkaloids
3.5. Pesticides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Analytical Pesticide | Detection Threshold | LWYP | LWYC | LBYC | LWYG | SWYP | SWYC | SBYC | SWYG |
|---|---|---|---|---|---|---|---|---|---|
| Acephate | <0.05 | - | - | - | - | - | - | - | - |
| Alachlor | <0.01 | - | - | - | - | - | - | - | - |
| Aldrin + dieldrin (sum) | <0.01 | - | - | - | - | - | - | - | - |
| Azinphos ethyl | <0.05 | - | - | - | - | - | - | - | - |
| Azinphos methyl | <0.05 | - | - | - | - | - | - | - | - |
| Alpha-cypermethrin | <0.05 | - | - | - | - | - | - | - | - |
| Atrazine | <0.05 | - | - | - | - | - | - | - | - |
| Bromophos ethyl | <0.05 | - | - | - | - | - | - | - | - |
| Bromophos methyl | <0.05 | - | - | - | - | - | - | - | - |
| Bromopropylate | <0.01 | - | - | - | - | - | - | - | - |
| Beta-cyfluthrin | <0.05 | - | - | - | - | - | - | - | - |
| Chlordane (sum of cis, trans isomers, oxychlordane) | <0.02 | - | - | - | - | - | - | - | - |
| Chlorpyrifos ethyl | <0.01 | - | - | - | - | - | - | - | - |
| Chlorpyrifos methyl | <0.01 | - | - | - | - | - | - | - | - |
| Chlorthalodimethyl | <0.01 | - | - | - | - | - | - | - | - |
| Cyfluthrin (sum of isomers) | <0.01 | - | - | - | - | - | - | - | - |
| λ–cyhalothrin | <0.01 | - | - | - | - | - | - | - | - |
| Cypermethrin (sum of α, β and z- isomers) | <0.05 | - | - | - | - | - | - | - | - |
| DDT (sum of p,p’-DDT, o,p’-DDT, o,p’- DDE, p,p’- DDE, o,p’ -TDE, p,p’-TDE) | <0.01 | - | - | - | - | - | - | - | - |
| Deltamethrin | <0.05 | - | - | - | - | - | - | - | - |
| Diazinon | <0.02 | - | - | - | - | - | - | - | - |
| Dichlorfluanid | <0.05 | - | - | - | - | - | - | - | - |
| Dichlorvos | <0.05 | - | - | - | - | - | - | - | - |
| Dicofol | <0.01 | - | - | - | - | - | - | - | - |
| Dimethoate + omethoate | <0.01 | - | - | - | - | - | - | - | - |
| Dieldrin | <0.01 | - | - | - | - | - | - | - | - |
| Difenoconazole | <0.05 | - | - | - | - | - | - | - | - |
| Dimethomorph | <0.05 | - | - | - | - | - | - | - | - |
| Endosulfan (sum of α, β endosulfan and endosulfan sulfate) | <0.01 | - | - | - | - | - | - | - | - |
| Endrin | <0.005 | - | - | - | - | - | - | - | - |
| Ethion | <0.02 | - | - | - | - | - | - | - | - |
| Etrimphos | <0.05 | - | - | - | - | - | - | - | - |
| Fenchlorophos | <0.05 | - | - | - | - | - | - | - | - |
| Fenitrothion | <0.005 | - | - | - | - | - | - | - | - |
| Fenpropathrin | <0.01 | - | - | - | - | - | - | - | - |
| Fensulfothion (sum) | <0.05 | - | - | - | - | - | - | - | - |
| Fenthion (sum) | <0.05 | - | - | - | - | - | - | - | - |
| Fenvalerate | <0.05 | - | - | - | - | - | - | - | - |
| Flucitrinate | <0.05 | - | - | - | - | - | - | - | - |
| τ-fluvalinate | <0.01 | - | - | - | - | - | - | - | - |
| Fonofos | <0.05 | - | - | - | - | - | - | - | - |
| Fentin acetate | <0.05 | - | - | - | - | - | - | - | - |
| Fentin Hydroxide | <0.05 | - | - | - | - | - | - | - | - |
| Fluazifop-P-butyl | <0.05 | - | - | - | - | - | - | - | - |
| Flutriafol | <0.01 | - | - | - | - | - | - | - | - |
| Flutricinate | <0.05 | - | - | - | - | - | - | - | - |
| Fozalon | <0.05 | - | - | - | - | - | - | - | - |
| Heptachlor (sum of heptachlor, heptachlor epoxide) | <0.01 | - | - | - | - | - | - | - | - |
| Hexachlorobenzene | <0.01 | - | - | - | - | - | - | - | - |
| Hexachorcyclohexane (sum of α, β, δ, d- isomers) | <0.01 | - | - | - | - | - | - | - | - |
| Imidacloprid | <0.05 | - | - | - | - | - | - | - | - |
| Iprodione | <0.01 | - | - | - | - | - | - | - | - |
| Lambda cyhalothrin | <0.01 | - | - | - | - | - | - | - | - |
| Lindane (γ-hexachorcyclohexane) | <0.01 | - | - | - | - | - | - | - | - |
| Malathion + malaoxon | <0.05 | - | - | - | - | - | - | - | - |
| Mecarbam | <0.05 | - | - | - | - | - | - | - | - |
| Metacryphos | <0.05 | - | - | - | - | - | - | - | - |
| Metalaxyl | <0.05 | - | - | - | - | - | - | - | - |
| Methamidophos | <0.05 | - | - | - | - | - | - | - | - |
| Metamitron | <0.01 | - | - | - | - | - | - | - | - |
| Metazachlor | <0.01 | ||||||||
| Metribuzin | <0.05 | - | - | - | - | - | - | - | - |
| Methidathion | <0.05 | - | - | - | - | - | - | - | - |
| Methoxychlor | <0.01 | - | - | - | - | - | - | - | - |
| Mirex | <0.01 | - | - | - | - | - | - | - | - |
| Monocrotophos | <0.05 | - | - | - | - | - | - | - | - |
| Parathion ethyl + paraoxon ethyl | <0.01 | - | - | - | - | - | - | - | - |
| Parathion methyl + Paraoxon methyl | <0.01 | - | - | - | - | - | - | - | - |
| Pendimethalin | <0.01 | - | - | - | - | - | - | - | - |
| Pentachloroanisole | <0.01 | - | - | - | - | - | - | - | - |
| Permethrin (sum of isomers) | <0.01 | - | - | - | - | - | - | - | - |
| Fozolone | <0.05 | - | - | - | - | - | - | - | - |
| Fosmet | <0.05 | - | - | - | - | - | - | - | - |
| Piperonyl butoxide | <0.05 | - | - | - | - | - | - | - | - |
| Pirimifos ethyl | <0.05 | - | - | - | - | - | - | - | - |
| Pirimiphosmethyl (pirimiphosmethyl+N-desethyl-pirimiphosmethyl) | <0.05 | - | - | - | - | - | - | - | - |
| Promethrin | <0.01 | - | - | - | - | - | - | - | - |
| Propiconazole | <0.01 | - | - | - | - | - | - | - | - |
| Procimidone | <0.05 | - | - | - | - | - | - | - | - |
| Profenofos | <0.05 | - | - | - | - | - | - | - | - |
| Prothiophos | <0.05 | - | - | - | - | - | - | - | - |
| Pyrethrins (pyrethrins 1 and 2, cinerins 1 and 2, | <0.01 | - | - | - | - | - | - | - | - |
| the sum of jasmolins 1 and 2) | <0.05 | - | - | - | - | - | - | - | - |
| Quinalphos | <0.05 | - | - | - | - | - | - | - | - |
| Quintocene (sum of quintocene, pentachloroaniline, methyl pentachlorophenyl sulfide) | <0.001 | - | - | - | - | - | - | - | - |
| Kaptan | <0.02 | - | - | - | - | - | - | - | - |
| S-421 | <0.05 | - | - | - | - | - | - | - | - |
| Simazine | <0.05 | - | - | - | - | - | - | - | - |
| Teknazen | <0.05 | - | - | - | - | - | - | - | - |
| Tetradifon | <0.01 | - | - | - | - | - | - | - | - |
| Tau-fluvinate | <0.005 | - | - | - | - | - | - | - | - |
| Thiamethoxam | <0.05 | - | - | - | - | - | - | - | - |
| Triadimefon | <0.005 | - | - | - | - | - | - | - | - |
| Triadimenol | <0.01 | - | - | - | - | - | - | - | - |
| Trifluralin | <0.01 | - | - | - | - | - | - | - | - |
References
- Novel Food. Available online: https://food.ec.europa.eu/safety/novel-food_en (accessed on 16 May 2023).
- Safety of Dried Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6343 (accessed on 22 October 2023).
- Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food under Regulation (EU) 2015/2283 of the Euro-pean Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg_impl/2022/169/oj (accessed on 23 October 2023).
- Young, K.S.; Geun, K.H.; Yong, L.K.; Joo, Y.H.; Jung, K.N. Effects of Brewer’s spent grain (BSG) on larval growth of mealworms, Tenebrio molitor (Coleoptera: Tenebrionidae). Int. J. Ind. Entomol. 2016, 32, 41–48. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Severini, C. Understanding the Drying Kinetic and Hygroscopic Behaviour of Larvae of Yellow Mealworm (Tenebrio molitor) and the Effects on Their Quality. J. Insects Food Feed 2016, 2, 233–243. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable Farming of the Mealworm Tenebrio molitor for the Production of Food and Feed. Z. Naturforschung C 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Aguilar-Miranda, E.D.; López, M.G.; Escamilla-Santana, C.; Barba de la Rosa, A.P. Characteristics of Maize Flour Tortilla Supplemented with Ground Tenebrio molitor Larvae. J. Agric. Food Chem. 2002, 50, 192–195. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the Nutritional Value of Mysore Thorn Borer (Anoplophora chinensis) and Mealworm Larva (Tenebrio molitor): Amino Acid, Fatty Acid, and Element Profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Tiwari, R.; Yadav, A.; Hamsa, S.; Dhewa, T. Food Metabolism and Chronic Diseases. In Nutritional Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 355–381. ISBN 978-1-394-22911-6. [Google Scholar]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Tao, X.; Liu, L.; Ma, P.; Hu, J.; Ming, Z.; Dang, K.; Zhang, Y.; Li, Y. Association of Circulating Very Long-Chain Saturated Fatty Acids with Cardiovascular Mortality in NHANES 2003–2004, 2011–2012. J. Clin. Endocrinol. Metab. 2023, dgad561. [Google Scholar] [CrossRef]
- Rich, M.W.; Nease, R.F. Cost-Effectiveness Analysis in Clinical Practice: The Case of Heart Failure. Arch. Intern. Med. 1999, 159, 1690–1700. Available online: https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/1105639 (accessed on 9 December 2023). [CrossRef]
- Thévenot, A.; Rivera, J.L.; Wilfart, A.; Maillard, F.; Hassouna, M.; Senga-Kiesse, T.; Le Féon, S.; Aubin, J. Mealworm Meal for Animal Feed: Environmental Assessment and Sensitivity Analysis to Guide Future Prospects. J. Clean. Prod. 2018, 170, 1260–1267. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Kejonen, A. Could Western Attitudes towards Edible Insects Possibly Be Influenced by Idioms Containing Unfavourable References to Insects, Spiders and Other Invertebrates? Foods 2020, 9, 172. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional Consumption of and Rearing Edible Insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Hunts, H.J.; Dunkel, F.V.; Thienes, M.J.; Carnegie, N.B. Gatekeepers in the Food Industry: Acceptability of Edible Insects. J. Insects Food Feed 2020, 6, 231–243. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-Reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Dagevos, H. A Literature Review of Consumer Research on Edible Insects: Recent Evidence and New Vistas from 2019 Studies. J. Insects Food Feed 2021, 7, 249–259. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Frozen and Dried Formulations from Whole House Crickets (Acheta Domesticus) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06779. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2021.6779 (accessed on 19 May 2023). [CrossRef]
- Noyens, I.; Schoeters, F.; Van Peer, M.; Berrens, S.; Goossens, S.; Van Miert, S. The Nutritional Profile, Mineral Content and Heavy Metal Uptake of Yellow Mealworm Reared with Supplementation of Agricultural Sidestreams. Sci. Rep. 2023, 13, 11604. [Google Scholar] [CrossRef]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of Hazardous Chemicals in Edible Insects and Insect-Based Food Intended for Human Consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef]
- Mlček, J.; Adamek, M.; Adámková, A.; Borkovcová, M.; Bednářová, M.; Skácel, J. Detection of Selected Heavy Metals and Micronutrients in Edible Insect and Their Dependency on the Feed Using XRF Spectrometry. Potravin. Slovak J. Food Sci. 2017, 11, 725–730. [Google Scholar] [CrossRef]
- Martin, S. Mean Growth Rates (in %) of Mealworms at Three Different Temperatures. Available online: https://www.researchgate.net/figure/Mean-growth-rates-in-of-mealworms-at-three-different-temperatures-20-C-25-C-and_fig4_359453872 (accessed on 22 October 2023).
- Murugan, P.; Han, L.; Gan, C.-Y.; Maurer, F.H.J.; Sudesh, K. A New Biological Recovery Approach for PHA Using Mealworm, Tenebrio molitor. J. Biotechnol. 2016, 239, 98–105. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as Food in Sub-Saharan Africa. Int. J. Trop. Insect Sci. 2003, 23, 163–185. [Google Scholar] [CrossRef]
- Harsányi, E.; Juhász, C.; Kovács, E.; Huzsvai, L.; Pintér, R.; Fekete, G.; Varga, Z.I.; Aleksza, L.; Gyuricza, C. Evaluation of Organic Wastes as Substrates for Rearing Zophobas Morio, Tenebrio molitor, and Acheta domesticus Larvae as Alternative Feed Supplements. Insects 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Y.; Ding, M.-Q.; Li, M.-X.; Ding, J.; Bai, S.-W.; Wu, Q.-L.; Zhao, L.; Cao, G.-L.; Ren, N.-Q.; et al. Sustainable Strategy for Lignocellulosic Crop Wastes Reduction by Tenebrio molitor Linnaeus (Mealworm) and Potential Use of Mealworm Frass as a Fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former Foodstuff Products in Tenebrio molitor Rearing: Effects on Growth, Chemical Composition, Microbiological Load, and Antioxidant Status. Animals 2019, 9, 484. [Google Scholar] [CrossRef]
- Hillier, K. Bran—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/bran (accessed on 22 October 2023).
- Papageorgiou, M.; Skendi, A. 1—Introduction to Cereal Processing and by-Products. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Galanakis, C.M., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–25. ISBN 978-0-08-102162-0. [Google Scholar]
- Mincyte, D. Unusual Ingredients: Gastronationalism, Globalization, Technology, and Zeppelins in the Lithuanian Imagination. Anthropol. East Eur. Rev. 2011, 29, 1–21. [Google Scholar]
- Beals, K.A. Potatoes, Nutrition and Health. Am. J. Potato Res. 2019, 96, 102–110. [Google Scholar] [CrossRef]
- Food Waste. Available online: https://food.ec.europa.eu/safety/food-waste_en (accessed on 9 December 2023).
- Natural Toxicants Originating from Food/Diet|SpringerLink. Available online: https://link-springer-com.ezproxy.dbazes.lsmuni.lt/chapter/10.1007/978-981-19-0872-9_4 (accessed on 22 October 2023).
- Ko, H.; Kim, Y.; Kim, J. The Produced Mealworm Meal through Organic Wastes as a Sustainable Protein Source for Weanling Pigs. J. Anim. Sci. Technol. 2020, 62, 365–373. [Google Scholar] [CrossRef]
- Full Article: Solanaceae Glycoalkaloids: α-Solanine and α-Chaconine Modify the Cardioinhibitory Activity of Verapamil. Available online: https://www-tandfonline-com.ezproxy.dbazes.lsmuni.lt/doi/full/10.1080/13880209.2022.2094966 (accessed on 22 October 2023).
- Spochacz, M.; Chowański, S.; Szymczak, M.; Lelario, F.; Bufo, S.A.; Adamski, Z. Sublethal Effects of Solanum Nigrum Fruit Extract and Its Pure Glycoalkaloids on the Physiology of Tenebrio molitor (Mealworm). Toxins 2018, 10, 504. [Google Scholar] [CrossRef]
- Insect Protein Solutions. Available online: https://www.divaks.com/ (accessed on 23 October 2023).
- Geresnis Maistas Geresnei Ateičiai|Kauno Grūdai. Available online: https://www.kauno-grudai.lt/ (accessed on 23 October 2023).
- Fasma—Fasma.Lt EN. Available online: https://fasma.lt/en/ (accessed on 23 October 2023).
- Sausų Alaus Mielių Gamyba—EKOPRODUKTAS. Available online: https://ekoproduktas.com/lt/ (accessed on 23 October 2023).
- Prekyba Grūdais, Pašarais Ir trąšomisEurokorma.Lt. Available online: https://www.eurokorma.lt/ (accessed on 23 October 2023).
- Sanitex Grupė—Sanitex Lithuania. Available online: https://sanitex.eu/ (accessed on 23 October 2023).
- Carl Roth—International|Homepage. Available online: https://www.carlroth.com/com/en/ (accessed on 23 October 2023).
- Alytaus Kolegija. Homepage. Available online: https://alytauskolegija.lt/lt/ (accessed on 23 October 2023).
- Available online: https://en.ktu.edu/ (accessed on 23 October 2023).
- Media, F. LAMMC. Available online: https://www.lammc.lt/en (accessed on 23 October 2023).
- ISO 13859:2014. Available online: https://www.iso.org/standard/54337.html (accessed on 23 October 2023).
- QTM PAH Mix Certified Reference Material, 2000 Μg/mL Each Component in Dichloromethane, Ampule of 1 mL|Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/ (accessed on 20 November 2023).
- BS EN 16170:2016; Sludge, Treated Biowaste and Soil. Determination of Elements Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). European Standards: Plzen, Czech Republic, 2016. Available online: https://www.en-standard.eu/bs-en-16170-2016-sludge-treated-biowaste-and-soil-determination-of-elements-using-inductively-coupled-plasma-optical-emission-spectrometry-icp-oes/ (accessed on 23 October 2023).
- BS EN 15621:2017; Animal Feeding Stuffs: Methods of Sampling and Analysis. Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Sulphur, Iron, Zinc, Copper, Manganese and Cobalt after Pressure Digestion by ICP-AES. European Standards: Plzen, Czech Republic, 2017. Available online: https://www.en-standard.eu/bs-en-15621-2017-animal-feeding-stuffs-methods-of-sampling-and-analysis-determination-of-calcium-sodium-phosphorus-magnesium-potassium-sulphur-iron-zinc-copper-manganese-and-cobalt-after-pressure-digestion-by-icp-aes/ (accessed on 23 October 2023).
- 1.11355.0100; ICP Multi-Element Standards, CertiPUR®, Supelco®. Supelco: Bellefonte, PA, USA, 2008. Available online: https://dk.vwr.com/store/product/8831666/icp-multi-element-standards-certipur-supelco (accessed on 20 November 2023).
- Hydrochloric Acid|30721|Honeywell Research Chemicals. Available online: https://lab.honeywell.com/shop/hydrochloric-acid-30721 (accessed on 20 November 2023).
- Nitric Acid. Available online: https://lab.honeywell.com/shop/nitric-acid-30709 (accessed on 20 November 2023).
- Institut für Boden und Umwelt. Available online: https://www.lufa-nord-west.de/index.cfm/article/3.html (accessed on 23 October 2023).
- EURL|Single Residue Methods|QuPPe Method. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=887&LabID=200&Lang=EN (accessed on 23 October 2023).
- BVL L 00.00-115:2018-10; Fachdaten Einzelsicht Norm—Beuth.De. Beuth Verlag: Berlin, Germany, 2018. Available online: https://www.beuth.de/de/technische-regel/bvl-l-00-00-115/296997774 (accessed on 23 October 2023).
- CSN EN 12393-2; Foods of Plant Origin—Multiresidue Methods for the Determination of Pesticide Residues by GC or LC-MS/MS—Part 2: Methods for Extraction and Clean-Up. European Standards: Plzen, Czech Republic, 2013. Available online: https://www.en-standard.eu/csn-en-12393-2-foods-of-plant-origin-multiresidue-methods-for-the-determination-of-pesticide-residues-by-gc-or-lc-ms-ms-part-2-methods-for-extraction-and-clean-up/ (accessed on 23 October 2023).
- Hexane Laboratory Reagent, ≥95%, 110-54-3. Available online: https://www.sigmaaldrich.com/LT/en/product/sigald/208752 (accessed on 20 November 2023).
- Acetonitrile. Available online: https://lab.honeywell.com/shop/acetonitrile-34851 (accessed on 20 November 2023).
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The Biogenic Amines Putrescine and Cadaverine Show in Vitro Cytotoxicity at Concentrations That Can Be Found in Foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic Amines in Foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Önal, A. A Review: Current Analytical Methods for the Determination of Biogenic Amines in Foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of Biogenic Amines in Food—Existing and Emerging Approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, L.S. Amino Acids and Biogenic Amines as Food Quality Factors. Pure Appl. Chem. 2019, 91, 289–300. [Google Scholar] [CrossRef]
- Muthukumar, J.; Selvasekaran, P.; Lokanadham, M.; Chidambaram, R. Food and Food Products Associated with Food Allergy and Food Intolerance—An Overview. Food Res. Int. 2020, 138, 109780. [Google Scholar] [CrossRef]
- Li, L.; Xie, B.; Dong, C.; Wang, M.; Liu, H. Can Closed Artificial Ecosystem Have an Impact on Insect Microbial Community? A Case Study of Yellow Mealworm (Tenebrio molitor L.). Ecol. Eng. 2016, 86, 183–189. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0925857415301439 (accessed on 23 October 2023). [CrossRef]
- Savio, C.; Mugo-Kamiri, L.; Upfold, J.K. Bugs in Bugs: The Role of Probiotics and Prebiotics in Maintenance of Health in Mass-Reared Insects. Insects 2022, 13, 376. [Google Scholar] [CrossRef]
- Barberis, M.; Calabrese, D.; Galloni, M.; Nepi, M. Secondary Metabolites in Nectar-Mediated Plant-Pollinator Relationships. Plants 2023, 12, 550. [Google Scholar] [CrossRef]
- Monastirioti, M. Biogenic Amine Systems in the Fruit Fly Drosophila Melanogaster. Microsc. Res. Tech. 1999, 45, 106–121. [Google Scholar] [CrossRef]
- Roshchina, V.V. New Trends and Perspectives in the Evolution of Neurotransmitters in Microbial, Plant, and Animal Cells. In Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health; Lyte, M., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–77. ISBN 978-3-319-20215-0. [Google Scholar]
- Schnedl, W.J.; Enko, D. Considering Histamine in Functional Gastrointestinal Disorders. Crit. Rev. Food Sci. Nutr. 2021, 61, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, J.M. Histamine and Scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Radley-Gardner, O.; Beale, H.; Zimmermann, R. (Eds.) Fundamental Texts on European Private Law; Hart Publishing: Oxford, UK, 2016; ISBN 978-1-78225-864-3. [Google Scholar]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. (Text with EEA Relevance). 2005; Volume 338. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601&rid=1 (accessed on 23 October 2023).
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2011.2393 (accessed on 23 October 2023). [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The Problem of Biogenic Amines in Fermented Foods and the Use of Potential Biogenic Amine-Degrading Microorganisms as a Solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA Panel); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of UV-Treated Powder of Whole Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2023, 21, e08009. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to the Potential Increase of Consumer Health Risk by a Possible Increase of the Existing Maximum Levels for Aflatoxins in Almonds, Hazelnuts and Pistachios and Derived Products. EFSA J. 2007, 5, 446. [Google Scholar] [CrossRef]
- Linares, D.M.; del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Alvarez, M.A. Factors Influencing Biogenic Amines Accumulation in Dairy Products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef]
- Diaz, M.; Ladero, V.; Redruello, B.; Sanchez-Llana, E.; del Rio, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. A PCR-DGGE Method for the Identification of Histamine-Producing Bacteria in Cheese. Food Control 2016, 63, 216–223. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on an Update on the Present Knowledge on the Occurrence and Control of Foodborne Viruses. EFSA J. 2011, 9, 2190. [Google Scholar] [CrossRef]
- Hui, J.Y.; Taylor, S.L. Inhibition of in Vivo Histamine Metabolism in Rats by Foodborne and Pharmacologic Inhibitors of Diamine Oxidase, Histamine N-Methyltransferase, and Monoamine Oxidase. Toxicol. Appl. Pharmacol. 1985, 81, 241–249. [Google Scholar] [CrossRef]
- Berg, T.; Piercey, B.W.; Jensen, J. Role of Β1–3-Adrenoceptors in Blood Pressure Control at Rest and during Tyramine-Induced Norepinephrine Release in Spontaneously Hypertensive Rats. Hypertension 2010, 55, 1224–1230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldstein, D.S.; Eisenhofer, G.; Kopin, I.J. Sources and Significance of Plasma Levels of Catechols and Their Metabolites in Humans. J. Pharmacol. Exp. Ther. 2003, 305, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.; Marcinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food Sources and Biomolecular Targets of Tyramine. Nutr. Rev. 2019, 77, 107–115. Available online: https://academic.oup.com/nutritionreviews/article/77/2/107/5084469?login=false (accessed on 23 October 2023). [CrossRef]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Frozen and Dried Formulations from Whole Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06778. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A Physiological Autophagy Inducer Acting as an Anti-Aging Vitamin in Humans? Autophagy 2018, 15, 165–168. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Starkute, V.; Zokaityte, G.; Kaminskaite, A.; Mockus, E.; Klupsaite, D.; Cernauskas, D.; Rocha, J.M.; Özogul, F.; et al. Crickets (Acheta domesticus) as Wheat Bread Ingredient: Influence on Bread Quality and Safety Characteristics. Foods 2023, 12, 325. [Google Scholar] [CrossRef]
- Plaza-Bolaños, P.; Frenich, A.G.; Vidal, J.L.M. Polycyclic Aromatic Hydrocarbons in Food and Beverages. Analytical Methods and Trends. J. Chromatogr. A 2010, 1217, 6303–6326. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Polycyclic Aromatic Hydrocarbons in Foods: A Critical Review. Curr. Nutr. Food Sci. 2020, 16, 866–873. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Öberg, L.; Haglund, P.; Tysklind, M. Sources, Fate, and Toxic Hazards of Oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH- Contaminated Sites. AMBIO A J. Hum. Environ. 2007, 36, 475–485. [Google Scholar] [CrossRef]
- Mackiewicz-Walec, E.; Krzebietke, S.J.; Borowik, A.; Klasa, A. The Effect of Spring Barley Fertilization on the Content of Polycyclic Aromatic Hydrocarbons, Microbial Counts and Enzymatic Activity in Soil. Int. J. Environ. Res. Public Health 2023, 20, 3796. Available online: https://www.mdpi.com/1660-4601/20/5/3796 (accessed on 20 November 2023). [CrossRef] [PubMed]
- Chinarak, K.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae. Foods 2022, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Scientific Publications—University of Bacau [Revues] (Scientific Reviews, Abstracts and Articles). Available online: https://pubs.ub.ro/?pg=revues&rev=cscc6&num=202201&vol=1&aid=5388 (accessed on 19 May 2023).
- Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in foodstuffsText with EEA Relevance. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0004:0008:En:PDF (accessed on 23 October 2023).
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks to Public Health Related to the Presence of Chromium in Food and Drinking Water. EFSA J. 2014, 12, 3595. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3595 (accessed on 23 October 2023).
- Van der Fels-Klerx, H.J.; Camenzuli, L.; van der Lee, M.K.; Oonincx, D.G.A.B. Uptake of Cadmium, Lead and Arsenic by Tenebrio Molitor and Hermetia Illucens from Contaminated Substrates. PLoS ONE 2016, 11, e0166186. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. (Text with EEA Relevance). 2006; Volume 364. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 23 October 2023).
- Knuthsen, P.; Jensen, U.; Schmidt, B.; Larsen, I.K. Glycoalkaloids in Potatoes: Content of Glycoalkaloids in Potatoes for Consumption. J. Food Compos. Anal. 2009, 22, 577–581. [Google Scholar] [CrossRef]
- Machado, R.M.D.; Toledo, M.C.F.; Garcia, L.C. Effect of Light and Temperature on the Formation of Glycoalkaloids in Potato Tubers. Food Control 2007, 18, 503–508. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk Assessment of Glycoalkaloids in Feed and Food, in Particular in Potatoes and Potato-derived Products. EFSA J. 2020, 18, e06222. [Google Scholar] [CrossRef] [PubMed]
- Toxic Glycoalkaloids in Potatoes. Available online: https://www.cfs.gov.hk/english/multimedia/multimedia_pub/multimedia_pub_fsf_112_01.html (accessed on 23 October 2023).
- Houbraken, M.; Spranghers, T.; De Clercq, P.; Cooreman-Algoed, M.; Couchement, T.; De Clercq, G.; Verbeke, S.; Spanoghe, P. Pesticide Contamination of Tenebrio molitor (Coleoptera: Tenebrionidae) for Human Consumption. Food Chem. 2016, 201, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pesticide Half-Life. Available online: http://npic.orst.edu/factsheets/half-life.html (accessed on 23 October 2023).
- Pedersen, K.E.; Pedersen, N.N.; Meyling, N.V.; Fredensborg, B.L.; Cedergreen, N. Differences in Life Stage Sensitivity of the Beetle Tenebrio molitor towards a Pyrethroid Insecticide Explained by Stage-Specific Variations in Uptake, Elimination and Activity of Detoxifying Enzymes. Pestic. Biochem. Physiol. 2020, 162, 113–121. [Google Scholar] [CrossRef] [PubMed]
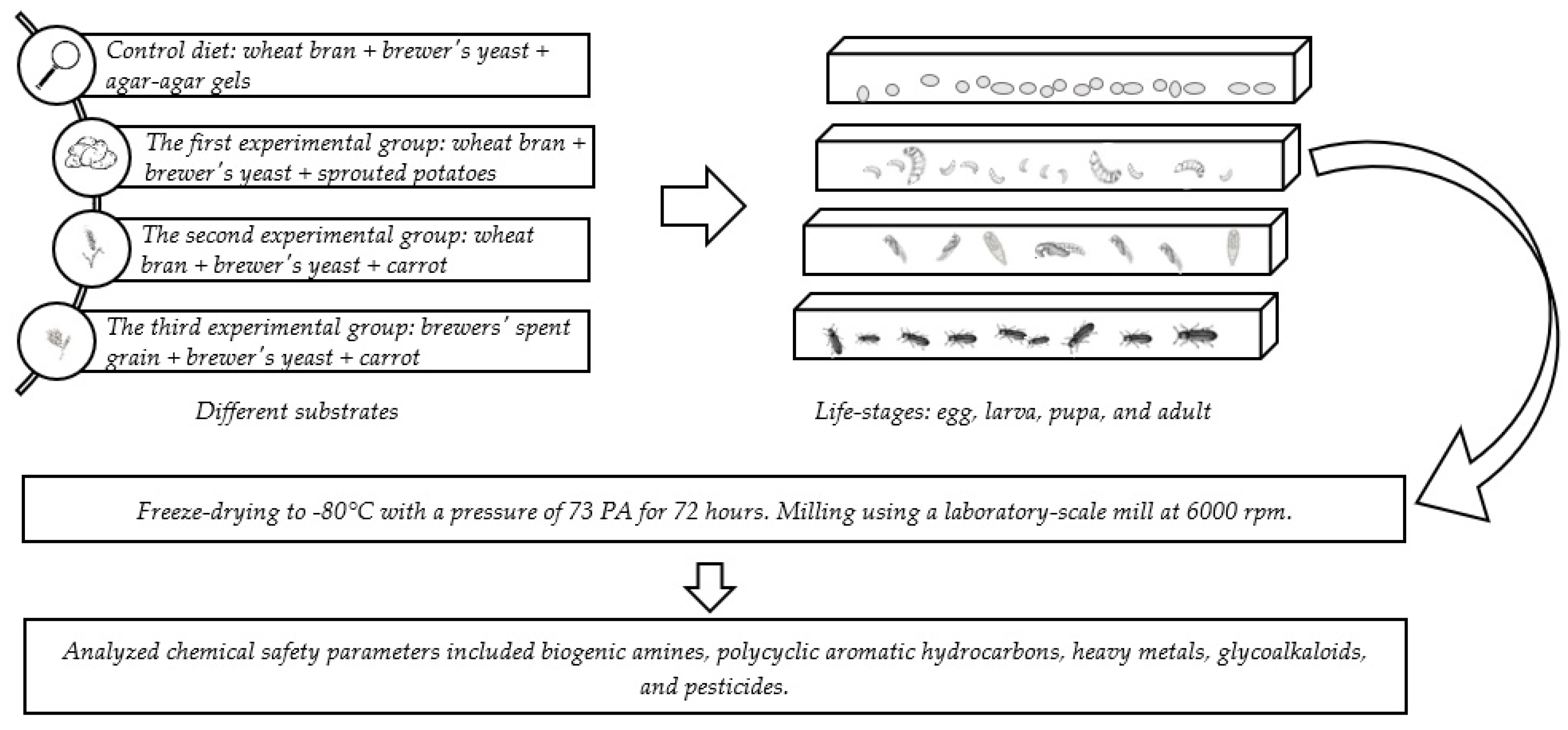
| Control Diet | The First Experiment | The Second Experiment | The Third Experiment | |
|---|---|---|---|---|
| Proportions used for rearing larvae | wheat bran 3600 g | wheat bran 3600 g | wheat bran 3600 g | brewers’ spent grain 3600 g |
| yeast 400 g | yeast 400 g | yeast 400 g | yeast 400 g | |
| agar-agar gels 2750 g | sprouted potatoes 2750 g | carrot 3450 g | carrot 3450 g | |
| Proportions used for an experiment | wheat bran 100 g | wheat bran 100 g | wheat bran 100 g | brewers’ spent grain 100 g |
| yeast 11.11 g | yeast 11.11 g | yeast 11.11 g | yeast 11.11 g | |
| agar-agar gels 76.39 g | sprouted potatoes 76.39 g | carrot 95.83 g | carrot 95.83 g | |
| Proportions used for an experiment after lyophilization | wheat bran 100 g | wheat bran 100 g | wheat bran 100 g | brewers’ spent grain 100 g |
| yeast 11.11 g | yeast 11.11 g | yeast 11.11 g | yeast 11.11 g | |
| agar-agar gels 0.76 g | sprouted potatoes 18.33 g | carrot 11.50 g | carrot 11.50 g | |
| (lost 99% of total weight) | (lost 76% total weight) | (lost 88% total weight) | (lost 88% total weight) | |
| Substrate abbreviation Larvae abbreviation | SWYG | SWYP | SWYC | SBYC |
| LWYG | LWYP | LWYC | LBYC |
| Material | Histamine | Cadaverine | Putrescine | Tyramine | Spermine | Spermidine | |
|---|---|---|---|---|---|---|---|
| LWYG (control) | 1.72 ± 0.24 a | 56.57 ± 2.41 b | 2042.47 ± 37.37 d | 10.51 ± 0.67 a | 235.65 ± 43.25 ab | 203.85 ± 7.24 a | |
| Mealworms | LWYP | 1.48 ±0.20 a | 14.72 ± 1.19 a | 1475.29 ± 20.14 a | 7.44 ± 1.73 a | 98.79 ± 20.57 a | 210.44 ± 22.3 a |
| LWYC | 1.53 ±0.16 a | 15.65 ± 0.60 a | 1260.06 ± 13.28 b | 10.54 ± 0.66 a | 163.57 ± 97.98 a | 193.72 ± 3.34 a | |
| LBYC | 3.20 ± 0.19 b | 13.39 ± 0.30 a | 1070.40 ± 37.80 c | 16.69 ± 1.41 b | 370.54 ± 42.63 b | 205.62 ± 3.70 a | |
| Substrate | SWYG (control) | 17.23 ± 1.09 a | 22.52 ± 3.93 a | 153.47 ± 8.93 a | 8.33 ± 0.66 a | 20.16 ± 1.02 a | 119.68 ± 8.53 b |
| SWYP | 15.97 ± 1.21 a | 29.41 ± 14.38 a | 160.03 ± 6.76 a | 7.92 ± 0.83 a | 19.19 ± 0.70 a | 131.08 ± 4.31 ab | |
| SWYC | 15.96 ± 0.47 a | 24.60 ± 9.04 a | 150.04 ± 5.17 a | 7.38 ± 0.87 a | 19.61 ± 1.07 a | 141.16 ± 5.58 a | |
| SBYC | 16.27 ± 0.37 a | 42.84 ± 6.31 a | 193.01 ± 5.78 b | 15.68 ± 0.63 b | 41.47 ± 1.11 b | 133.67 ± 6.86 ab | |
| Mealworms | 1.98 ± 0.76 | 25.03 ± 18.95 a | 1462.05 ± 381.45 a | 11.3 ± 3.66 a | 217.14 ± 116.65 a | 203.41 ± 12.04 a | |
| Substrate | 16.36 ± 0.91 | 25.03 ± 18.95 a | 164.16 ± 18.72 b | 9.83 ± 3.60 a | 25.11 ± 9.91 b | 131.4 ± 9.78 b | |
| LWYG (Control) | LWYP | LWYC | LBYC | SWYG | SWYP | SWYC | SBYC | |
|---|---|---|---|---|---|---|---|---|
| Naphthalene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Acenaphthene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Fluorene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Phenanthrene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Anthracene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Fluoranthene | N.D. | N.D. | N.D. | 0.026 ± 0.001 | N.D. | N.D. | 0.019 ± 0.002 | 0.034 ± 0.002 |
| Pyrene | N.D. | N.D. | N.D. | 0.032 | N.D. | N.D. | N.D. | 0.044 ± 0.005 |
| Benz(a)anthracene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Chrysene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Benzo(b)fluoranthene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.004 ± 0.001 |
| Benzo(k)fluoranthene | N.D. | N.D. | N.D. | 0.0007 ± 0.0002 | N.D. | N.D. | N.D. | 0.002 ± 0.001 |
| Benzo(a)pyrene | N.D. | N.D. | N.D. | 0.0007 ± 0.0001 | N.D. | N.D. | N.D. | 0.006 ± 0.002 |
| Benzo(g. h,i)perylene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| dibenzo(a,h)anthracene | N.D. | N.D. | N.D. | 0.003 ± 0.0004 | N.D. | N.D. | N.D. | 0.012 ± 0.001 |
| Indene(1,2,3-cd)pyrene | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Sum of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene | N.D. | N.D. | N.D. | 0.0007 ± 0.0003 | N.D. | N.D. | N.D. | 0.01 ± 0.003 |
| LWYP | LWYC | LBYC | LWYG | SWYP | SWYC | SBYC | SWYG | |
|---|---|---|---|---|---|---|---|---|
| Cadmium | 0.06 ± 0.005 a | 0.05 ± 0.001 a | 0.08 ± 0.003 b | 0.03 ± 0.004 c | 0.04 ± 0.002 a | 0.04 ± 0.05 a | 0.09 ± 0.003 b | 0.04 ± 0.34 ab |
| Chrome | <1 | <1 | <1 | <1 | <1 | <1 | 1.45 ± 0.02 | <1 |
| Nickel | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 0.65 ± 0.06 | <0.5 |
| Lead | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Manganese | 8.91 ± 0.04 a | 9.43 ± 0.09 b | 5.62 ± 0.08 c | 7.95 ± 0.88 ab | 72.34 ± 0.79 a | 69.34 ± 0.28 b | 52.49 ± 0.18 c | 71.76 ± 0.77 a |
| Material | Tomatidin | Tomatin | α-Solanine | α-Chaconine | Solanidin | Sum |
|---|---|---|---|---|---|---|
| Potatoes | <0.0100 | <0.0100 | 62.54 ± 0.19 a | 145.01 ± 0.01 a | 15.52 ± 0.12 a | 225 ± 0.32 a |
| LWYP | <0.0100 | <0.0100 | 175.12 ± 0.21 b | 139.32 ± 0.32 b | 3.59 ± 0.02 b | 317.87 ± 0.55 b |
| SWYP | <0.0100 | <0.0100 | 58.41 ± 0.22 c | 116.44 ± 0.11 c | 0.39 ± 0.01 c | 175.24 ± 0.34 c |
| LWYG | <0.0100 | <0.0100 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankauskienė, A.; Aleknavičius, D.; Antanaitis, Š.; Kiseliovienė, S.; Wedi, P.; Šumskienė, M.; Juknienė, I.; Gaižauskaitė, Ž.; Kabašinskienė, A. The Impact of Farm and Industrial Feed Waste on the Safety Parameters of Tenebrio molitor Larvae. Processes 2024, 12, 37. https://doi.org/10.3390/pr12010037
Jankauskienė A, Aleknavičius D, Antanaitis Š, Kiseliovienė S, Wedi P, Šumskienė M, Juknienė I, Gaižauskaitė Ž, Kabašinskienė A. The Impact of Farm and Industrial Feed Waste on the Safety Parameters of Tenebrio molitor Larvae. Processes. 2024; 12(1):37. https://doi.org/10.3390/pr12010037
Chicago/Turabian StyleJankauskienė, Agnė, Dominykas Aleknavičius, Šarūnas Antanaitis, Sandra Kiseliovienė, Philipp Wedi, Marijona Šumskienė, Ignė Juknienė, Žydrūnė Gaižauskaitė, and Aistė Kabašinskienė. 2024. "The Impact of Farm and Industrial Feed Waste on the Safety Parameters of Tenebrio molitor Larvae" Processes 12, no. 1: 37. https://doi.org/10.3390/pr12010037
APA StyleJankauskienė, A., Aleknavičius, D., Antanaitis, Š., Kiseliovienė, S., Wedi, P., Šumskienė, M., Juknienė, I., Gaižauskaitė, Ž., & Kabašinskienė, A. (2024). The Impact of Farm and Industrial Feed Waste on the Safety Parameters of Tenebrio molitor Larvae. Processes, 12(1), 37. https://doi.org/10.3390/pr12010037






