Generation of Hydrogen Peroxide and Phenolic Content in Plant-Material-Based Beverages and Spices
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Materials and Equipment
2.2. Extracts and Solutions
2.3. Estimation of the Phenolic Content
2.4. Estimation of Antioxidant Capacity
2.5. Generation and Scavenging of Hydrogen Peroxide
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Lan, A.N.B.; Hsuan, F.T.Y.; Halliwell, B. Generation of hydrogen peroxide by “antioxidant” beverages and the effect of milk addition. Is cocoa the best beverage? Free Radic. Res. 1999, 31, 67–71. [Google Scholar] [CrossRef]
- Fujita, Y.; Wakabayashi, K.; Nagao, M.; Sugimura, T. Implication of hydrogen peroxide in the mutagenicity of coffee. Mutat. Res. Lett. 1985, 144, 227–230. [Google Scholar] [CrossRef]
- Nagao, M.; Fujita, Y.; Wakabayashi, K.; Nukaya, H.; Kosuge, T.; Sugimura, T. Mutagens in coffee and other beverages. Environ. Health Perspect. 1986, 67, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Tsiju, S.; Shibata, T.; Ohara, K.; Okada, N.; Ito, Y. Studies on the factors affecting the formation of hydrogen peroxide in coffee. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1991, 32, 504–512. [Google Scholar]
- Tama, A.; Bartosz, G.; Sadowska-Bartosz, I. Is hydrogen peroxide generated in wine? Food Biosci. 2022, 45, 101487. [Google Scholar] [CrossRef]
- Bartosz, G.; Rajzer, K.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Hydrogen peroxide is formed upon cooking of vegetables. Acta Biochim. Pol. 2022, 69, 471–474. [Google Scholar] [CrossRef]
- Bartosz, G.; Baran, S.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. The Antioxidant capacity and hydrogen peroxide formation by black and orange carrots: Black and orange carrots. Agric. Food Sci. 2022, 31, 71–77. [Google Scholar] [CrossRef]
- Tama, A.; Pieńkowska, N.; Stefaniuk, I.; Bartosz, G.; Kapusta, I.; Sadowska-Bartosz, I. Is hydrogen peroxide generated in infusions of medicinal herbs? Processes 2023, 11, 2855. [Google Scholar] [CrossRef]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Ferreira, I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Faller, A.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Gündeşli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Kirsch, M.; De Groot, H. NAD (P) H, a directly operating antioxidant? FASEB J. 2001, 15, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, L.-M.; Yang, L.; Liu, Z.-L. Evidence for α-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamazaki, S.-I.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta Gen. Sub. 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, X.; Yu, Y.; Li, W.; Mou, Y.; Chen, F.; Hu, X.; Ji, J.; Ma, L. From polyphenol to o-quinone: Occurrence, significance, and intervention strategies in foods and health implications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3254–3291. [Google Scholar] [CrossRef]
- Sang, S.; Yang, I.; Buckley, B.; Ho, C.-T.; Yang, C.S. Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (-)-epigallocatechin-3-gallate: Studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radic. Biol. Med. 2007, 43, 362–371. [Google Scholar] [CrossRef]
- Wagner, M.; Nicell, J.A. Detoxification of phenolic solutions with horseradish peroxidase and hydrogen peroxide. Water Res. 2002, 36, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Yvonne, O.; Driss, F.; Dang, P.M.-C.; Elbim, C.; Gougerot-Pocidalo, M.-A.; Pasquier, C.; El-Benna, J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004, 68, 2003–2008. [Google Scholar]
- Eskandari, M.; Rembiesa, J.; Startaitė, L.; Holefors, A.; Valančiūtė, A.; Faridbod, F.; Ganjali, M.R.; Engblom, J.; Ruzgas, T. Polyphenol-hydrogen peroxide reactions in skin: In vitro model relevant to study ROS reactions at inflammation. Anal. Chim. Acta 2019, 1075, 91–97. [Google Scholar] [CrossRef]
- Sroka, Z.; Fecka, I.; Cisowski, W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z. Nat. C 2005, 60, 826–832. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. A novel hydrogen peroxide scavenging assay of phenolics and flavonoids using cupric reducing antioxidant capacity (CUPRAC) methodology. J. Food Compos. Anal. 2010, 23, 689–698. [Google Scholar] [CrossRef]
- Mansouri, A.; Makris, D.P.; Kefalas, P. Determination of hydrogen peroxide scavenging activity of cinnamic and benzoic acids employing a highly sensitive peroxyoxalate chemiluminescence-based assay: Structure–activity relationships. J. Pharm. Biomed. Anal. 2005, 39, 22–26. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Dong, J.; Qian, W. Determination of hydrogen peroxide scavenging activity of phenolic acids by employing gold nanoshells precursor composites as nanoprobes. Food Chem. 2011, 126, 698–704. [Google Scholar] [CrossRef]
- Kılıçgün, H.; Altıner, D. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn. Mag. 2010, 6, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.-T.; Gan, R.-Y.; Zhang, Y.; Xu, X.-R.; Xia, E.-Q.; Li, H.-B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A modification of the ABTS● decolorization method and an insight into its mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Bochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Gay, C.A.; Gebicki, J.M. Measurement of protein and lipid hydroperoxides in biological systems by the ferric–xylenol orange method. Anal. Biochem. 2003, 315, 29–35. [Google Scholar] [CrossRef]
- Vignoli, J.; Bassoli, D.; Benassi, M.D.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Presler, A.; Michel, P. Profiling of phenolic compounds and antioxidant activity of dry extracts from the selected Sorbus species. Molecules 2012, 17, 3093–3113. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Souza, J.N.; Silva, E.M.; Loir, A.; Rees, J.F.; Rogez, H.; Larondelle, Y. Antioxidant capacity of four polyphenol-rich Amazonian plant extracts: A correlation study using chemical and biological in vitro assays. Food Chem. 2008, 106, 331–339. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Gordon, M.H.; Pérez-Coello, M.S. A study of the antioxidant capacity of oak wood used in wine ageing and the correlation with polyphenol composition. Food Chem. 2011, 128, 997–1002. [Google Scholar] [CrossRef]
- Konan, Y.; Witabouna, K.M.; Bassirou, B.; Kagoyire, K. Antioxidant activity and total phenolic content of nine plants from Côte d’Ivoire (West Africa). J. Appl. Pharm. Sci. 2014, 4, 036–041. [Google Scholar]
- Pu, F.; Ren, X.-L.; Zhang, X.-P. Phenolic compounds and antioxidant activity in fruits of six Diospyros kaki genotypes. Eur. Food Res. Technol. 2013, 237, 923–932. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Kreicbergs, V.; Dimins, F.; Mikelsone, V.; Cinkmanis, I. Biologically active compounds in roasted coffee. In Proceedings of the 6th Baltic Conference on Food Science and Technology FOODBALT, Jelgava, Latvia, 5–6 May 2011; pp. 110–115. [Google Scholar]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Masuda, T. Identification of pyrogallol in the ethyl acetate-soluble part of coffee as the main contributor to its xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2016, 64, 7743–7749. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Pyrogallol: A competent therapeutic agent of the future. Biotechnol. Environ. Sci. 2021, 23, 213–217. [Google Scholar]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Olk, M.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Formation of hydrogen peroxide in cell culture media by apple polyphenols and its effect on antioxidant biomarkers in the colon cell line HT-29. Mol. Nutr. Food Res. 2009, 53, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Hoi, A.; Halliwell, B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys. 2010, 501, 162–169. [Google Scholar] [CrossRef]
- Mu, K.; Kitts, D.D. Hydrogen peroxide produced from selective phenolic acids in cell culture underlies Caco-2 changes in cell proliferation parameters. J. Agric. Food Chem. 2023, 71, 3022–3032. [Google Scholar] [CrossRef]
- Long, L.H.; Clement, M.V.; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin,(−)-epigallocatechin gallate,(+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000, 273, 50–53. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Oh, S.; Kim, S.W.; Kim, J.-W.; Kim, Y.S.; Woo, J.-T. Prooxidative effects of green tea polyphenol (−)-epigallocatethin-3-gallate on the HIT-T15 pancreatic beta cell line. Cell Biol. Toxicol. 2010, 26, 189–199. [Google Scholar] [CrossRef]
- Valdés, A.; García-Cañas, V.; Koçak, E.; Simó, C.; Cifuentes, A. Foodomics study on the effects of extracellular production of hydrogen peroxide by rosemary polyphenols on the anti-proliferative activity of rosemary polyphenols against HT-29 cells. Electrophoresis 2016, 37, 1795–1804. [Google Scholar] [CrossRef]
- Chai, P.C.; Long, L.H.; Halliwell, B. Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem. Biophys. Res. Commun. 2003, 304, 650–654. [Google Scholar] [CrossRef]



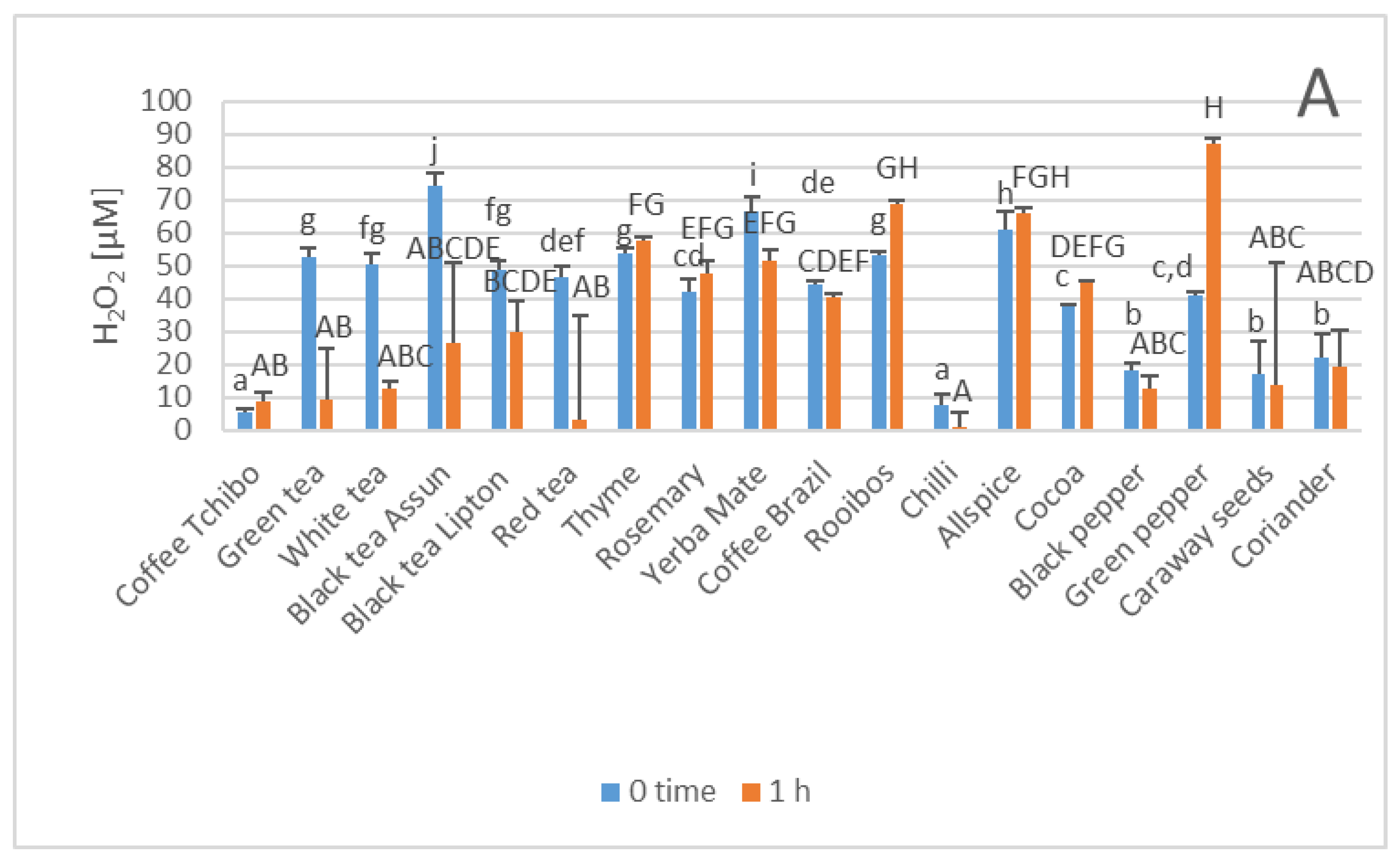
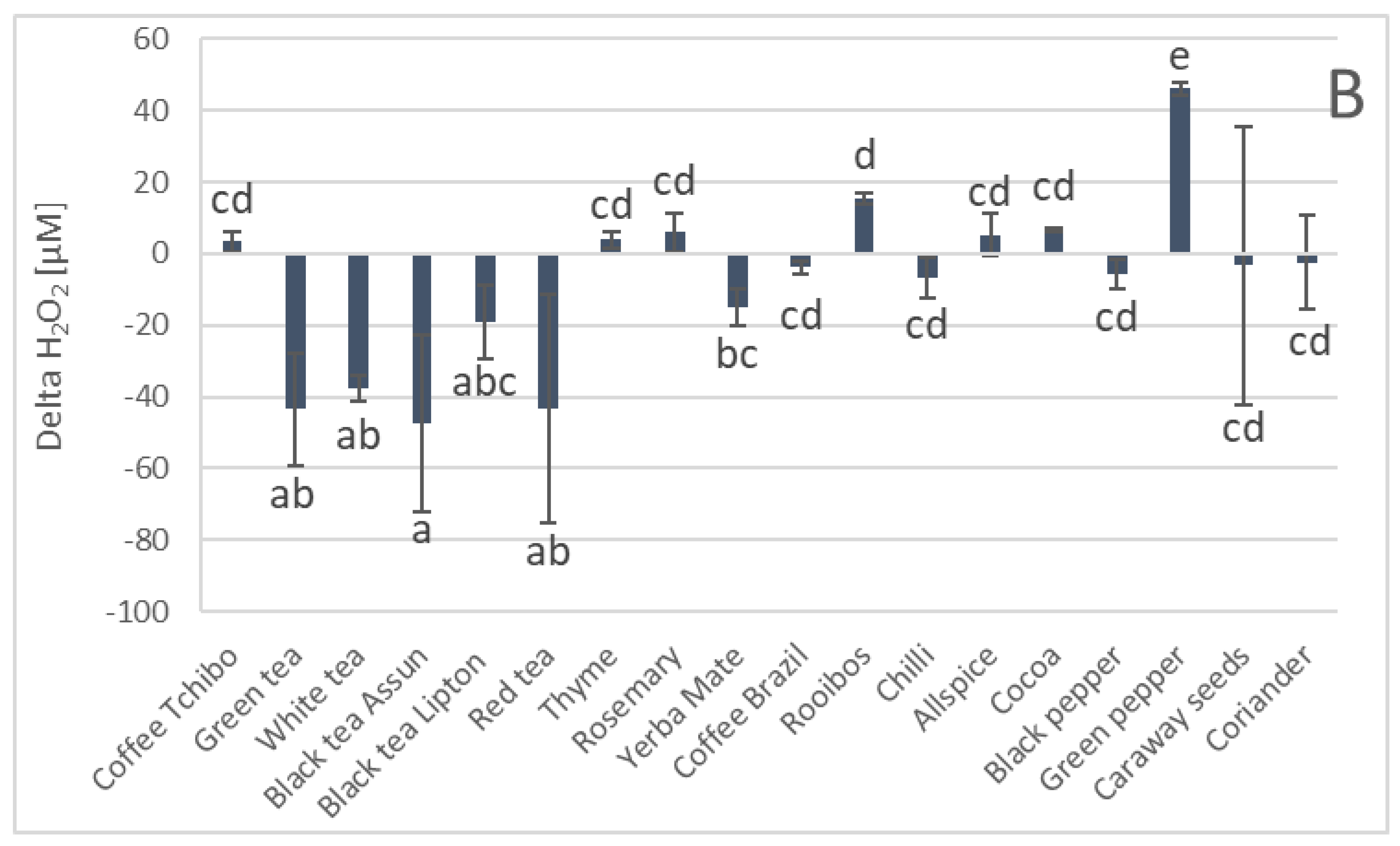
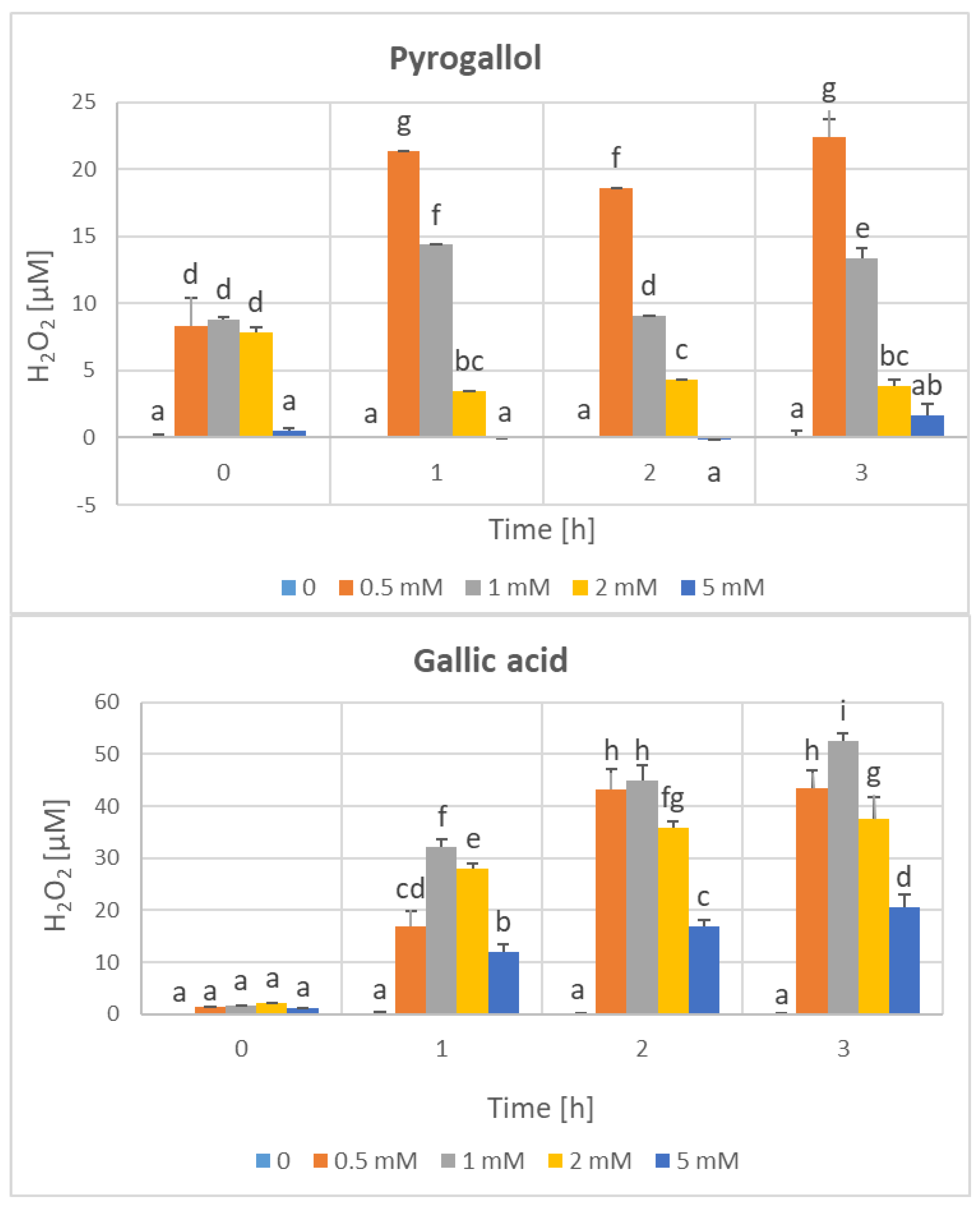
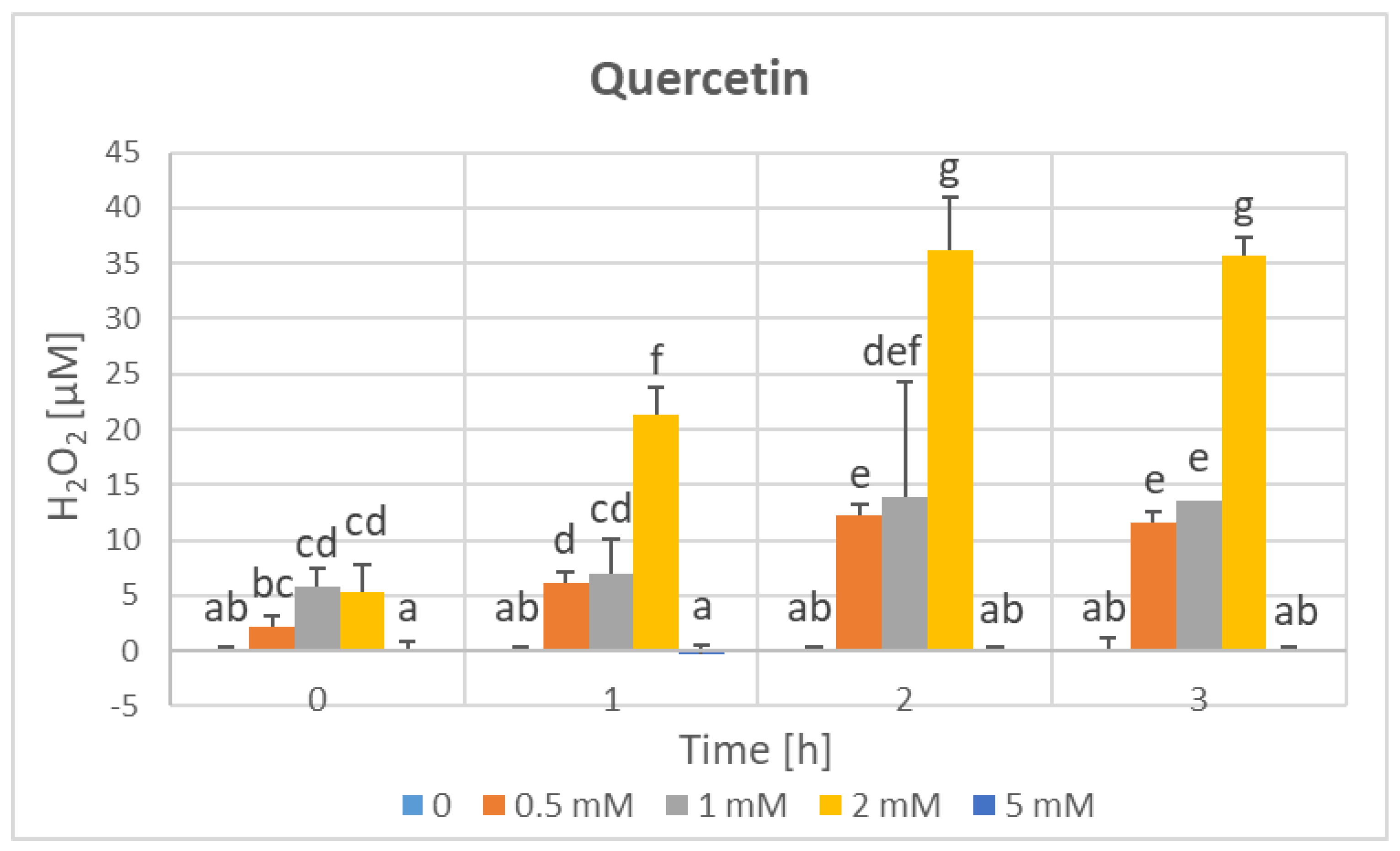
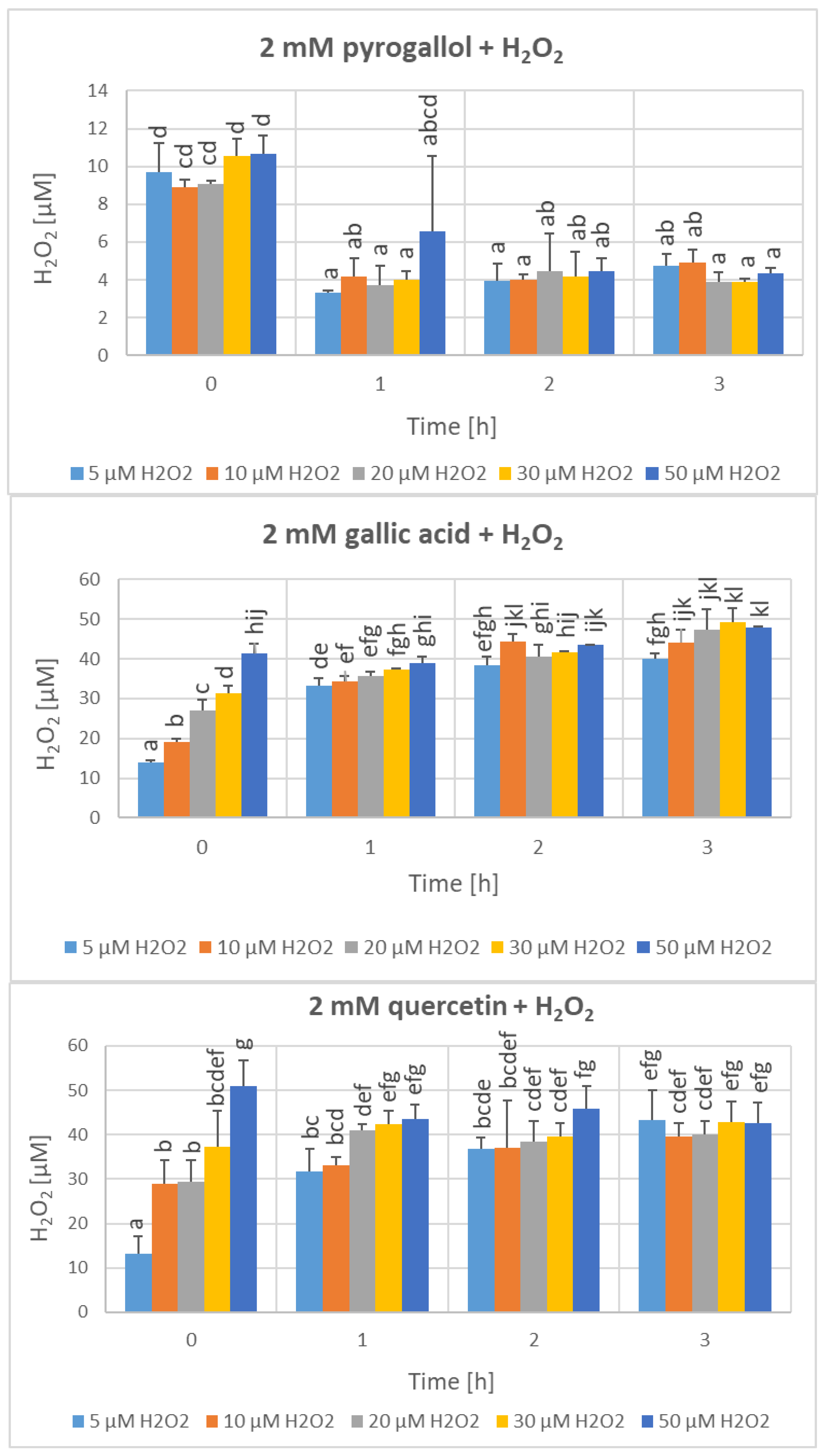
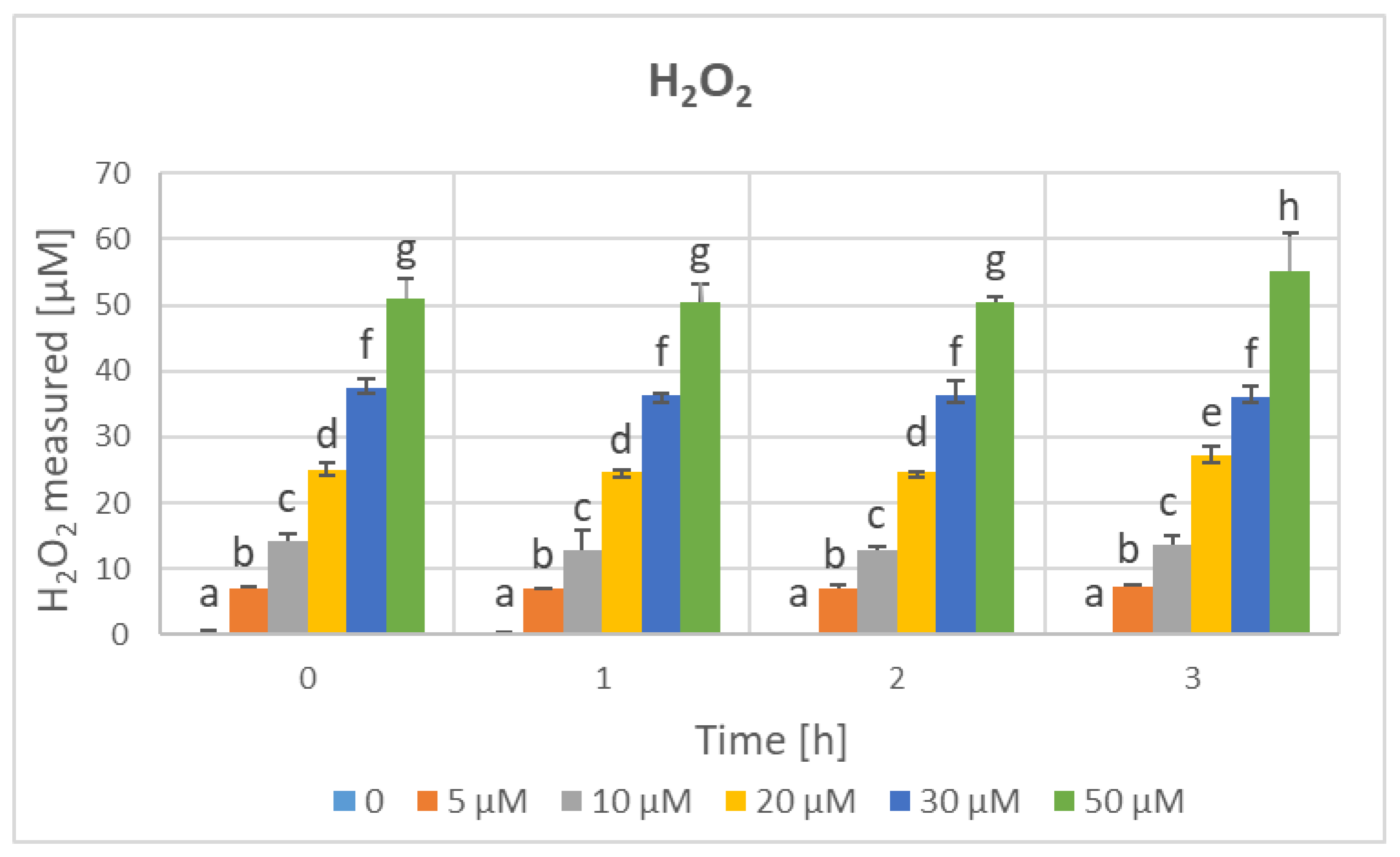
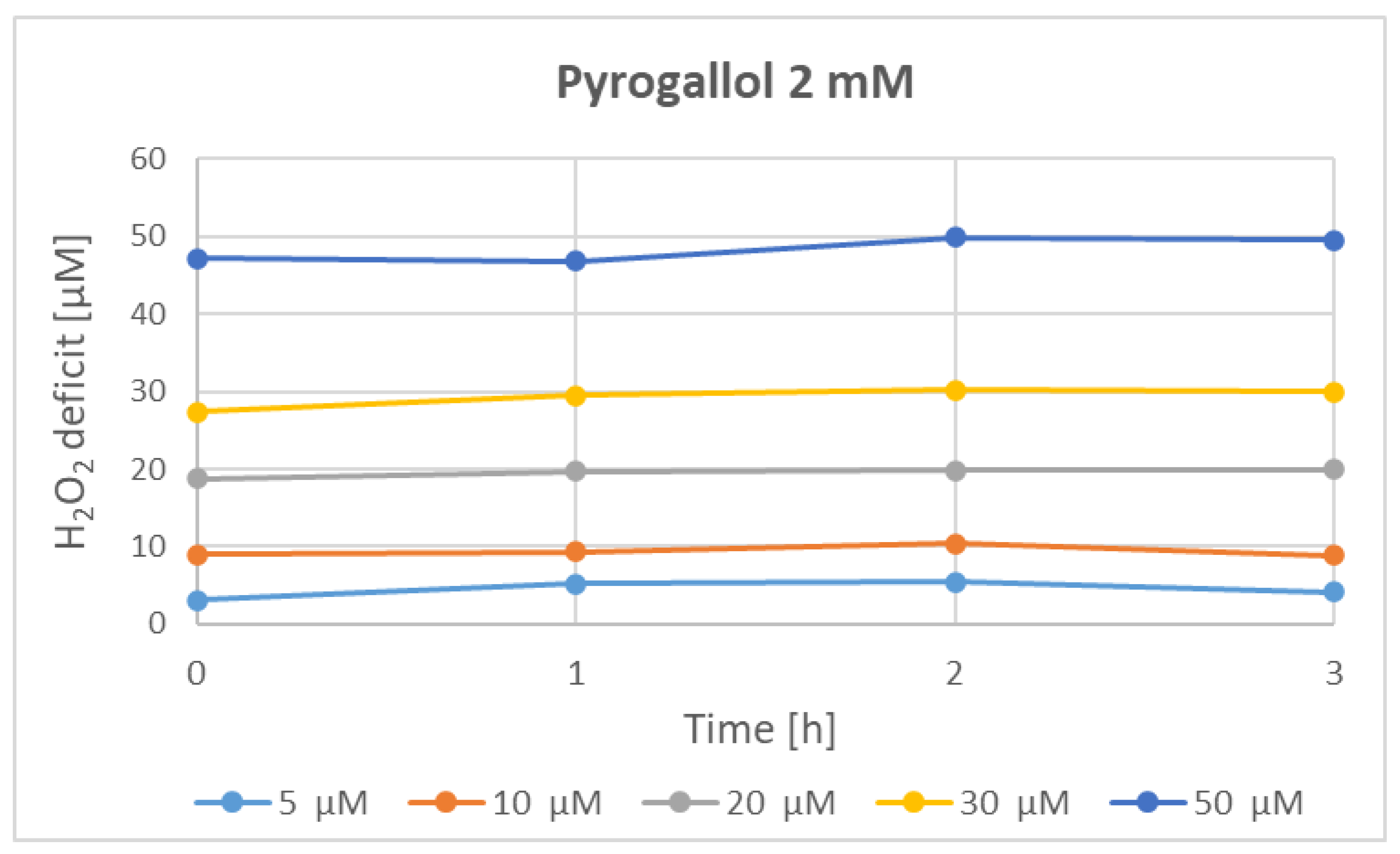
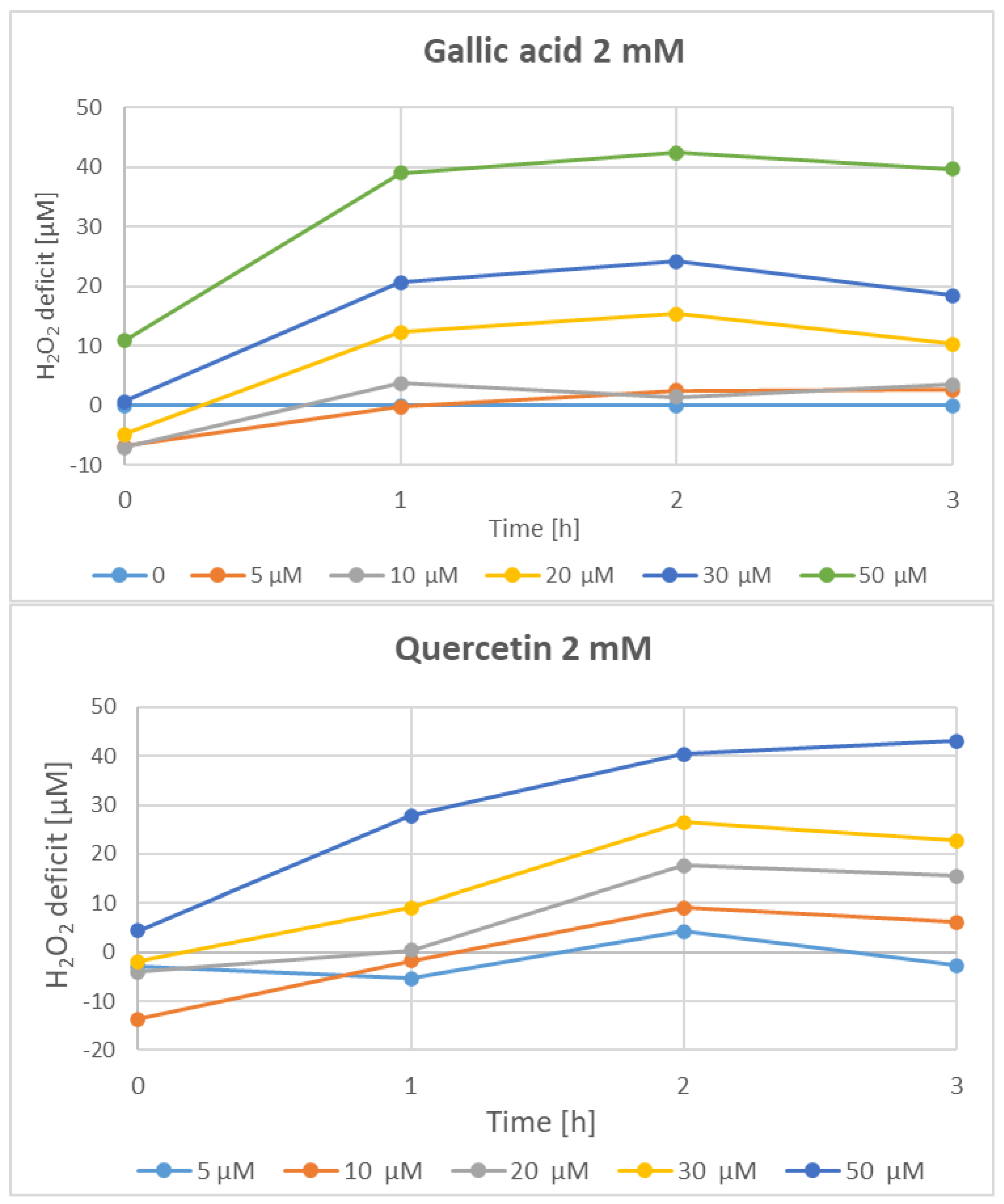
| Parameter | Phenolics [μg GAE/mL] | TAC-ABTS● [μmol TE/mL] | TAC-FRAP [μmol TE/mL] | TAC-CUPRAC [μmol TE/mL] | TAC-DPPH● [μmol TE/mL] | H2O2 Time “0” [μM] |
|---|---|---|---|---|---|---|
| TAC-ABTS● | 0.94 *** | |||||
| TAC-FRAP | 0.90 *** | 0.83 *** | ||||
| TAC-CUPRAC | 0.93 *** | 0.88 *** | 0.95 *** | |||
| TAC-DPPH● | 0.78 *** | 0.74 *** | 0.63 ** | 0.68 *** | ||
| H2O2 time “0” | 0.25 | 0.40 | 0.13 | 0.21 | 0.31 | |
| H2O2 1 h | −0.37 | −0.37 | −0.31 | −0.30 | −0.40 | 0.48 * |
| Delta H2O2 | −0.61 ** | 0.73 *** | 0.45 * | 0.49 * | 0.69 *** | 0.16 |
| Correlation between Total Phenolic Content and: | Material | Correlation Coefficient r | Reference |
|---|---|---|---|
| TAC/DPPH● decolorization | Coffee | 0.88 | [39] |
| TAC/ABTS● decolorization | Coffee | 0.82 | [39] |
| TAC/Crocin bleaching | Sicilian wines | 0.98 | [40] |
| TAC/FRAP | Millefiori honeys | 0.97 | [41] |
| TAC/DPPH● decolorization | Slovenian honeys | 0.93 | [42] |
| TAC/FRAP | Slovenian honeys | 0.97 | [42] |
| TAC/DPPH● decolorization | European honeydew honeys | 0.86 | [43] |
| TAC/ABTS● decolorization | Extracts of 23 Bulgarian plants | 0.92 | [44] |
| TAC/ABTS● decolorization | Extracts of inflorescences and/or leaves of seven Sorbus species | 0.80 | [45] |
| TAC/DPPH● decolorization | Extracts of inflorescences and/or leaves of seven Sorbus species | 0.74 | [45] |
| TAC/FRAP | Extracts of inflorescences and/or leaves of seven Sorbus species | 0.65 | [45] |
| TAC/DPPH● decolorization | Extracts of grape seeds | 0.99 | [46] |
| TAC/FRAP | Extracts of various parts of four Amazonian plants | 0.80 | [47] |
| TAC/ABTS● decolorization | Extracts of oak wood used in wine aging | 0.95 | [48] |
| TAC/DPPH● decolorization | Extracts of oak wood used in wine aging | 0.97 | [48] |
| TAC/FRAP | Extracts of oak wood used in wine aging | 0.96 | [48] |
| TAC/DPPH● decolorization | Chosen Côte d’Ivoire plants | 0.97 | [49] |
| TAC/ABTS● decolorization, | Various persimmon genotypes | 0.91 | [50] |
| TAC/DPPH● decolorization | Various persimmon genotypes | 0.96 | [50] |
| TAC/FRAP | Various persimmon genotypes | 0.97 | [50] |
| TAC/ABTS● decolorization | Nine tomato varieties | 0.42 | [51] |
| TAC/DPPH● decolorization | Green peppers | 0.84 | [52] |
| TAC/DPPH● decolorization | Red peppers | 0.59 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kut, K.; Tama, A.; Furdak, P.; Bartosz, G.; Sadowska-Bartosz, I. Generation of Hydrogen Peroxide and Phenolic Content in Plant-Material-Based Beverages and Spices. Processes 2024, 12, 166. https://doi.org/10.3390/pr12010166
Kut K, Tama A, Furdak P, Bartosz G, Sadowska-Bartosz I. Generation of Hydrogen Peroxide and Phenolic Content in Plant-Material-Based Beverages and Spices. Processes. 2024; 12(1):166. https://doi.org/10.3390/pr12010166
Chicago/Turabian StyleKut, Kacper, Anna Tama, Paulina Furdak, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2024. "Generation of Hydrogen Peroxide and Phenolic Content in Plant-Material-Based Beverages and Spices" Processes 12, no. 1: 166. https://doi.org/10.3390/pr12010166
APA StyleKut, K., Tama, A., Furdak, P., Bartosz, G., & Sadowska-Bartosz, I. (2024). Generation of Hydrogen Peroxide and Phenolic Content in Plant-Material-Based Beverages and Spices. Processes, 12(1), 166. https://doi.org/10.3390/pr12010166









