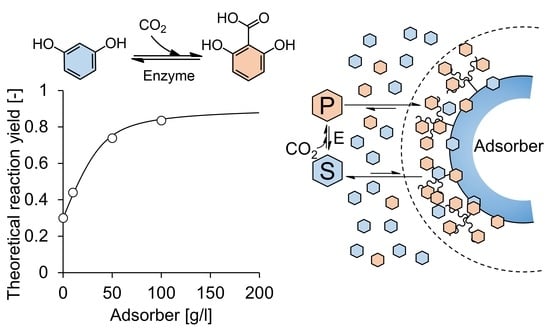
Synthesis of 2,6-Dihydroxybenzoic Acid by Decarboxylase-Catalyzed Carboxylation Using CO2 and In Situ Product Removal
Abstract
:1. Introduction
2. Results and Discussion
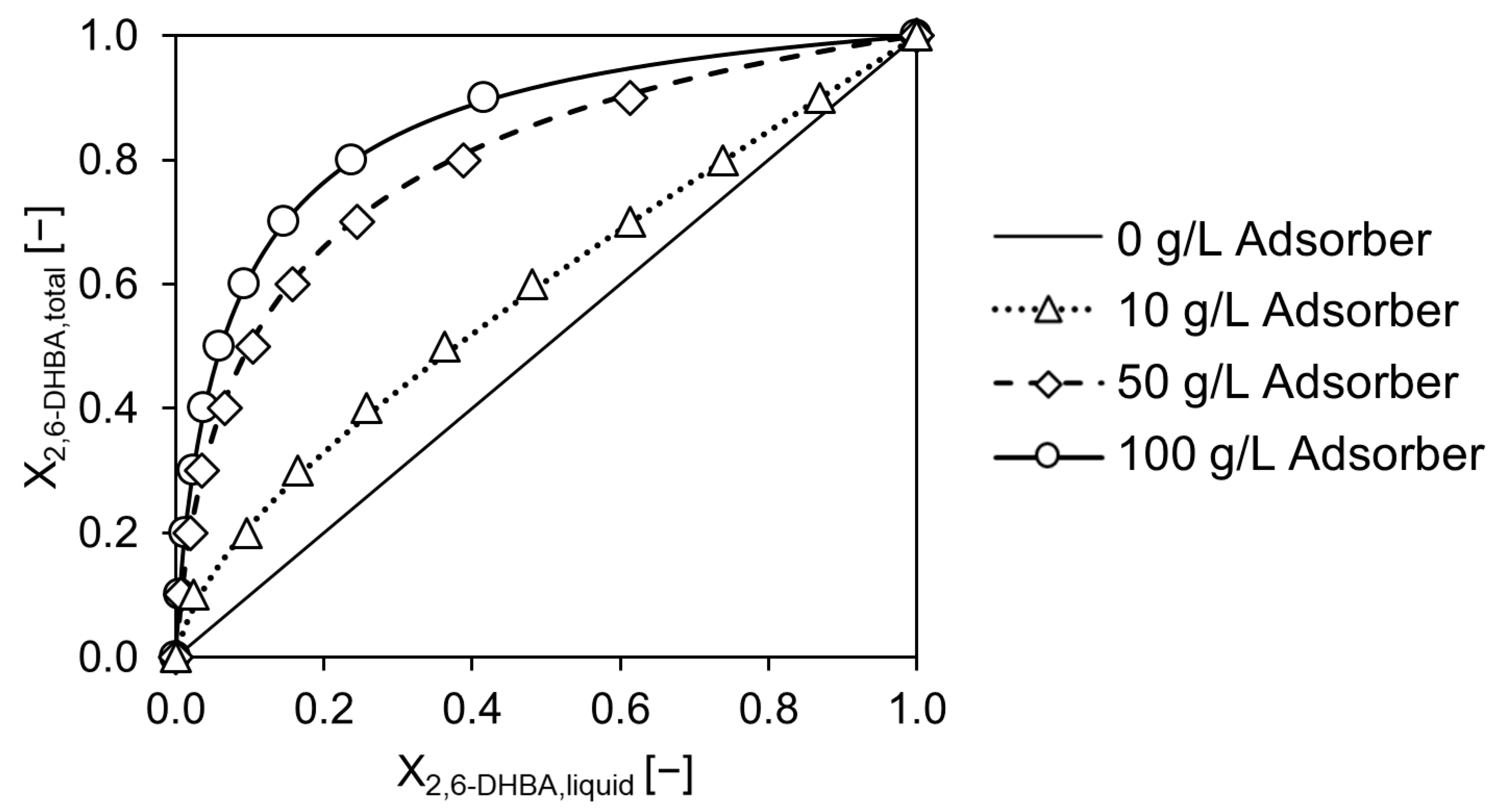
2.1. Adsorber Affinity Investigation for Resorcinol and 2,6-DHBA
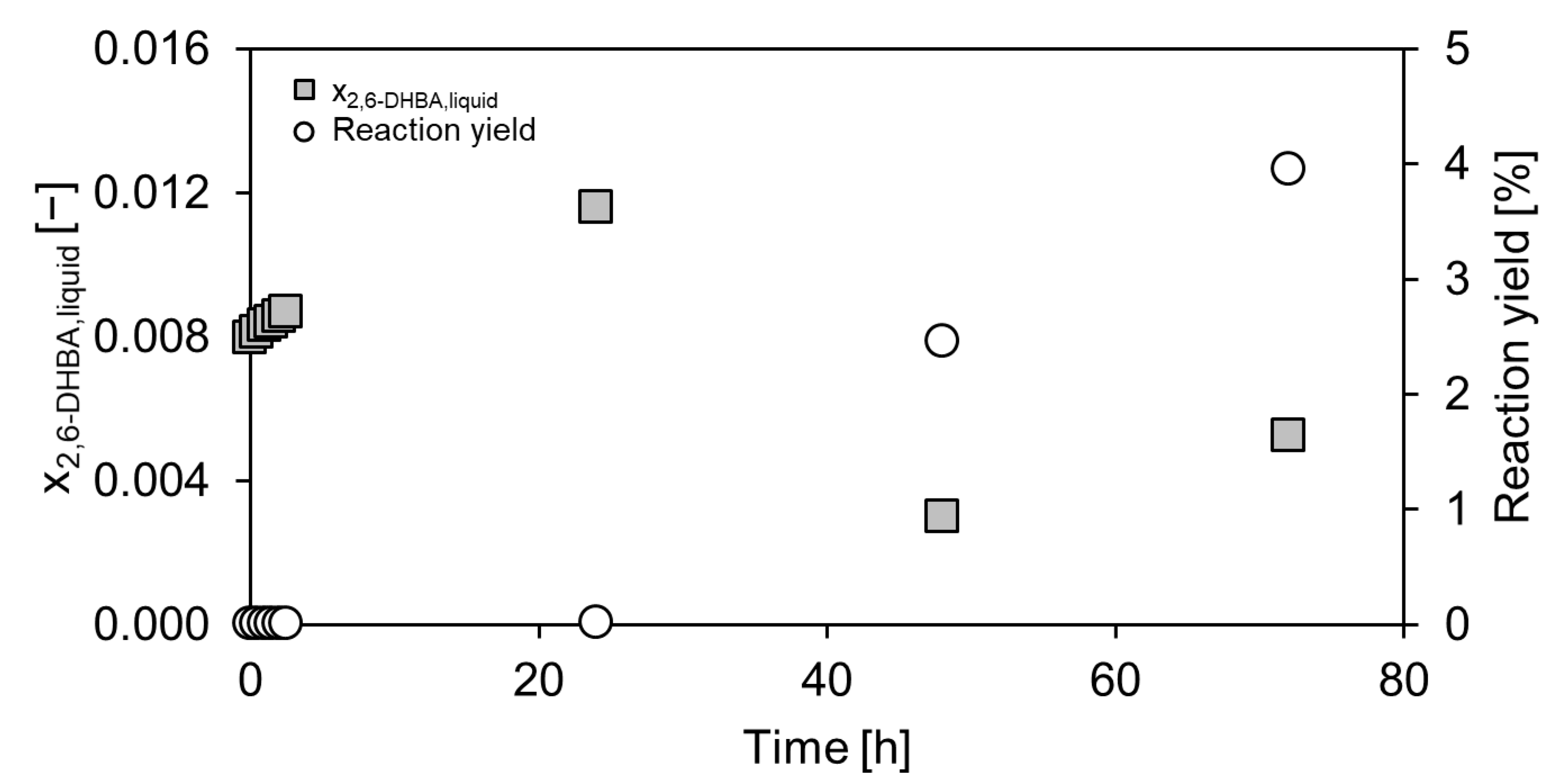
2.2. Product Elution and Purification in a Dowex®-Based Downstream Process
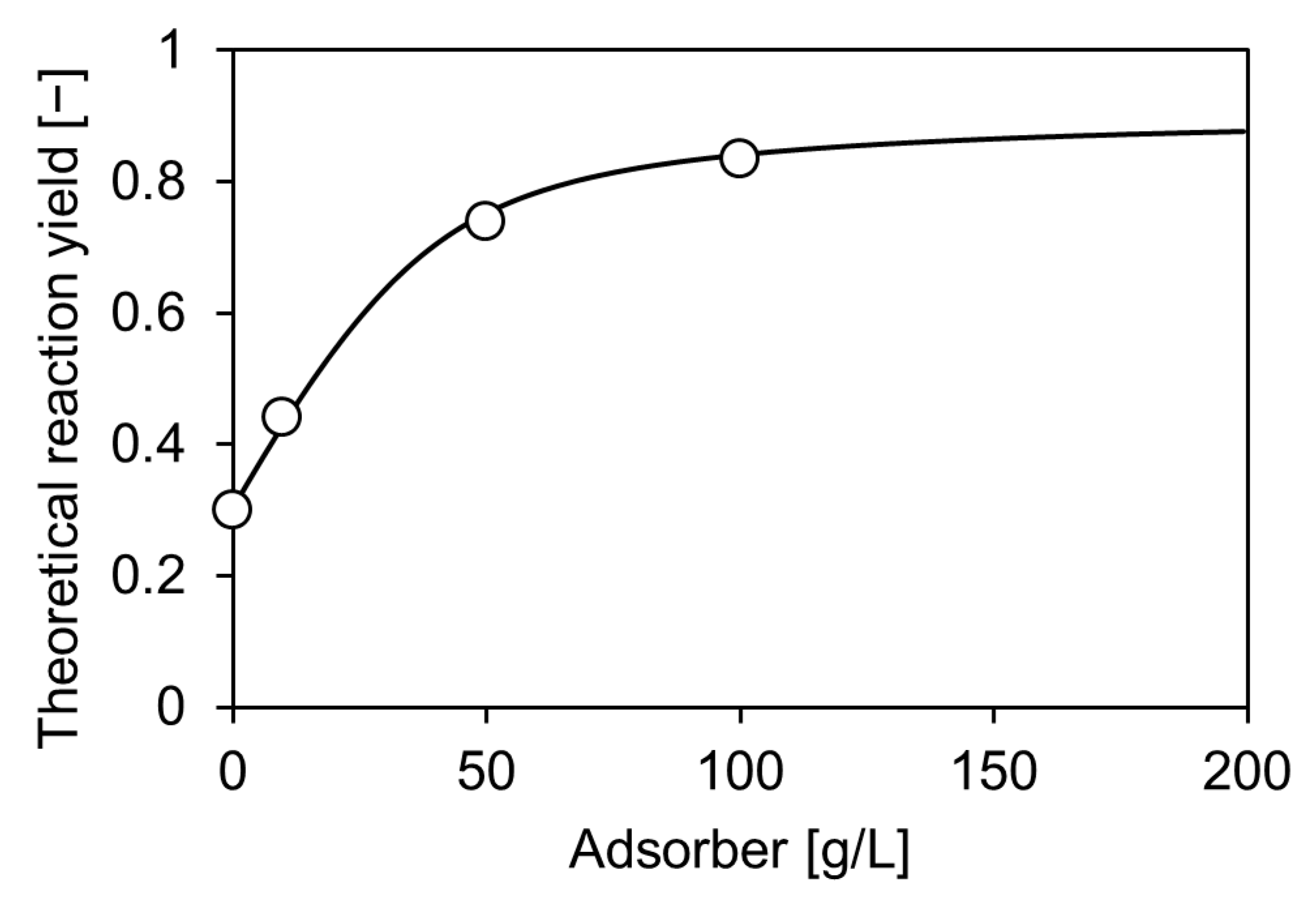
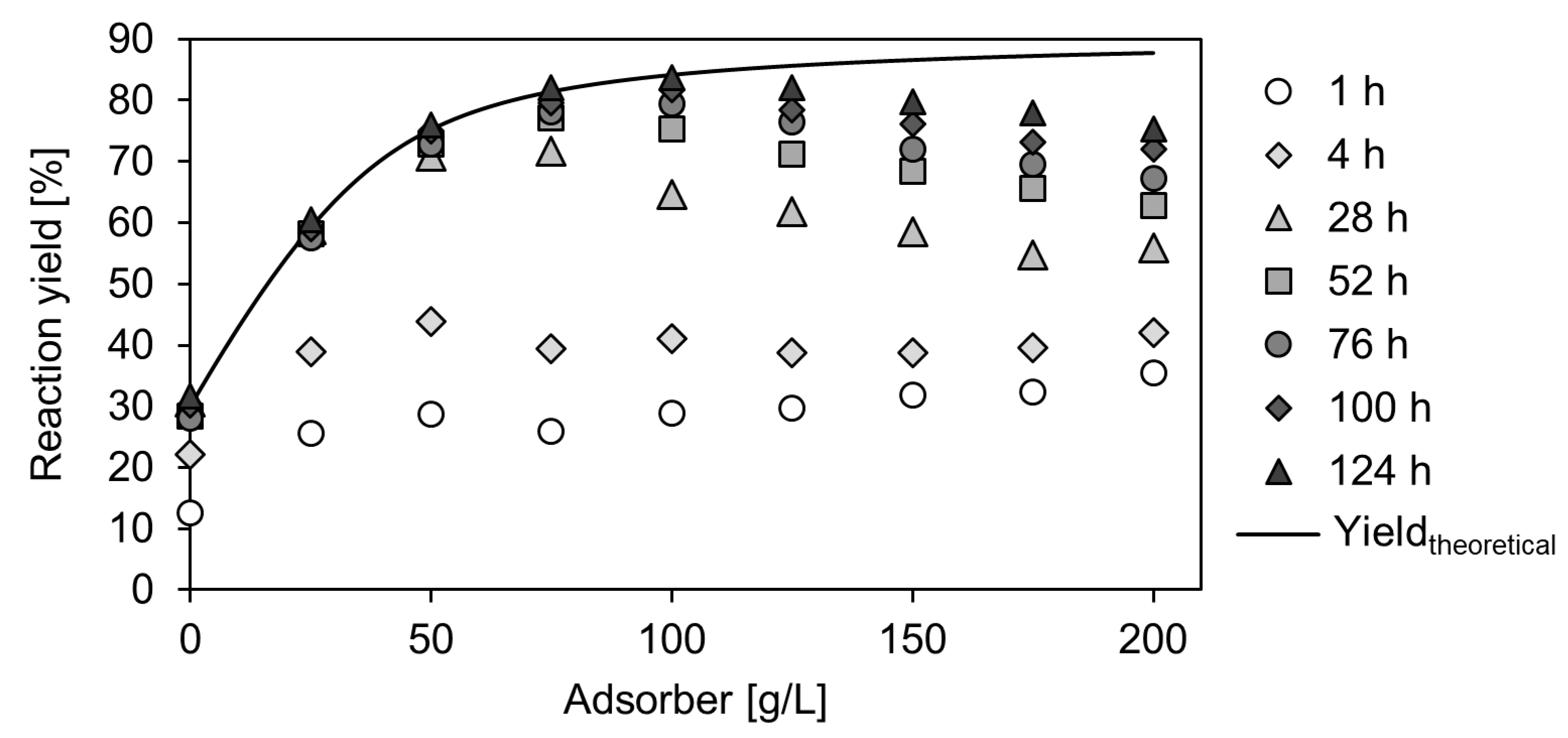
2.3. Enzymatic Carboxylation of Resorcinol on Lab-Scale
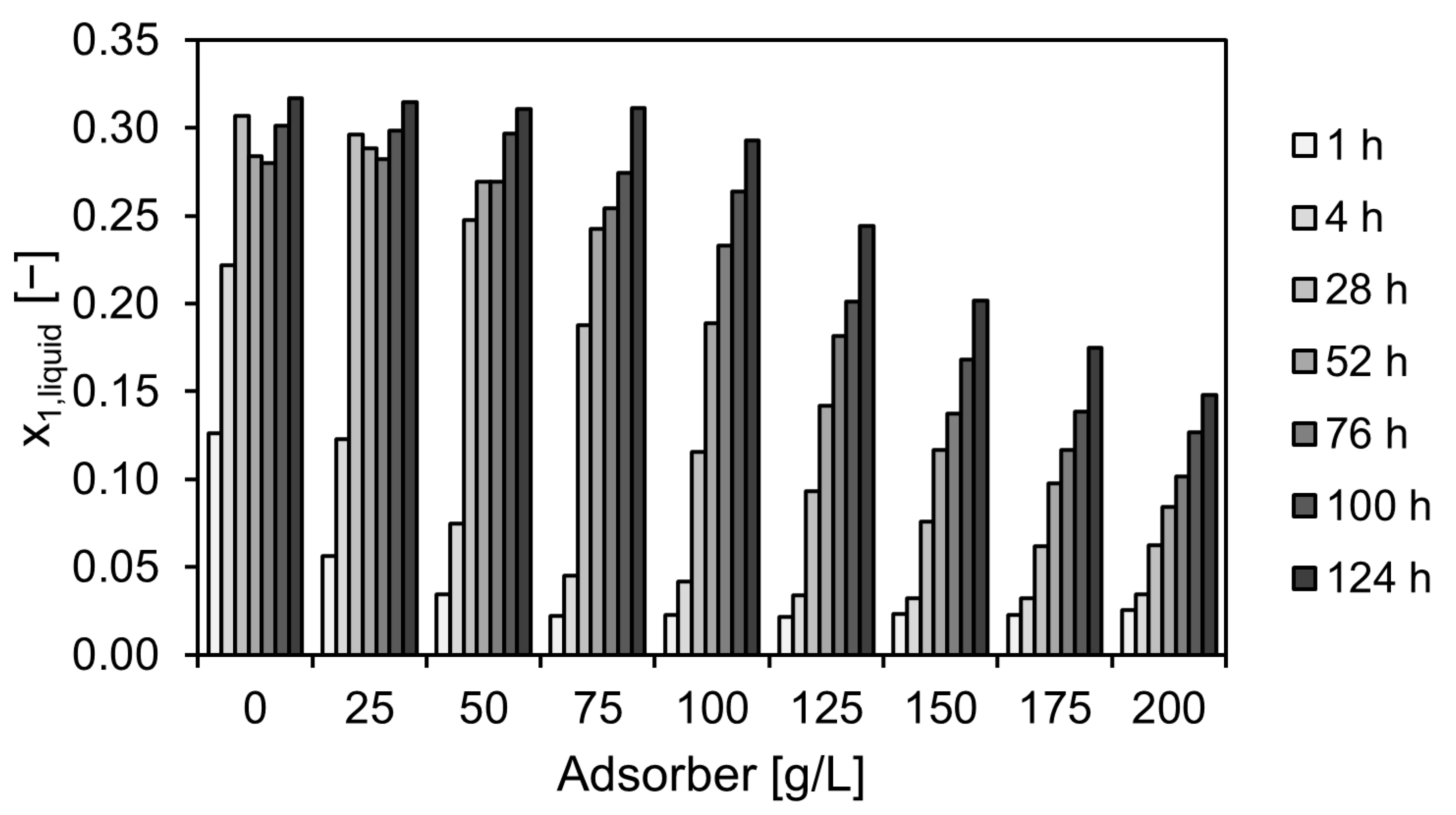
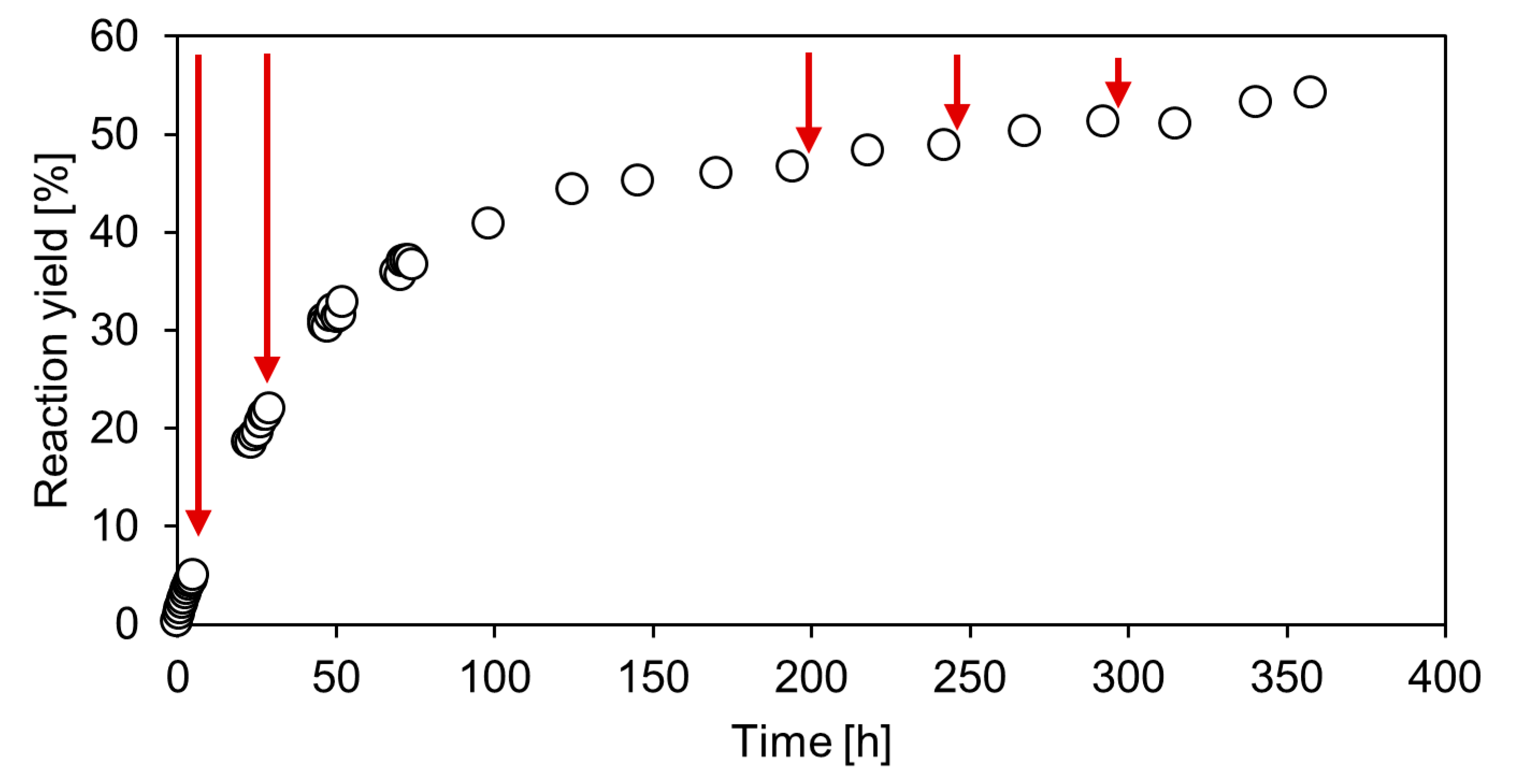
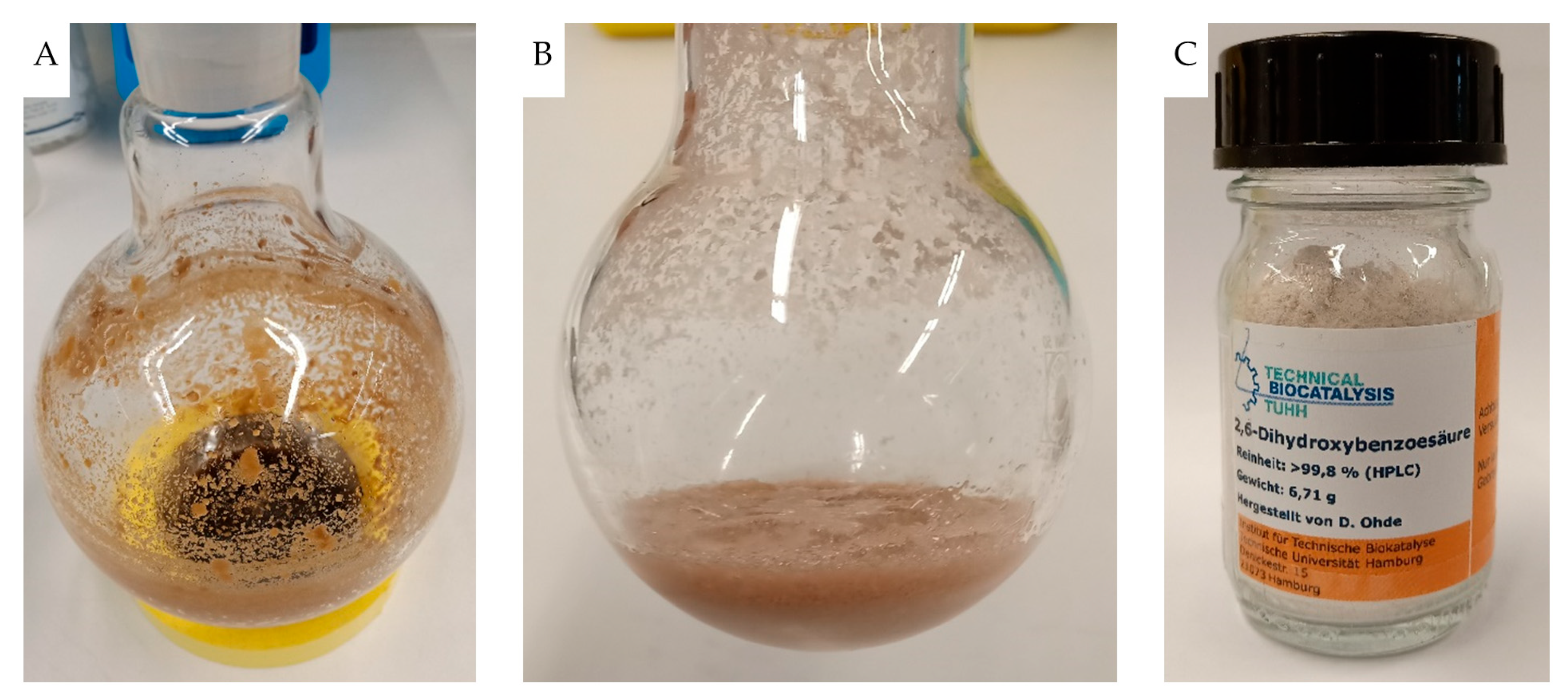
2.4. Repetitive Batch Operation
3. Materials and Methods
3.1. General
3.2. Biocatalyst Preparation
3.3. Adsorber Characterization without Enzyme Addition
3.4. Small-Scale Enzymatic Reactions with Adsorber
3.5. Product Purification of the Reaction Medium
3.6. Lab-Scale Carboxylation with Product Purification
3.7. Sampling Procedure and RP-HPLC Method
3.8. Adsorber Characterization and Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Iglesias, J.; Martínez-Salazar, I.; Maireles-Torres, P.; Martin Alonso, D.; Mariscal, R.; López Granados, M. Advances in catalytic routes for the production of carboxylic acids from biomass: A step forward for sustainable polymers. Chem. Soc. Rev. 2020, 49, 5704–5771. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Kucmierczyk, P.; Dühren, R.; Razzaq, R.; Dong, K.; Liu, J.; Franke, R.; Jackstell, R.; Beller, M. Synthesis of Carboxylic Acids by Palladium-Catalyzed Hydroxycarbonylation. Angew. Chem. 2019, 131, 14503–14511. [Google Scholar] [CrossRef]
- Aleku, G.A.; Roberts, G.W.; Titchiner, G.R.; Leys, D. Synthetic Enzyme-Catalyzed CO2 Fixation Reactions. ChemSusChem 2021, 14, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, I.C. Carboxylation of Hydroxyaromatic Compounds with HCO3− by Enzyme Catalysis: Recent Advances Open the Perspective for Valorization of Lignin-Derived Aromatics. Catalysts 2019, 9, 37. [Google Scholar] [CrossRef]
- Pesci, L.; Glueck, S.M.; Gurikov, P.; Smirnova, I.; Faber, K.; Liese, A. Biocatalytic carboxylation of phenol derivatives: Kinetics and thermodynamics of the biological Kolbe-Schmitt synthesis: Kinetics and thermodynamics of the biological Kolbe-Schmitt synthesis. FEBS J. 2015, 282, 1334–1345. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, J.; Yang, M.; Tan, X.; Fan, H.; Guo, M.; Wang, B.; Xue, S. CO2 (aq) concentration–dependent CO2 fixation via carboxylation by decarboxylase. Green Chem. 2021, 23, 4403–4409. [Google Scholar] [CrossRef]
- Wuensch, C.; Schmidt, N.; Gross, J.; Grischek, B.; Glueck, S.M.; Faber, K. Pushing the equilibrium of regio-complementary carboxylation of phenols and hydroxystyrene derivatives. J. Biotechnol. 2013, 168, 264–270. [Google Scholar] [CrossRef]
- Pesci, L.; Gurikov, P.; Liese, A.; Kara, S. Amine-Mediated Enzymatic Carboxylation of Phenols Using CO2 as Substrate Increases Equilibrium Conversions and Reaction Rates. Biotechnol. J. 2017, 12, 1700332. [Google Scholar] [CrossRef]
- Ohde, D.; Thomas, B.; Matthes, S.; Tanaka, S.; Bubenheim, P.; Terasaka, K.; Schlüter, M.; Liese, A. Microbubble enhanced mass transfer efficiency of CO2 capture utilizing aqueous triethanolamine for enzymatic resorcinol carboxylation. RSC Adv. 2021, 11, 4087–4096. [Google Scholar] [CrossRef]
- Ohde, D.; Thomas, B.; Bubenheim, P.; Liese, A. Enhanced CO2 fixation in the biocatalytic carboxylation of resorcinol: Utilization of amines for amine scrubbing and in situ product precipitation. Biochem. Eng. J. 2021, 166, 107825. [Google Scholar] [CrossRef]
- Kertes, A.S.; King, C.J. Extraction chemistry of fermentation product carboxylic acids. Biotechnol. Bioeng. 1986, 28, 269–282. [Google Scholar] [CrossRef] [PubMed]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Pesci, L. Biocatalytic (de)carboxylation of Phenolic Compounds: Fundamentals and Applications. Ph.D. Thesis, TUHH Universitätsbibliothek, Hamburg, Germany, 2017. [Google Scholar]
- Ren, J.; Yao, P.; Yu, S.; Dong, W.; Chen, Q.; Feng, J.; Wu, Q.; Zhu, D. An Unprecedented Effective Enzymatic Carboxylation of Phenols. ACS Catal. 2016, 6, 564–567. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, J.; Yao, P.; Gong, R.; Wang, M.; Wu, Q.; Zhu, D. Biochemical characterization and substrate profiling of a reversible 2,3-dihydroxybenzoic acid decarboxylase for biocatalytic Kolbe-Schmitt reaction. Enzyme Microb. Technol. 2018, 113, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.-E.; Plasch, K.; Kragl, U.; von Langermann, J. Adsorbent-Based Downstream-Processing of the Decarboxylase-Based Synthesis of 2,6-Dihydroxy-4-methylbenzoic Acid. Org. Process Res. Dev. 2018, 22, 963–970. [Google Scholar] [CrossRef]
- Yoshida, M.; Fukuhara, N.; Oikawa, T. Thermophilic, reversible gamma-resorcylate decarboxylase from Rhizobium sp. strain MTP-10005: Purification, molecular characterization, and expression. J. Bacteriol. 2004, 186, 6855–6863. [Google Scholar] [CrossRef]
- Goto, M.; Hayashi, H.; Miyahara, I.; Hirotsu, K.; Yoshida, M.; Oikawa, T. Crystal structures of nonoxidative zinc-dependent 2,6-dihydroxybenzoate (gamma-resorcylate) decarboxylase from Rhizobium sp. strain MTP-10005. J. Biol. Chem. 2006, 281, 34365–34373. [Google Scholar] [CrossRef]
- Wuensch, C.; Glueck, S.M.; Gross, J.; Koszelewski, D.; Schober, M.; Faber, K. Regioselective enzymatic carboxylation of phenols and hydroxystyrene derivatives. Org. Lett. 2012, 14, 1974–1977. [Google Scholar] [CrossRef]
- Wuensch, C.; Gross, J.; Steinkellner, G.; Lyskowski, A.; Gruber, K.; Glueck, S.M.; Faber, K. Regioselective ortho-carboxylation of phenols catalyzed by benzoic acid decarboxylases: A biocatalytic equivalent to the Kolbe–Schmitt reaction: A biocatalytic equivalent to the Kolbe–Schmitt reaction. RSC Adv. 2014, 4, 9673. [Google Scholar] [CrossRef]
- Jain, J.S.; Snoeyink, V.L. Adsorption from Bisolute Systems on Active Carbon. J. Water Pollut. Control Fed. 1973, 45, 2463–2479. [Google Scholar]
- Butler, J.A.V.; Ockrent, C. Adsorption from Solutions containing Two Solutes. Nature 1930, 125, 853–854. [Google Scholar] [CrossRef]
- Sheindorf, C.; Rebhun, M.; Sheintuch, M. A Freundlich-type multicomponent isotherm. J. Colloid Interface Sci. 1981, 79, 136–142. [Google Scholar] [CrossRef]
- Digiano, F.A.; Baldauf, G.; Frick, B.; Sontheimer, H. A simplified competitive equilibrium adsorption model. Chem. Eng. Sci. 1978, 33, 1667–1673. [Google Scholar] [CrossRef]
- Fritz, W.; Schluender, E.-U. Simultaneous adsorption equilibria of organic solutes in dilute aqueous solutions on activated carbon. Chem. Eng. Sci. 1974, 29, 1279–1282. [Google Scholar] [CrossRef]
- Jaroniec, M.; Töth, J. Adsorption of gas mixtures on heterogeneous solid surfaces: I. Extension of Tóth isotherm on adsorption from gas mixtures. Colloid Polym. Sci. 1976, 254, 643–649. [Google Scholar] [CrossRef]
- Laun, H.M. Rheological properties of aqueous polymer dispersions. Angew. Makromol. Chem. 1984, 123, 335–359. [Google Scholar] [CrossRef]
- Willenbacher, N.; Georgieva, K. Rheology of Disperse Systems. In Product Design and Engineering: Formulation of Gels and Pastes; Bröckel, U., Meier, W., Wagner, G., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 7–49. ISBN 978-3-527-65477-2. [Google Scholar]
- Gustafson, R.L.; Albright, R.L.; Heisler, J.; Lirio, J.A.; Reid, O.T. Adsorption of Organic Species by High Surface Area Styrene-Divinylbenzene Copolymers. Ind. Eng. Chem. Prod. Res. Dev. 1968, 7, 107–115. [Google Scholar] [CrossRef]
- Dias Gomes, M.; Woodley, J.M. Considerations when Measuring Biocatalyst Performance. Molecules 2019, 24, 3573. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Paye, M.F. Stabilizing biocatalysts. Chem. Soc. Rev. 2013, 42, 6534–6565. [Google Scholar] [CrossRef]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Charm, S.E.; Wong, B.L. Shear effects on enzymes. Enzyme Microb. Technol. 1981, 3, 111–118. [Google Scholar] [CrossRef]
- D’Imprima, E.; Floris, D.; Joppe, M.; Sánchez, R.; Grininger, M.; Kühlbrandt, W. Protein denaturation at the air-water interface and how to prevent it. Elife 2019, 8, e42747. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hayakawa, Y.; Matsui, T.; Nagasawa, T. Purification and characterization of 2,6-dihydroxybenzoate decarboxylase reversibly catalyzing nonoxidative decarboxylation. Arch. Microbiol. 2004, 181, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Staby, A.; Jensen, I.H.; Mollerup, I. Comparison of chromatographic ion-exchange resins. J. Chromatogr. A 2000, 897, 99–111. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Drews, A.; Kraume, M. Enzymatic membrane reactors: Designs, applications, limitations and outlook. Chem. Eng. Process. 2022, 180, 108729. [Google Scholar] [CrossRef]
- Ohde, D.; Thomas, B.; Matthes, S.; Percin, Z.; Engelmann, C.; Bubenheim, P.; Terasaka, K.; Schlüter, M.; Liese, A. Fine Bubble-based CO2 Capture Mediated by Triethanolamine Coupled to Whole Cell Biotransformation. Chem. Ing. Tech. 2019, 91, 1822–1826. [Google Scholar] [CrossRef]
- Keller, J.U.; Staudt, R. Gas Adsorption Equilibria: Experimental Methods and Adsorptive Isotherms; Springer: New York, NY, USA, 2005; ISBN 0387235981. [Google Scholar]
- Atkins, P.W. Physical Chemistry, 6th ed.; reprint; Oxford Univ. Press: Oxford, UK, 2001; ISBN 0198501013. [Google Scholar]



| Extended Langmuir isotherms | ||||||||
| Jain and Snoeyink (1973) [21] | Butler and Ockrent (1930) [22] | Single Solute | ||||||
| qm,1 | mol g−1 | 1.857 | qm,1 | mol g−1 | 1.838 | qm,1 | mol g−1 | 1.807 |
| qm,2 | mol g−1 | 1.806 | qm,2 | mol g−1 | 2.028 | qm,2 | mol g−1 | 1.946 |
| b1 | mM−1 | 0.597 | b1 | mM−1 | 0.589 | b1 | mM−1 | 0.560 |
| b2 | mM−1 | 0.038 | b2 | mM−1 | 0.032 | b2 | mM−1 | 0.026 |
| R2 | 0.989 | R2 | 0.989 | R2 | 0.959 | |||
| Extended Freundlich isotherms | ||||||||
| Sheindorf et al. (1981) [23] | DiGiano et al. (1978) [24] | Fritz and Schlünder (1974) [25] | ||||||
| K1 | 0.709 | K1 | 0.742 | b1,1 | 1.633 | |||
| K2 | 0.139 | K2 | 0.280 | b1,2 | 0.257 | |||
| n1 | 0.332 | n−1 | 2.959 | b2,1 | 1.180 | |||
| n2 | 0.577 | R2 | 0.978 | b2,2 | 0.214 | |||
| K1,2 | 0.044 | b2,2,1 | 1.880 | |||||
| R2 | 0.988 | b2,1,2 | 0.037 | |||||
| n1 | 0.911 | |||||||
| Extended Tóth isotherm | n2 | 0.996 | ||||||
| Jaroniec and Tóth (1976) [26] | m1 | 0.771 | ||||||
| qm | mol g−1 | 1.910 | m2 | 0.872 | ||||
| b1 | mM−1 | 1.685 | m1,2 | 0.991 | ||||
| b2 | mM−1 | 27.02 | m2,1 | 0.728 | ||||
| n | 0.985 | d1 | 1.217 | |||||
| b1,2 | 0.062 | d2 | 3.518 | |||||
| b2,1 | 16.03 | R2 | 0.989 | |||||
| R2 | 0.989 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohde, D.; Thomas, B.; Bubenheim, P.; Liese, A. Synthesis of 2,6-Dihydroxybenzoic Acid by Decarboxylase-Catalyzed Carboxylation Using CO2 and In Situ Product Removal. Processes 2024, 12, 10. https://doi.org/10.3390/pr12010010
Ohde D, Thomas B, Bubenheim P, Liese A. Synthesis of 2,6-Dihydroxybenzoic Acid by Decarboxylase-Catalyzed Carboxylation Using CO2 and In Situ Product Removal. Processes. 2024; 12(1):10. https://doi.org/10.3390/pr12010010
Chicago/Turabian StyleOhde, Daniel, Benjamin Thomas, Paul Bubenheim, and Andreas Liese. 2024. "Synthesis of 2,6-Dihydroxybenzoic Acid by Decarboxylase-Catalyzed Carboxylation Using CO2 and In Situ Product Removal" Processes 12, no. 1: 10. https://doi.org/10.3390/pr12010010
APA StyleOhde, D., Thomas, B., Bubenheim, P., & Liese, A. (2024). Synthesis of 2,6-Dihydroxybenzoic Acid by Decarboxylase-Catalyzed Carboxylation Using CO2 and In Situ Product Removal. Processes, 12(1), 10. https://doi.org/10.3390/pr12010010