Research and Evaluation of Foam-Drainage Corrosion-Inhibition Hydrate Anti-Aggregation Integrated Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Surface-Tension Measurement
2.3. Foaming-Capacity Evaluation
2.4. Salt-Resistance Evaluation
2.5. Temperature Resistance
2.6. Methanol Effect Evaluation
2.7. Liquid-Carrying-Capacity Test
2.8. Adsorption Experiment
2.9. Surface Micromorphology of Steel Sheet
2.10. Micro-Morphology of Hydrates
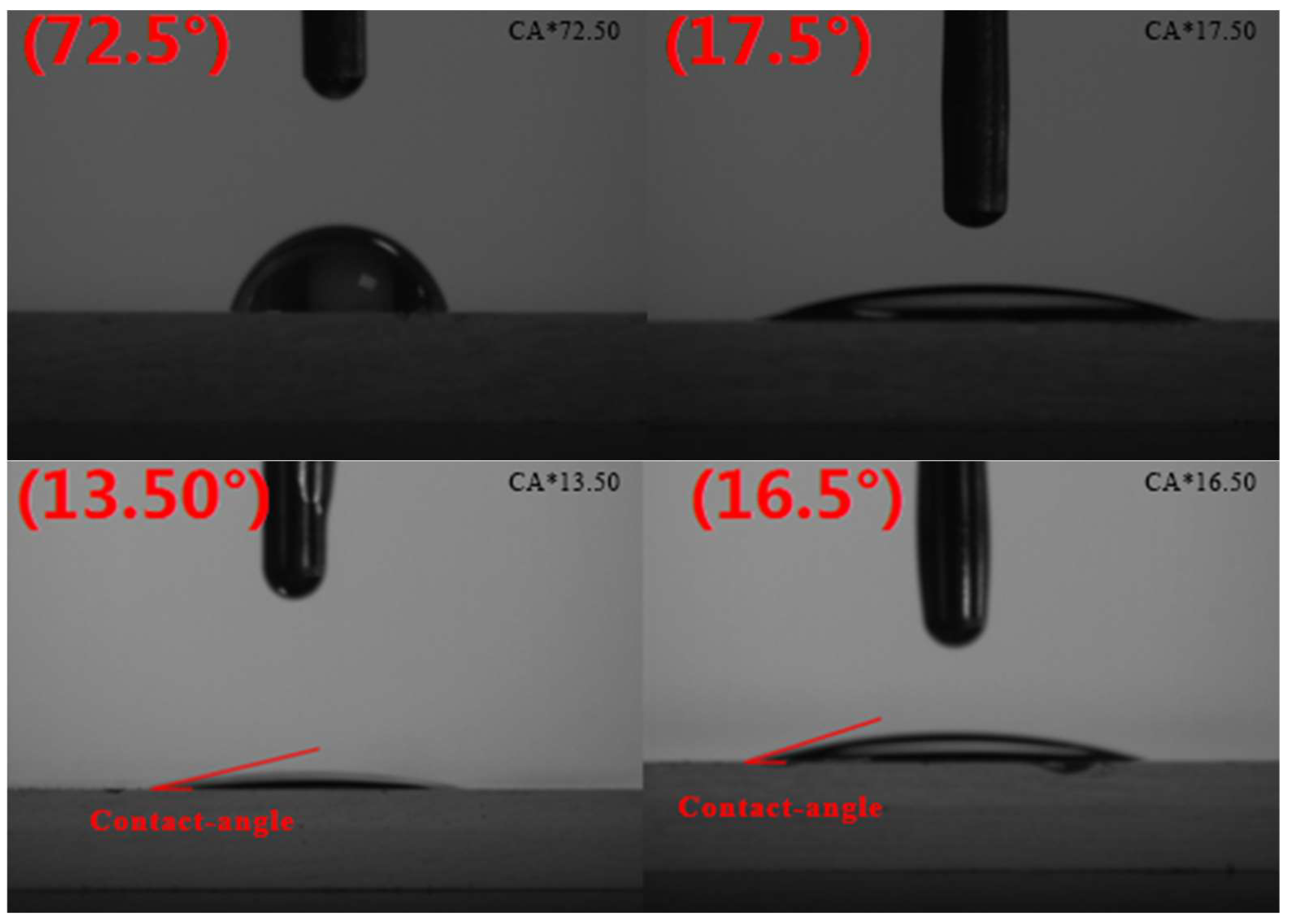
2.11. Contact-Angle Experiment
2.12. Thermodynamic Analysis of Hydrates
2.13. Emulsification Experiment and Microstructure Analysis after Emulsification
3. Results and Discussion
3.1. Measurement of Surface Tension
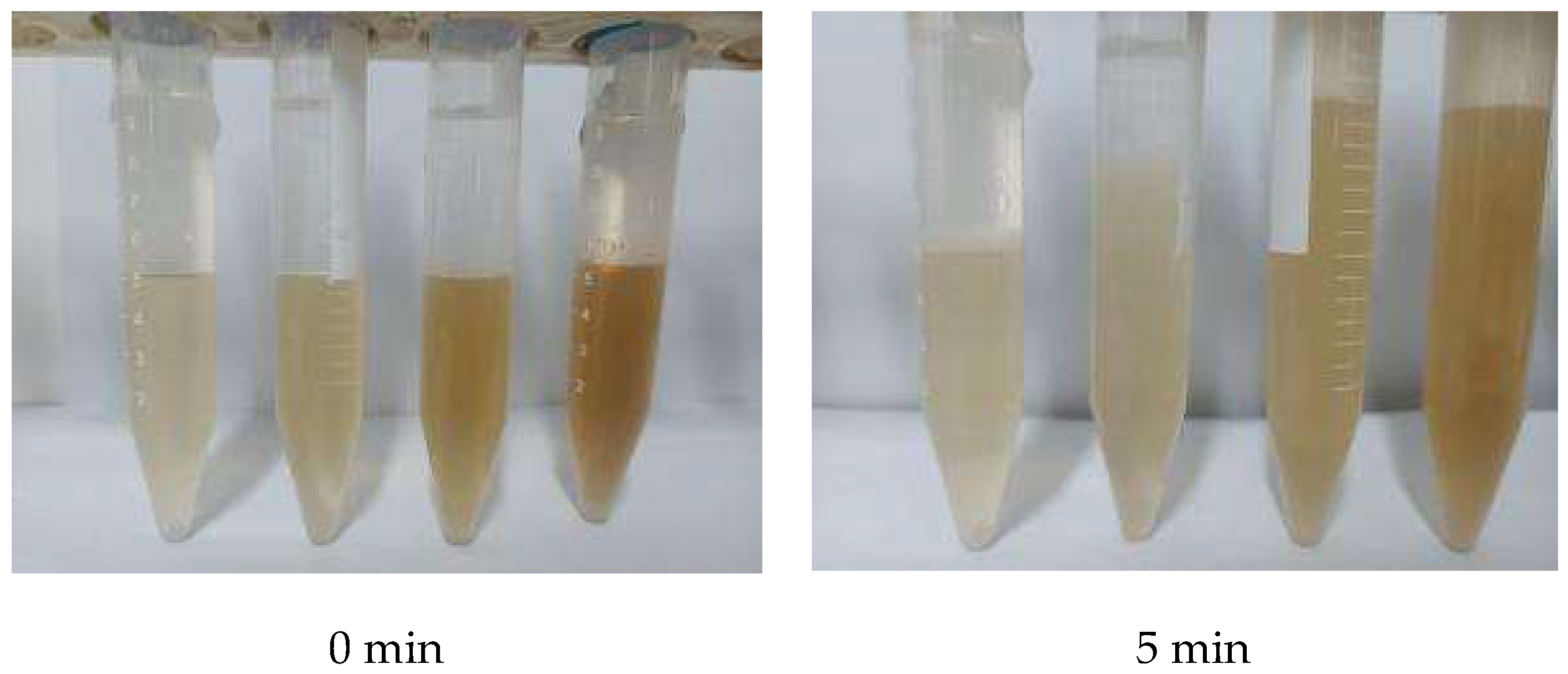
3.2. Mineral-Content Impact Test
3.3. Temperature-Resistance Evaluation
3.4. Methanol-Resistance Test
3.5. Analysis of Foam Microstructure
3.6. Liquid-Carrying Capacity
3.7. Viscoelasticity of Foam
3.8. Adsorption Experiment
3.9. Surface Micromorphology of Metal
3.10. Microscopic Morphology of Hydrate Growth
3.11. Contact-Angle Experiment
3.12. Thermodynamic Analysis of Hydrates
3.13. Emulsification Experiment and Microstructure Analysis after Emulsification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Wang, S.Z.; Dong, C.H. Experimental study on the emergency disposal agent for methanol leakage. Adv. Mat. Res. 2017, 1142, 306–313. [Google Scholar] [CrossRef]
- Gu, X.F.; Gao, L.; Li, Y.F.; Dong, K.; Zhang, J.; Du, W.C.; Qu, C.T.; Chen, G. Performance and mechanism of span surfactants as clean flow improvers for crude oil. Pet. Chem. 2020, 60, 140–145. [Google Scholar] [CrossRef]
- Behera, M.R.; Varade, S.R.; Ghosh, P.; Paul, P.; Negi, A.S. Foaming in micellar solutions: Effects of surfactant, salt, and oil concentrations. Ind. Eng. Chem. Res. 2014, 53, 18497–18507. [Google Scholar] [CrossRef]
- Qu, C.; Wang, J.; Yin, H.; Lu, G.; Li, Z.; Feng, Y. Condensate oil-tolerant foams stabilized by an anionic−sulfobetaine surfactant mixture. ACS Omega 2019, 4, 1738–1747. [Google Scholar] [CrossRef]
- Negm, N.A.; El Farargy, A.F.; Mohammed, D.E.; Mohamad, H.N. Environmentally friendly nonionic surfactants derived from tannic acid: Synthesis, characterization and surface activity. J. Surfactants Det. 2012, 15, 433–443. [Google Scholar] [CrossRef]
- Lee, J.J.; Nikolov, A.; Wasan, D. Surfactant micelles containing solubilized oil decrease foam film thickness stability. J Colloid Interface Sci. 2014, 415, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Ichinokawa, T.; Kaji, M.; Esumi, K. Synthesis and surface-active properties of sulfobetaine-type zwitterionic gemini surfactants. Colloids Surf. A 2006, 273, 208–212. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.; Li, Q.; Huang, H.; Ni, W.; Wang, Q.; Xin, X.; Zhao, B.; Chen, G. Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry. Molecules 2023, 28, 3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cheng, C.; Zhang, J.; Sun, Y.; Hu, Q.; Qu, C.T.; Dong, S.B. Synergistic effect of surfactant and alkali on the treatment of oil sludge. J. Petrol. Sci. Eng. 2019, 183, 106420. [Google Scholar] [CrossRef]
- Koczo, K.; Tselnik, O.; Falk, B. Silicon-based foamants for foam assisted lift of aqueous-hydrocarbon mixtures. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011; OnePetro: Richardson, TX, USA, 2011. [Google Scholar] [CrossRef]
- Chen, G.; Lin, J.; Liu, Q.N.; Zhang, J.; Wu, Y.; Li, H.; Ma, Y.; Qu, C.T.; Song, W.Q. Corrosion inhibition and the structure-efficiency relationship study of two cationic surfactants. Anti-Corros. Methods Mater. 2019, 66, 388–393. [Google Scholar] [CrossRef]
- Pavelyev, R.S.; Zaripova, Y.F.; Yarkovoi, V.V.; Vinogradova, S.S.; Razhabov, S.; Khayarov, K.R.; Nazarychev, S.A.; Stoporev, A.S.; Mendgaziev, R.I.; Semenov, A.P.; et al. Performance of Waterborne Polyurethanes in Inhibition of Gas Hydrate Formation and Corrosion: Influence of Hydrophobic Fragments. Molecules 2020, 25, 5664. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.A.; Hassan, M.K.; Al-Muhtaseb, A. Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques. Energies 2022, 15, 8551. [Google Scholar] [CrossRef]
- Mandal, S.; Bej, S.; Banerjee, P. Insights into the uses of two azine decorated d10-MOFs for corrosion inhibition application on mild steel surface in saline medium: Experimental as well as theoretical investigation. J. Mol. Liq. 2023, 381, 109846. [Google Scholar] [CrossRef]
- Raviprabha, K.; Bhat, R.S. Corrosion inhibition of mild steel in 0.5 M HCL by substituted 1,3,4-oxadiazole. Egypt. J. Pet. 2023, 32, 1–10. [Google Scholar] [CrossRef]
- Pesha, T.; Mulaudzi, V.L.; Cele, M.L.; Mothapo, M.P.; Ratshisindi, F. Evaluation of corrosion inhibition effect of glycerol stearate on aluminium metal by electrochemical techniques. Arab. J. Chem. 2023, 16, 104798. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, X.; Gao, K. Localized CO2 corrosion of carbon steel with different microstructures in brine solutions with an imidazoline-based inhibitor. Appl. Surf. Sci. 2018, 442, 446–460. [Google Scholar] [CrossRef]
- Christogonus, A.O.; Arinze, C.M. Experimental and DFT evaluation of adsorption and inhibitive properties of Moringa oliefera extract on mild steel corrosion in acidic media. Arab. J. Chem. 2020, 13, 9270–9282. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Dong, S.; Li, J.; Zhang, R.; Pu, C.; Chen, G. Effect of anion on the corrosion inhibition of cationic surfactants and a mechanism study. Desalination Water Treat. 2020, 188, 130–139. [Google Scholar] [CrossRef]
- Enabulele, D.O.; Bamigboye, G.O.; Solomon, M.M.; Durodola, B. Exploration of the Corrosion Inhibition Potential of Cashew Nutshell on Thermo-Mechanically Treated Steel in Seawater. Arab. J. Sci. Eng. 2022, 48, 223–237. [Google Scholar] [CrossRef]
- Hussain, H.H.; Husin, H. Review on Application of Quaternary Ammonium Salts for Gas Hydrate Inhibition. Appl. Sci. 2020, 10, 1011. [Google Scholar] [CrossRef]
- Paz, P.; Netto, T.A. On the Rheological Properties of Thermodynamic Hydrate Inhibitors Used in Offshore Oil and Gas Production. J. Mar. Sci. Eng. 2020, 8, 878. [Google Scholar] [CrossRef]
- Habib, S.; Nawaz, M.; Kahraman, R.; Ahmed, E.M.; Shakoor, R.A. Effect of the modified hybrid particle on the corrosion inhibition performance of polyolefin based coatings for carbon steel. J. Sci. Adv. Mater. Devices 2022, 7, 100466. [Google Scholar] [CrossRef]
- Xu, X.; Wei, H.Y.; Liu, M.G.; Zhou, L.S.; Shen, G.Z.; Li, Q.; Hussain, G.; Yang, F.; Fathi, R.; Chen, H.G.; et al. Nitrogen-doped carbon quantum dots for effective corrosion inhibition of Q235 steel in concentrated sulphuric acid solution. Mater. Today Commun. 2021, 29, 102872. [Google Scholar] [CrossRef]
- Ulhaq, M.I.; Saleem, Q.; Ajwad, H.; Aleisa, R.M.; Alanazi, N.M.; Leoni, M.; Zahrani, I.; Makogon, T. Corrosion inhibition of carbon steel in a sour (H2S) environment by an acryloyl-based polymer. ACS Omega 2023, 8, 18047–18057. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2009, 52, 198–204. [Google Scholar] [CrossRef]
- Jiang, L.L.; Xu, N.; Liu, Q.B.; Cheng, Z.C.; Liu, Y.; Zhao, J.F. Review of Morphology Studies on Gas Hydrate Formation for Hydrate-Based Technology. Cryst. Growth Des. 2020, 20, 8148–8161. [Google Scholar] [CrossRef]
- Zandi, M.S.; Hasanzadeh, M. The self-healing evaluation of microcapsule-based epoxy coatings applied on AA6061 Al alloy in 3.5% NaCl solution. Anti-Corros. Methods Mater. 2017, 64, 225–232. [Google Scholar] [CrossRef]
- Sliem, M.H.; El Basiony, N.M.; Zaki, E.G.; Sharaf, M.A.; Abdullah, A.M. Corrosion Inhibition of Mild Steel in Sulfuric Acid by a Newly Synthesized Schiff Base: An Electrochemical, DFT, and Monte Carlo Simulation Study. Electroanalysis 2020, 32, 3145–3158. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Ech-Chihbi, E.; Rezki, N.; Benhiba, F.; Taleb, M.; Chauhan, D.S.; Quraishi, M. Electrochemical and theoretical insights on the adsorption and corrosion inhibition of novel pyridinium-derived ionic liquids for mild steel in 1 M HCl. J. Mol. Liq. 2020, 314, 113737. [Google Scholar] [CrossRef]
- Navidfar, A.; Bulut, O.; Baytak, T.; Iskender, H.; Trabzon, L. Boosted viscoelastic and dynamic mechanical behavior of binary nanocarbon based polyurethane hybrid nanocomposite foams. J. Compos. Mater. 2022, 56, 2907–2920. [Google Scholar] [CrossRef]
- Alaboalirat, M.; Qi, L.; Arrington, K.J.; Qian, S.; Keum, J.K.; Mei, H.; Littrell, K.C.; Sumpter, B.G.; Carrillo, J.M.; Verduzco, R.; et al. Amphiphilic Bottlebrush Block Copolymers: Analysis of Aqueous Self-Assembly by Small-Angle Neutron Scattering and Surface Tension Measurements. Macromolecules 2019, 52, 465–476. [Google Scholar] [CrossRef]
- Dong, S.B.; Liu, C.W.; Han, W.W.; Li, M.Z.; Zhang, J.; Chen, G. The Effect of the Hydrate Antiagglomerant on Hydrate Crystallization at the Oil-Water Interface. ACS Omega 2020, 5, 3315–3321. [Google Scholar] [CrossRef]














| Concentration/% | 0.00001 | 0.0001 | 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | 0.8 | 1.2 |
| Tension value/mN/m | 32.6 | 31.3 | 30.1 | 26.1 | 25.4 | 23.5 | 23.9 | 24.7 | 24.3 | 24.8 |
| Concentration/% | 0.024 | 0.06 | 0.12 | 0.24 | 0.6 | 1.2 |
| Tension value/mN/m | 2.12 | 1.244 | 7.1 × 10−1 | 2.3 × 10−2 | 1.2 × 10−1 | 1.5 × 10−1 |
| Concentration of NaCl/% | Initial Height of the Foam/cm | Concentration of KCl/% | Initial Height of the Foam/cm | Concentration of CaCl2/% | Initial Height of the Foam/cm | Concentration of MgCl2/% | Initial Height of the Foam/cm |
|---|---|---|---|---|---|---|---|
| 0 | 21.3 | 0 | 21.3 | 0 | 21.3 | 0 | 21.3 |
| 2.5 | 16.7 | 2.5 | 17.8 | 2.5 | 8.3 | 2.5 | 14.8 |
| 5 | 15.6 | 5 | 18.0 | 5 | 5.9 | 5 | 10.1 |
| 10 | 12.3 | 10 | 17.5 | 10 | 4.2 | 10 | 6.6 |
| 20 | 3.9 | 20 | 8.7 | 20 | 2.5 | 20 | 3.2 |
| Temperature/°C | 0 min/cm | 5 min/cm | 10 min/cm | 15 min/cm | 20 min/cm |
|---|---|---|---|---|---|
| 30 | 21.3 | 21.7 | 21.7 | 21.3 | 21.0 |
| 40 | 21.5 | 22.0 | 21.9 | 20.5 | 20.3 |
| 50 | 21.9 | 22.3 | 20.0 | 13.8 | 7.9 |
| 60 | 22.3 | 22.9 | 12.5 | 7.8 | 5.4 |
| 70 | 22.6 | 23.6 | 7.3 | 3.9 | 3.0 |
| Concentration/% | 0 min/cm | 5 min/cm | 10 min/cm | 15 min/cm | 20 min/cm |
|---|---|---|---|---|---|
| 5 | 18.5 | 18.5 | 18.0 | 18.0 | 17.9 |
| 10 | 17.0 | 17.0 | 16.9 | 16.9 | 16.7 |
| 20 | 14.0 | 13.9 | 13.8 | 13.5 | 13.3 |
| 30 | 14.0 | 13.5 | 13.3 | 13.2 | 12.9 |
| Content | Changqing Gas Field (Yulin)/mL | Changqing Gas Field (Shenmu)/mL | One-Piece Agent/mL |
|---|---|---|---|
| Foam height (0 min) | 120 | 110 | 215 |
| Foam height (5 min) | 70 | 60 | 245 |
| Liquid-carrying capacity (0~15 min) | 120 | 110 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, W.; Yang, G.; Dong, J.; Pan, Y.; Chen, G.; Gu, X. Research and Evaluation of Foam-Drainage Corrosion-Inhibition Hydrate Anti-Aggregation Integrated Agent. Processes 2023, 11, 2745. https://doi.org/10.3390/pr11092745
Ni W, Yang G, Dong J, Pan Y, Chen G, Gu X. Research and Evaluation of Foam-Drainage Corrosion-Inhibition Hydrate Anti-Aggregation Integrated Agent. Processes. 2023; 11(9):2745. https://doi.org/10.3390/pr11092745
Chicago/Turabian StyleNi, Weijun, Guohao Yang, Jie Dong, Yansong Pan, Gang Chen, and Xuefan Gu. 2023. "Research and Evaluation of Foam-Drainage Corrosion-Inhibition Hydrate Anti-Aggregation Integrated Agent" Processes 11, no. 9: 2745. https://doi.org/10.3390/pr11092745
APA StyleNi, W., Yang, G., Dong, J., Pan, Y., Chen, G., & Gu, X. (2023). Research and Evaluation of Foam-Drainage Corrosion-Inhibition Hydrate Anti-Aggregation Integrated Agent. Processes, 11(9), 2745. https://doi.org/10.3390/pr11092745








