Oxidation-Induced Changes in the Lattice Structure of YSZ Deposited by EB-PVD in High-Vacuum Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results
4. Discussion
5. Conclusions
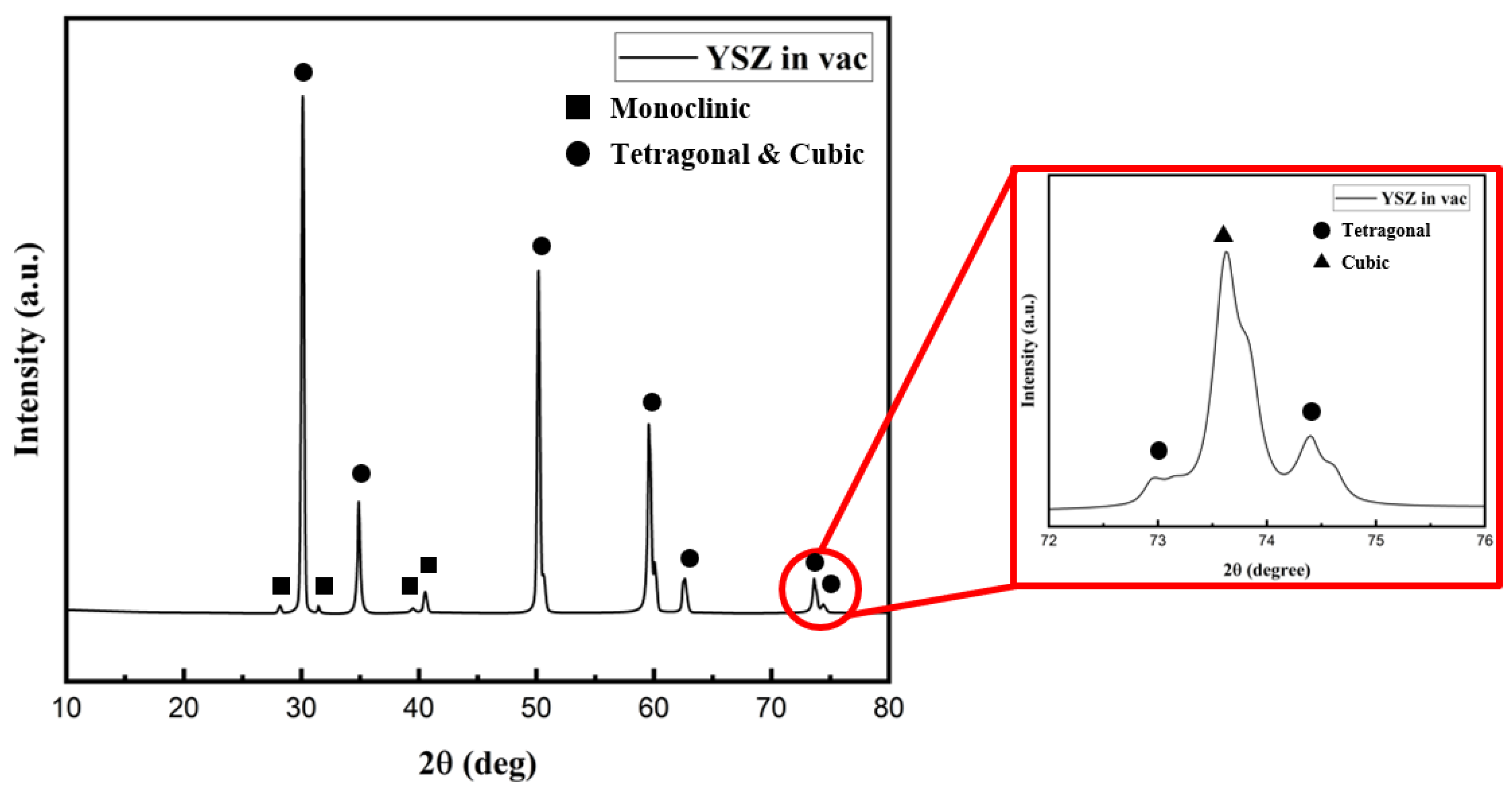
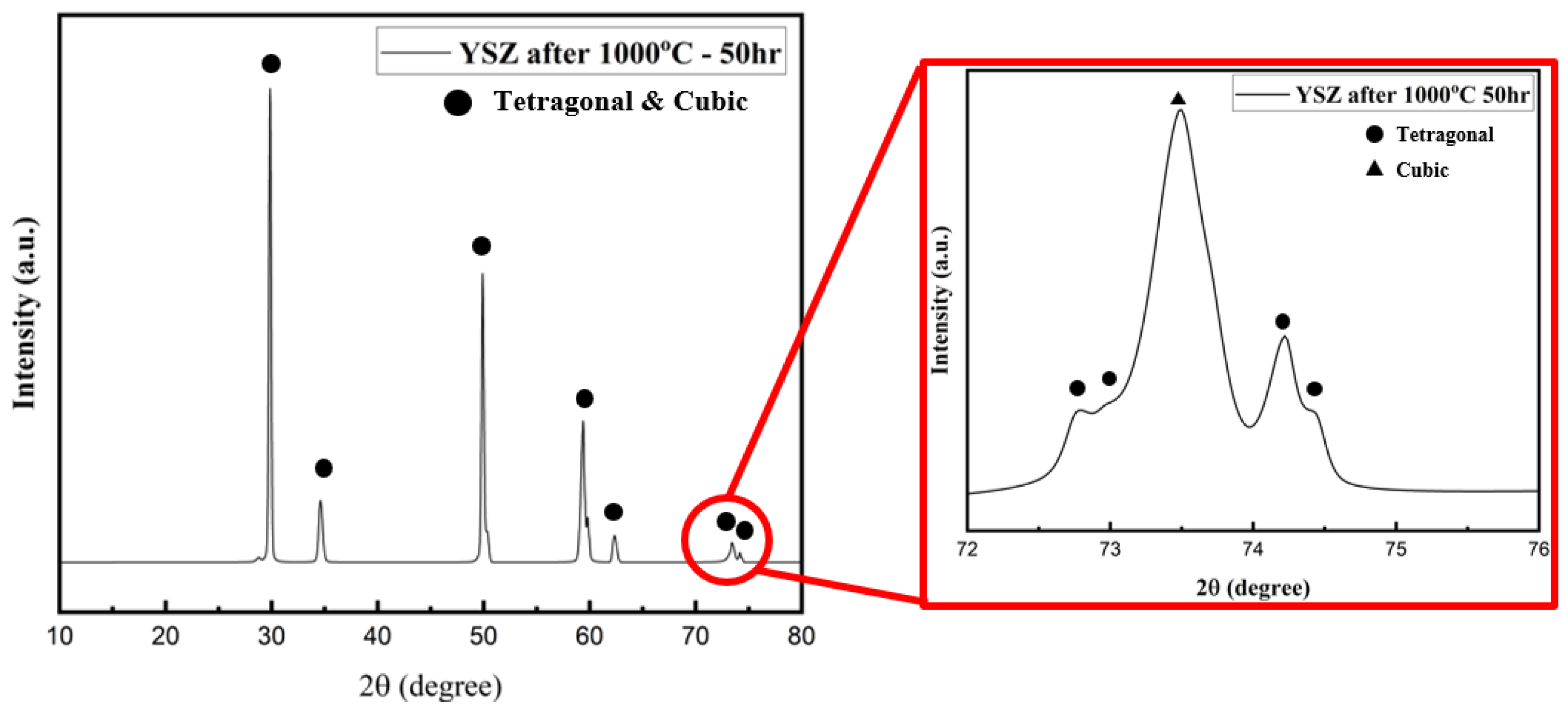
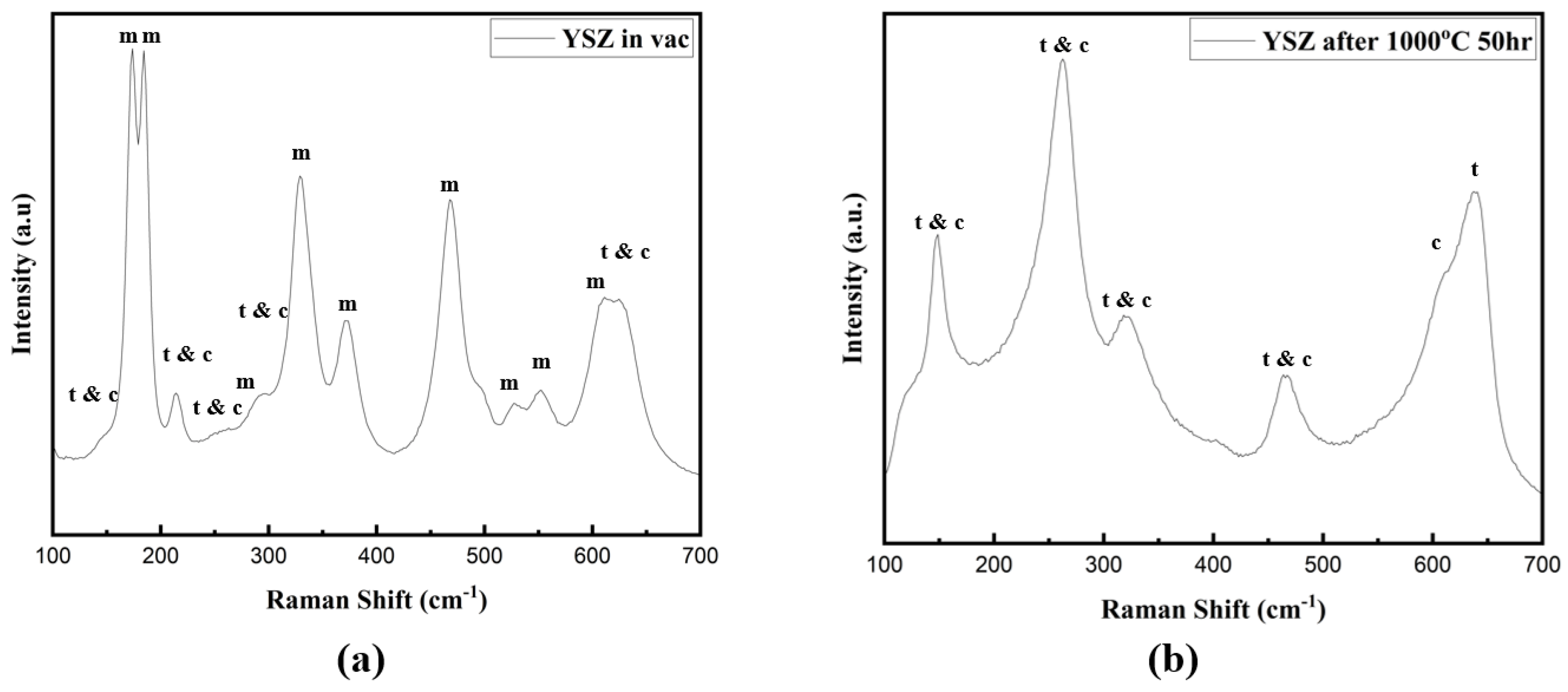
- Upon conducting a thorough analysis of the bulk YSZ sample before and after the vacuum heat treatment, distinct differences emerged. Initially, only the tetragonal phase was identified in the untreated sample. However, after subjecting it to a vacuum heat treatment, the sample exhibited the presence of not only a tetragonal phase but also monoclinic and cubic phases. Notably, variations in color were observed in various areas of the sample, with those highly influenced by the vacuum heat treatment atmosphere appearing darker (average L* of 64.93) and those less affected appearing relatively lighter (average L* of 39.5). Subsequently, through the oxidation heat treatment, the color of the bulk YSZ sample was restored to a light white hue. This treatment also led to the disappearance of the monoclinic phase of zirconia, indicating a transformation back to the original state. However, it is worth noting that the cubic phase formed during the vacuum heat treatment did not completely revert to the tetragonal phase.
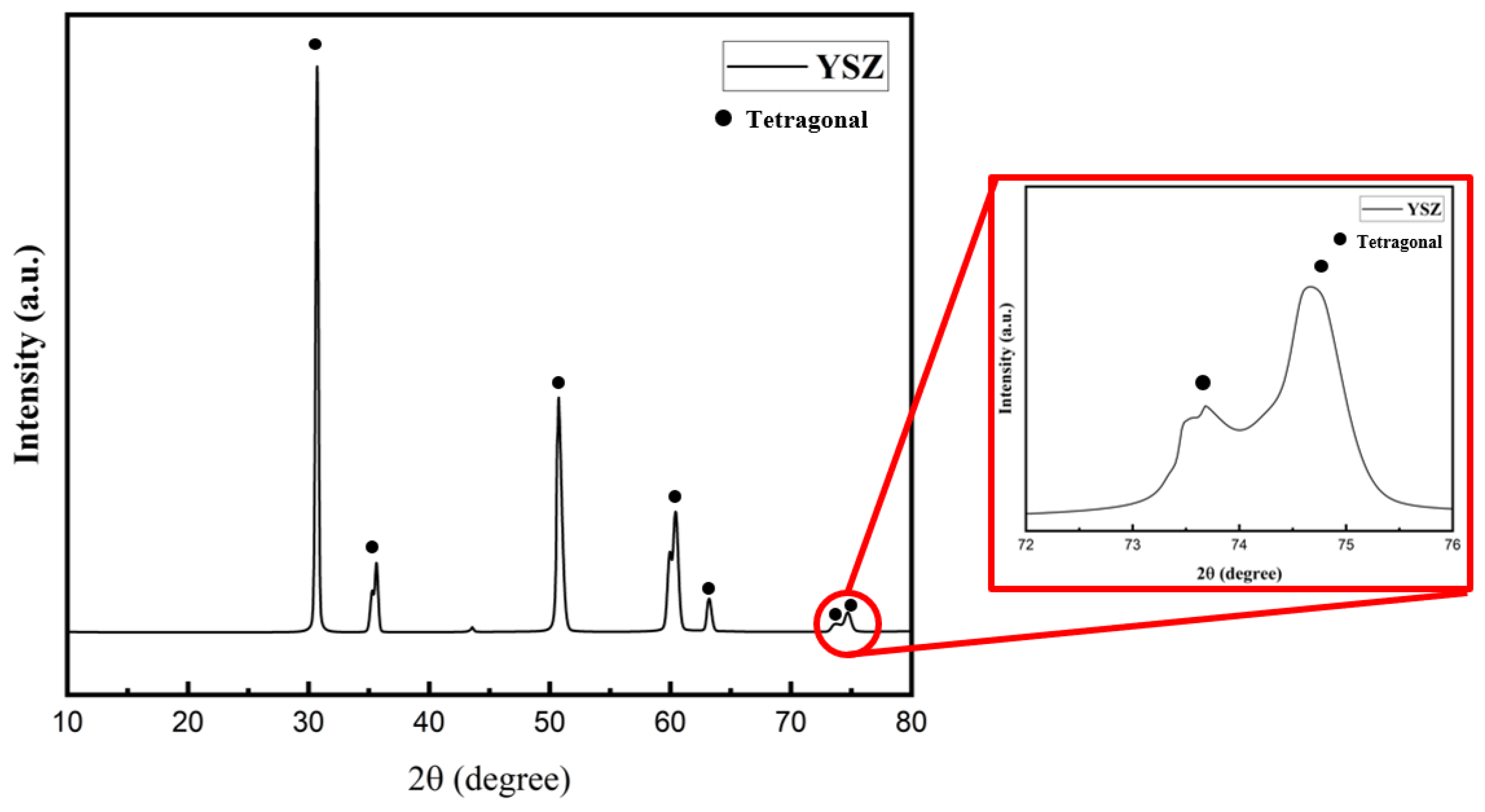
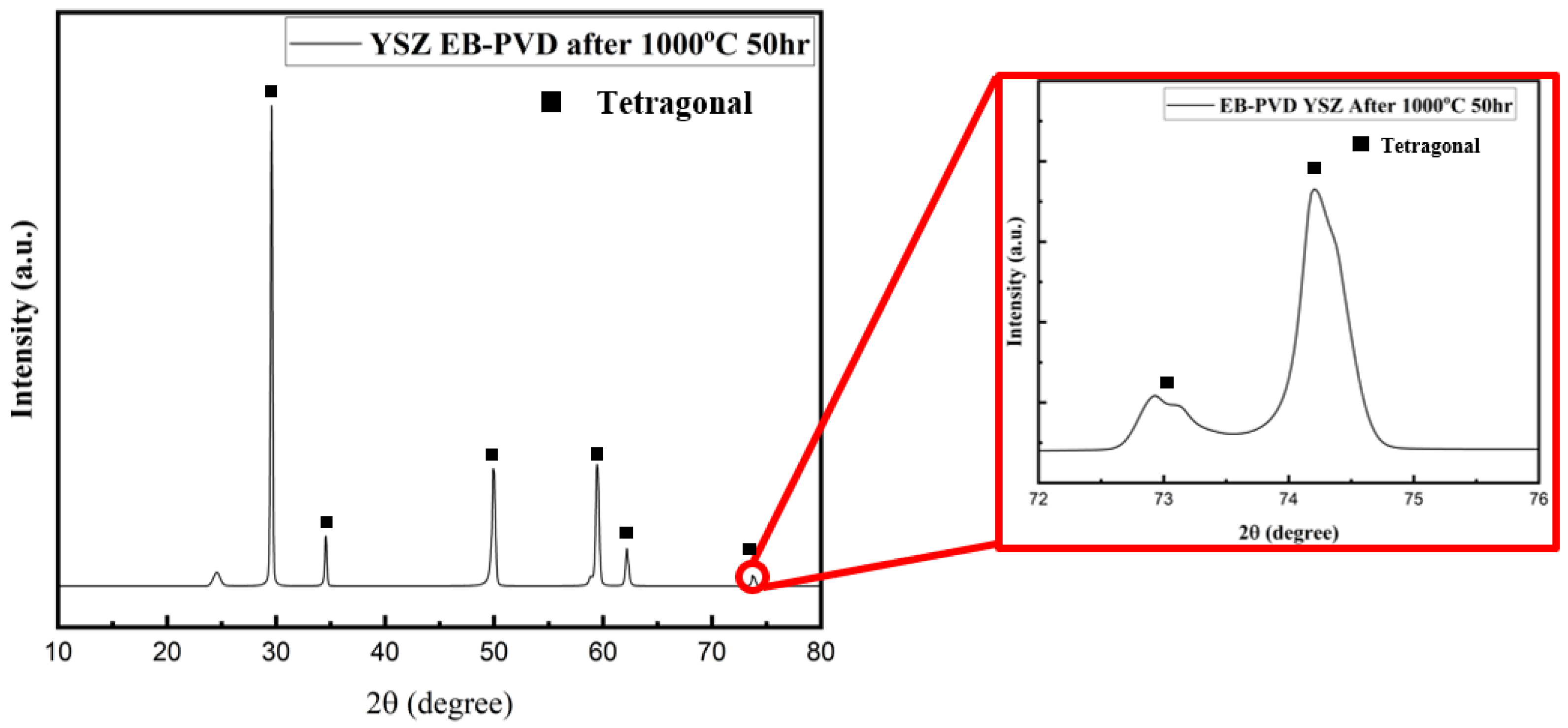
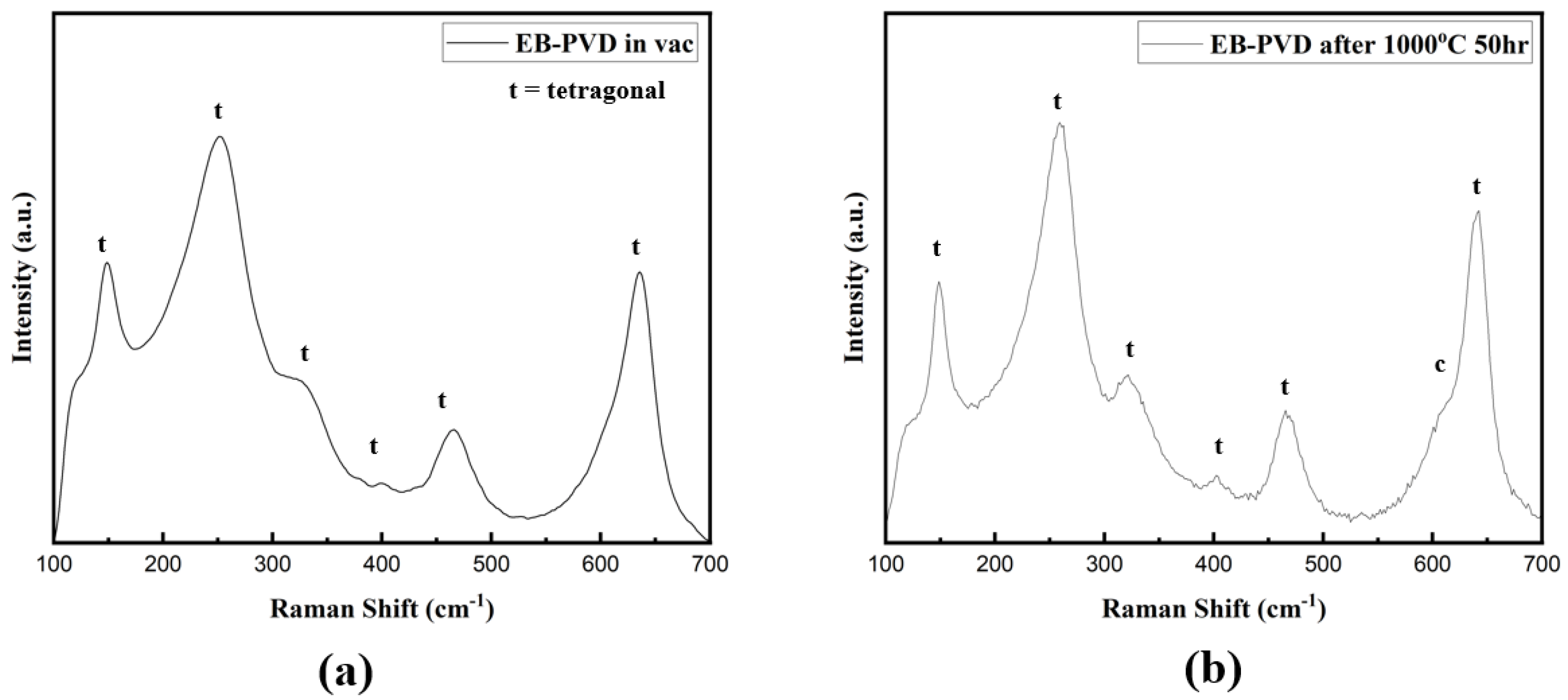
- Intriguingly, X-ray diffraction (XRD) analyses performed in the 72–76° range on the YSZ samples produced by EB-PVD in the high vacuum environment detected only the cubic phase. Conversely, Raman analysis confirmed the presence of the tetragonal phase, suggesting that the crystal structure of the YSZ sample deposited in this high vacuum atmosphere is in the t′ phase. Subsequent XRD and Raman analyses of EB-PVD samples after the oxidation heat treatment indicated the existence of only the tetragonal phase. This intriguingly suggests that even if the crystal structure of YSZ undergoes changes due to deposition in a high vacuum environment, it can be restored to its original phase through oxidation heat treatment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, R.A. Thermal barrier coatings for aircraft engines: History and directions. J. Therm. Spray Technol. 1997, 6, 35–42. [Google Scholar] [CrossRef]
- Xu, L.; Bo, S.; Hongde, Y.; Lei, W. Evolution of Rolls-Royce air-cooled turbine blades and feature analysis. Procedia Eng. 2015, 99, 1482–1491. [Google Scholar] [CrossRef]
- Younossi, O.; Arena, M.V.; Moore, R.M.; Lorell, M.; Mason, J.; Graser, J.C. Military Jet Engine Acquisition: Technology Basics and Cost-Estimating Methodology; Rand: Santa Monica, CA, USA, 2002. [Google Scholar]
- Toriz, F.C.; Thakker, A.B.; Gupta, S.K. Flight service evaluation of thermal barrier coatings by physical vapor deposition at 5200 H. Surf. Coat. Technol. 1989, 39–40, 161–172. [Google Scholar] [CrossRef]
- Rigney, D.V.; Viguie, R.; Wortman, D.J.; Skelly, D.W. PVD thermal barrier coating applications and process development for aircraft engines. J. Therm. Spray Technol. 1997, 6, 167–175. [Google Scholar] [CrossRef]
- Bobzin, K.; Bagcivan, N.; Brögelmann, T.; Yildirim, B. Influence of temperature on phase stability and thermal conductivity of single- and double-ceramic-layer EB–PVD TBC top coats consisting of 7YSZ, Gd2Zr2O7 and La2Zr2O7. Surf. Coat. Technol. 2013, 237, 56–64. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and economical aspects of current thermal barrier coating systems for gas turbine engines by thermal spray and EBPVD: A review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Huang, G.; Mu, R.; He, L. Thermal cycling behavior of EB-PVD TBCs on CVD platinum modified aluminide coatings. J. Alloys Compd. 2015, 637, 226–233. [Google Scholar] [CrossRef]
- Xu, Z.; He, L.; Mu, R.; Zhong, X.; Zhang, Y.; Zhang, J.; Cao, X. Double-ceramic-layer thermal barrier coatings of La2Zr2O7/YSZ deposited by electron beam-physical vapor deposition. J. Alloys Compd. 2009, 473, 509–515. [Google Scholar] [CrossRef]
- Guo, S.; Kagawa, Y. Isothermal and cycle properties of EB-PVD yttria-partially-stabilized zirconia thermal barrier coatings at 1150 and 1300 °C. Ceram. Int. 2007, 33, 373–378. [Google Scholar] [CrossRef]
- Audigié, P.; Put, A.R.-V.; Malié, A.; Thouron, C.; Monceau, D. High-temperature cyclic oxidation of Pt-rich γ-γ’bond-coatings. Part II: Effect of Pt and Al on TBC system lifetime. Corros. Sci. 2019, 150, 1–8. [Google Scholar] [CrossRef]
- Barjesteh, M.M.; Abbasi, S.M.; Zangeneh Madar, K.; Shirvani, K. Correlation between platinum–aluminide coating features and tensile behavior of nickel-based superalloy Rene® 80. Rare Met. 2019, 1–12. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Sarkar, R.; Jayaram, V.; Alam, M.Z. Fatigue behavior of a freestanding Pt-aluminide (PtAl) bond coat at ambient temperature. Surf. Coat. Technol. 2021, 427, 127787. [Google Scholar] [CrossRef]
- Lakiza, S.; Hrechanyuk, M.; Red’ko, V.; Ruban, O.; Tyshchenko, J.S.; Makudera, A.; Dudnik, O. The role of hafnium in modern thermal barrier coatings. Powder Metall. Met. Ceram. 2021, 60, 78–89. [Google Scholar] [CrossRef]
- Texier, D.; Monceau, D.; Selezneff, S.; Longuet, A.; Andrieu, E. High temperature micromechanical behavior of a pt-modified nickel aluminide bond-coating and of its interdiffusion zone with the superalloy substrate. Metall. Mater. Trans. A 2020, 51, 1475–1480. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, Z.; Huang, G.; Mu, R.; He, L.; Xu, Z. Thermal cycling of EB-PVD TBCs based on YSZ ceramic coat and diffusion aluminide bond coat. J. Alloys Compd. 2021, 873, 159720. [Google Scholar] [CrossRef]
- Viazzi, C.; Bonino, J.-P.; Ansart, F.; Barnabé, A. Structural study of metastable tetragonal YSZ powders produced via a sol–gel route. J. Alloys Compd. 2008, 452, 377–383. [Google Scholar] [CrossRef]
- Tsipas, S.A. Effect of dopants on the phase stability of zirconia-based plasma sprayed thermal barrier coatings. J. Eur. Ceram. Soc. 2010, 30, 61–72. [Google Scholar] [CrossRef]
- Krogstad, J.A.; Lepple, M.; Gao, Y.; Lipkin, D.M.; Levi, C.G.; Green, D.J. Effect of yttria content on the zirconia unit cell parameters. J. Am. Ceram. Soc. 2011, 94, 4548–4555. [Google Scholar] [CrossRef]
- Borik, M.A.; Bublik, V.T.; Vilkova, M.Y.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, P.O.; Myzina, V.A.; Ryabockina, P.A.; Tabachkova, N.Y.; Ushakov, S.N. Structure, phase composition and mechanical properties of ZrO2 partially stabilized with Y2O3. Mod. Electron. Mater. 2015, 1, 26–31. [Google Scholar] [CrossRef]
- Srinivasan, R.; De Angelis, R.J.; Ice, G.; Davis, B.H. Identification of tetragonal and cubic structures of zirconia using synchrotron X-radiation source. J. Mater. Res. 1991, 6, 1287–1292. [Google Scholar] [CrossRef]
- Muraleedharan, K.; Subrahmanyam, J.; Bhaduri, S.B. Identification of t’phase in ZrO2-7.5 wt% Y2O3 Thermal-Barrier Coatings. J. Am. Ceram. Soc. 1988, 71, C-226–C-227. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M.; Shoja Razavi, R.; Loghman-Estarki, M.R. Synthesis and characterization of non-transformable tetragonal YSZ nanopowder by means of Pechini method for thermal barrier coatings (TBCs) applications. J. Sol. Gel Sci. Technol. 2014, 70, 6–13. [Google Scholar] [CrossRef]
- Bakan, E.; Vaßen, R. Ceramic top coats of plasma-sprayed thermal barrier coatings: Materials, processes, and properties. J. Therm. Spray Technol. 2017, 26, 992–1010. [Google Scholar] [CrossRef]
- Dudnik, E.V.; Lakiza, S.N.; Hrechanyuk, I.N.; Ruban, A.K.; Redko, V.P.; Marek, I.O.; Shmibelsky, V.B.; Makudera, A.A.; Hrechanyuk, N.I. Thermal barrier coatings based on ZrO2 solid solutions. Powder Metall. Met. Ceram. 2020, 59, 179–200. [Google Scholar] [CrossRef]
- Yang, S. Multiscale modeling of chemo-thermo-mechanical damage of EB-PVD thermal barrier coatings. J. Mech. Phys. Solids 2022, 158, 104667. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Z.; Mu, R.; He, L.; Liu, G. Y–Er–ZrO2 thermal barrier coatings by EB-PVD: Thermal conductivity, thermal shock life and failure mechanism. Appl. Surf. Sci. Adv. 2021, 3, 100043. [Google Scholar] [CrossRef]
- Vasile, B.S.; Birca, A.C.; Surdu, V.A.; Neacsu, I.A.; Nicoară, A.I. Ceramic composite materials obtained by electron-beam physical vapor deposition used as thermal barriers in the aerospace industry. Nanomaterials 2020, 10, 370. [Google Scholar] [CrossRef]
- Singh, J.; Quli, F.; Wolfe, D.E.; Schriempf, J.T.; Singh, J. An Overview: Electron Beam-Physical Vapor Deposition Technology-Present and Future Applications; Applied Research Laboratory, Pennsylvania State University: University Park, PA, USA, 1999. [Google Scholar]
- Suk, O.Y.; Chae, J.-M.; Ryu, H.-l.; Han, Y.; Ahn, J.-K.; Son, M.-S.; Kim, H.-K. Deposition uniformity of 7 wt% YSZ as a thermal barrier coating with different configurational arrangement for turbine blade shape mock-up by electron beam physical vapor deposition. J. Korean Cryst. Growth Cryst. Technol. 2019, 29, 308–316. [Google Scholar]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Donovan, T.E.; Vallittu, P.K.; Narhi, T.O.; Lassila, L.V. The effect of staining and vacuum sintering on optical and mechanical properties of partially and fully stabilized monolithic zirconia. Dent. Mater. J. 2015, 34, 605–610. [Google Scholar] [CrossRef]
- Borik, M.A.; Chislov, A.S.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Ryabochkina, P.A.; Sidorova, N.V.; Tabachkova, N.Y. Effect of heat treatment on the structure and mechanical properties of partially gadolinia-stabilized zirconia crystals. J. Asian Ceram. Soc. 2021, 9, 559–569. [Google Scholar] [CrossRef]
- Orera, V.M.; Merino, R.I.; Peña, F. Ce3+ ↔ Ce4+ conversion in ceria-doped zirconia single crystals induced by oxido-reduction treatments. Solid State Ion. 1994, 72, 224–231. [Google Scholar] [CrossRef]
- Orera, V.M.; Merino, R.I.; Chen, Y.; Cases, R.; Alonso, P.J. Intrinsic electron and hole defects in stabilized zirconia single crystals. Phys. Rev. B Condens. Matter 1990, 42, 9782–9789. [Google Scholar] [CrossRef] [PubMed]
- Seok, C.Y.; Gye Won, L.; Woo, J.C.; Sahn, N.; Suk, O.Y. Analysis of phase formation behavior of YSZ-based composites according to rare earth and other oxide doping amounts. J. Surf. Sci. Eng. 2022, 55, 368–375. [Google Scholar]



| Material Group | Example |
|---|---|
| RE-doped ZrO2 | Nd2O3-Yb2O3-Y2O3-ZrO2 Yb2O3-Gd2O3-Y2O3-ZrO2 |
| Perovskites | BaZrO3 SrZrO3 CaZrO3 |
| Hexa-aluminates | LaMgAl11O19 GdMgAl11O19 |
| Pyrochlores | La2Zr2O7 Nd2Zr2O7 Sm2Zr2O7 Eu2Zr2O7 Gd2Zr2O7 |
| L* Result Number | Sample Top Surface | Sample Bottom Surface |
|---|---|---|
| 1 | 40.72 | 64.5 |
| 2 | 39.16 | 64.93 |
| 3 | 38.62 | 65.36 |
| Average | 39.5 | 64.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-W.; Lee, I.-H.; Oh, Y.-S. Oxidation-Induced Changes in the Lattice Structure of YSZ Deposited by EB-PVD in High-Vacuum Conditions. Processes 2023, 11, 2743. https://doi.org/10.3390/pr11092743
Lee G-W, Lee I-H, Oh Y-S. Oxidation-Induced Changes in the Lattice Structure of YSZ Deposited by EB-PVD in High-Vacuum Conditions. Processes. 2023; 11(9):2743. https://doi.org/10.3390/pr11092743
Chicago/Turabian StyleLee, Gye-Won, In-Hwan Lee, and Yoon-Suk Oh. 2023. "Oxidation-Induced Changes in the Lattice Structure of YSZ Deposited by EB-PVD in High-Vacuum Conditions" Processes 11, no. 9: 2743. https://doi.org/10.3390/pr11092743
APA StyleLee, G.-W., Lee, I.-H., & Oh, Y.-S. (2023). Oxidation-Induced Changes in the Lattice Structure of YSZ Deposited by EB-PVD in High-Vacuum Conditions. Processes, 11(9), 2743. https://doi.org/10.3390/pr11092743







