1. Introduction
Rice (
Oryza sativa L.) constitutes a fundamental food product for almost half of the world’s population mainly due to its nutritive properties and low cost [
1,
2,
3]. It is a complex matrix composed of carbohydrates, proteins, fibers, vitamins, and numerous essential elements including Ca, Cu, Fe, Mg, Mn, and Zn [
3,
4,
5]. Due to its composition, rice is not only a popular food product but also a promising ingredient in skin or haircare products and cosmetics [
6,
7]. Moreover, it can also be applied as a source of plant nutrients [
8]. Truly, this is not surprising because the healing/medicinal properties of rice have been known for centuries. Components of rice like phenolic compounds, betaine, squalene, tricin, and rice bran are recognized to have anti-aging, anti-inflammatory, whitening, photoprotective, and moisturizing properties [
6,
7]. Additionally, rice-derived ingredients are demonstrated to possess a variety of dermatological benefits [
7]. For example, rice starch (the main ingredient of rice grains) is recommended to be added to the bath to repair the skin barrier and alleviate atopic dermatitis symptoms [
9].
One of the oldest rice-based natural products used as a cosmetic is rice water (RW), i.e., a milky-colored suspension of starch obtained by draining boiled rice or boiling rice until it completely dissolves in water [
10]. Rice water is also called water obtained by rinsing/washing rice before cooking or soaking its grains in cold or hot water [
6,
10]. It can be obtained from various types of commonly consumed rice (white and brown) but also from rice residues generated in the rice industry [
6]. Therefore, it means that RW is a food processing waste, normally discarded during the raw rice preparation before its consumption. It appears that this waste can have several useful benefits, e.g., as a medicine (it may work as a digestible healthy drink for diarrhea patients), skincare (helps to create fair, smooth, and toned skin), or protective agent against dandruff-causing fungi [
10,
11]. Lately, the biological (anti-aging and anti-oxidant) properties of RW have been proven; therefore, its usage as an ingredient in skincare cosmetics should be considered [
6]. It is also worth mentioning that RW was used in daily haircare treatment in medieval Japan [
12]. It was considered “a key component” responsible for increasing hair elasticity, reducing surface friction, moisturizing the scalp, adding some volume and shine, and strengthening the hair in general [
12]. Interestingly, the effect of such water on skin and hair can be improved by using fermented RW, i.e., RW left to ferment. The fermentation process lowers the pH of such RW to make it slightly acidic and close to the skin and hair pH. Hence, RW processed in this way may be successfully used as a face care, skin tonic, or hair conditioner [
13]. In view of this, it is not surprising that there is a growing interest in the use of RW by cosmetics manufacturers.
Among various ingredients extracted from rice grains during their washing/rinsing/soaking/boiling, there are also minerals. It was confirmed in several works focusing on the removal of essential elements from various types of rice during their pre-cooking and cooking with different methods [
14,
15,
16,
17,
18,
19]. On the other hand, these processes could be beneficial in eliminating non-essential/toxic elements (As, Cd, and Pb) accumulated in rice grains [
20,
21,
22,
23]. Hence, one should be aware that the traditional rice preparation prior to its consumption can affect the concentration of various elements in cooked rice. In this regard, there is much interest in rice impurities, namely As, which the rice plant can easily accumulate in the grains [
24]. The concentration of As in rice is generally an order of magnitude greater than in other cereal crops [
24]. The best way to reduce As in rice (and in rice-containing dishes) is to properly wash/rinse and cook it in excess water (mainly 6:1, water: rice), which should be discarded at the end [
20,
21,
22]. Paradoxically, this post-process water is this precious RW, whose beneficial properties were described above. Unfortunately, there are no studies on the mineral composition, i.e., the content of essential and toxic elements (especially As), in RW. The papers published so far have been focused only on the physico-chemical properties of a different kind of home-made (natural) RW and its effect on various ailments [
6,
7,
10,
12].
Therefore, to fill the gap associated with the lack of comprehensive information about the mineral composition of differently prepared RW, this work was aimed at answering the question: is the use of natural RW as a cosmetic product and health-promoting drink safe for health, and does it pose a risk of As poisoning or not? To carry out this experiment, five procedures were applied to prepare natural, home-made RW (RWMs) including washing/rinsing, soaking, boiling, and fermentation steps. The three most popular types of rice, i.e., white (WR), brown (BR), and jasmine (JR), of two brands were used to produce these RWMs. Next, all of them were analyzed on the total content of both essential and non-essential elements (Al, As Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Zn) by using ICP OES after the wet sample decomposition in the presence of concentrated HNO3. In the case of As, the hydride generation (HG) technique was used as a sample introduction system for the ICP OES instrument. The corresponding As hydride was generated in the reaction with sodium borohydride (NaBH4) and HCl after the pre-reduction of As(V) to As(III) using a KI-ascorbic acid-HCl mixture. The advantages (the As content reduction) and disadvantages (the reduction in the content of the essential elements) of the tested procedures were pointed out to find the optimal procedure. Additionally, the quality (in terms of the elemental composition) of RWMs was compared with that assessed for commercialized rice-based cosmetic products (RWPs) for skin/haircare.
2. Materials and Methods
2.1. Samples
Three different types of conventional rice, i.e., white (WR), brown (BR), and jasmine (JR), of two brands, packed in 4 × 100 g bags, commercially available and sold in Poland, were bought in a local market (Wroclaw). In total, six (n = 6) rice samples were selected and coded as WR1 and WR2 (for WR), BR1 and BR2 (for BR), and JR1 and JR2 (for JR). The analyzed rice originated from Mjanma/Birma (WR1), Birma (WR2), Vietnam (BR1, BR2), Greece (RJ1), and Paraguay (RJ2). For each type of rice, three different packages of the same product series were purchased and mixed before the analysis. Hence, the total amount of each respective rice type was about 1.2 kg. Prior to the sampling (the case of the total element content determination), portions of rice were homogenized to a fine powder using an electric mill and then kept in closed plastic containers in the dark.
Additionally, four different RWPs were also analyzed. These were a rice face toner for stabilizing and nourishing (RWP1), rice micellar water for removing make-up and cleansing (RWP2), a rice water make-up remover (RWP3), and a concentrated bio-fermented rice extract for the skin, hair, and body care (RWP4). Importantly, all of these products contained natural rice extract (the rice ferment filtrate (sake)) and/or rice seed protein. A standard reference material (SRM), 156b Rice Flour from NIST, was used to verify the trueness of the results of the multi-element analysis obtained through ICP OES as well as the determination of the total content of As by using HG-ICP OES, both after the microwave-assisted (MW) wet digestion. To express the results on the dry weight basis, the SRM, according to the manufacturer’s recommendation, was dried to a constant weight at 105 °C, employing thermogravimetric measurements (a halogen moisture analyzer was used).
2.2. Reagents and Solutions
All reagents used were of analytical grade or better. A 65% (m/v) HNO3 solution from Merck (Merck, KGaA, Darmstadt, Germany) was used for the MW wet digestion. A Merck Certipur® multi-elemental stock (100 mg L−1) ICP stock solution No. XVI was used for the calibration of the ICP OES instrument. Single-element stock solutions (1000 mg L−1) of As(III) and As(V) from Inorganic Ventures (Christiansburg, VA, USA) and Merck, respectively, were used to determine the As content by using HG-ICP OES. Working standard solutions were prepared by using stepwise dilutions of the stock standards with deionized water. To acidify the sample and standard solutions (to 3 mol L−1, S) and prepare an additional acid solution (10 mol L−1, A) in the case of the HG reaction, a 37% (m/v) HCl solution from Sigma-Aldrich (St. Louis, MO, USA) was used. Both L(+)-ascorbic acid (AA) and potassium iodide (KI) from Avantor Performance Reagents (Gliwice, Poland) served as pre-reducing agents for As(V). A mixed concentrated solution of these two pre-reducing agents, i.e., 2.5% (m/v) KI-10.0% (m/v) AA, was prepared by dissolving the respective solid reagents in deionized water. To neutralize residual HNO3 after the wet digestion, hydroxylamine hydrochloride (HH) was employed. A 40% (m/v) aqueous solution of HH was made from its solid reagent (Avantor Performance Reagents). A 1.0% (m/v) NaBH4 (stabilized with a 0.10% (m/v) NaOH) reductant (R) solution was used to generate arsenic hydride (AsH3). The R solution was prepared before the measurements by dissolving an appropriate amount of powdered NaBH4 (Sigma-Aldrich) in a NaOH solution (Sigma-Aldrich) and filtering through a hard filter paper (type: 3H, Ahlstrom & Munktell, Bärenstein, Germany). Deionized water (18.3 MΩ cm−1), from a Barnstead™ (Boston, MA, USA) EASYpure RF purification system (model D7033), was used throughout.
2.3. Instrumentation
All measurements were made using the Agilent (Agilent Technologies Inc., Santa Clara, CA, USA) simultaneous ICP OES instrument of the axially viewed Ar plasma, model 720. The spectrometer was equipped with a 4-channel peristaltic pump, a high-resolution Echelle-type polychromator with temperature-controlled optics, a VistaChip II CCD detector, and a one-piece quartz torch (2.4 mm ID injector tube) for sustaining the plasma. Sample and standard solutions were introduced using a OneNeb
® pneumatic concentric nebulizer (Agilent Technologies Inc., Santa Clara, CA, USA) and a single-pass glass cyclonic spray chamber (Agilent). In the case of the As determination, the continuous flow HG technique with gas–liquid phase separation was applied. The HG system consisted of a modified cyclonic spray chamber that acted as a gas–liquid phase separator; the OneNeb
® nebulizer; two Y-shaped polypropylene (PP) connectors (3.0 mm ID) to mix the S and A solutions (1) and then the acidified S solution with the R solution (2); appropriate delivery PVC pump tubing for the S, A, and R solutions and wastes; mixing/reaction coil tubing, i.e., a PTFE capillary tubing (15 cm long × 0.5 mm ID) and a reaction coil (a PTFE capillary tubing (5 cm long × 0.5 mm ID)); and an additional peristaltic pump to drain the resulting post-reaction solution. Optimized working parameters for the HG reaction and the ICP OES detection are listed in
Table S1. To calculate the concentrations of elements in analyzed sample solutions, the background-corrected net intensities (I
net) of the As, Al, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Zn emission analytical lines were used. Every time, the acquired line intensity was a mean of 3–5 repeated measurements (
n = 3–5). To obtain I
nets, a fitted background mode with 7 points per emission line profile was applied to obtain the background intensity at the analyte wavelength, which was then subtracted from the acquired analyte signals.
All samples were digested with the aid of a Multiwave PRO microwave reaction system (Anton Paar GmbH, Graz, Austria), equipped with a 24HVT50 rotor and 50 mL PTFE-TFM pressure-activated-venting vessels. A halogen moisture analyzer (type HE53) from Mettler Tolledo (Greifensee, Switzerland) was used to measure the moisture content of the SRM samples.
2.4. Experimental Procedures
The MW closed-vessel wet digestion with concentrated HNO3 was applied before the determination of total As and other elements by using HG- and PN-ICP OES, respectively, in rice (solid) and RW (liquid) samples. In the case of the latter samples, these analyses included both home-made (RWMs) and commercial (RWPs) products. RWMs were prepared for all studied rice samples according to five different procedures.
2.4.1. Total Content of Elements—Microwave-Assisted Closed-Vessel Wet Digestion
Appropriate portions of samples, i.e., 0.5 g of milled rice, 10.0 mL (RWM), or 5.0 mL (RWP), were weighed into PTFE vessels and treated with 6.0 mL (rice) or 5.0 mL (RWM, RWP) of concentrated HNO3. Next, the vessels were closed, shielded, inserted into the rotor, and subjected to a 7-step MW heating program with a maximum temperature of 190 °C for 60 min as follows. Step 1: 90 °C (5 min, ramp); step 2: 90 °C (15 min, hold); step 3: 130 °C (5 min, ramp); step 4: 130 °C (15 min, hold); step 5: 190 °C (5 min, ramp); step 6: 190 °C (15 min, hold); and step 7: 55 °C (cooling). Afterward, the resultant sample digests were allowed to cool down to room temperature then quantitatively transferred into 30 mL PP screw-capped containers (Equimed, Kraków, Poland), diluted with deionized water to 20.0 g (rice, RWM) or 15.0 g (RWP), mixed, and kept at 4 °C until the HG-ICP and PN-ICP OES analyses.
2.4.2. Home-Made Rice Water Preparation
Five different procedures were used for the preparation of RWMs, including washing/rinsing, soaking, boiling, and fermentation steps.
No. 1—Water resulting from washing/rinsing rice with cold water (RWM1): A 100 g portion of rice grains, weighed into a glass beaker (1 L), was poured with 600 mL of cold (room temperature) deionized water and stirred for about 3 min. Finally, the resultant RWM1 was separated from the rice grains using a plastic sieve and collected into a clean beaker.
No. 2—Water resulting from soaking rice in cold water (RWM2): A portion of 100 g of pre-washed rice grains (see No. 1) was mixed with 600 mL of cold (room temperature) deionized water and left for 30 min for soaking. Afterward, the resultant RWM2 was drained into a clean beaker.
No. 3—Water resulting from soaking rice in hot water (instant water, RWM3): A 100 g portion of pre-washed rice grains (see No. 1) was mixed with 600 mL of hot deionized water (90 °C) and soaked for 15 min, stirring every 5 min. After this time, the resultant RWM3 was separated from the rice grains into a clean beaker and cooled to room temperature.
No. 4—Water resulting from boiling rice in water (boiled water, RWM4): A 100 g portion of pre-washed rice grains (see No. 1) was mixed with 600 mL of cold (room temperature) deionized water and then brought to a boil on an electric heating plate. The mixture was stirred from time to time to prevent sticking of the rice grains to the bottom of the beaker. The beaker was removed from the hot plate after ~30 min, i.e., when the water began to boil. Next, the rice grains were drained, and the resulting RWM4 was cooled to room temperature.
No. 5—Water resulting from boiling rice in water and the fermenting process (fermented water, RWM5): In the beginning, the preparation of this RWM proceeded as above (No. 4). After cooling, the resultant RWM4 was sealed in the beaker and left for 48 h at room temperature to ferment, giving RWM5.
Samples were analyzed in triplicate (n = 3). To avoid differences in densities, all sample solutions were prepared using the weight method. With each set of digested solid and liquid samples, respective procedural blanks were simultaneously prepared and considered in the final results. In the case of liquid samples, appropriate portions of deionized water instead of RWM or RWP were used when preparing the above-mentioned procedural blanks. These procedural blanks were also used to prepare the matrix-matched standard solutions, which were used to measure the studied elements by using PN-ICP OES and As by using HG-ICP OES. The concentration of HNO3 in these blanks was 3.5 (RWM), 4.2 (rice), and 4.7 (RWP) mol L−1. The concentrations of the studied elements were determined in undiluted sample solutions. The only exception was Mg; to measure its concentration, the prepared sample solutions were diluted at least 10 times. Five-point calibration curves were considered to quantify the concentrations of the analytes by using HG-ICP and PN-ICP OES. The external HNO3 content-matching standards of As(V) (prepared at concentrations up to 20 ng g−1) and of other elements (prepared at concentrations up to 5.0 mg kg−1 from the multi-element standard solution) were applied.
For a better overview, a schematic diagram of the methodology used, covering both the sample preparation procedures before the spectrometric measurements and the preparation procedures of RMWs, is shown in
Figure 1.
2.5. Pre-Reduction of As and Generation of AsH3
The total As content was determined in all solutions of the digested samples as As(III) after the pre-reduction of As(V) using a 0.5% (m/v) KI and 2.0% (m/v) AA solution in the acidic medium (3 mol L−1 HCl). To prevent a negative effect of residual HNO3 originating from the sample digestion (~1.8–2.1 mol L−1 after the pre-reduction) in the presence of 3 mol L−1 HCl (likely due to the aqua regia formation), respective sample solutions were initially neutralized with a concentrated HH solution (to reach its final concentration equal to 2.0%) before the subsequent pre-reduction of As. Accordingly, 2.0 g portions of the solutions of the digested samples were transferred into 12 mL screw-capped PP tubes (Equimed, Kraków, Poland), into which 0.2 g of the 40% (m/v) HH solution was added, followed by the addition of 0.8 g of the 2.5% (m/v) KI-10.0% (m/v) AA mixed solution, and completed to the final mass of 4.0 g with contracted HCl. Next, the tubes were capped, their contents mixed, and finally left to react at room temperature for 30 min before the measurements. In the same way as the samples, the respective blanks and the standard solutions were pre-reduced.
AsH3 was generated in a continuous flow system with gas–liquid phase separation that was directly coupled to the ICP OES instrument by the reaction between the pre-reduced sample (S) solution and the 1.0% NaBH4 (R) solution in the acidic medium (an additional 10 mol L−1 HCl (A) solution was used). For this purpose, all of these solutions were simultaneously pumped in separate streams by using a spectrometer peristaltic pump. At first, the S and A solutions were mixed in the 1st Y-connector before being mixed with the incoming R solution in the 2nd Y connector. The resulting heterogeneous reaction mixture was introduced through the reaction coil to the spray chamber to separate the volatile species from the spent liquid phase. AsH3 and other gaseous co-products were swept by a carrier Ar stream (introduced through the nebulizer gas inlet) and transported to the plasma torch. A post-reaction solution was constantly removed from the spray chamber by using the additional peristaltic pump.
2.6. Enrichment of As
In the case of RWM1 and RWM2, the digested samples were concentrated (5-fold) before the total As content determination using HG-ICP OES. This was because the As concentration in these samples was below its method limit of detection (MLOD), i.e., 0.13 ng g−1. For this purpose, the 10.0 g sample portions were weighed into 50 mL beakers and evaporated to about 1.0 g by gentle heating on a heating plate. Next, the concentrated samples were cooled to room temperature, quantitatively transferred into screw-capped PP tubes with deionized water to reach the final mass of 2.0 g, and then subjected to the pre-reduction procedure.
3. Results and Discussion
3.1. Verification of the Trueness of the Results
The trueness of the spectrometric measurements was verified through the analysis of the NIST SRM (1568b, rice flour). The obtained results were statistically compared with the assigned certified values by using a suitable statistical test at the 95% significance level (α = 0.05) [
25]. In this case, the Student
t-test with a critical value (
tcritical) of 2.776 was used to compare the respective mean concentrations of the studied elements. All of these results, i.e., mean concentrations of elements (
n = 3) with their SDs (both determined and certified), as well as the
tcalculated values, are collected in
Table 1.
The values of the
t-test for all studied elements were lower than the critical value (
tcalculated <
tcritical), which meant that there were no statistically significant differences between the determined concentrations of the elements and their certified values. Additionally, the recoveries obtained for all elements (
Table 2) were very high, i.e., 93.3–102%. For the remaining elements, i.e., not present in the SRM (Al, Cr, Ni, and Pb), the validity of the results was verified through the spike-and-recovery experiments using the standard addition method. To carry this out, the selected rice samples (WR2, BR2, and JR2) were spiked with proper amounts of the single element standards to achieve concentrations of 10 and 20 ng g
−1 (Cd, Cr, and Ci), 20 and 40 ng g
−1 (Pb), and 0.200 and 0.400 mg kg
−1 (Al) in the final sample solutions. Then, these sample solutions were subjected to the wet digestion procedure and finally analyzed using ICP OES to assess the recoveries of the elements. The concentration levels of the additions depended on the concentrations of elements determined in chosen rice samples (Al, Cr, and Ni) and their MLODs (Cd and Pb). As a result, quantitative recoveries were obtained, i.e., at the levels of 98.1–102% (Al), 92.8–105% (Cd), 95.6–103% (Cr), 95.1–97.9% (Ni), and 94.8–105% (Pb). All of these measures proved that the results obtained in this work are accurate, meaning precise and true.
3.2. Multi-Element Analysis of Raw Rice
3.2.1. Total Element Content in Raw Rice—General Characteristic
The total concentrations of Al, As Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Zn determined by using (HG)-ICP OES in six rice samples are collected in
Table 2. The results are expressed as mean values (
n = 3) along with %RSDs in the brackets.
As shown in
Table 2, the content of Cd and Pb was below their MLODs in all analyzed rice samples. The precision of the measurements of the remaining elements was good and varied from 0.57 to 5.3%.
Independent of the rice type, the highest values were obtained for both macroelements, i.e., Ca (35.5–87.5 mg kg
−1) and Mg (92.0–995 mg kg
−1), but the content of Mg was always higher than that of Ca (Mg > Ca). For WRs and JRs, the difference was 2.6–4.3-fold, while for BRs, the difference was higher, i.e., 1 order of magnitude (~12-fold). Considering the essential trace elements (Cu, Fe, Mn, and Zn), their content in all rice samples varied from 1.15 to 22.5 mg kg
−1. For WRs and JRs, they could be arranged as follows: Zn > Mn > Cu > Fe. As for Mg and Ca, a quite different relation was noticed for BRs, i.e., Mn > Zn > Fe > Cu. In the case of WRs and JRs, the contents of Zn (11.5–15.6 mg kg
−1) and Mn (6.29–8.75 mg kg
−1) were 3.6–13 times higher than those of Cu (1.48–3.44 mg kg
−1) and Fe (1.12–1.67 mg kg
−1). Differences between the concentrations of Cu and Fe or Mn and Zn were much lower (1.3–2.3 times). In the case of BRs, a similar relation was observed between the concentrations of Mn, Zn, and Fe against the concentration of Cu. The content of these three elements, i.e., 14.4–16.1 mg kg
−1 (Zn), 15.6–29.4 mg kg
−1 (Mn), and 11.1–12.1 mg kg
−1 (Fe), was 3.5–12 times higher as compared to that of Cu, i.e., 2.53–3.44 mg kg
−1. Except for Al, the concentrations of the rest of the non-essential and/or toxic trace elements (As, Cr, and Ni) were low (0.039–1.11 mg kg
−1). The content of Al (9.94–90.2 mg kg
−1) was significantly higher as compared to those determined for the aforementioned elements. The observed differences may result from the country of origin and the environmental conditions, including soil and water, which commonly affect the degree of the element assimilation by the rice plant. The method of the subsequent grain processing may also matter in this case [
26].
3.2.2. Total Element Content in Raw Rice—Effect of the Brand
Comparing two different suppliers of a given type of rice, the concentrations of individual elements were quite similar (
Table 2). The group with low variability of the results included As, Ca, Fe, Mg, Mn, Mn, and Zn, for which the coefficient of variation (%CV) was within the range of 0.54–15%. More differentiated results were noted for Cu (21–27%). In contrast, in the case of Al, Cr, and Ni, the discrepancy in the concentration between both rice samples was significant (26–100%). However, what was noticeable was that the content of Ni and Al was higher in the rice of the first supplier (WR1, BR1, and JR1).
3.2.3. Total Element Content in Raw Rice—Effect of the Type of Rice
Considering the type of rice, it was observed that BRs were a better source of elements than WRs and JRs. Nevertheless, the higher content of elements in BR is not surprising. This is due to the minerally rich husk of this rice, which is polished to obtain WR. Accordingly, the average concentrations of elements in BRs were 1.3–7.5 times and 1.3–10 times higher as compared to such average concentrations for WRs and JRs, respectively. Few exceptions were noted, i.e., for Zn (JRs) as well as Cr and Ni (WRs). The content of these elements was similar (Zn) or ~30% higher (Cr and Ni) compared to the results established for BRs. On the other hand, except for Cr, Ni, and Mg, the mean concentrations of most elements were quite comparable between BRs and JRs.
It must be commented that, as expected, all rice samples contained toxic As in the range of 144–209 ng g
−1, and, for the same reason as above, its average content in BRs was the highest. The mean concentrations of this element in both WRs and JRs were close (156–161 ng g
−1) and lower by about 30% than those determined in BRs. Concerning the literature available, the obtained results for the studied elements are within the ranges reported for them in all three rice types studied here [
24].
3.3. Multi-Element Analysis of Home-Made Rice Water
3.3.1. Total Element Content—General Characteristic
The total contents of the studied elements in differently prepared RWMs are presented in
Table 3.
Similarly to raw rice, the content of Cd and Pb was below their MLODs. Also, Cr was not detected in any of the investigated RWMs. Probably, it was due to the very low content of this element in raw rice (0.039–0.308 mg kg−1). The precision of measurements was satisfactory and ranged between 0.19 and 7.7%.
It should be commented that the first water examined here (RWM1) is typically discarded and not used as a cosmetic. However, it allowed us to check the effect of the pre-washing/rinsing of the rice grains on the elemental content of the water used for this purpose. This is important because it is standard practice to wash/rinse rice before it is further processed by cooking or making RW. Since all other RWMs (RWM2–RWM5) were prepared from washed rice, it was decided to compare them first.
Considering the essential macro- and microelements (Ca, Mg, Cu, Fe, Mn, and Zn), their behavior in all RWMs was similar to that in raw rice. Accordingly, RWMs were characterized by the highest Ca (0.291–1.52 mg kg−1) and Mg (1.05–9.38 mg kg−1) contents, and the level of Mg was higher than that of Ca (6.1–10 times). In the case of Cu, Fe, Mn, and Zn, their concentrations had ranges of 0.0037–0.030 mg kg−1 (Cu), 0.0079–0.121 mg kg−1 (Fe), 0.056–0.515 mg kg−1 (Mn), and 0.026–0.221 mg kg−1 (Zn), Additionally, the same, i.e., Zn > Mn > Cu > Fe (JRs), or close, i.e., Mn > Zn > Fe > Cu (WRs) and Mn > Fe > Zn > Cu (BRs), relations were observed for the corresponding RWMs. Also, similarly to the relation found in raw rice, for RWMs prepared from WRs and JRs, the level of Zn and Mn was higher (2.8–18 times) than that of Cu and Fe, while in the case of RWMs prepared from BRs, the content of Cu was significantly lower (4.9–31 times) than the content of Mn, Zn, and Fe. The composition closeness of RWMs with raw rice was also observed for the non-essential and/or toxic trace elements. Accordingly, the concentrations of As and Ni were low, i.e., 0.0011–0.018 mg kg−1 (As) and 0.0093–0.070 mg kg−1 (Ni) (their level was close to that of Cu and Fe), and the content of Al (0.047–311 mg kg−1) was much higher as compared to those determined for both elements (its level was close to that of Zn/Mn (WRs and JRs) and Ca (BRs)).
3.3.2. Total Element Content—Effect of the Procedure of RWMs Preparation
Considering the results detailed in
Table 3, it was observed that both the water temperature and the contact time between rice grains and water influenced the levels of elements in differently prepared RWMs. In general, a higher content of elements was found in RWMs prepared using hot water (RWM3) or cold water brought to a boil (RWM4 and RWM5) in comparison to RWMs prepared using cold water (RWM2). Importantly, for all three procedures based on hot water (RWM3–RWM5), a positive effect of time on the final content of elements in the obtained RWMs was observed. On the other hand, considerable differences between boiled water (RWM4) and fermented water (RWM5) were not found. It seemed that the fermenting process had little effect on the content of the studied elements; changes in their level were rather minor (±15%). Consequently, the mineral content in the examined RWMs could be arranged as follows: RWM2 < RWM3 < RWM4 ≤ RWM5. All of the above-mentioned findings were proved by the statistical analysis. Accordingly, considering all of the analyzed types of rice (WR1, WR2, BR1, BR2, JR1, and JR2) and all preparation procedures that were applied to obtain RMWs, i.e., RWM1 (No. 1), RWM2 (No. 2), RWM3 (No. 3), RWM4 (No. 4), and RWM5 (No. 5), the statistical difference between these treatments (used as the grouping variable) according to the concentration of the studied elements, i.e., Al, Ca, Cu, Fe, Mg, Mn, Ni, Zn, and As (used as the descriptive variables), were evaluated by using the two-side one-way analysis of variance (ANOVA) and the post hoc Fisher least significant difference (LSD) test. Since the variance of the concentrations of the studied elements within the groups formed by the analyzed rice samples was unequal, the ANAOVA with the Welch correction was used. For both tests, the 95% significance level (α = 0.05) was assumed. The results of this comparison are given in
Table S2. Although the comparison made for each element gave the possibility to maximally find 10 differences in its content due to the home-made RW preparation procedure applied, the highest number of such differences was established in the case of Ca (7) and As (6) as well as Fe, Mg, Mn, and Ni (4). There were no such differences found for Cu and only one such difference in the case of Al and Zn (1), likely due to the strong binding of these elements to the rice organic matrix. The evident differences in the concentration of the studied elements in the resulting RWMs were found between the treatments in which cold water was only used for washing (RWM1, No. 1) or soaking (RWM2, No.2) the rice portions, on the one hand, and the treatments in which cold water was brought with rice portions to boil (RWM4, No. 4) or the RWMs from the treatment No. 4 were eventually further fermented (RWM5, No. 5), on the other hand. In this case, the number of differences in the content of the studied elements for the pairs No. 1–No. 4, No. 1–No. 5, No. 2–No. 4, and No. 2–No. 5 was the highest, i.e., 16 out of 36 possible. The effect of hot water used for soaking the rice portions (RWM3, No. 3) was also confirmed since five differences for the No. 1–No. 3 pair were established (out of nine possible). The treatment in which portions of rice were boiled with water, after which this water was left to be fermented (RWM5, No. 5), did not result in increased concentrations of the studied elements; there was no difference established for the pair No. 4–No. 5.
The average content of the essential and non-essential elements in boiled water (RWM4) was 1.6–3.3 and 2.3–7.6 times higher, respectively, compared to RW obtained by soaking rice in cold water (RWM2). Also, a nearly two times longer time applied in the 4th procedure (RWM4) resulted in more efficient leaching of the elements from rice than during the RWM3 preparation when hot water was used for soaking the rice grains. The differences between these two RWMs were 1.1–1.8- (essential elements) and 1.1–2.7-fold on average (non-essential elements).
3.3.3. Total Element Content—Effect of the Rice Type
Surprisingly, despite the higher content of the investigated elements in BRs (see
Section 3.2.1), it was found that except for Al, Fe, and Mn, RWMs made from WRs or JRs are a better source of the elements as compared to those prepared from BRs. Changes in the average content of other elements ranged from 1.1 to 4.6 times and were the most significant in the case of As, Cu, and Zn. In addition, when comparing WRs and JRs, it was noticed that the concentrations of most elements in RWMs prepared from WRs were higher than those determined in RWMs prepared from JRs.
3.3.4. Solubility of Elements
Based on the average concentrations of the studied elements in RWMs (
Table 3,
Section 3.3.1) and raw rice (
Table 2,
Section 3.2.1), it was possible to estimate to what extent the elements were leached from rice grains during the preparation of RWMs by a given procedure (Nos. 1–5). This allowed us to assess the solubility of the elements in water depending on the time, the temperature, and the type of rice used in the procedures applied. The results concerning the extraction percentage of each element are collected in
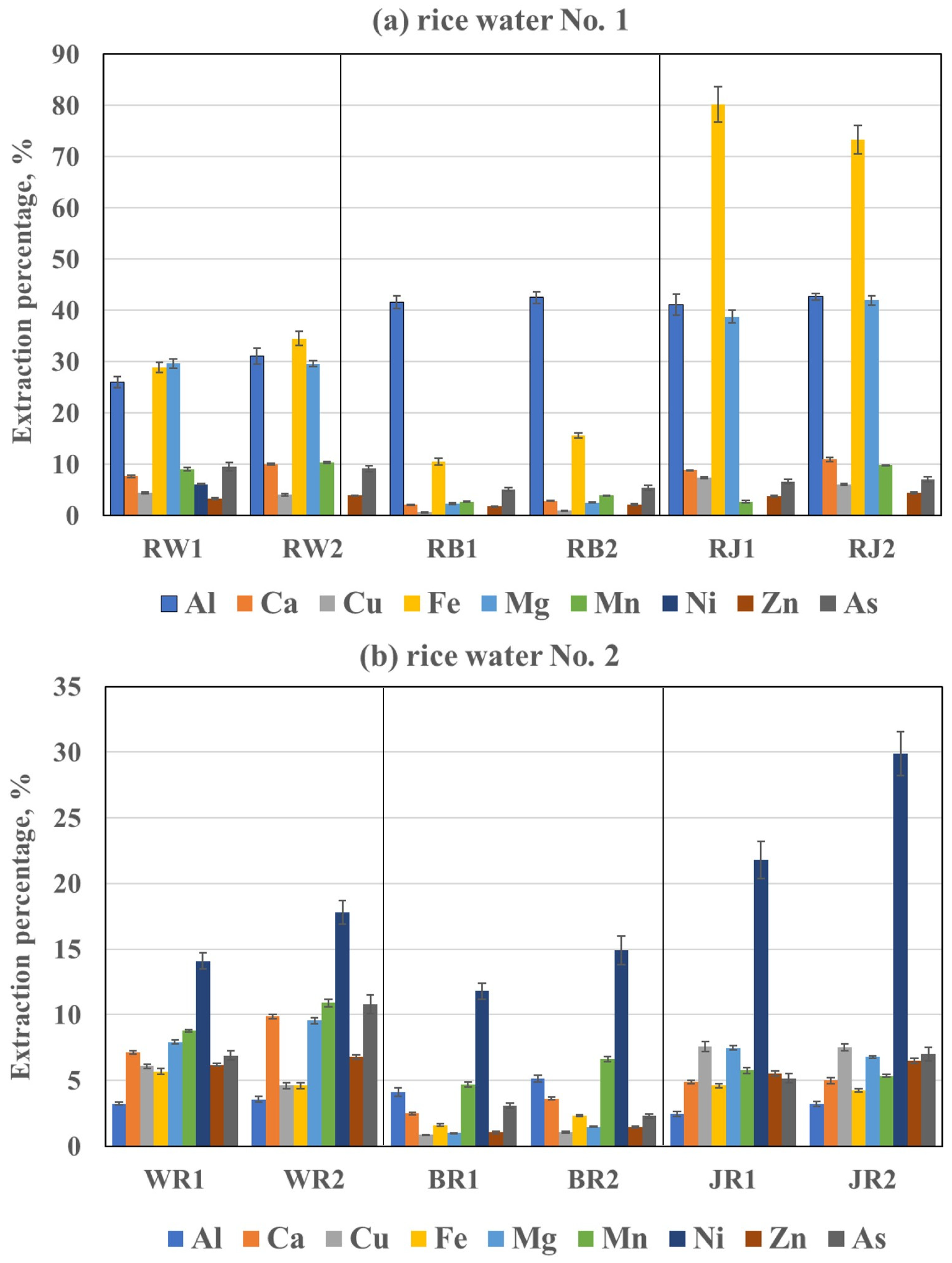
Figure 2a–e.
The results obtained showed that RWMs prepared with hot water (RWM3–RWM5,
Figure 2c–e) are characterized by higher extraction percentages of elements from rice grains due to the higher solubilities of their species at higher temperatures. Simultaneously, the longer contact time of water with the rice grains enhances the solubility of the element species as well. Because of this, the highest solubility of the elements species from the rice grains could be achieved when preparing boiled (RWM4) or fermented (RWM5) RWs. In addition, the type of rice affected the solubility of the elements species as well and changed as follows: WRs > JRs > BRs. Satisfactorily, it agreed with the relation found for the concentrations of elements determined in the respective RWMs. However, it must be noticed that the extraction percentages of the elements did not follow the same order as the content of these elements determined in RWMs or raw rice. Accordingly, taking into account the highest average extraction percentage (case of boiled/fermented RWMs), the following elements were classified as follows:
Highly soluble: As (64% for WRs and 56% for JRs);
Medium-soluble: Ni > Mg > Ca > Al > Fe > Mn (16–34% for WRs and 12–35% for JRs), or Ni > As > Al (18–34% for BRs);
Low- or extremely low-soluble: Zn > Cu (8.6–10% for WRs and 7.7–8.6% for JRs) or Mn > Ca > Fe > Mg > Cu~Zn (2.6–10% for BRs).
Given this, the percentages for the elements extracted from BRs explain their low content in the prepared RWs compared to their content in raw rice. Consequently, except for Al and Ni, the remaining elements were less extracted from this rice than from WRs or JRs. A reduction in the transfer of elements into the water from 50% (Mn) through 2–5 washes (As, Ca, Cu, Fe, and Zn) up to 7 times (Mg) on average was noted.
The observed relationships between RWs prepared from rice coming from two different brands were quite similar; therefore, the degree of the element transfer from rice grains to water (see
Figure 2) was not accidental and depended to a greater extent on the method of water preparation than on the initial amount of elements in rice.
Interestingly and importantly, the previous washing/rinsing of rice (RWM1) with cold water resulted in a partial removal of the elements from its grains (see
Table 3,
Section 3.3.1). Accordingly, the concentrations of the studied elements were as follows: 0.308–0.658 mg kg
−1 (Ca), 3.72–9.31 mg kg
−1 (Mg), 0.0028–0.026 mg kg
−1 (Cu), 0.080–0.317 mg kg
−1 (Fe), 0.095–0.133 mg kg
−1 (Mn), 0.042–0.108 mg kg
−1 (Zn), 0.654–6.25 mg kg
−1 (Al), n.d.–0.011 mg kg
−1 (Ni), and 0.0016–0.0028 mg kg
−1 (Al). It was observed that during the short washing of rice (3 min), relatively soluble elements like Al, Fe, and Mg (WRs and JRs) or Al and Fe (BRs) can be extracted in the average range of 29–32% (WRs), 40–77% (JRs), and 13–42% (BRs). The average extraction percentage for the remaining elements was lower and had ranges of 3.6–9.3% (WRs), 4.1–9.9% (JRs), and 0.83–5.3% (BRs). The exception was Ni, which, except for WR1, was not determined in RWM1 probably due to the too short time applied during this procedure and a quite low content of this element in rice samples. Again, the average extraction percentage of elements obtained for both WRs and JRs was higher than that obtained for BRs. In the case of WRs and JRs, very high removal of Fe from JRs (77% on average) and Mg from WRs and JRs (30–40% on average) deserves attention. On the other hand, it allows for a significant (Al, 29–42% on average) or small (As, 5.3–9.3% on average) removal of non-essential/toxic elements from the rice grains. This agreed with the literature data, where washing the rice grains three or five times resulted in a transfer of up to 8% of their total As to water [
18]. Therefore, it should be taken into account that the pre-washing/rinsing of rice can affect the original content of the elements in its grains and may change the quality of the prepared RWMs. Consequently, in the absence of this step, the extraction percentage of the elements, and thus their content in RW, would be higher. As a result, in the case of boiled/fermented RWMs (RWM4 and RWM5), the classification of elements according to their solubility would change and could be arranged as follows:
(Very) highly soluble: As > Mg > Fe > Al (47–73% for WRs) or Fe > Mg > As > Al (54–93% for JRs) or Al (60% for BRs);
Medium-soluble: Ca > Ni > Mn (25–38% for WRs and 25–35% for JRs) or As > Ni (28–34% for BRs);
Low-soluble: Zn~Cu (13–14% for WRs and JRs) or Fe > Mn > Ca (11–18% for BRs);
Extremely low-soluble: Zn > Cu (3.3–4.6% for BRs).
Simultaneously, the average levels of the studied elements in these waters would be increased as follows:
WRs: 0.93 mg kg−1 (Al), 2.0 mg kg−1 (Ca), 0.036 mg kg−1 (Cu), 0.12 mg kg−1 (Fe), 17 mg kg−1 (Mg), 0.34 mg kg−1 (Mn), 0.049 mg kg−1 (Ni), 0.25 mg kg−1 (Zn) and 0.019 mg kg−1 (As);
JRs: 1.1 mg kg−1 (Al), 1.6 mg kg−1 (Ca), 0.043 mg kg−1 (Cu), 0.17 mg kg−1 (Fe), 9.9 mg kg−1 (Mg), 0.28 mg kg−1 (Mn), 0.014 mg kg−1 (Ni), 0.30 mg kg−1 (Zn) and 0.015 mg kg−1 (As);
BRs: 5.6 mg kg−1 (Al), 1.4 mg kg−1 (Ca), 0.016 mg kg−1 (Cu), 0.36 mg kg−1 (Fe), 9.0 mg kg−1 (Mg), 0.47 mg kg−1 (Mn), 0.026 mg kg−1 (Ni), 0.11 mg kg−1 (Zn) and 0.0094 mg kg−1 (As).
3.3.5. Preparation of Healthy RW
Regarding the mineral content of differently prepared RWs (see
Section 3.3.1), it could be concluded that, except for Mg and Ca, they are rather a poor source of elements. Hence, the best option to extract the highest amounts of the essential macro- and microelements (Ca, Mg, Cu, Fe, Mn, and Zn) into RWMs is boiling (No. 4) of unwashed rice grains (mainly WRs). Unfortunately, along with the elements mentioned above, the non-essential/toxic Al, Ni, and As would also be present in the prepared RWs. Accordingly, compared to WRs, by choosing JRs or BRs, the amount of Ni in RWs could be reduced. On the other hand, choosing BRs instead of WRs or JRs would result in a higher Al content in the prepared RWs. What is worse, in contact with hot water, As would be leached to a large extent from BRs and WRs (55–65%). This results in obtaining the concentration of this element at 15–19 ng g
−1 in RWs. The leaching degree of As from WR is similar to the literature value (74%) obtained for rice washed and cooked in a six-fold excess of water [
27]. Satisfactorily, it could be reduced by half (to ~9 ng g
−1) when BRs are selected for the RW preparation due to a much lower extraction percentage of As (~20%) for this type of rice. It is in agreement with already published results showing that when rice is three times rinsed and then cooked in a six-fold excess of water, As is removed in 31% [
18]. A lower degree of the As transfer for BR (despite a higher As content in raw rice) compared to WR was also observed [
27]. Thus, BRs appear to be the best choice for the proposed procedure that aims at preparing RW by boiling unwashed rice. Although the permissible amount of As in cosmetics is 3 μg g
−1 [
28], and theoretically, all RWMs can be safely used for skin and hair, the As limit in drinking water is only 10 ng mL
−1 [
29]. It means that these RWMs should not be drunk, especially those obtained from WR and JR. In the case of WRs and JRs, the addition of an initial washing step (+No. 1) could be a good option. However, while it can conveniently remove Al (29–42%) and As (7–9%) from rice grains, it can also significantly remove Mg, Fe, or Ca from them. Therefore, by examining the levels of all tested elements vs. the RW preparation procedure and the rice type, it was possible to find the optimal one. In view of this, it seems that the preparation of RW by soaking the rice grains in cold water (RWM2, No. 2) but without their washing should be recommended. Except for Al, this preparation procedure guarantees even better results (an increase in the average element content) than these obtained for pre-washed rice and then boiled in a six-fold excess of water (RWM4, No. 1 + No. 4), i.e.:
WRs: 0.65 mg kg−1 (Al), 1.2 mg kg−1 (Ca), 0.030 mg kg−1 (Cu), 0.096 mg kg−1 (Fe), 11 mg kg−1 (Mg), 0.27 mg kg−1 (Mn), 0.024 mg kg−1 (Ni), 0.20 mg kg−1 (Zn) and 0.0048 mg kg−1 (As);
JRs: 1.0 mg kg−1 (Al), 0.88 mg kg−1 (Ca), 0.043 mg kg−1 (Cu), 0.15 mg kg−1 (Fe), 7.4 mg kg−1 (Mg), 0.16 mg kg−1 (Mn), 0.015 mg kg−1 (Ni), 0.25 mg kg−1 (Zn) and 0.0034 mg kg−1 (As);
BRs: 4.2 mg kg−1 (Al), 0.76 mg kg−1 (Ca), 0.0092 mg kg−1 (Cu), 0.30 mg kg−1 (Fe), 6.9 mg kg−1 (Mg), 0.32 mg kg−1 (Mn), 0.011 mg kg−1 (Ni), 0.083 mg kg−1 (Zn) and 0.0028 mg kg−1 (As).
It must be commented that this RW contains a lower content of the essential elements than RWM4 proceeded without the initial washing step. However, this procedure allowed for a reduction in the amounts of Al, Ni, and As. For the latter element, the reduction was evident (3.4–4.6 fold), i.e., to a concentration <5 ng g−1 (2.8–3.4 ng g−1). It indicates that this RW is safe and suitable for consumption. On the other hand, the recommended procedure is simple and quick, which is an additional advantage.
3.4. Total Element Content in Commercial Rice-Based Cosmetics Products
The total contents of the studied elements in the selected cosmetic commercial products based on RW and a rice extract, including RWP1, RWP2, RWP3, and RWP4 (see their names in
Section 2.1), are presented in
Table 4.
The content of Cd and Pb was below their MLODs in all analyzed products. Similarly, As was determined in just one (RWP2) out of four. The precision of measurements was better than 6.0% (0.50–5.9%). The only exception was found for Cr and Cu (RWP1), for which RSDs were higher (6.7–7.9%). However, this was justified due to the very low concentrations of these elements present in these samples. In general, the highest concentrations of the studied elements were found in RWP4, while the lowest were in RWP3. In the case of RWP4, based on the rice extract, the very high concentrations of the elements were expected because it is a concentrated product and must be diluted before use at least 20 times, i.e., to a concentration of 5%. The main elements in the analyzed commercial cosmetics were Ca and Mg. Their average concentrations fulfilled the following order: Ca > Mg (RWP1 and RWP3) or Mg > Ca (RWP2 and RWP4). The differences between their concentrations varied and could reach up to six times (RWP1). The lowest difference (two-fold) was observed for RMP4. Regarding the essential elements (Fe, Cu, Mn, and Zn), their average concentrations could be arranged as follows: Fe > Zn > Cu > Mn. The relation was quite different as compared to that found for RWMs. In general, the level of Fe was 2.4–7.9 times and 10–45 times higher than that of Zn. Discrepancies between Fe and Cu were much higher (30–190 times). For the non-essential elements, the following relation was ascribed: Al > Ni ≫ Cr > As. Concentrations of Al were close to those obtained for Ca (RWP1, RWP3, and RWP4) or Mg (RWP2) and 3.2–13 times higher than those determined for Ni. Except for RWP4, the content of Cr was significantly reduced (by about 1–2 orders of magnitude) compared to those established for the aforementioned elements.
Finally, considering the determined concentrations of the studied elements, RWMs prepared according to the recommended procedure, i.e., by soaking rice grains in cold water (RWM2, No. 2) with no initial washing of the rice grains, were compared with RWPs. Accordingly, it was concluded that the contents of Al (0.65–4.2 mg kg−1) and Ca (0.76–1.2 mg kg−1) in RWMs were within the concentration ranges established for the ready-to-use cosmetics (RWP1–RWP3). Satisfactorily, the content of Cu (9.2–43 ng g−1), Fe (0.096–0.30 mg kg−1), Mg (6.9–11 mg kg−1), Mn (160–320 ng g−1), and Zn (83–250 ng g−1) in RWMs was higher than that found in these three commercial products. Importantly, the content of Ni (0.011–0.024 mg kg−1) was significantly lowered (by about 1 order of magnitude). Although RWMs contained As, its level (2.8–4.8 ng g−1) was lower than that determined in RWP2. Since RWP4 should be at least 20 times diluted before use (it is recommended to use a 1–5% solution), it seems that, only for this product, the concentrations of Al, Ca, Cu, Fe, and Ni are close to those obtained for RWMs. Otherwise, the content of Mg, Mn, and Zn is significantly lower in this product. In the case of a larger dilution (1% solution), the concentration of all elements will be meaningfully reduced and much lower than that of RWMs.
4. Conclusions
This work demonstrates for the first time the results on the mineral content of home-made (natural) rice water (RWM) determined by using ICP OES (Al, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Zn) and HG-ICP OES (As). Five different procedures and three types of rice (white, brown, and jasmine) were used to prepare RWMs. Satisfactorily, all types of RWMs were free from Cd and Pb. In the case of the remaining elements, their content in the final product depended on the water temperature used at the step of the RWM preparation, the extraction time, and the type of rice. The lowest solubility of elements was observed for brown rice. The best results (the highest contents of the studied elements) were obtained for RWM prepared by boiling rice grains with no initial washing. Unfortunately, besides the essential elements (Ca, Cu, Fe, Mg, and Zn), all RWMs contained As (2–20 ng g−1). The permissible content of As in cosmetics is set to be 3 μg g−1; therefore, all produced RWMs can be safely used for skin and hair. In contrast, this contamination limited the consumption of selected RWMs (especially those obtained from white and jasmine rice) due to the As limit in drinking water, which is 10 ng mL−1. Fortunately, we managed to find the optimal procedure (soaking unwashed rice grains in cold water for 30 min), which guarantees the obtainment of an appropriate content of the essential elements and reduces the concentration of As to a minimum (<5 ng g−1). As a result, such rice water is safe for human health and suitable for consumption.
Finally, the comparison of prepared RWMs with commercial rice-based cosmetics (RWPs) showed that the level of the essential elements in the home-made products was higher. Hence, their use as a raw material also on the skin and hair seems justified.