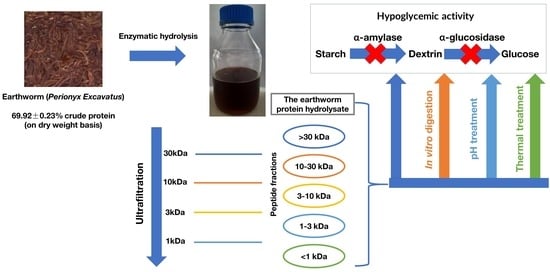
Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Earthworm Protein Hydrolysates
2.2.2. Effect of Hydrolysis Condition on α-AIA and α-GIA of the Earthworm Protein Hydrolysate
2.2.3. Determination of α-AIA and α-GIA of the Earthworm Protein Hydrolysates and Their 5 Peptide Fractions
2.2.4. Fractionation of Earthworms Protein Hydrolysate
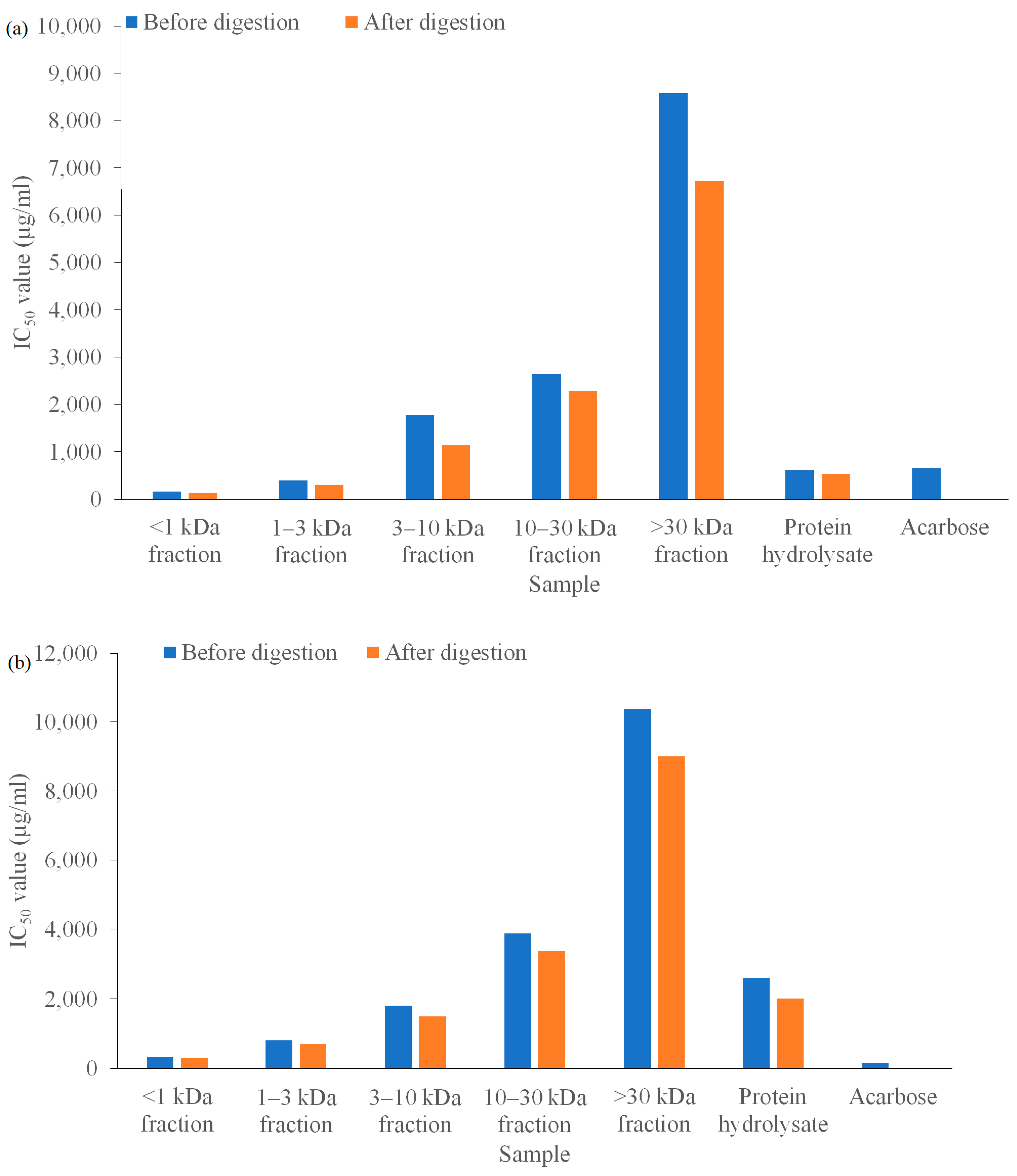
2.2.5. In Vitro Digestion
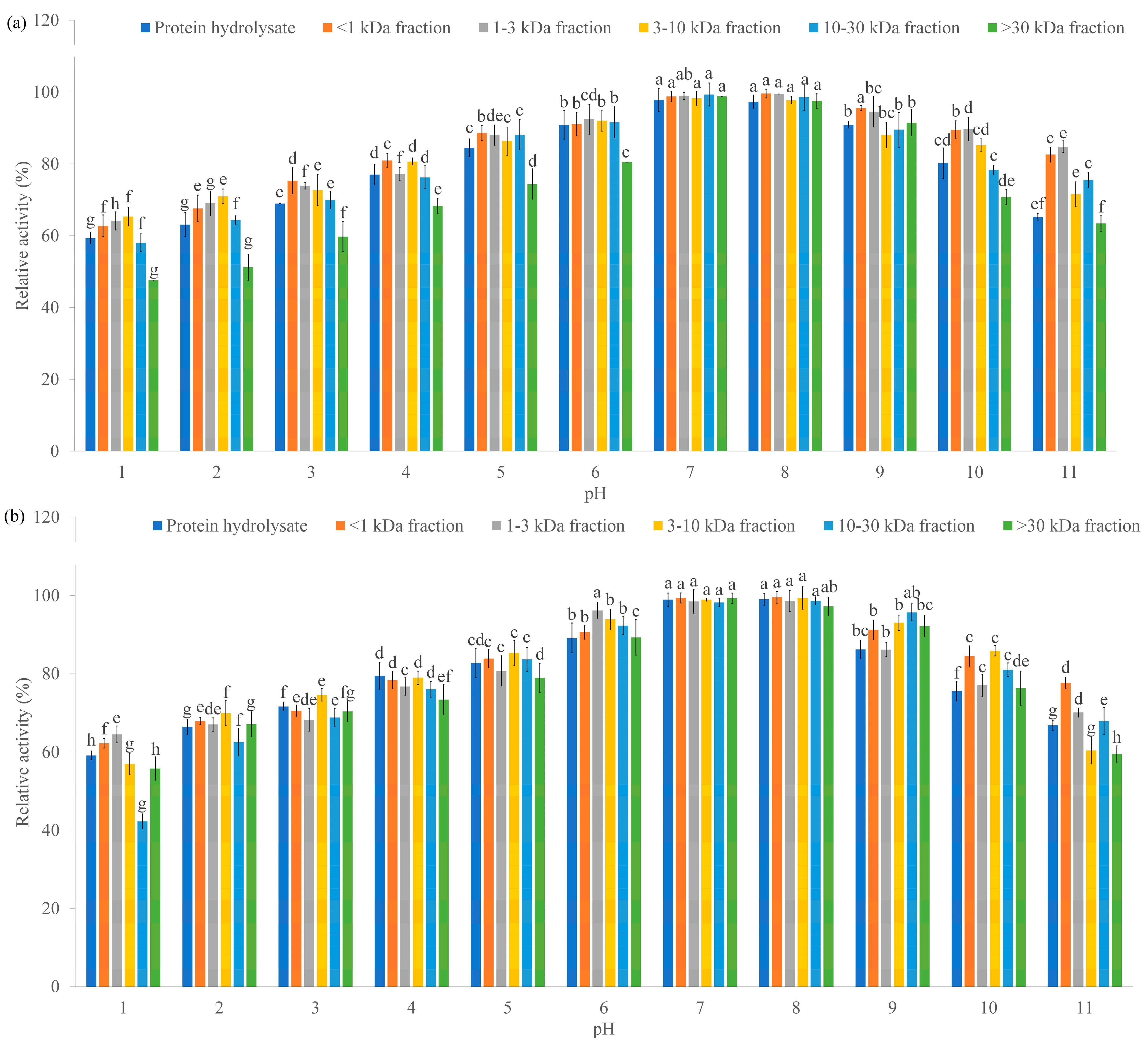
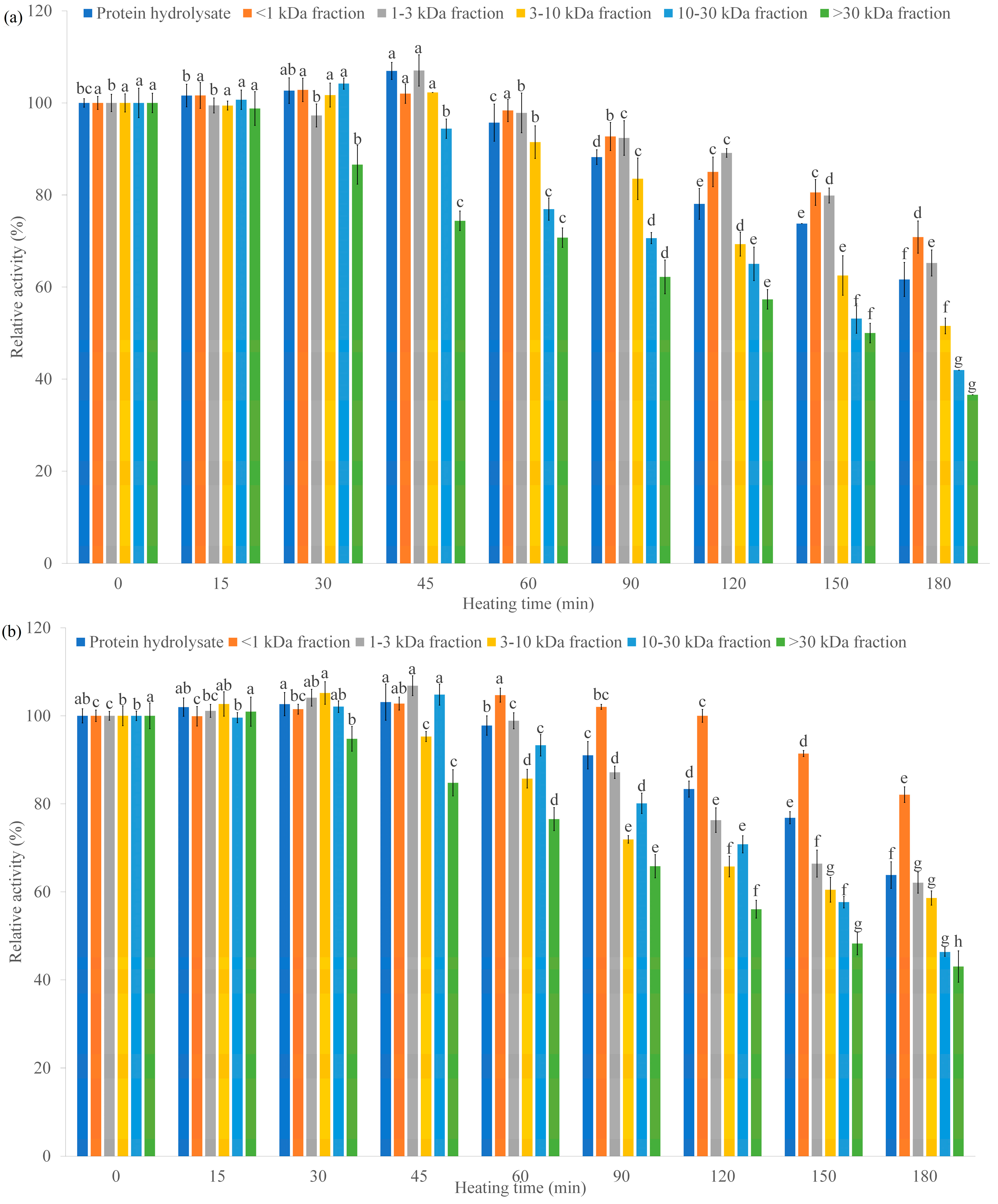
2.2.6. Thermal and pH Stability
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Investigation Results of α-AIA and α-GIA of the Earthworm Hydrolysate under the Influence of Hydrolysis Condition
3.1.1. Effect of Hydrolysis Enzyme
3.1.2. Effect of Earthworm: Phosphate Buffer Ratio
3.1.3. Effect of Hydrolysis Temperature
3.1.4. Effect of pH
3.1.5. Effect of E:S Ratio
3.1.6. Effect of Hydrolysis Time
3.2. α-AIA and α-GIA of Peptide Fractions
3.3. Investigation Results of In Vitro Digestion Stability of α-AIA and α-GIA of the Hydrolysates and Their Peptide Fractions
3.4. Investigation Results of Thermal and pH Stability of α-AIA and α-GIA of the Hydrolysates and Their Peptide Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, N.; Gupta, R.K.; Singh, D.; Shashank, S. Comparative study of antibacterial activity of two different earthworm species, Perionyx excavatus and Pheretima posthuma against pathogenic bacteria. J. Appl. Nat. Sci. 2015, 7, 666–671. [Google Scholar] [CrossRef]
- Ngoc, T.N.; Pucher, J.; Becker, K.; Focken, U. Earthworm powder as an alternative protein source in diets for common carp (Cyprinus Carpio L.). Aquac. Res. 2016, 47, 2917–2927. [Google Scholar] [CrossRef]
- Williamson, E.M.; Lorenc, A.; Booker, A.; Robinson, N. The rise of traditional Chinese medicine and its materia medica: A comparison of the frequency and safety of materials and species used in Europe and China. J. Ethnopharmacol. 2013, 149, 453–462. [Google Scholar] [CrossRef]
- Prakash, M.; Gunasekaran, G.; Elumalai, K. Effect of earthworm powder on antioxidant enzymes in alcohol induced hepatotoxic rats. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 237–243. [Google Scholar] [PubMed]
- Ding, S.; Lin, X.; He, S. Earthworms: A Source of Protein. J. Food Sci. Eng. 2019, 9, 159–170. [Google Scholar] [CrossRef][Green Version]
- Zhao, Q.; Wei, G.; Li, K.; Duan, S.; Ye, R.; Huang, A. Identification and molecular docking of novel α-glucosidase inhibitory peptides from hydrolysates of Binglangjiang buffalo casein. LWT—Food Sci. Technol. 2022, 156, 113062. [Google Scholar] [CrossRef]
- Baba, W.N.; Mudgil, P.; Kamal, H.; Kilari, B.P.; Gan, C.-Y.; Maqsood, S. Identification and characterization of novel α-amylase and α-glucosidase inhibitory peptides from camel whey proteins. J. Dairy Sci. 2021, 104, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Luo, J.; Liu, Y.; Wang, B.; Liang, Z.; Li, L. Insight into the binding modes and mechanisms of inhibition between soybean-peptides and α-amylase based on spectrofluorimetry and kinetic analysis. LWT—Food Sci. Technol. 2021, 142, 110977. [Google Scholar] [CrossRef]
- Tseng, P.-S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. Int. Ed. 2023, 62, e202217809. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G.; Noroozi, M. Protein nutritional quality, amino acid profile, anti-amylase and anti-glucosidase properties of microalgae: Inhibition and mechanisms of action through in vitro and in silico studies. LWT—Food Sci. Technol. 2021, 150, 112023. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. Biology and Ecology of Earthworms, 4th ed.; Springer Nature: New York, NY, USA, 2022; p. 578. [Google Scholar] [CrossRef]
- Verma, M.K.; Pulicherla, K.K. Enzyme promiscuity in earthworm serine protease: Substrate versatility and therapeutic potential. Amino Acids 2016, 48, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Castrica, M.; Balzaretti, C.M.; Tedesco, D.E.A. Edible earthworms in a food safety perspective: Preliminary data. Ital. J. Food Saf. 2019, 8, 7695. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.E.A.; Castrica, M.; Tava, A.; Panseri, S.; Balzaretti, C.M. From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects 2020, 11, 293. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Conti, C.; Lovarelli, D.; Biazzi, E.; Bacenetti, J. Bioconversion of fruit and vegetable waste into earthworms as a new protein source: The environmental impact of earthworm meal production. Sci. Total Environ. 2019, 683, 690–698. [Google Scholar] [CrossRef]
- Kostecka, J.; Garczyńska, M.; Pączka, G.; Mazur-Pączka, A. Chemical Composition of Earthworm (Eisenia Fetida Sav.) Biomass and Selected Determ for its Production. J. Ecol. Eng. 2022, 23, 169–179. [Google Scholar] [CrossRef]
- Rodrigues, M.; Carlesso, W.M.; Kuhn, D.; Altmayer, T.; Martini, M.C.; Tamiosso, C.D.; Mallmann, C.A.; Souza, C.F.V.D.; Ethur, E.M.; Hoehne, L. Enzymatic hydrolysis of the Eisenia andrei earthworm: Characterization and evaluation of its properties. Biocatal. Biotransformation 2017, 35, 110–119. [Google Scholar] [CrossRef]
- Mir, M.A.; Upadhyay, S.; Mir, B.A. Inhibition of Alpha Amylase and Alpha Glycosidase Enzymes by Various Earth Worm Extracts. Biomed. Pharmacol. J. 2018, 11, 1261–1268. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelfattah, M.A.; Bahaaeldine, M.A.; Rashed, A.R.; Mohamed, A.S.; Ali, M.F.; Elbatran, M.M.; Saad, D.Y. Earthworm Extract Enhanced Organ Functions in Diabetic Rats by Ameliorating Physiological and Structural Changes. Biointerface Res. Appl. Chem. 2023, 13, 445. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.; Ma, H. Production, bioactivities and bioavailability of bioactive peptides derived from walnut origin byproducts: A review. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Patil, P.J.; Usman, M.; Zhang, C.; Mehmood, A.; Zhou, M.; Teng, C.; Li, X. An updated review on food-derived bioactive peptides: Focus on the regulatory requirements, safety, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1732–1776. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Reappraisal and perspectives of clinical drug-drug interaction potential of α-glucosidase inhibitors such as acarbose, voglibose and miglitol in the treatment of type 2 diabetes mellitus. Xenobiotica 2018, 48, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.-X.; Wang, B.-K.; Wu, Y.-C.; Li, Q.-Y.; Qin, B.-W.; Li, H.-J. Release of antidiabetic peptides from Stichopus japonicas by simulated gastrointestinal digestion. Food Chem. 2020, 315, 126273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.-M.; Elahi, F.; Yeon, S.-J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.-H. The Role of Bioactive Peptides in Diabetes and Obesity. Foods 2021, 10, 2220. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Félix-Medina, J.V.; Sepúlveda-Haro, A.G.; Quintero-Soto, M.F. Stability of antioxidant and hypoglycemic activities of peptide fractions of Maize (Zea mays L.) under different processes. J. Food Meas. Charact. 2023, 17, 362–370. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Liu, J.; Meng, Z.; Huang, A.; Xu, F.; Wang, X. Identification, characterization and in vitro activity of hypoglycemic peptides in whey hydrolysates from rubing cheese by-product. Food Res. Int. 2023, 164, 112382. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Xu, F.; Xie, J.; Gao, X.; Li, L.; Tian, Y.; Sheng, J. Characterization of the structure, stability, and activity of hypoglycemic peptides from Moringa oleifera seed protein hydrolysates. Food Funct. 2022, 13, 3481–3494. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 1990; pp. 69–79. [Google Scholar]
- Vo, T.D.L.; Pham, K.T.; Le, V.M.V.; Lam, H.H.; Huynh, O.N.; Vo, B.C. Evaluation of iron-binding capacity, amino acid composition, functional properties of Acetes japonicus proteolysate and identification of iron-binding peptides. Process Biochem. 2020, 91, 374–386. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-Glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Kang, B.; Skonberg, D.I.; Myracle, A.D. Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes. Foods 2020, 9, 258. [Google Scholar] [CrossRef]
- Sripokar, P.; Benjakul, S.; Klomklao, S. Antioxidant and functional properties of protein hydrolysates obtained from starry triggerfish muscle using trypsin from albacore tuna liver. Biocatal. Agric. Biotechnol. 2019, 17, 447–454. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavanoe, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Marson, G.V.; Castro, R.J.S.d.; Machado, M.T.d.C.; Zandonadi, F.d.S.; Barros, H.D.d.F.Q.; Júnior, M.R.M.; Sussulini, A.; Hubinger, M.D. Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem. 2020, 91, 34–45. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Y.; Xiao, C.; Sun-Waterhouse, D.; Zhao, M.; Su, G. Mechanism of the discrepancy in the enzymatic hydrolysis efficiency between defatted peanut flour and peanut protein isolate by Flavorzyme. Food Chem. 2015, 168, 100–106. [Google Scholar] [CrossRef]
- Fernández, A.; Kelly, P. pH-stat vs. free-fall pH techniques in the enzymatic hydrolysis of whey proteins. Food Chem. 2016, 199, 409–415. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Hansen, E.T.; Bredie, W.L.P.; Lametsch, R. Structural characteristics of low bitter and high umami protein hydrolysates prepared from bovine muscle and porcine plasma. Food Chem. 2018, 257, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Willmore, W.G.; Tsopmo, A. Peptidomic analysis of hydrolyzed oat bran proteins, and their in vitro antioxidant and metal chelating properties. Food Chem. 2019, 279, 49–57. [Google Scholar] [CrossRef]
- Balderas-León, I.; Baigts-Allende, D.; Cardador-Martínez, A. Antioxidant, angiotensin-converting enzyme, and α-amylase inhibitory activities of protein hydrolysates of Leucaena leucocephala seeds. CYTA—J. Food 2021, 19, 349–359. [Google Scholar] [CrossRef]
- Qiao, H.; Bi, X.; Zhang, Y.; Liu, M.; Zu, S.; Jia, N.; Jiang, S.; Lu, Q.; Zu, Y.; Bao, Y. Enzymic polypeptide antioxidant activity and inhibitory activity on α-glucosidase and α-amylase from Paeonia ostii cake. Ind. Crops Prod. 2020, 146, 112158. [Google Scholar] [CrossRef]
- Feng, J.; Ma, Y.-L.; Sun, P.; Thakur, K.; Wang, S.; Zhang, J.-G.; Wei, Z.-J. Purification and characterization of α-glucosidase inhibitory peptides from defatted camellia seed cake. Int. J. Food Sci. Technol. 2021, 56, 138–147. [Google Scholar] [CrossRef]
- Ujiroghene, O.J.; Liu, L.; Zhang, S.; Jing, L.; Pang, X.; Lv, J. α-glucosidase and ACE Dual Inhibitory Protein Hydrolysates and Peptide Fractions of Sprouted Quinoa Yoghurt Beverages Inoculated with Lactobacillus casei. Food Chem. 2019, 299, 124985. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Arise, R.O.; Idi, J.J.; Mic-Braimoh, I.M.; Korode, E.; Ahmed, R.N.; Osemwegie, O. In vitro Angiotesin-1-converting enzyme, α-amylase and α-glucosidase inhibitory and antioxidant activities of Luffa cylindrical (L.) M. Roem seed protein hydrolysate. Heliyon 2019, 5, e01634. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Yang, Q.; Sun, J.; Bi, J.; Liu, S.; Zhang, C.; Tang, L. Optimization of a Microwave-Coupled Enzymatic Digestion Process to Prepare Peanut Peptides. Molecules 2012, 17, 5661–5674. [Google Scholar] [CrossRef]
- Shu, G.; Zhang, B.; Zhang, Q.; Wan, H.; Li, H. Effect of Temperature, pH, Enzyme to Substrate Ratio, Substrate Concentration and Time on the Antioxidative Activity of Hydrolysates from Goat Milk Casein by Alcalase. Acta Univ. Cibiniensis. Ser. E Food Technol. 2017, 20, 29–38. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the Preparation of Fish Protein Anti-Obesity Hydrolysates Using Response Surface Methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139. [Google Scholar] [CrossRef]
- Vo, T.D.L.; Pham, K.T.; Doan, K.T. Identification of Copper-Binding Peptides and Investigation of Functional Properties of Acetes japonicus Proteolysate. Waste Biomass Valorization 2021, 12, 1565–1579. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem. 2007, 103, 1385–1394. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Jiménez-Morales, K.; Quintal-Novelo, C.; Moo-Puc, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Enzymatic protein hydrolysates and ultrafiltered peptide fractions from Cowpea Vigna unguiculata L. bean with in vitro antidiabetic potential. J. Iran. Chem. Soc. 2019, 16, 1773–1781. [Google Scholar] [CrossRef]
- Suwanangul, S.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Ruttarattanamongkol, K. Inhibition of α-amylase, α-glucosidase and pancreatic lipase activities in vitro by sacha inchi (Plukenetia volubilis L.) protein hydrolysates and their fractionated peptides. Maejo Int. J. Sci. Technol. 2021, 15, 13–26. [Google Scholar]
- Jiang, M.; Yan, H.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro activities of a-amylase, a-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Ayaz, M.; Ghufran, M.; Ullah, F.; Sadiq, A.; Ullah, I.; Haq, I.U.; et al. Phytochemical Analysis, α-Glucosidase and Amylase Inhibitory, and Molecular Docking Studies on Persicaria hydropiper L. Leaves Essential Oils. Evid.-Based Complement. Altern. Med. 2022, 2022, 7924171. [Google Scholar] [CrossRef]
- Hasan, T.; Islam, A.; Riva, R.a.k.; Rahman, N.; Ahmed, S.; Islam, A.; Daula, A.F.M.S.U. Phytochemicals from Zingiber capitatum rhizome as potential a-glucosidase, a-amylase, and glycogen phosphorylase inhibitors for the management of Type-II diabetes mellitus: Inferences from in-vitro, in-vivo and in-silico investigations. Arab. J. Chem. 2023, 16, 105128. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.; Cao, J.; Li, Y.; Jin, X.; He, C.; Wang, M. Inhibition mechanism of α-glucosidase inhibitors screened from Tartary buckwheat and synergistic effect with acarbose. Food Chem. 2023, 420, 136102. [Google Scholar] [CrossRef]
- Mudgil, P.; Alblooshi, M.; Singh, B.P.; Devarajan, A.R.; Maqsood, S. Pearl millet protein hydrolysates exhibiting effective in-vitro antioxidant, antidiabetic and anti-lipidemic properties as potential functional food ingredient. Int. J. Food Sci. Technol. 2023, 58, 3264–3272. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Rane, A.S.; Joshi, R.S.; Giri, A.P. Molecular determinant for specificity: Differential interaction of α-amylases with their proteinaceous inhibitors. Biochim. Biophys. Acta BBA—Gen. Subj. 2020, 1864, 129703. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R.M. Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed. Pharmacother. 2018, 107, 234–242. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Gill, S.; Wilcox, M.D.; Pearson, J.P.; Chater, P.I. Starch Digestion in the Upper Gastrointestinal Tract of Humans. Starch—Stärke 2018, 70, 1700111. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Ponce-Alquicira, E.; Pedroza-Islas, R.; de la Pena-Díaz, A.; Soriano-Santos, J. Effects of heat and pH treatments and in vitro digestion on the biological activity of protein hydrolysates of Amaranthus hypochondriacus L. grain. J. Food Sci. Technol. 2016, 53, 4298–4307. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Zhang, Y.; Dong, Y.; Hu, X. Structural characteristics and stability of salmon skin protein hydrolysates obtained with different proteases. LWT—Food Sci. Technol. 2022, 153, 112460. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Kilari, B.P.; Salim, M.A.S.M.; Gan, C.-Y.; Maqsood, S. Simulated gastrointestinal digestion of camel and bovine casein hydrolysates: Identification and characterization of novel anti-diabetic bioactive peptides. Food Chem. 2021, 353, 129374. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Ina, S.; Hamada, A.; Yamaguchi, Y.; Akao, M.; Shinmachi, F.; Kumagai, H.; Kumagai, H. Suppressive Effect of the α-Amylase Inhibitor Albumin from Buckwheat (Fagopyrum esculentum Moench) on Postprandial Hyperglycaemia. Nutrients 2018, 10, 1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry; CRC Press: New York, NY, USA, 2017; pp. 284–297. [Google Scholar] [CrossRef]
- Ketnawa, S.; Benjakul, S.; Martinez-Alvarez, O.; Rawdkuen, S. Fish skin gelatin hydrolysates produced by visceral peptidase and bovine trypsin: Bioactivity and stability. Food Chem. 2017, 215, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Klomklao, S.; Benjakul, S. Protein Hydrolysates Prepared from the Viscera of Skipjack Tuna (Katsuwonus pelmamis): Antioxidative Activity and Functional Properties. Turk. J. Fish. Aquat. Sci. 2018, 18, 69–79. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Wali, A.; Ma, H.; Shahnawaz, M.; Hayat, K.; Xiaong, J.; Jing, L. Impact of Power Ultrasound on Antihypertensive Activity, Functional Properties, and Thermal Stability of Rapeseed Protein Hydrolysates. J. Chem. 2017, 2017, 4373859. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; SadeghiMahoonak, A.; Alami, M.; Ghorbani, M. Amino acid composition and antioxidative properties of hydrolysed pumpkin (Cucurbita Pepo L.) oil cake protein. Int. J. Food Prop. 2017, 20, 3244–3255. [Google Scholar] [CrossRef]


| Tested Parameter | X1 * | X2 * | X3 * | X4 * | X5 * | X6 * |
|---|---|---|---|---|---|---|
| Investigation 1 | 4 proteases ** | 1:6 | Optimal ** | Optimal ** | 500 | 4 |
| Investigation 2 | X *** | Tested range | Optimal ** | Optimal ** | 500 | 4 |
| Investigation 3 | X *** | X *** | Tested range | Optimal ** | 500 | 4 |
| Investigation 4 | X *** | X *** | X *** | Tested range | 500 | 4 |
| Investigation 5 | X *** | X *** | X *** | X *** | Tested range | 4 |
| Investigation 6 | X *** | X *** | X *** | X *** | X *** | Tested range |
| Enzyme Preparation | Optimal pH | Optimal Temperature |
|---|---|---|
| Alcalase® 2.5 L | 8.0 | 55 °C |
| Neutrase® 0.8 L | 7.0 | 50 °C |
| Protamex® | 6.5 | 55 °C |
| Flavourzyme® 500 MG | 7.0 | 50 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, P.T.; Pham, K.T.; Vo, T.D.L. Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions. Processes 2023, 11, 2490. https://doi.org/10.3390/pr11082490
Bui PT, Pham KT, Vo TDL. Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions. Processes. 2023; 11(8):2490. https://doi.org/10.3390/pr11082490
Chicago/Turabian StyleBui, Phong T., Khoa T. Pham, and Tam D. L. Vo. 2023. "Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions" Processes 11, no. 8: 2490. https://doi.org/10.3390/pr11082490
APA StyleBui, P. T., Pham, K. T., & Vo, T. D. L. (2023). Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions. Processes, 11(8), 2490. https://doi.org/10.3390/pr11082490