Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol)
Abstract
1. Introduction
2. Materials
3. Methods
3.1. Molecular Weights of PVA
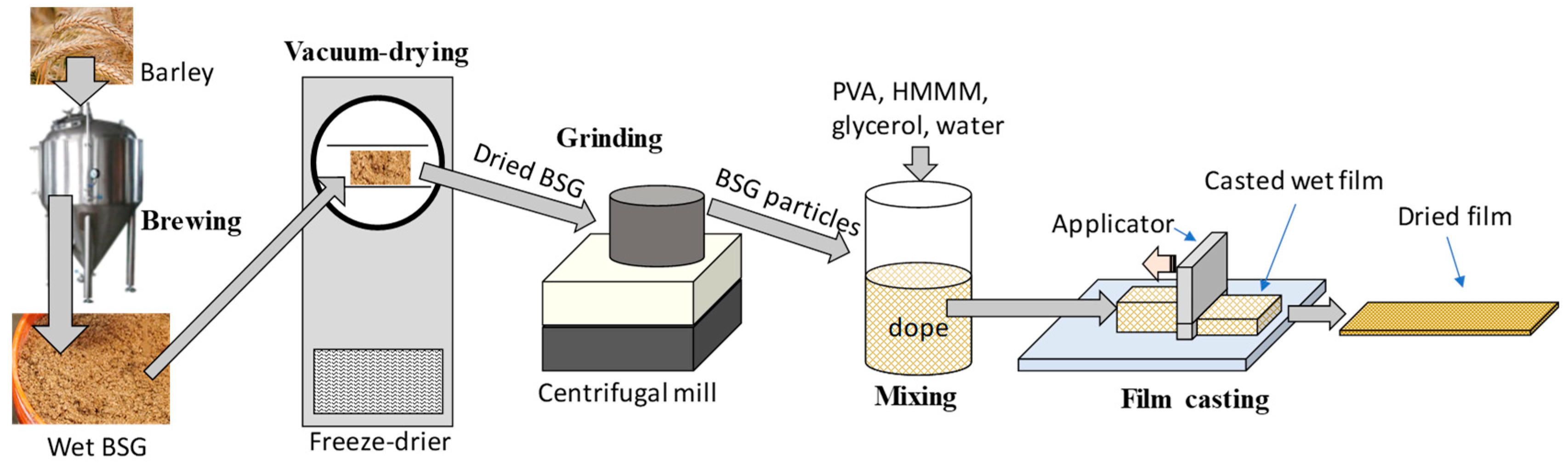
3.2. BSG Particles Preparation
3.3. Film Casting and Thickness Measurement
3.4. Tensile Tests
3.5. Hydrophilicity and Moisture Uptake
3.6. Statistical Analysis
4. Results and Discussion
4.1. Particle Size Distribution of Milled BSG Particles
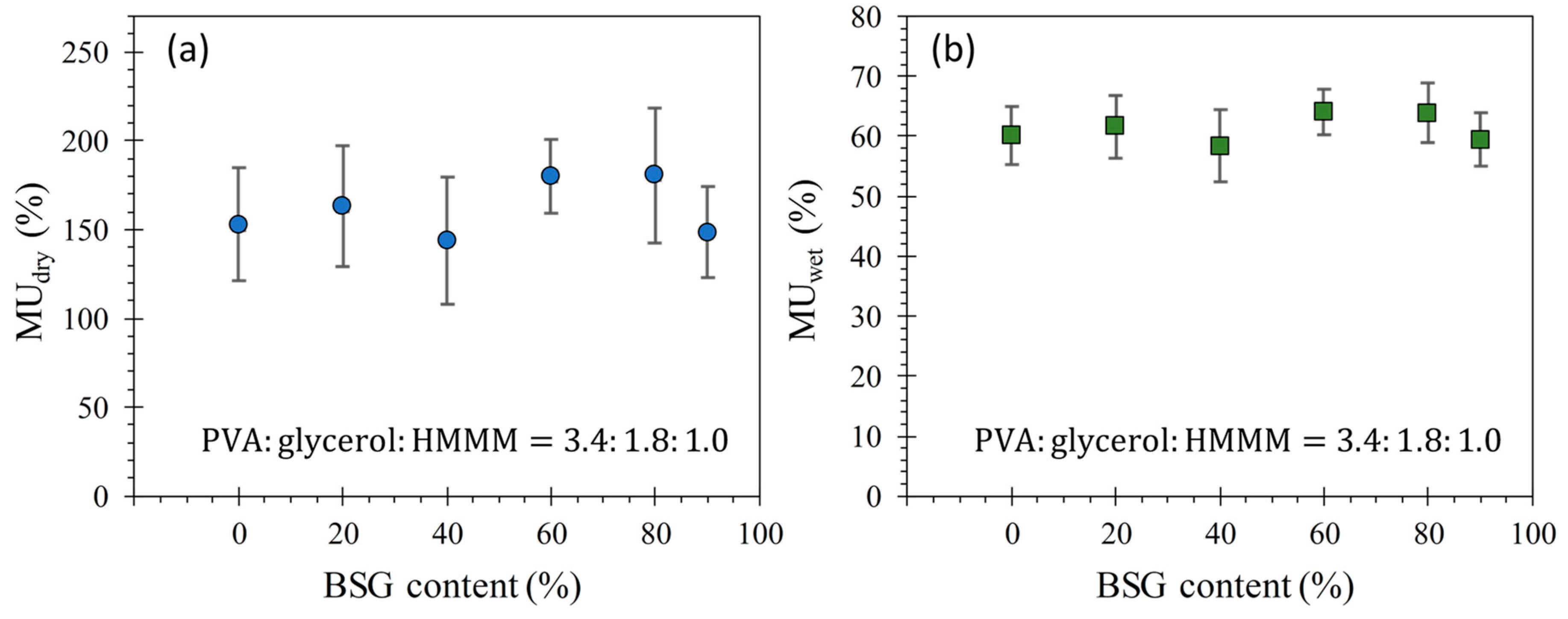
4.2. Moisture Uptake
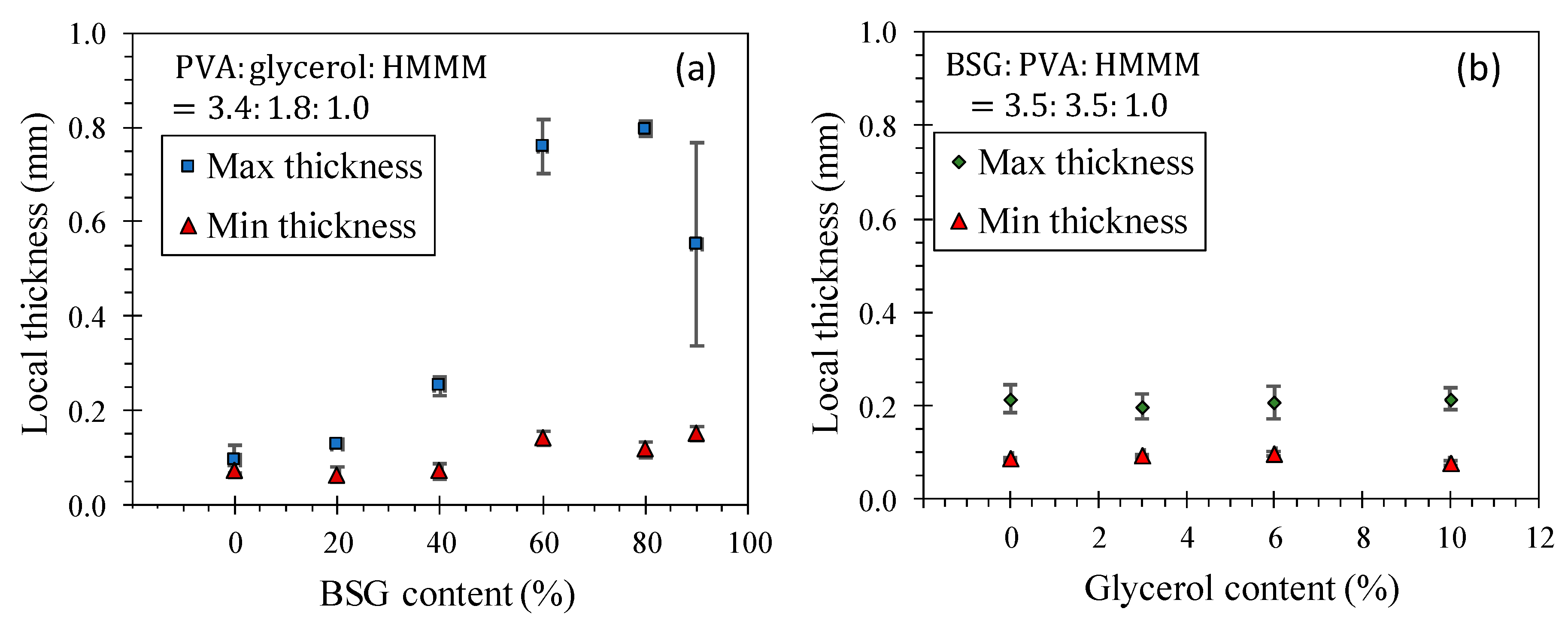
4.3. Film Morphology and Thickness
4.4. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Chang. Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Mazlan, N.; Lin, L.; Park, H. Microplastics in the New Zealand Environment. Processes 2022, 10, 265. [Google Scholar] [CrossRef]
- Ahmed, S. (Ed.) Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Maria, T.M.C.; de Carvalho, R.A.; Sobral, P.J.A.; Habitante, A.M.B.Q.; Solorza-Feria, J. The effect of the degree of hydrolysis of the PVA and the plasticizer concentration on the color, opacity, and thermal and mechanical properties of films based on PVA and gelatin blends. J. Food Eng. 2008, 87, 191–199. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, S.; Yan, J.; Wang, Z.; Ullah, I.; Ahmad, Z.; Zhang, Y.; Cao, Y.; Wang, L.; Mansoorianfar, M.; et al. Electrospun tannin-rich nanofibrous solid-state membrane for wastewater environmental monitoring and remediation. Chemosphere 2022, 307, 135810. [Google Scholar] [CrossRef]
- Fărcaş, A.; Tofană, M.; Socaci, S.; Mudura, E.; Scrob, S.; Salanţă, L.; Mureşan, V. Brewers’ spent grain—A new potential ingredient for functional foods. J. Agroaliment. Process. Technol. 2014, 20, 137–141. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Emmanuel, J.K.; Nganyira, P.D.; Shao, G.N. Evaluating the potential applications of brewers’ spent grain in biogas generation, food and biotechnology industry: A review. Heliyon 2022, 8, e11140. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Estévez, A.; Padrell, L.; Iñarra, B.; Orive, M.; San Martin, D. Brewery by-products (yeast and spent grain) as protein sources in rainbow trout (Oncorhynchus mykiss) feeds. Front. Mar. Sci. 2022, 9, 862020. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef] [PubMed]
- Mirani, A.; Goli, M. Optimization of cupcake formulation by replacement of wheat flour with different levels of eggplant fiber using response surface methodology. Food Sci. Technol. 2022, 42, e52120. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Anti-Corrosive Potential of the Sustainable Corrosion Inhibitors Based on Biomass Waste: A Review on Preceding and Perspective Research. J. Phys. Conf. Ser. 2021, 2267, 012079. [Google Scholar] [CrossRef]
- Lauria, G.A.; Cendejas, J.F.; Malpica, M.A.; Joshina, J.K. Edible Multi-Ring Can-Holder and Methods for Manufacturing Edible Can-Holders. U.S. 15/590,754, 18 January 2018. [Google Scholar]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Finch, C.A. Polyvinyl Alcohol—Development; Wiley: Hoboken, NJ, USA, 1992. [Google Scholar]
- Goodship, V.; Daniel, J. Polyvinyl Alcohol: Materials, Processing and Applications; Smithers Rapra: Shrewsbury, UK, 2009. [Google Scholar]
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Aruldass, S.; Mathivanan, V.; Mohamed, A.R.; Tye, C.T. Factors affecting hydrolysis of polyvinyl acetate to polyvinyl alcohol. J. Environ. Chem. Eng. 2019, 7, 103238. [Google Scholar] [CrossRef]
- Sakurada, I. Polyvinyl Alcohol Fibers; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Tan, B.; Ching, Y.; Poh, S.; Abdullah, L.; Gan, S. A Review of Natural Fiber Reinforced Poly(Vinyl Alcohol) Based Composites: Application and Opportunity. Polymers 2015, 7, 2205–2222. [Google Scholar] [CrossRef]
- Mahardika, M.; Asrofi, M.; Amelia, D.; Syafri, E.; Rangappa, S.M.; Siengchin, S. Tensile Strength and Moisture Resistance Properties of Biocomposite Films Based on Polyvinyl Alcohol (PVA) with Cellulose as Reinforcement from Durian Peel Fibers. E3S Web Conf. 2021, 302, 02001. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2015, 53, 1608–1619. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-Polyvinyl Alcohol Film for Tissue Engineering: A Concise Review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Li, L.; Li, Y.; Wang, Q. Water-Soluble Poly(vinyl alcohol)/Biomass Waste Composites: A New Route toward Ecofriendly Materials. ACS Omega 2022, 7, 42515–42523. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Imam, S.H.; Mao, L. Composite Films Based on Biorelated Agro-Industrial Waste and Poly(vinyl alcohol). Preparation and Mechanical Properties Characterization. Biomacromolecules 2001, 2, 1029–1037. [Google Scholar] [CrossRef]
- Lin, L.; Lee, Y.; Park, H.E. Recycling and rheology of poly(lactic acid) (PLA) to make foams using supercritical fluid. Phys. Fluids 2021, 33, 067119. [Google Scholar] [CrossRef]
- Chen, L.; Imam, S.H.; Gordon, S.H.; Greene, R.V. Starch- polyvinyl alcohol crosslinked film— performance and biodegradation. J. Environ. Polym. Degrad. 1997, 5, 111–117. [Google Scholar] [CrossRef]
- Regubalan, B.; Pandit, P.; Maiti, S.; Nadathur, G.T.; Mallick, A. Potential Bio-Based Edible Films, Foams, and Hydrogels for Food Packaging. In Bio-Based Materials for Food Packaging; Springer: Singapore, 2018; pp. 105–123. [Google Scholar]
- Liu, Y.; Zhang, Y.; An, Z.; Zhao, H.; Zhang, L.; Cao, Y.; Mansoorianfar, M.; Liu, X.; Pei, R. Slide-Ring Structure-Based Double-Network Hydrogel with Enhanced Stretchability and Toughness for 3D-Bio-Printing and Its Potential Application as Artificial Small-Diameter Blood Vessels. ACS Appl. Bio Mater. 2021, 4, 8597–8606. [Google Scholar] [CrossRef]
- Prasad, R. Fertilizer urea, food security, health and the environment. Curr. Sci. 1998, 75, 677–683. [Google Scholar]
- Sarria, M.; Gonzales, J.M.; Gerrity, D.; Batista, J. Biological Reduction of Nitrate and Perchlorate in Soil Microcosms: An Electron Donor Comparison of Glycerol, Emulsified Oil, and Mulch Extract. Groundw. Monit. Remediat. 2018, 39, 32–42. [Google Scholar] [CrossRef]
- Westhoff, R.P.; Kwolek, W.F.; Otey, F.H. Starch-Polyvinyl Alcohol Films–Effect of Various Plasticizers. Starch—Stärke 1979, 31, 163–165. [Google Scholar] [CrossRef]
- Lawton, J.W.; Fanta, G.F. Glycerol-plasticized films prepared from starch—Poly(vinyl alcohol) mixtures: Effect of poly(ethylene-co-acrylic acid). Carbohydr. Polym. 1994, 23, 275–280. [Google Scholar] [CrossRef]
- Mitsoulis, E. Chapter 11—Calendering of polymers. In Advances in Polymer Processing: From Macro- to Nano- Scales; Thomas, S., Weimin, Y., Eds.; Woodhead Publishing: Athens, Greece, 2009; pp. 312–351. [Google Scholar]
- Chen, N.; Li, L.; Wang, Q. New technology for thermal processing of poly(vinyl alcohol). Plast. Rubber Compos. 2013, 36, 283–290. [Google Scholar] [CrossRef]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of plasticizer type on tensile property and in vitro indomethacin release of thin films based on low-methoxyl pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Rouder, J.N.; Engelhardt, C.R.; McCabe, S.; Morey, R.D. Model comparison in ANOVA. Psychon. Bull. Rev. 2016, 23, 1779–1786. [Google Scholar] [CrossRef]
- Lv, C.; Liu, D.; Tian, H.; Xiang, A. Non-isothermal crystallization kinetics of polyvinyl alcohol plasticized with glycerol and pentaerythritol. J. Polym. Res. 2020, 27, 66. [Google Scholar] [CrossRef]
- Porta, R.; Sabbah, M.; Di Pierro, P. Bio-Based Materials for Packaging. Int. J. Mol. Sci. 2022, 23, 3611. [Google Scholar] [CrossRef]
- Lim, L.Y.; Wan, L.S.C. The Effect of Plasticizers on the Properties of Polyvinyl Alcohol Films. Drug Dev. Ind. Pharm. 2008, 20, 1007–1020. [Google Scholar] [CrossRef]
- Kim, E.; Park, H.; Lopez-Barron, C.; Lee, P. Enhanced Foamability with Shrinking Microfibers in Linear Polymer. Polymers 2019, 11, 211. [Google Scholar] [CrossRef]


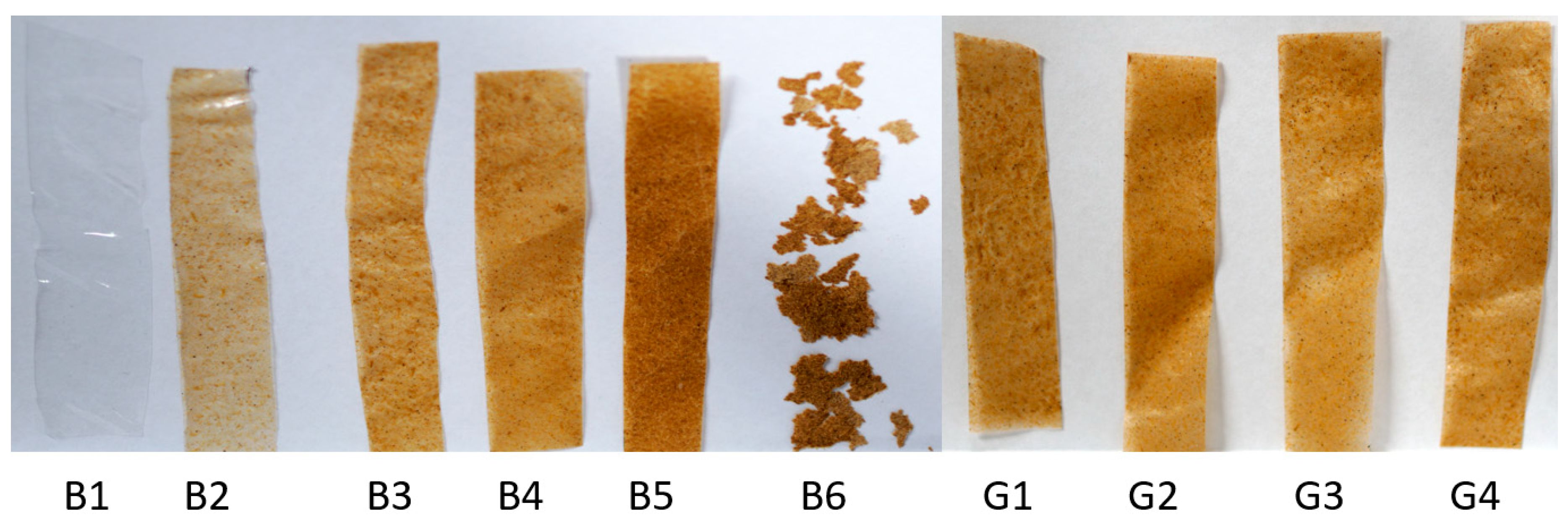


| Code | BSG (mg) | PVA (mg) | Glycerol (mg) | HMMM (mg) | DI Water (mL) | Ratio |
|---|---|---|---|---|---|---|
| B1 | 0.0 | 54.8 | 29.0 | 16.1 | 0.9 |
PVA:glycerol:HMMM = 3.4:1.8:1.0 |
| B2 | 20.0 | 43.9 | 23.2 | 12.9 | 0.9 | |
| B3 | 40.0 | 32.9 | 17.4 | 9.7 | 0.9 | |
| B4 | 60.0 | 21.9 | 11.6 | 6.5 | 0.9 | |
| B5 | 80.0 | 11.0 | 5.8 | 3.2 | 0.9 | |
| B6 | 90.0 | 5.5 | 2.9 | 1.6 | 0.9 | |
| G1 | 43.8 | 43.8 | 0.0 | 12.5 | 0.9 |
BSG:PVA:HMMM = 3.5:3.5:1.0 |
| G2 | 42.4 | 42.4 | 3.0 | 12.1 | 0.9 | |
| G3 | 41.1 | 41.1 | 6.0 | 11.8 | 0.9 | |
| G4 | 39.4 | 39.4 | 10.0 | 11.3 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Mirkin, S.; Park, H.E. Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol). Processes 2023, 11, 2400. https://doi.org/10.3390/pr11082400
Lin L, Mirkin S, Park HE. Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol). Processes. 2023; 11(8):2400. https://doi.org/10.3390/pr11082400
Chicago/Turabian StyleLin, Lilian, Sarah Mirkin, and Heon E. Park. 2023. "Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol)" Processes 11, no. 8: 2400. https://doi.org/10.3390/pr11082400
APA StyleLin, L., Mirkin, S., & Park, H. E. (2023). Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol). Processes, 11(8), 2400. https://doi.org/10.3390/pr11082400







